Abstract
Simple Summary
Immune checkpoint inhibitors are widely used in clinical practice and have demonstrated remarkable efficacy in advanced non-small cell lung cancer (NSCLC). However, their use is also commonly accompanied by immune-related adverse events (irAEs), and markers that are able to predict the onset of irAEs represent an urgent need. In this study, we found that the baseline level of peripheral CD8+ T lymphocytes was the independent risk factor of the onset of irAEs, and it was associated with longer survival in advanced NSCLC patients treated with PD-1/PD-L1 inhibitors. Furthermore, the study showed the combinational predictive value of baseline CD8+ T lymphocytes and the onset of irAEs for the clinical outcomes.
Abstract
Immune checkpoint inhibitors (ICIs) therapy has revolutionized the treatment patterns of non-small cell lung cancer (NSCLC). However, patients treated with ICIs may experience immune-related adverse events (irAEs). Markers that could predict the onset of irAEs are still unclear. Here, we report the possible correlation of baseline peripheral lymphocytes with irAEs and clinical outcomes in advanced NSCLC patients receiving ICIs. A total of 109 advanced NSCLC patients treated with ICIs from April 2017 to January 2021 were analyzed retrospectively. Logistic and Cox regression analyses was applied to evaluate independent risk factors for irAEs, progression-free survival (PFS), and overall survival (OS). Among these patients, 55 (50.5%) patients experienced irAEs. The level of CD8+ T lymphocytes at baseline was the independent risk factor for the onset of irAEs (p = 0.008). A higher level of CD8+ T lymphocytes was associated with longer PFS (11.0 months vs. 3.0 months, p < 0.001) and OS (27.9 months vs. 11.7 months, p = 0.014). Furthermore, patients who had higher baseline CD8+ T lymphocytes and experienced irAEs had a longer PFS (18.4 months vs. 2.2 months, p < 0.001) and OS (32.8 months vs. 9.0 months, p = 0.001) than those who had lower CD8+ T lymphocytes and no irAEs. Our study highlights the value of baseline peripheral CD8+ T lymphocytes as a predictive factor for irAEs in advanced NSCLC patients receiving ICIs. In addition, patients who have higher baseline CD8+ T lymphocytes and experience irAEs would have a superior PFS and OS.
Keywords: immune-related adverse events, baseline peripheral CD8+ T lymphocytes, non-small cell lung cancer, prognostic
1. Introduction
Lung cancer is a commonly diagnosed cancer and the leading cause of cancer-related death worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all cases, and more than half of these are diagnosed after the cancer has already metastasized [2]. In recent years, immune checkpoint inhibitors (ICIs), including antibodies to programmed cell death-1 (PD-1) and programmed cell death ligand 1 (PD-L1), have revolutionized the treatment of several types of malignancy, including head and neck carcinoma [3], hepatocellular carcinoma [4], esophageal carcinoma [5], colorectal carcinoma, [6] and NSCLC [7,8], and show superior median overall survival (OS) and long-term survival compared to standard chemotherapy for some special groups [9,10]. Although ICIs therapy is better tolerated in NSCLC patients than traditional chemotherapy, patients treated with ICIs may still experience a wide range of side effects, which are called immune-related adverse events (irAEs). IrAEs occur in any organ, most frequently involving skin, endocrine organs, the lung, and the liver [11]. The incidence of all grade irAEs (according to Common Terminology Criteria for Adverse Events (CTCAE)) has been reported from 58% to 69%. Most of the irAEs are mild or moderate (grade 1 or grade 2), and the incidence of grade 3–5 irAEs is about 7–13% in NSCLC [12,13,14,15]. Although severe irAEs remain rare, they could become life-threatening if not recognized early and accurately and managed appropriately [16]. Therefore, effective predictive markers for identify risk factors of irAEs represent an urgent need in clinic.
The mechanisms for irAEs have still not been elucidated. Some potential mechanisms include increasing T-cell activity against antigens that are present in tumors and healthy tissue, increasing levels of preexisting autoantibodies, and an increase in the level of inflammatory cytokines [17]. The enhancement of systemic T-cell activity by ICIs causes a loss of immune tolerance in various organs, resulting in irAEs [17,18]. There is a complex interplay between the immune system and tumor. It has been reported that T-cell tumor infiltrating lymphocytes (TILs) and circulating T-cell subpopulations are associated with clinical outcomes in many cancer types, including NSCLC [19,20,21,22,23]. Up to now, markers that could predict the onset of irAEs are still unclear. It is suspected that several factors including tumor mutational burden, TILs, PD-L1, gut bacterial diversity, and cytokines may be linked to the occurrence of irAEs [24]. Baseline peripheral lymphocytes have shown a great potential as a predictor of irAEs because of the advantages in practically noninvasive and dynamic monitoring. However, studies in this area are still limited.
The present study explored the potential predictive value of clinical characteristics, especially baseline subsets of lymphocytes on the onset of irAEs, and investigated the factors associated with clinical outcomes in advanced NSCLC patients receiving ICIs-based therapy.
2. Materials and Methods
2.1. Patients and Data Collection
Patients histologically or cytologically confirmed advanced NSCLC (IIIB/IV), treated with ICIs at a single institution from April 2017 to January 2021, were analyzed retrospectively. Other inclusion criteria were as follow: (1) Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2; (2) complete medical records, including baseline level of lymphocytes subsets; (3) treatment with anti-PD1/anti-PD-L1 drugs or anti-PD1/anti-PD-L1 drugs in combination with chemotherapy or antiangiogenesis therapy; and (4) all patients received at least 3 months of immunotherapy unless disease progression or unacceptable toxicity occurred. The PD1/PD-L1 inhibitors were administered intravenously, according to the instructions.
Information including patient demographics, clinical characteristics, treatment patterns, and baseline subsets of lymphocytes were collected. The baseline levels of lymphocytes subsets (defined as the most recent records within 1 week before immunotherapy initiation), including CD4+ T lymphocytes, CD8+ T lymphocytes, and regulatory T lymphocytes, were recorded. A total of 109 advanced NSCLC patients treated with ICIs were included in the study. Overall, six ICIs were assessed, namely pembrolizumab (n = 46), nivolumab (n = 28), durvalumab (n = 5), sintilimab (n = 11), toripalimab (n = 14), and atelizumab (n = 5). At the time of analysis, the median follow-up time was 9.0 months (0.1–47.4), and the median treatment duration was 3.6 months (0.1–39.5). The clinical characteristics of the enrolled patients are summarized in Table 1. The median age was 65 years (36–85), 19.3% of participants were female, 42.2% of patients were never-smokers, and 84.4% of patients had an ECOG PS of 0–1. Most patients had adenocarcinoma (55.0%), and most had no or undetected common driver gene (including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and V-ros UR2 sarcoma virus oncogene homolog 1 (ROS1)) alteration (91.7%). The disease stage was IIIB in 6.4% of the patients and IV in 93.6% of the patients. In this cohort, 73 (67.0%) patients were tested for PD-L1 expression in tumor tissue, and 76.7% of those were PD-L1 positive. A total of 41 (37.6%) patients accepted ICIs as first-line treatment. About 42.2% of patients received ICIs monotherapy, while 57.8% of patients received ICIs combined with chemotherapy or antiangiogenesis therapy. The median baseline level of the CD4+ T lymphocytes count was 266 (range 37–1008) M/L, the median baseline level of the CD8+ T lymphocytes count was 288 (range 25–1195) M/L, and the median baseline level of regulatory T lymphocytes count was 17 (range 3–63) M/L.
Table 1.
Baseline characteristics of 109 advanced non-small cell lung cancer patients.
| Characteristics | Subsets | No. | % |
|---|---|---|---|
| Gender | Male | 88 | 80.7% |
| Female | 21 | 19.3% | |
| Age (years) | Median | 65 | |
| Range | 36–85 | ||
| ECOG PS score | 0–1 | 92 | 84.4% |
| 2 | 17 | 15.6% | |
| Smoking status | Current/former | 63 | 57.8% |
| Never | 46 | 42.2% | |
| Histology | Adenocarcinoma | 60 | 55.0% |
| Squamous carcinoma | 46 | 42.2% | |
| Large cell carcinoma | 3 | 2.8% | |
| Stage | Ⅳ | 102 | 93.6% |
| ⅢB | 7 | 6.4% | |
| PD-L1 states test | ≥50% | 23 | 21.1% |
| 1–49% | 33 | 30.3% | |
| <1% | 17 | 15.6% | |
| Unknown | 36 | 33.0% | |
| Driver gene alteration | EGFR/ALK/ROS1 positive | 9 | 8.3% |
| Negative/unknown | 100 | 91.7% | |
| Treatment line | First | 41 | 37.6% |
| Second | 39 | 35.8% | |
| Third | 15 | 13.8% | |
| Fourth or later line | 14 | 12.8% | |
| Combination treatment | Yes | 63 | 57.8% |
| No | 46 | 42.2% |
ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death ligand 1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, V-ros UR2 sarcoma virus oncogene homolog 1.
2.2. Study Assessment
Tumor response evaluation was performed every 6–9 weeks, according to the response evaluation criteria in solid tumor (RECIST) (version 1.1; https://recist.eortc.org/recist-1-1-2/, accessed on 26 April 2017). Baseline measurements were defined as those taken within 4 weeks before receiving ICIs. Progression-free survival (PFS) was defined as the time from the date of initial administration of ICIs to first disease progression or death due to any cause, censored at the date of the latest survival information available or initiation of other treatment. OS was defined as time from the first day of ICIs to death by any cause, or censored at the date of the latest survival information available.
Toxicity was assessed by CTCAE (version 5.0; https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf, accessed on 6 March 2018). IrAEs were defined as AEs with a potential immunologic basis that required more frequent monitoring and potential intervention with corticosteroids and other immunomodulatory agents, such as pneumonitis, hepatic dysfunction, thyroid disorder, rash, and other conditions. Improvement or resolution of irAEs was also assessed by the investigators.
2.3. Statistical Analysis
In the statistical analysis, descriptive statistics were calculated for categorical variables and continuous variables. A univariate logistic regression model (Enter) was applied to explore the correlation of the demographic, clinical data, baseline level of lymphocytes subsets, and the onset of irAEs. We examined the Pearson’s correlation between the median baseline level of CD8+ T lymphocytes and the incidence of irAEs. Univariate Cox proportional hazard regression model (Enter) was performed to clarify the risk factors for PFS and OS. Multivariate logistic and Cox analysis were performed by using the logistic/Cox regression model (Enter) containing all of the variables that attained or trended toward univariate statistical significance (p < 0.10). PFS and OS curves were calculated by using the Kaplan–Meier method, and the log-rank test was employed to assess differences. The results were considered statistically significant when the two-sided p-value was < 0.05. All data analyses were performed with SPSS version 21.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Inc., La Jolla, CA, USA).
3. Results
3.1. Profiles of IrAEs
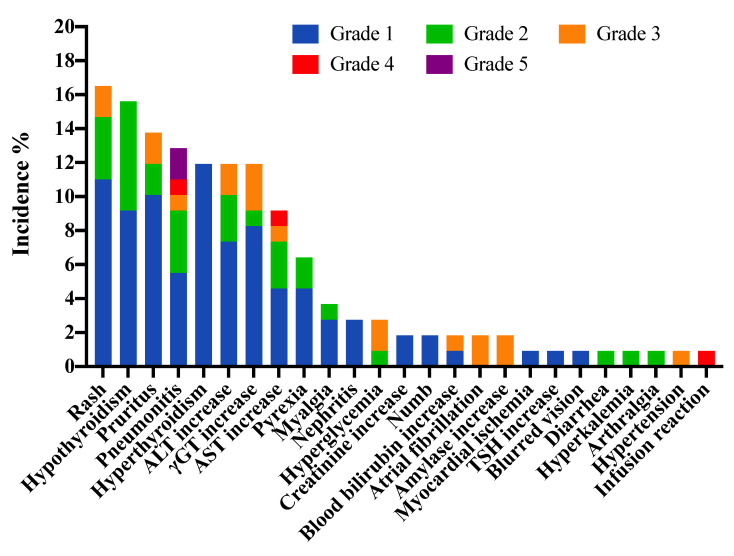
A total of 55 patients (50.5%) experienced at least one type of irAE, and 38 patients (34.9%) developed multiple irAEs. Ninety-three patients (85.3%) experienced mild and/or moderate (grade 1 and/or grade 2) irAEs, while grade 3–5 irAEs occurred in 16 patients (14.7%) in the study. Treatment related death was observed in 2 patients (1.8%). A total of 148 irAEs were observed in all patients, and 104 irAEs (70.3%) were resolved by the end of the observation. The most common any-grade irAEs were rash (16.5%), hypothyroidism (15.6%), pruritus (13.8%), pneumonitis (12.8%), hyperthyroidism (11.9%), alanine transaminase increase (11.9%), gamma-glutamyltransferase increase (11.9%), aspartate aminotransferase increase (9.2%), and pyrexia (6.4%). The most common grade 3–5 irAEs were pneumonitis in four patients (3.7%) and gamma-glutamyltransferase increase in three patients (2.8%) (Figure 1 and Table 2). The median time to onset was 6.9 weeks and median time to resolution was 3.4 weeks for all irAEs (Supplementary Figure S1). The majority of new irAEs occurred from cycles 1 to 3 in all patients, and few new events occurred after 15 months (Supplementary Figure S2).
Figure 1.
Profiles of immune-related adverse events among 109 advanced non-small cell lung cancer patients. Abbreviation: ALT, alanine transaminase; AST, aspartate aminotransferase; γGT, gamma-glutamyltransferase.
Table 2.
Immune-related adverse events (irAEs) by characteristics and resolution rates.
| IrAEs Category | No. of Patients, n = 109 | No. of Resolved Events, n = 148, (%) | |
|---|---|---|---|
| Any Grade, n (%) | Grade 3–5, n (%) | ||
| Any irAEs | 55 (50.5%) | 16 (14.7%) | 104 (70.3%) |
| Single site irAEs | 17 (15.6%) | / | |
| Multiple site irAEs | 38 (34.9%) | / | |
| Pulmonary | |||
| Pneumonitis | 14 (12.8%) | 4 (3.7%) | 8 (57.1%) |
| Cardiovascular | |||
| Myocardial ischemia | 1 (0.9%) | 0 | 1 (100.0%) |
| Atrial fibrillation | 2 (1.8%) | 2 (1.8%) | 1 (50.0%) |
| Hypertension | 1 (0.9%) | 1 (0.9%) | 0 |
| Gastrointestinal | |||
| Diarrhea | 1 (0.9%) | 0 | 1 (100.0%) |
| Amylase increase | 2 (1.8%) | 2 (1.8%) | 1 (50.0%) |
| Hepatic | |||
| ALT increase | 13 (11.9%) | 2 (1.8%) | 11 (84.6%) |
| AST increase | 10 (9.2%) | 2 (1.8%) | 7 (70.0%) |
| γGT increase | 13 (11.9%) | 3 (2.8%) | 10 (76.9%) |
| Blood bilirubin increase | 2 (1.8%) | 1 (0.9%) | 2 (100.0%) |
| Renal | |||
| Nephritis | 3 (2.8%) | 0 | 1 (33.3%) |
| Creatinine increase | 2 (1.8%) | 0 | 1 (50.0%) |
| Hyperkalemia | 1 (0.9%) | 0 | 1 (100.0%) |
| Musculoskeletal | |||
| Myalgia | 4 (3.7%) | 0 | 3 (75.0%) |
| Arthralgia | 1 (0.9%) | 0 | 1 (100.0%) |
| Endocrine | |||
| Hypothyroidism | 17 (15.6%) | 0 | 4 (23.5%) |
| TSH increase | 1 (0.9%) | 0 | 1 (100.0%) |
| Hyperthyroidism | 13 (11.9%) | 0 | 12 (92.3%) |
| Hyperglycemia | 3 (2.8%) | 2 (1.8%) | 1 (33.3%) |
| Skin | |||
| Rash | 18 (16.5%) | 2 (1.8%) | 15 (83.3%) |
| Pruritus | 15 (13.8%) | 2 (1.8%) | 13 (86.7%) |
| Eye | |||
| Blurred vision | 1 (0.9%) | 0 | 1 (100.0%) |
| Neurology | |||
| Numb | 2 (1.8%) | 0 | 0 |
| Others | |||
| Infusion reaction | 1 (0.9%) | 1 (0.9%) | 1 (100.0%) |
| Pyrexia | 7 (6.4%) | 0 | 7 (100.0%) |
ALT, Alanine transaminase; AST, Aspartate aminotransferase; γGT, Gamma-Glutamyltransferase; TSH, Thyroid Stimulating Hormone.
3.2. Association of CD8+ T Lymphocytes at Baseline with the Occurrence of IrAEs
Demographic, clinical, treatment characteristics and baseline levels of lymphocytes subsets were analyzed under univariate and multivariate logistics analyses as candidate factors predicting irAEs. Patients were divided into high and low groups according to the median value of CD4+ T lymphocytes (266 M/L), CD8+ T lymphocytes (288 M/L), and regulatory T lymphocytes (17 M/L). According to the univariate logistics analysis, the high-CD8+-T-lymphocytes group had a significantly higher incidence of irAEs compared with the low-CD8+-T-lymphocytes group (OR = 2.975, 95% CI = 1.365–6.484, and p = 0.006). On the contrary, patients with an ECOG PS of 2 tended to have a lower incidence of irAEs than those with an ECOG PS of 0 or 1 (OR = 0.350, 95% CI = 0.114–1.074, and p = 0.066). Similarly, patients who harbored common driver gene alterations tended to have a lower incidence of irAEs (OR = 0.253, 95% CI = 0.050–1.280, and p = 0.097). No significant correlations were observed between irAEs and other lymphocytes’ subsets and clinical characteristics (Table 3).
Table 3.
Univariate and multivariate analyses for the risk factors of immune-related adverse events (irAEs).
| Variable | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Gender | Male | 1.151 | 0.444–2.985 | 0.772 | |||
| Age (years) | ≥65 years | 1.292 | 0.606–2.752 | 0.507 | |||
| Histology | Non-adenocarcinoma | 1.630 | 0.762–3.487 | 0.208 | |||
| ECOG PS score | 2 | 0.350 | 0.114–1.074 | 0.066 | 0.355 | 0.109–1.163 | 0.087 |
| Smoking status | Former/current | 0.656 | 0.305–1.410 | 0.280 | |||
| Treatment line | ≥Second | 0.593 | 0.271–1.299 | 0.192 | |||
| Driver gene alterlation | EGFR/ALK/ROS1 positive | 0.253 | 0.050–1.280 | 0.097 | 0.308 | 0.055–1.709 | 0.178 |
| Combination treatment | Yes | 0.833 | 0.389–1.784 | 0.639 | |||
| PD-L1 states test | ≥50% | 1.362 | 0.539–3.440 | 0.513 | |||
| CD4+ T lymphocytes | ≥266 M/L | 1.392 | 0.655–2.957 | 0.390 | |||
| CD8+ T lymphocytes | ≥288 M/L | 2.975 | 1.365–6.484 | 0.006 | 2.953 | 1.324–6.587 | 0.008 |
| Regulatory T lymphocytes | ≥17 M/L | 0.773 | 0.364–1.640 | 0.502 | |||
ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death ligand 1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, V-ros UR2 sarcoma virus oncogene homolog 1.
The multivariate logistic regression analysis showed that the level of CD8+ T lymphocytes was the independent predictor for the occurrence of irAEs (OR = 2.953, 95% CI = 1.324–6.587, and p = 0.008; Table 3). The incidence of irAEs was more frequent in the high CD8+ T lymphocytes group (63.6%) than in the group with low CD8+ T lymphocytes (37.0%, p < 0.001). Furthermore, we divided patients into six groups according to the baseline level of CD8+ T lymphocytes (20 cases per group in Groups 1 to 5, and 9 cases in Group 6). Our analysis revealed a significant positive correlation between the incidence of irAEs and the median baseline level of CD8+ T lymphocytes (Pearson correlation coefficient, R = 0.817; p = 0.047; Supplementary Figure S3).
3.3. Factors Predictive of Clinical Outcomes
Among the overall population, the median PFS and median OS were 5.5 months (95% CI = 3.2–7.8 months) and 16.8 months (95% CI = 9.5–24.1 months), respectively. The univariable and multivariable analysis results are showed in Table 4A,B. In the univariate analysis, the group with high CD8+ T lymphocytes had a significantly longer PFS and OS than that of the group with the low CD8+ T lymphocytes (HR = 0.386, 95% CI = 0.237–0.629, and p < 0.001 vs. HR = 0.485, 95% CI = 0.273–0.862, and p = 0.014, respectively). Patients with an ECOG PS of 2 had a shorter PFS and OS than those with an ECOG PS of 0 or 1 (HR = 2.146, 95% CI = 1.208–3.812, and p = 0.009 vs. HR = 1.948, 95% CI = 1.004–3.781, and p = 0.049). Patients who received ICIs as later-line therapy had a shorter PFS and OS than those receiving ICIs as first-line therapy (HR = 3.211, 95% CI = 1.857–5.551, and p < 0.001 vs. HR = 2.413, 95% CI = 1.234–4.715, and p = 0.010). Patients with irAEs had a significantly longer PFS and OS compared with those without irAEs (HR = 0.368, 95% CI = 0.227–0.596, and p < 0.001 vs. HR = 0.439, 95% CI = 0.247–0.782, and p = 0.005). In addition, the administration of combination therapy was associated with a longer PFS (HR = 0.636, 95% CI = 0.385–1.053, and p = 0.078), but there was no significant difference in OS. Patients who harbored common driver gene alterations had a shorter OS (HR = 2.474, 95% CI = 1.042–5.872, and p = 0.040).
Table 4.
(A) Cox analysis of influencing factors for progression-free survival of 109 lung cancer patients treated with immunotherapy. (B) Cox analysis of influencing factors for overall survival of 109 lung cancer patients treated with immunotherapy.
| (A) | |||||||
| Variable | Category | Univariate | Multivariate | ||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Gender | Male | 1.091 | 0.584–2.040 | 0.785 | |||
| Age (years) | ≥65 years | 0.975 | 0.612–1.551 | 0.913 | |||
| Histology | Non-adenocarcinoma | 1.114 | 0.700–1.774 | 0.648 | |||
| ECOG PS score | 2 | 2.146 | 1.208–3.812 | 0.009 | 1.882 | 1.036–3.420 | 0.038 |
| Smoking status | Former/current | 1.284 | 0.793–2.080 | 0.309 | |||
| Treatment line | ≥Second | 3.211 | 1.857–5.551 | <0.001 | 3.479 | 1.906–6.349 | <0.001 |
| Driver gene alterlation | EGFR/ALK/ROS1 positive | 1.934 | 0.831–4.500 | 0.126 | |||
| Combination treatment | Yes | 0.636 | 0.385–1.053 | 0.078 | 0.638 | 0.369–1.102 | 0.107 |
| PD-L1 states test | ≥50% | 0.877 | 0.714–1.078 | 0.213 | |||
| CD4+ T lymphocytes | ≥266 M/L | 0.850 | 0.533–1.356 | 0.496 | |||
| CD8+ T lymphocytes | ≥288 M/L | 0.386 | 0.237–0.629 | <0.001 | 0.364 | 0.217–0.612 | <0.001 |
| Regulatory T lymphocytes | ≥17 M/L | 0.911 | 0.572–1.452 | 0.696 | |||
| IrAEs | Yes | 0.368 | 0.227–0.596 | <0.001 | 0.344 | 0.204–0.578 | <0.001 |
| (B) | |||||||
| Variable | Category | Univariate | Multivariate | ||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Gender | Male | 1.379 | 0.615–3.094 | 0.436 | |||
| Age (years) | ≥65 years | 0.958 | 0.548–1.673 | 0.879 | |||
| Histology | Non-adenocarcinoma | 1.095 | 0.627–1.910 | 0.750 | |||
| ECOG PS score | 2 | 1.948 | 1.004–3.781 | 0.049 | 1.596 | 0.796–3.200 | 0.188 |
| Smoking status | Former/current | 1.248 | 0.692–2.252 | 0.462 | |||
| Treatment line | ≥Second | 2.413 | 1.234–4.715 | 0.010 | 1.952 | 0.977–3.902 | 0.058 |
| Driver gene alterlation | EGFR/ALK/ROS1 positive | 2.474 | 1.042–5.872 | 0.040 | 1.890 | 0.775–4.609 | 0.162 |
| Combination treatment | Yes | 0.693 | 0.367–1.310 | 0.259 | |||
| PD-L1 states test | ≥50% | 0.883 | 0.702–1.110 | 0.287 | |||
| CD4+ T lymphocytes | ≥266 M/L | 0.674 | 0.385–1.178 | 0.166 | |||
| CD8+ T lymphocytes | ≥288 M/L | 0.485 | 0.273–0.862 | 0.014 | 0.647 | 0.348–1.202 | 0.169 |
| Regulatory T lymphocytes | ≥17 M/L | 0.655 | 0.373–1.149 | 0.140 | |||
| IrAEs | Yes | 0.439 | 0.247–0.782 | 0.005 | 0.599 | 0.320–1.120 | 0.109 |
ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death ligand 1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, V-ros UR2 sarcoma virus oncogene homolog 1; IrAEs, immune-related adverse events.
The multivariate analysis was performed to identify independent prognostic factors related to PFS and OS (Table 4A,B). Variables with a p-value ≤ 0.10 in the univariate analysis were included in the multivariable analyses. High-CD8+-T-lymphocytes group still had a longer PFS than the low-CD8+-T-lymphocytes group (HR = 0.364, 95% CI = 0.217–0.612, and p < 0.001). Patients with a poor ECOG PS had a shorter PFS than those with a good ECOG PS (HR = 1.882, 95% CI = 1.036–3.420, and p = 0.038). Receiving ICIs as later-line therapy was associated with a shorter PFS (HR = 3.479, 95% CI = 1.906–6.349, and p < 0.001) than receiving ICIs as the first-line ICIs treatment. Patients with irAEs had a significantly longer PFS compared with those without irAEs (HR = 0.344, 95% CI = 0.204–0.578, and p < 0.001). However, there were no prognostic factors of OS according to the multivariate Cox analysis.
3.4. Association among Baseline CD8+ T Lymphocytes, IrAEs and Clinical Outcomes
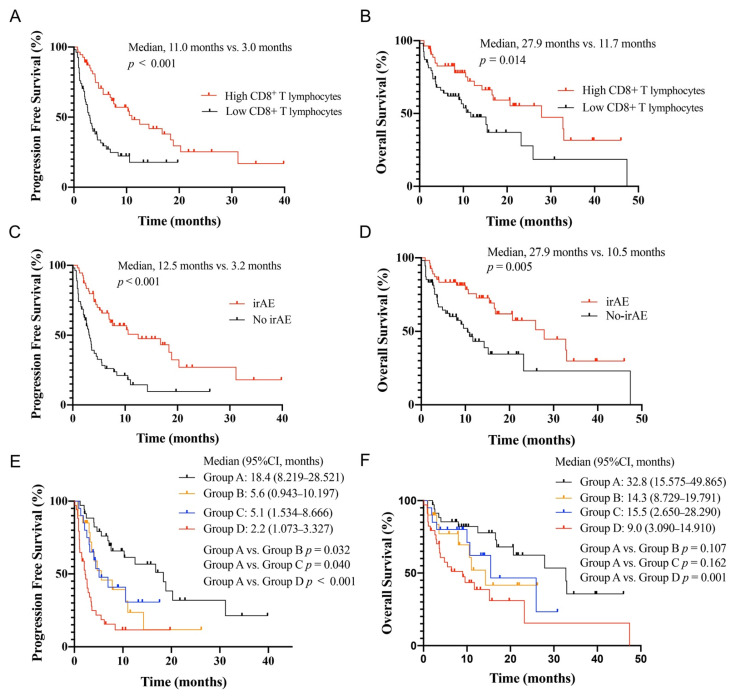
The Kaplan–Meier curve analysis demonstrated a significantly higher median PFS and OS in the group with high CD8+ T lymphocytes than in the group with low CD8+ T lymphocytes (median, 11.0 months vs. 3.0 months, p < 0.001; 27.9 months vs. 11.7 months, p = 0.014) (Figure 2A,B). Patients with irAEs had a significantly longer PFS and OS compared with the no-irAEs group (median, 12.5 months vs. 3.2 months, p < 0.001; 27.9 months vs. 10.5 months, p = 0.005) (Figure 2C,D). We explored the OS and PFS by high CD8+ T lymphocytes and/or the occurrence of irAEs (Figure 2E,F). All patients were divided into four groups: patients who developed irAE with high or low CD8+ T lymphocytes were assigned to Group A or B, while patients not presenting irAE with high or low CD8+ T lymphocytes were assigned to Group C or D. The PFS of Group A was significantly longer than that of Groups B, C, and D (p = 0.032, p = 0.040, and p < 0.001). However, the difference in OS was observed only between Groups A and D (p = 0.001), and there was no difference in the OS between patients with one favorable factor (high CD8+ T lymphocytes or irAEs) and those have both favorable factors.
Figure 2.
Association between baseline peripheral blood CD8+ T lymphocytes, immune-related adverse events (irAEs), and survival in non-small cell lung cancer patients treated with ICIs. PFS (A) and OS (B) curves of patients according to baseline peripheral blood CD8+ T lymphocytes. PFS (C) and OS (D) curves of patients stratified according to the onset of irAEs. PFS (E) and OS (F) curves of patients stratified according to baseline peripheral blood CD8+ T lymphocytes and irAEs. Group A, high CD8+ T lymphocytes and irAEs; Group B, low CD8+ T lymphocytes and irAEs; Group C, high CD8+ T lymphocytes and no irAEs; and Group D, low CD8+ T lymphocytes and no irAEs. Abbreviation: ICIs, immune checkpoint inhibitors; PFS, progression-free survival; OS, overall survival.
4. Discussion
Historically, survival rates for advanced NSCLC were disappointingly low, and treatment-related toxicities were extremely significant. Although ICIs have achieved important progress in driver-gene negative advanced NSCLC patients with acceptable adverse events in recent years, grade 3–5 irAEs could still occur [15,25]. Therefore, effective predictive factors are urgently needed for the identification of advanced NSCLC patients who may suffer from irAEs. Herein, our study demonstrated that baseline peripheral CD8+ T lymphocytes were significantly associated with occurrence of irAEs. Meanwhile, the median PFS and OS of the patients with higher baseline CD8+ T lymphocytes was significantly better than that of patients with lower baseline CD8+ T lymphocytes. Furthermore, the study also showed that the combination of baseline CD8+ T lymphocytes and the onset of irAEs could be a more valuable prognostic factor for the clinical outcomes.
In our study, approximately 50% of patients presented any-grade irAEs, and about 15% developed ≥ grade 3 irAEs. Skin-, endocrine-, hepatic-, and pulmonary-related toxicities were the most commonly reported irAEs, while pneumonitis was the most common grade 3–5 irAE. These findings are similar to those of the existing literature on the lung cancer patients treated with ICIs [12,13,14,15]. A variety of markers from the tumor microenvironment, circulating blood, target organs, or clinical factors have been reported to be associated with the onset of irAEs. However, none of them has been identified as a definitive predictive marker. Previous studies indicated that cytokines were most likely responsible for irAEs, as the inhibition of cytokines could rapidly reverse that condition [26]. A recent study showed that baseline and dynamic IL-10 levels were significantly and independently related to a higher incidence of irAEs (OR = 5.318, 95% CI = 1.174–24.081, and p = 0.030) [27]. In addition, the literature showed that autoantibodies, including thyroglobulin antibody [28], and gut microbiota such as Faecalibacterium [29] were also associated with the occurrence of irAEs. Moreover, the neutrophil lymphocyte ratio (NLR) (OR = 1.046; CI 95% = 1.006–1.088), a higher level of PD-L1 expression (OR = 2.009; 95% CI = 1.03–3.921), and smoking status (OR = 1.249; 95% CI = 1.021–1.528) were significantly associated with irAEs in a meta-analyses [30]. However, in our study, no statistical difference was observed between smoking status, expression of PD-L1, and irAEs. Possible reasons are the partial loss of PD-L1 data and selection bias of patients for receiving ICIs therapy in the real world.
Interestingly, we observed that baseline peripheral CD8+ T lymphocytes were associated with irAEs. Although the mechanisms underlying the onset of irAEs remain unclear, some studies have revealed that the potential mechanism is related to T cells. One study showed that, in patients treated with ICIs, the expansion of specific CD8+ T cell clones was associated with CTCAE grade 2 and 3 irAEs [31]. Furthermore, a recent study research reported a significant accumulation of CD8+ T cells with markers of cytotoxicity and proliferation in tissue biopsy samples from patients with colitis, suggesting that a significant subset of T-cell clones associated with irAEs were pre-existent [32]. Additional studies reported T-cell TILs were associated with hepatitis and pneumonitis [33,34]. It may be hypothesized that activated T-cell TILs are a hallmark of irAEs, and activated T cells kill tumor cells and may attack normal human tissue cells, forming ICIs-related toxicities. Compared with TILs, peripheral lymphocytes are more convenient and noninvasive. To our knowledge, this is the first study to investigate the predictive value of peripheral CD8+ T cells for irAEs. Of course, these preliminary results warrant further research on a larger patient cohort.
It is now widely recognized that there is the correlation between T lymphocytes and clinical outcomes in cancer patients treated with immunotherapy. IMpassion130, a phase III multicenter, randomized, double-blind, placebo-controlled trial in metastatic triple-negative breast cancer patients showed that high CD8+ TILs expression was significantly associated with improvements in OS and PFS [19]. A meta-analysis encompassing 14,395 NSCLC patients revealed that high CD8+ TILs were associated with improved prognosis predictions for OS (3-year OS AUC: 0.659; 5-year OS AUC: 0.665) [21]. In a study of patients with melanoma (n = 43) and non-squamous NSCLC (n = 40), patients with high circulating central memory/effector T-cell ratios experienced extended PFS compared with patients with low ratios [22]. In a study of 29 NSCLC patients treated with ICIs, the early proliferation of PD-1+ CD8+ T cells following ICIs treatment may be associated with clinical response [23]. Similarly, in our study, we observed that high baseline peripheral CD8+ T lymphocytes were associated with improved PFS and OS. Although baseline CD8+ T lymphocytes were not an independent prognostic factor of OS, these results also indicate that ICIs therapy should be used with caution in patients with low baseline peripheral CD8+ T lymphocytes. Certainly, the molecular feature of CD8+ T lymphocytes’ subsets may have preferable predictive value, such as its subsets proportion and PD-L1 expression status, which would be of further concern in the future. Furthermore, existing evidence has showed the prognosis value of irAEs in NSCLC patients treated with ICIs. A retrospective study of 134 patients with advanced or recurrent NSCLC who were treated with nivolumab revealed that the development of irAEs was positively associated with PFS (p = 0.03) and OS (p = 0.003) [35]. A prospective observational study of 38 NSCLC patients reported a correlation between irAEs and efficacy in NSCLC patients treated with nivolumab [36]. Similarly, the current study has provided evidence of the correlation between irAEs and survival in NSCLC treated with ICIs. In addition, our data revealed detailed information about the onset and resolution time of irAEs, which may provide important clues about the mechanisms linking irAEs with the efficacy of ICIs. The relationship of irAEs’ site, grade, timing of onset, and clinical outcomes deserves a further analysis in the future. Moreover, the current study has also explored the combined predictive effect of irAEs and the peripheral blood CD8+ T lymphocytes and found that patients with both high CD8+ T lymphocytes and irAEs have the best PFS. Thus, an evaluation of baseline CD8+ T lymphocytes and irAEs may provide meaningful information for efficacy prediction in immunotherapy.
We acknowledge a number of potential limitations in this study. Firstly, the cohort population was heterogenous in the disease context (first-line treatment vs. later-line treatment), and this might affect the outcomes [37]. Furthermore, the retrospective nature and the relatively small sample size limit the generalizability of the study. Although the results of our study are interesting, the combination predictive value of the CD8+ T lymphocytes and irAEs requires further validation by prospective large sample studies. Fortunately, there was no missing survival and lymphocytes test data in our analysis. Despite these limitations, the study is unique as the first to explore the predictive value of peripheral CD8+ T cells for irAEs and the combinational predictive value for the clinical outcomes in advanced NSCLC treated with ICIs.
5. Conclusions
Our data indicated that the baseline peripheral CD8+ T lymphocytes were correlated with the onset of irAEs and clinical outcomes. Importantly, the study revealed the combinational prognostic value of peripheral CD8+ T lymphocytes and irAEs in advanced NSCLC patients treated with PD-1/PD-L1 inhibitors. An evaluation of baseline CD8+ T lymphocytes and irAEs may be a convenient method to identify clinical outcomes in a timely manner. Certainly, these preliminary results warrant further research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14153568/s1, Figure S1: Onset and resolution time of immune-related adverse events (irAEs); Figure S2: Any-grade immune-related adverse events (irAEs); Figure S3: Association between median baseline level of CD8+ T lymphocytes and incidence of immune-related adverse events (irAEs) during immunotherapy.
Author Contributions
Conceptualization, S.M. and X.C.; investigation, resources, and data curation, K.W., B.X., J.Z., X.L., S.Y., M.Z., L.Z., B.W., X.X. and X.C.; methodology and performed data analysis, J.Z., L.Z. and B.W.; writing—original draft preparation, K.W. and B.X.; writing—review and editing, S.M. and X.C.; supervision, S.M. and X.C.; project administration, S.M. and X.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was carried out in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of Hangzhou Cancer Hospital (IRB No. HZCH-2020-019).
Informed Consent Statement
Informed consent was obtained from all patients who could be followed up in the study.
Data Availability Statement
Data are not publicly available due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Natural Science Foundation of Zhejiang Province (Grant Nos. LQ20H160019 and LY21H310002).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N.A., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., et al. SEER Cancer Statistics Review 1975–2018. [(accessed on 15 April 2021)]; Available online: https://seer.cancer.gov/csr/1975_2018/
- 3.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K.J., Kasper S., Vokes E.E., Even C., et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 5.Kato K., Cho B.C., Takahashi M., Okada M., Lin C.Y., Chin K., Kadowaki S., Ahn M.J., Hamamoto Y., Doki Y., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 6.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H., Gettinger S., Vokes E.E., Chow L.Q.M., Burgio M.A., de Castro Carpeno J., Pluzanski A., Arrieta O., Frontera O.A., Chiari R., et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021;39:723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Socinski M.A., Jotte R.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., Rodríguez-Abreu D., Moro-Sibilot D., Thomas C.A., Barlesi F., et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 9.Garon E.B., Hellmann M.D., Rizvi N.A., Carcereny E., Leighl N.B., Ahn M.J., Eder J.P., Balmanoukian A.S., Aggarwal C., Horn L., et al. Five-Year Overall Survival for Patients with Advanced Non-Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019;37:2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mok T.S.K., Wu Y.L., Kudaba I., Kowalski D.M., Cho B.C., Turna H.Z., Castro G., Jr., Srimuninnimit V., Laktionov K.K., Bondarenko I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y.H., Zang X.Y., Wang J.C., Huang S.S., Xu J., Zhang P. Diagnosis and Management of Immune Related Adverse Events (irAEs) in Cancer Immunotherapy. Biomed. Pharmacother. 2019;120:109437. doi: 10.1016/j.biopha.2019.109437. [DOI] [PubMed] [Google Scholar]
- 12.Fehrenbacher L., Spira A., Ballinger M., Kowanetz M., Vansteenkiste J., Mazieres J., Park K., Smith D., Artal-Cortes A., Lewanski C., et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 13.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst R.S., Baas P., Kim D.W., Felip E., Pérez-Gracia J.L., Han J.Y., Molina J., Kim J.H., Arvis C.D., Ahn M.J., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 16.Li L., Li G., Rao B., Dong A.H., Liang W., Zhu J.X., Qin M.P., Huang W.W., Lu J.M., Li Z.F., et al. Landscape of immune checkpoint inhibitor-related adverse events in Chinese population. Sci. Rep. 2020;10:15567. doi: 10.1038/s41598-020-72649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postow M.A., Sidlow R., Hellmann M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl. J. Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 18.Khoja L., Day D., Wei-Wu Chen T., Siu L.L., Hansen A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017;28:2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 19.Emens L., Loi S., Rugo H., Schneeweiss A., Diéras V., Iwata H., Barrios C., Nechaeva M., Molinero L., Nguyen Duc A., et al. Abstract GS1-04: IMpassion130: Efficacy in immune biomarker subgroups from the global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab + nab-paclitaxel in patients with treatment-naïve, locally advanced or metastatic triple-negative breast cancer. Cancer Res. 2019;79:GS1-04. doi: 10.1158/1538-7445.Sabcs18-gs1-04. [DOI] [Google Scholar]
- 20.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y., Zeng D., Ou Q., Liu S., Li A., Chen Y., Lin D., Gao Q., Zhou H., Liao W., et al. Association of Survival and Immune-Related Biomarkers with Immunotherapy in Patients with Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis. JAMA Netw. Open. 2019;2:e196879. doi: 10.1001/jamanetworkopen.2019.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manjarrez-Orduño N., Menard L.C., Kansal S., Fischer P., Kakrecha B., Jiang C., Cunningham M., Greenawalt D., Patel V., Yang M., et al. Circulating T Cell Subpopulations Correlate With Immune Responses at the Tumor Site and Clinical Response to PD1 Inhibition in Non-Small Cell Lung Cancer. Front. Immunol. 2018;9:1613. doi: 10.3389/fimmu.2018.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamphorst A.O., Pillai R.N., Yang S., Nasti T.H., Akondy R.S., Wieland A., Sica G.L., Yu K., Koenig L., Patel N.T., et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. USA. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y., Fu Y., Zhu B., Wang J., Zhang B. Predictive Biomarkers of Immune Checkpoint Inhibitors-Related Toxicities. Front. Immunol. 2020;11:2023. doi: 10.3389/fimmu.2020.02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horn L., Spigel D.R., Vokes E.E., Holgado E., Ready N., Steins M., Poddubskaya E., Borghaei H., Felip E., Paz-Ares L., et al. Nivolumab Versus Docetaxel in Previously Treated Patients with Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes from Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057) J. Clin. Oncol. 2017;35:3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verheijden R.J., May A.M., Blank C.U., Aarts M.J.B., van den Berkmortel F., van den Eertwegh A.J.M., de Groot J.W.B., Boers-Sonderen M.J., van der Hoeven J.J.M., Hospers G.A., et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020;26:2268–2274. doi: 10.1158/1078-0432.CCR-19-3322. [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Zhou F., Zhao C., Cheng L., Zhou C., Qiao M., Li X., Chen X. Interleukin-10 Is a Promising Marker for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving Immunotherapy. Front. Immunol. 2022;13:840313. doi: 10.3389/fimmu.2022.840313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maekura T., Naito M., Tahara M., Ikegami N., Kimura Y., Sonobe S., Kobayashi T., Tsuji T., Minomo S., Tamiya A., et al. Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. In Vivo. 2017;31:1035–1039. doi: 10.21873/invivo.11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaput N., Lepage P., Coutzac C., Soularue E., Le Roux K., Monot C., Boselli L., Routier E., Cassard L., Collins M., et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017;28:1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 30.Suazo-Zepeda E., Bokern M., Vinke P.C., Hiltermann T.J.N., de Bock G.H., Sidorenkov G. Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2021;70:3069–3080. doi: 10.1007/s00262-021-02996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subudhi S.K., Aparicio A., Gao J., Zurita A.J., Araujo J.C., Logothetis C.J., Tahir S.A., Korivi B.R., Slack R.S., Vence L., et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc. Natl. Acad. Sci. USA. 2016;113:11919–11924. doi: 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luoma A.M., Suo S., Williams H.L., Sharova T., Sullivan K., Manos M., Bowling P., Hodi F.S., Rahma O., Sullivan R.J., et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell. 2020;182:655–671.e622. doi: 10.1016/j.cell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suresh K., Naidoo J., Zhong Q., Xiong Y., Mammen J., de Flores M.V., Cappelli L., Balaji A., Palmer T., Forde P.M., et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J. Clin. Investig. 2019;129:4305–4315. doi: 10.1172/JCI128654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zen Y., Yeh M.M. Hepatotoxicity of immune checkpoint inhibitors: A histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod. Pathol. 2018;31:965–973. doi: 10.1038/s41379-018-0013-y. [DOI] [PubMed] [Google Scholar]
- 35.Haratani K., Hayashi H., Chiba Y., Kudo K., Yonesaka K., Kato R., Kaneda H., Hasegawa Y., Tanaka K., Takeda M., et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato K., Akamatsu H., Murakami E., Sasaki S., Kanai K., Hayata A., Tokudome N., Akamatsu K., Koh Y., Ueda H., et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Blumenthal G.M., Zhang L., Zhang H., Kazandjian D., Khozin S., Tang S., Goldberg K., Sridhara R., Keegan P., Pazdur R. Milestone Analyses of Immune Checkpoint Inhibitors, Targeted Therapy, and Conventional Therapy in Metastatic Non-Small Cell Lung Cancer Trials: A Meta-analysis. JAMA Oncol. 2017;3:e171029. doi: 10.1001/jamaoncol.2017.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available due to privacy and ethical restrictions.