Abstract
The fungus Neotyphodium lolii is an endophytic symbiont. It grows in the intercellular spaces of the perennial ryegrass Lolium perenne, producing secondary metabolites which enhance the fitness of the association over that of uninfected L. perenne. We report that the average number of hyphal strands in a given section of a leaf remains constant during the life of a leaf, indicating synchrony of leaf and hyphal extension, including cessation of hyphal extension when leaf extension ceases. We used a constitutively expressed reporter gene as an indicator of the mycelium's metabolic activity during and after hyphal extension. Reporter gene activity decreased when the mycelium stopped extending in liquid culture but not in planta. This indicates that in planta endophyte hyphae remain metabolically highly active when extension has ceased and throughout the life of the leaf they are colonizing. The behavior of the fungus in planta indicates the existence of signaling pathways which (i) synchronize the extension of leaf and hypha by regulating hyphal extension, (ii) suppress hyphal branching, and (iii) stop apical extension of fungal hyphae, without reducing the mycelium's metabolic activity. These signals may be crucial for the symbiosis, by allowing the endophyte to switch the focus of its metabolic activity from extension to the production of secondary metabolites.
Neotyphodium endophytes are mutualistic symbionts of grasses, living in their intercellular spaces. Their presence confers upon the grass a number of physiological characteristics such as drought resistance, enhanced growth, and protection from herbivores, through the synthesis of feeding deterrents and toxins. In return, the plant provides nutrients and propagates the endophyte via its seeds (17, 28, 34, 35, 46, 47).
We have recently used a Neotyphodium endophyte, transformed with the Escherichia coli β-glucuronidase gene (GUS reporter gene system [14]), under the control of a heterologous constitutive promoter, to estimate the in planta distribution of endophyte metabolic activity (as GUS activity) (12). We found that GUS activity followed well-defined patterns, established during the growth of ryegrass leaves (12). The endophyte metabolic activity in plant tissue measured in such experiments depends on (i) the number of endophyte hyphae present, (ii) the rate of gene expression in these hyphae, and (iii) the half-life of the GUS enzyme. Thus, while the existence of these patterns was intriguing, these experiment allowed only limited conclusions as to how they are generated. We have now determined directly the in planta distribution of endophyte hyphal biomass. In parallel, we have determined the endophyte's constitutive GUS expression in the same ryegrass tillers. We have also determined GUS activity in exponentially growing and stationary laboratory cultures of the same endophyte strain. Together, these data allow us to draw conclusions regarding the endophyte's metabolic state in different parts of the ryegrass tiller.
MATERIALS AND METHODS
Strains and plant growth conditions.
All experiments were carried out with strain KS1, created by cotransformation of Neotyphodium lolii strain Lp19 (7) with the hygromycin resistance plasmid pAN7-1 (29) (kindly provided by D. B. Scott) and plasmid pFG-gpd, containing the GUS reporter gene under the control of a constitutive heterologous promoter, the Aspergillus nidulans gpdA promoter. Plasmid pFG-gpd was constructed as follows (21). Initially, the Escherichia coli β-glucuronidase gene was obtained as a 1.9-kb fragment by sequential digestion of plasmid pRAJ275 (14)with EcoRI, the Klenow fragment of DNA polymerase I and SalI. The A. nidulans trpC terminator was then obtained as a 0.6-kb fragment by sequential digestion of plasmid pNOM-102 (29) with BamHI, the Klenow fragment, and HindIII. The two fragments were ligated into a position adjacent to the multicloning site of pGem-1 (Promega), following digestion of the vector with HindIII and SalI, yielding the plasmid pFunGus. The latter was digested with SmaI and NcoI, and the A. nidulans gpdA promoter was ligated into it; the promoter was obtained by sequential digestion of pNom-102 with EcoRI, the Klenow fragment and NcoI. For cotransformation, protoplasts were generated by digestion of cell walls with Novozyme 234 (Novo Industri A/S) according to the method of Murray et al. (22) except that the MgSO4 concentration in the osmotic medium was raised to 1.5 M, transformed by the method of Itoh et al. (13), and plated on osmotically stabilized CM medium (23), containing 100 μg of hygromycin B/ml. Selected hygromycin-resistant transformants were assessed for GUS expression by placing them in microtiter wells containing 4-methylumbelliferyl glucuronide (MUG) in GUS extraction buffer (14) at room temperature for 3 h and monitoring the emergence of fluorescence brought about by the conversion of MUG into methylumbelliferone (MU) on a transilluminator (31). The transformant chosen for further work, KS1, contains a single copy of the GUS reporter gene (31). KS1 could not be purified by spore purification (it does not sporulate), but we have evidence that it is a homokaryon: Lp19 is a monokaryotic fungus (33), and heterokaryons are thus expected to form pellets in liquid culture with clearly defined GUS-negative sectors (38); none were observed among 104 pellets of transformant KS1 assessed by using the dye Imagene Green, according to the methods described by Herd et al. (12). KS1 was introduced into perennial ryegrass seedlings (cultivar Nui) 1 year prior to the beginning of the study, by the method of Latch and Christensen (18). Its in planta behavior is indistinguishable from that of its untransformed parent (49).
Grass plants originating from a single infected seedling were maintained at 15°C in a growth cabinet with “12/24 h” illumination (296 μmol of photons per m2 per s) in 1.4-liter pots containing potting mix to which Osmocote (Grace Sierra, Australia) slow-release fertilizer had been added. After growth for 1 month, the plants were fertilized weekly (Thrive, Yates, New Zealand). Plants were watered to saturation twice weekly and transferred to new pots once the plants contained ca. 65 tillers (roughly every 2 months).
Counting of hyphae in cross sections.
Over a period of 9 months, single tillers were harvested and dissected as shown in Fig. 1. Hyphae were counted in aniline blue-stained transverse cuts taken from tissue samples 0.5 cm in length (marked by shading in Fig. 1). For staining, samples were incubated in a solution containing 63% ethanol, 7% lactic acid, and 16% glycerol for 2 h at room temperature or, in case of the leaf blades, at 100°C for 1 to 2 min. Samples were then placed in 95% ethanol for 15 min and rinsed in distilled water for 1 min. Subsequently, all tissues except the lowest part of the emerging leaf were placed into a solution of 2.5 mg of chloral hydrate per ml of water for 1 to 2 h (most parts of the emerging leaf and the leaf sheath) or 20 h (blades), followed by 15 min in 50% ethanol and a 1 min rinse in distilled water. Subsequently, the samples were placed in an aqueous solution containing 27% lactic acid and 33% glycerol for 15 min. Transverse slices for microscopy were then cut by hand with a scalpel and placed in a drop of a solution containing 0.1% aniline blue and 2.5 g of chloral hydrate per ml of water. After 15 to 20 min, excess staining solution was removed with no. 1 Whatman paper, and sections were mounted on a microscope slide in a solution containing equal parts of 80% lactic acid, glycerol, and water, covered with a coverslip, and hyphae were counted at ×400 magnification on a Zeiss KM microscope.
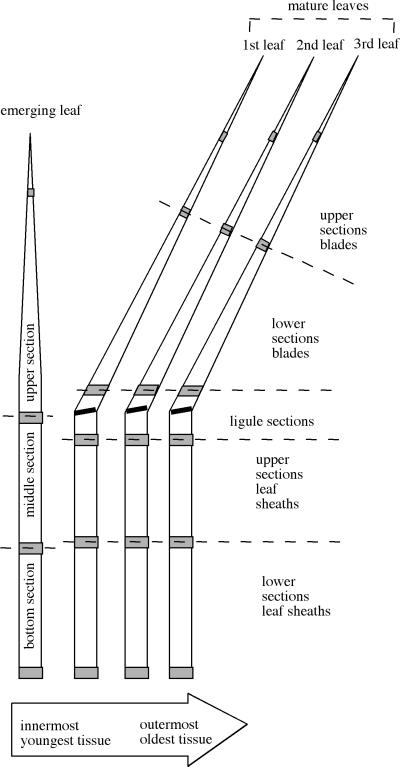
FIG. 1.
Expanded view of a ryegrass tiller showing tissues used for hyphal counts (shaded areas) and for GUS assays (unshaded areas). In the uppermost sections, the tissue removed for counting was 0.5 cm below the tip of the leaf. Shown is a three-leaf tiller, i.e., a tiller with three mature, nonextending leaves in which the ligular zone (marked by a thick black line) is visible, separating blade and sheath. Some of the tillers only had two mature leaves, lacking the third leaf, and others had only one mature leaf, lacking the second and third leaves. The dashed lines indicate the borders of sections for which results are reported in Table 1 and Fig. 2 and 3.
Determination of hyphal diameters in planta
Transverse plant tissue segments, 1 to 2 mm thick, were fixed in 3% glutaraldehyde and 2% formaldehyde in 0.1 M phosphate buffer (pH 7.2) (15) and then prepared for transmission electron microscopy as described by Spiers and Hopcroft (39). Electron micrographs (at either ×31,800 or ×48,600 magnification) were photocopied in duplicate onto Reflex A4 paper. Images of hyphal cross sections were then cut out and weighed on an Ohaus Explorer Balance. Squares of known size of the same paper were also weighed and used as standards to calculate the area of the cross sections from the weight of the cut-out images. Areas of magnified hyphal cross sections thus obtained were divided by the square of the magnification factor to obtain the area of the original hyphal cross sections.
Endophyte GUS activity determination.
Endophyte GUS expression in grass tissue was determined in the same tillers used for hyphal counts. The length and the fresh weight of each piece of tissue taken for GUS assays (unshaded areas in Fig. 1) was determined. The material was then ground in liquid nitrogen and freeze-dried, and its dry weight was determined. The material was then extracted by sonication and analyzed for GUS activity by a fluorometric assay based on the conversion of MUG to MU (12). After subtraction of background (0.25 pmol of MU min−1 per mg of dry weight [12]) and correction for the interference of plant material with the GUS assay by using a calibration curve (38; data not shown), the concentration of endophyte metabolic activity in plant material was calculated as the picomoles of MU per minute per milligram of dry weight. Because the grinding step could lead to substantial losses of material when small pieces of tissue were processed, a calibration curve (38; data not shown) was used to determine the expected dry weight of the entire tissue sampled, based on its fresh weight. Expected dry weights were then used to calculate the amount of GUS activity (in picomoles of MU per minute) in the entire tissue sampled. To calculate the GUS activity in the sections shown in Fig. 3, corrections were made for removal of part of the tissue for hyphal counting, based on the length of the tissue removed for hyphal counting.
FIG. 3.
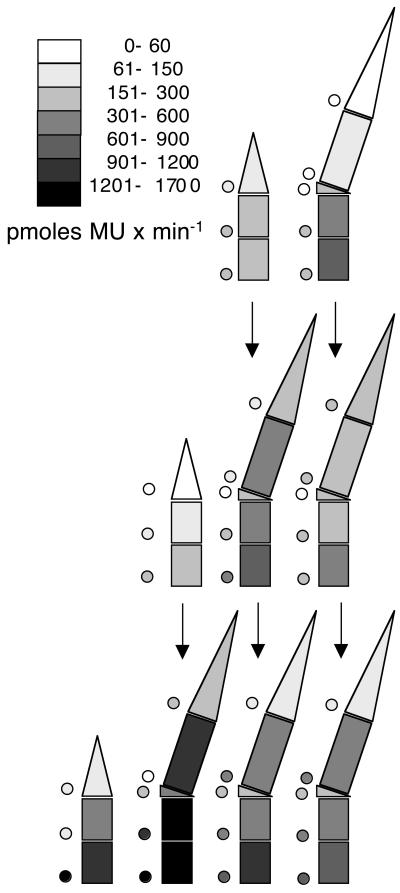
Distribution of GUS activity throughout ryegrass tillers infected with endophyte KS1, constitutively expressing the GUS gene. GUS activity was analyzed in extracts of the same sections of the same tillers whose hyphal counts are shown in Fig. 2. Tillers are represented schematically in a side view, with age of leaves increasing from left to right; the emerging leaves, which are still growing, are on the very left. Shading of the sections of the individual leaves indicates the average GUS activity in a section, according to the key provided as part of the figure (for instance, the shading of the top section of the emerging leaf in the uppermost row indicates that the tips of emerging leaves contain on average a GUS activity that falls between 61 and 150 pmol of MU per min). The shaded circles next to the sections indicate the standard deviation, according to the same key. The vertical arrows indicate the developmental fate of tissues as tillers develop more leaves (see Results for details).
The GUS activity of endophyte mycelium in liquid cultures was determined on filtered, washed mycelia grown with shaking at 15°C in YEG medium (0.5% yeast extract plus 2% glucose) and at 25°C in potato dextrose broth (Difco) by using the assay and extraction protocol as described above with one exception: based on estimates of extraction efficiency carried out as described by Herd et al. (12; data not shown), sonication was prolonged 2.5 times to achieve the same extraction efficiency as in the plant tissue.
RESULTS
The average number of hyphal strands in a given section of a leaf remains constant during the life of a leaf, indicating synchrony of leaf and hyphal extension.
To get an indication of the distribution of endophyte biomass in ryegrass tissue, we determined the average number of hyphal strands in different parts of ryegrass tillers with one, two, and three mature leaves (mature leaves are leaves that have stopped growing and have exposed the ligular zone, which separates the blade from the leaf sheath [37]). Figure 2 gives a graphic overview of average numbers, listed also in Table 1. In the figure, the leaves of the different tillers are aligned so that by following the vertical arrows, one can follow hyphal counts throughout the developmental history of an average leaf (for example, the emerging leaf in tillers with one mature leaf will become the innermost mature leaf in tillers with two mature leaves and then the second leaf in tillers with three mature leaves). Note that leaves elongate by basal addition of tissue (37); thus, the tip of the emerging leaf will become the tip of mature leaf, and the basal parts of the mature leaf are the last to be formed.
FIG. 2.
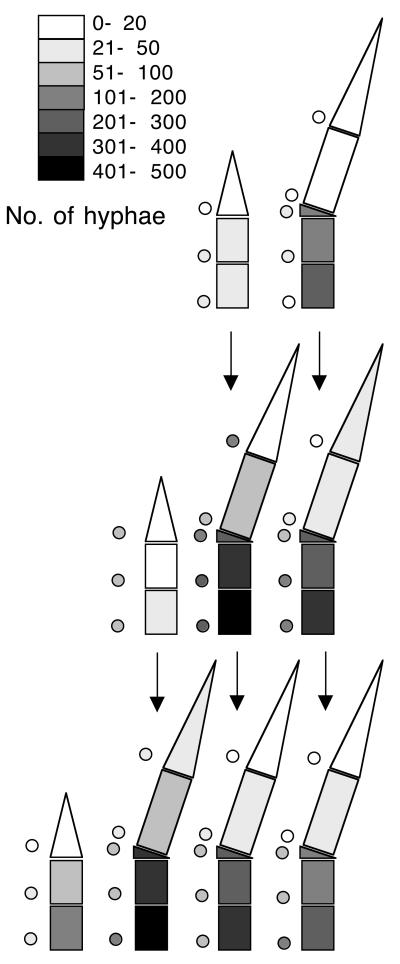
Numbers of hyphal strands in sections of ryegrass tillers infected with endophyte KS1. Three tillers with one mature leaf, three tillers with two mature leaves, and five tillers with three mature leaves were dissected and analyzed as described in Materials and Methods. Tillers are represented schematically in side view with age of leaves increasing from left to right; the emerging leaves, which are still growing, are on the very left. Shading of the sections of the individual leaves indicates the average numbers of hyphal strands per section, following the key provided as part of the figure (for instance, shading of the top section of the emerging leaf in the uppermost row indicates that the tips of emerging leaves have an average that falls between between 0 and 20 hyphal strands; the number of hyphae in a section of an individual tiller is the average of hyphal counts in cross sections taken from the top and the bottom of the section; marked by shading in Fig. 1). Shaded circles next to the sections indicate the standard deviation, according to the same key. The vertical arrows indicate the developmental fate of tissues as tillers develop more leaves (see Results for details).
TABLE 1.
Average number of hyphal strands and average GUS activities in sections of ryegrass tillers
| Tiller size (no. of mature leaves)a | Leafb (no.) | Sectionb | Avg no. of hyphal strands in section ± SD | Avg GUS activity in section (pmol of MU min−1) ± SD |
|---|---|---|---|---|
| 1 | Emerging | Bottom | 45 ± 28 | 223 ± 161 |
| Middle | 35 ± 32 | 192 ± 182 | ||
| Upper | 13 ± 14 | 68 ± 72 | ||
| 1 | Lower sheath | 270 ± 8 | 654 ± 181 | |
| Upper sheath | 191 ± 27 | 404 ± 199 | ||
| Ligule | 173 ± 35 | 163 ± 41 | ||
| Lower blade | 13 ± 8 | 79 ± 55 | ||
| Upper blade | 6 ± 5 | 7 ± 9 | ||
| 2 | Emerging | Bottom | 48 ± 69 | 152 ± 184 |
| Middle | 20 ± 32 | 88 ± 93 | ||
| Upper | 3 ± 5 | 15 ± 24 | ||
| 1 | Lower sheath | 464 ± 81 | 805 ± 400 | |
| Upper sheath | 320 ± 98 | 423 ± 289 | ||
| Ligule | 290 ± 97 | 258 ± 16 | ||
| Lower blade | 56 ± 35 | 377 ± 94 | ||
| Upper blade | 19 ± 14 | 160 ± 112 | ||
| 2 | Lower sheath | 340 ± 156 | 396 ± 209 | |
| Upper sheath | 275 ± 137 | 284 ± 190 | ||
| Ligule | 233 ± 96 | 207 ± 0 | ||
| Lower blade | 49 ± 38 | 295 ± 276 | ||
| Upper blade | 30 ± 30 | 247 ± 275 | ||
| 3 | Emerging | Bottom | 128 ± 38 | 985 ± 1248 |
| Middle | 53 ± 29 | 337 ± 136 | ||
| Upper | 17 ± 19 | 61 ± 63 | ||
| 1 | Lower sheath | 480 ± 138 | 1670 ± 1288 | |
| Upper sheath | 394 ± 97 | 1299 ± 985 | ||
| Ligule | 340 ± 79 | 528 ± 234 | ||
| Lower blade | 89 ± 35 | 1005 ± 809 | ||
| Upper blade | 26 ± 19 | 207 ± 164 | ||
| 2 | Lower sheath | 361 ± 78 | 953 ± 753 | |
| Upper sheath | 264 ± 60 | 558 ± 522 | ||
| Ligule | 228 ± 70 | 267 ± 163 | ||
| Lower blade | 40 ± 21 | 560 ± 520 | ||
| Upper blade | 12 ± 9 | 138 ± 112 | ||
| 3 | Lower sheath | 257 ± 125 | 639 ± 811 | |
| Upper sheath | 186 ± 99 | 451 ± 592 | ||
| Ligule | 167 ± 95 | 161 ± 160 | ||
| Lower blade | 37 ± 29 | 350 ± 498 | ||
| Upper blade | 16 ± 18 | 137 ± 134 |
Three tillers with one and two mature leaves, respectively, and five tillers with three mature leaves were analyzed.
The nomenclature for leaves and sections is identical to that presented in Fig. 1.
The figure shows that there are basal-apical gradients of similar steepness in all emerging and mature leaves and that the number of hyphae in a given section of the average leaf remained more or less constant throughout its development. (The statistical significance of basal-apical gradients was demonstrated by ranking the hyphal counts in sections of each individual leaf and applying the Kruskal-Wallis test to these ranking data for each type of leaf in each type of tiller shown in Fig. 1 [P < 0.05]; no statistically significant changes in hyphal counts in a given leaf section with developmental age were observed by using the t test, nor did we find any trends in the data indicative of changes in hyphal counts with age.) Given the mode of extension of the leaf, the simplest explanation of these data is that an increasing number of hyphae enters the leaf as it extends from a multibranched mycelium at its base (leading to the basal apical gradients) and that hyphae, once they are in the leaf, extend at the same rate as the surrounding leaf tissue and without forming significant numbers of branches (leading to a constant number of hyphae in each plant tissue section once it has formed). This synchrony of growth includes a more or less simultaneous cessation of leaf tissue and hyphal extension, leading to constant hyphal numbers after the leaf has stopped extending.
Estimates of the concentration of endophyte biomass.
To get an indication of the contribution of endophyte biomass to the biomass of the infected leaf, we determined the area of hyphal cross sections by using electron microscopy in various parts of a tiller with three mature leaves, and we used this value to estimate dry weight of the mycelium (Table 2). According to these estimates, even in the most densely colonized areas of the tiller, the endophyte did not constitute more than 0.2% of the biomass of the infected tissue. The data also suggest that endophyte biomass may increase approximately twofold after extension has ceased, due to an increase in hyphal thickness; such postextension increases in hyphal thickness have also been observed in other filamentous fungi (40).
TABLE 2.
Estimates of endophyte biomass in infected ryegrass tissue
| Tissue sectiona | Area (mm2) of cross section of individual hyphae (no. of observations)b | Estimated fungal dry wt/mg dry wt of grass tissue (μg)c |
|---|---|---|
| Lower emerging leaf | 1.95 × 10−6 (10) | 0.386 |
| Upper emerging leaf | 2.21 × 10−6 (9) | 0.120 |
| Blade innermost mature leaf | 2.73 × 10−6 (13) | 0.291 |
| Blade middle mature leaf | 3.58 × 10−6 (10) | 0.269 |
| Blade outermost mature leaf | 3.49 × 10−6 (9) | 0.443 |
| Sheath innermost mature leaf | 2.43 × 10−6 (34) | 1.080 |
| Sheath outermost mature leaf | 4.28 × 10−6 (42) | 2.045 |
All data were obtained by using tillers with three mature leaves.
Values were determined by using electron micrographs as described in Materials and Methods. The values are for individual hyphae.
This value was calculated as follows. The total length of mycelium in individual grass tissue sections (sections correspond to sections shown in Fig. 1) of tillers with three mature leaves was calculated as the number of hyphae in the section times the length of the section. These values were then multiplied first with the hyphal cross sections shown in this table and then with 1.1 g/cm3 (16) to arrive at an estimate of the fresh weight of the mycelium and finally with 0.3 (48) to convert the fresh weight to dry weight. These values were divided by the dry weight of the grass tissue section. The values shown for lower and upper emerging leaves are averages of all upper and lower emerging leaf sections of five tillers with three mature leaves. Blade values are averages of all lower- and upper-blade values from the same five tillers, and sheath values are averages of all lower- and upper-blade values from the same five tillers.
Evidence that nonextending endophyte hyphae maintain high metabolic rates in mature leaves.
In culture, cessation or slowing of biomass increase and hyphal extension in fungi occurs when environmental conditions curtail metabolic activity (1, 27). We wanted to investigate whether, in planta, the cessation of extension of N. lolii hyphae coincides with, or is brought about by, a curtailing of metabolic activity. The endophyte we used carries the GUS reporter gene under control of a constitutive heterologous promoter (see Materials and Methods for details). We could therefore assess endophyte metabolic activity as constitutive GUS reporter gene expression (12). Figure 3 shows the average distribution of reporter enzyme activity in the same tillers that were used for hyphal counts (data are also presented numerically in Table 1). No decrease of reporter gene activity after leaf maturation was apparent.
Figure 3 and Table 1 show activities in whole sections. Since the sizes of sections varied between tillers, we also calculated GUS activity per grass tissue dry weight and per length of mycelium in a section (data not shown) and likewise saw no evidence indicating a decline with leaf age. We verified this by t tests, in which we compared GUS activity values for the same section in mature leaves of different ages. Only in lower leaf sheath sections did we find statistically significant (P < 0.05) decreases with age, but only for GUS activities per dry weight. No trend of such a decrease was apparent when total GUS activity in sections or GUS activities per mycelial length were compared for these sections. Thus, the age-dependent differences in GUS activity per dry weight in lower leaf sheaths are more likely caused by age-dependent changes in the composition of the sheath tissue rather than by a decline in GUS activity of the mycelium within them.
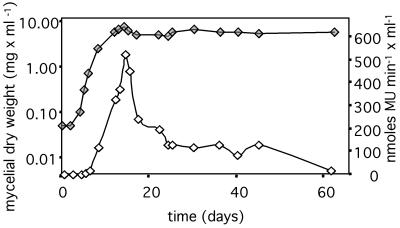
The levels of GUS activity in tissue depend not only on the rate of synthesis of the β-glucuronidase enzyme but also on the half-life of the enzyme after its synthesis. Thus, the data presented above would not necessarily preclude that hyphae reduce their metabolic rate, provided that β-glucuronidase molecules, once synthesized, do not decay significantly over the lifetime of a leaf. We therefore assessed the half-life of GUS activity in stationary-phase mycelium in liquid culture in YEG medium. The data shown in Fig. 4 suggest that the decay is a three-component process with an initial half-life of 5 days, followed by a plateau after activity has declined to 25% of the original, followed by another phase of decline with a half-life of the remaining GUS activity of 6 days. The half-life of GUS, averaged across the entire length of the experiment, was 15 days. Very similar data were obtained when the same experiment was conducted in another growth medium, potato dextrose broth, or when growth was inhibited in the latter medium by adding KCN: there was an initial decline with a half-life of 5 to 6 days until activity had fallen to <50% of the original level, followed by a phase of slower decline (in these experiments GUS activity was only followed for ≤17 days after growth had stopped).
FIG. 4.
GUS activity of endophyte mycelium in liquid cultures (YEG medium). Solid symbols show growth as the mycelial dry weight per milliliter of culture; open symbols show the GUS activity per milliliter culture. GUS activity was determined as described in Materials and Methods in harvested mycelium.
To estimate from these data whether a decrease of endophyte metabolic activity in planta, after completion of leaf extension, would lead to measurable differences between plant tissues of different ages, we next determined the age difference between leaves as follows. The extension rate of leaves was determined (0.94 mm per h, or 2.26 cm per day), as was the maximum length of a leaf (20 cm inclusive of the leaf sheath). We also determined that the next emerging leaf became visible once the youngest mature leaf had reached a length of ca. 11 cm. Based on these data, one can calculate that it takes ca. 9 days for a leaf to reach its final size and that each new leaf finishes its elongation ca. 4 days after the previous one. Thus, the outermost leaf of a tiller with two fully grown mature leaves will have been in a nonextending state for ca. 4 days and the outermost leaf of a tiller with three fully grown mature leaves will have been in a nonextending state for ca. 8 days. If the nonextending hyphae in planta enter a metabolic state comparable to what occurs in stationary cultures, GUS levels should be lower by 30% in outer leaves of tillers with two mature leaves than in the mature leaf of a tiller with one mature leaf. Likewise, GUS activity in the outermost leaf of a tiller with three mature leaves should be 30% lower than in the outermost leaf of a tiller with two mature leaves and 60 to 70% lower than in the mature leaf of a tiller with one mature leaf.
These calculations indicate that a decrease in metabolic activity of the endophyte in planta, after leaf extension has ceased, should be detectable as a substantial reduction of GUS activity in the older tissues of the tillers we observed. The absence of such a reduction is therefore evidence that nonextending hyphae in planta retain high metabolic activity.
DISCUSSION
In planta regulation of hyphal extension, hyphal branching, and metabolism of N. lolii.
Our data provide evidence that extension of endophyte hyphae and ryegrass tissue are highly coordinated, as indicated by constant endophyte biomass content of leaf tissue throughout its age. This conclusion is corroborated by recent observations by Christensen et al. (6) that endophyte extension ceases in mature leaves not only in the association we used but also in a variety of other Neotyphodium-grass associations examined.
Using the GUS reporter gene as an indicator of metabolic activity of the mycelium, we have provided evidence indicating that it is not the curtailing of metabolic activity of the endophyte that stops hyphal growth when the leaf stops extending. This conclusion is supported by evidence which suggests that the environment of the endophyte in mature leaves is conducive to its further growth. Christensen et al. (6) recently showed that in associations containing a mutant of the strain used here, as well as in some other N. lolii/ryegrass associations, older nonextending leaves can contain significantly higher number of endophyte hyphae than young nonextending leaves of the same tillers. The simplest explanation for these differences is the continued growth of endophyte hyphae in these leaves after completion of leaf extension.
The most likely reason for coordinated growth of the endophyte and plant tissue would thus be a signaling mechanism. The mechanism could make use of the movement of Neotyphodium hyphae versus surrounding plant tissue during extension. We have recently proposed (32, 33) that N. lolii colonizes extending leaves from a multibranched mycelium located in the meristematic tissue at the base of the leaf, with the endophyte mycelium growing by adding material to the hyphal apices as is customary for fungal hyphal extension (2, 11, 45). If the model is correct, the apically extending hyphae will continuously slide through the intercellular spaces of the extending leaf, which grows by addition of cells at its base.
Based on this model, one possible mechanism for synchronizing endophyte growth with that of the plant would be perception by the growing tip of the apically extending endophyte of biochemical changes of neighboring plant cells as they are separated farther and farther from the basally located meristem (25). An even more attractive (since it is conceptually simpler) mechanism would rely on the endophyte possessing mechanosensitive channels, homologous to those shown to be involved in apical extension in other fungi (19, 20, 26, 42). Located at the tip of the hypha, such channels could sense a differential in speed of extension of hypha and grass as friction as the two slide past each other. If the extension of both tissues occurs at identical rates, no net movement of tip versus surrounding tissue and no friction would occur. Signals from such channels could be used to regulate the speed of incorporation of new cellular material into the hyphal apex, including the cessation of hyphal elongation when leaf extension ceases. Likewise, mechanosensitive channels along distal parts of the hypha could sense continuing friction, due to movement of plant cells past the stationary parts of the hypha, and this signal could suppress branching; note that the suppression of branching in extending areas of the leaf is not only supported by the constancy of the number of hyphal strands reported here but also by direct microscopic assessment of in planta branching frequency in epidermal strips and leaf sheaths (6).
In culture, the cessation of growth of N. lolii is accompanied by a decline in GUS expression. The decline in expression of the GUS gene, controlled by a heterologous constitutive promoter, is to be expected as the mycelium enters stationary phase. Stationary phase, triggered through changes in the environment such as nutrient limitation, pH changes, or the accumulation of waste products (27), is accompanied in fungi and other organisms by reduced protein synthesis, even though specific pathways, such as secondary metabolite pathways, might become more active (1, 4, 8, 9, 24, 30, 36, 41, 43, 44). In other words, in culture N. lolii biomass increase, through hyphal apical extension, and metabolic activity are correlated and linked. The maintenance of high GUS levels in nonextending mycelium in mature leaves indicates that in planta apical extension can cease without inducing a decline in protein synthesis rates typical of the stationary phase. This suggests that in planta hyphal extension and metabolic activity can apparently be uncoupled, with hyphal extension ceasing, while high metabolic rates are maintained. This should benefit the symbiosis greatly, allowing the endophyte to utilize, in mature leaves, the equivalent of the biosynthetic capacity expended previously for rapid apical extension (or a large part thereof) for the production of secondary metabolites which protect its host. If undiminished biosynthetic capacity is indeed switched from biomass synthesis to secondary metabolite biosynthesis, the end of leaf extension should see the turning on of the respective pathways at high rates. While no in planta gene expression studies are yet available to ascertain that the pathways are switched on once extension ceases, the distribution of at least some of the known alkaloids in ryegrass tissue supports the idea of their predominant synthesis in mature, i.e., fully expanded leaf tissues (38).
Implications for biotechnology.
Our data suggest (i) that it is possible to stop the extension of a fungal mycelium without curtailing its metabolic activity, (ii) that N. lolii can turn on secondary metabolite pathways under favorable environmental conditions sustaining high metabolic rates, and (iii) the existence of plant signals that regulate mycelial morphology in terms of branching. These observations are of potential interest for biotechnology, given the rheological impact of mycelial morphology on biotechnological production and given that many biotechnological productions with fungi aim at secondary metabolites (3, 5, 10). If we could identify the N. lolii signaling mechanisms that regulate its in planta growth and morphology, it might be possible to apply this knowledge to filamentous fungi used in biotechnology to control mycelial morphology and to switch from fungal biomass synthesis to secondary metabolite synthesis without having to resort to creating adverse conditions to stop growth. An additional biotechnological application of the pathways regulating the speed of extension could be to use their homologues in human pathogenic fungi as targets for the development of novel therapeutic fungistatic agents.
ACKNOWLEDGMENTS
This work was supported by the New Zealand Foundation for Research Science and Technology. M.J.S. was supported by a Massey University Ph.D. scholarship.
REFERENCES
- 1.Alberghina L, Struani E, Costantini M G, Maregani E, Zippel R. Regulation of macromolecular composition during growth of Neurospora crassa. In: Burnett J H, Trinci A P J, editors. Fungal walls and hyphal growth. New York, N.Y: Cambridge University Press; 1979. pp. 295–318. [Google Scholar]
- 2.Bartnicki-Garcia S. Microbial differentiation: 23rd Symposium of the Society for General Microbiology. London, England: University Press, Imperial College; 1973. Fundamental aspects of hyphal morphogenesis; pp. 245–267. [Google Scholar]
- 3.Bennet J W, Klich M A E. Aspergillus: biology and industrial applications. Bio/Technology Series. Vol. 23. London, England: Butterworth-Heinemann; 1992. [Google Scholar]
- 4.Boucherie H. Protein synthesis during transition and stationary phases under glucose limitation in Saccharomyces cerevisiae. J Bacteriol. 1985;161:385–392. doi: 10.1128/jb.161.1.385-392.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calam C T. Process development in antibiotic fermentations. Vol. 4. Cambridge, England: Cambridge University Press; 1987. [Google Scholar]
- 6.Christensen, M., R. J. Bennett, and J. Schmid. Growth of Epichloë/Neotyphodium and p-endophytes in leaves of Lolium and Festuca grasses. Mycolog. Res., in press.
- 7.Christensen M J, Leuchtmann A, Rowan D D, Tapper B A. Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (F. pratensis), and perennial rye-grass (Lolium perenne) Mycolog Res. 1993;97:1083–1092. [Google Scholar]
- 8.Didec-Brumec M, Gaberc-Porekar V, Alacevic M. Relationship between the Claviceps life cycle and productivity of ergot alkaloids. Crit Rev Biotechnol. 1996;16:257–299. [Google Scholar]
- 9.Fracella F, Scholle C, Kallies A, Hafker T, Schroder T, Rensing L. Differential HSC70 expression during asexual development of Neurospora crassa. Microbiology. 1997;143:3615–3624. doi: 10.1099/00221287-143-11-3615. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs P A, Seviour R J, Schmid F. Growth of filamentous fungi in submerged culture: problems and possible solutions. Crit Rev Biotechnol. 2000;20:17–48. doi: 10.1080/07388550091144177. [DOI] [PubMed] [Google Scholar]
- 11.Gooday G W, Trinci A P J. Wall structure and biosynthesis in fungi. Symp Soc Gen Microbiol. 1980;30:207–251. [Google Scholar]
- 12.Herd S, Christensen M J, Saunders K, Scott D B, Schmid J. Quantitative assessment of in planta distribution of metabolic activity and gene expression of an endophytic fungus. Microbiology. 1997;143:267–275. doi: 10.1099/00221287-143-1-267. [DOI] [PubMed] [Google Scholar]
- 13.Itoh Y, Johnson R, Scott D B. Integrative transformation of the mycotoxin-producing fungus Penicillium paxilli. Curr Genet. 1994;25:508–513. doi: 10.1007/BF00351670. [DOI] [PubMed] [Google Scholar]
- 14.Jefferson R A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- 15.Karnovsky J E M. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol. 1965;27:137–138. [Google Scholar]
- 16.Kubitschek H E. Buoyant density variation during the cell cycle in microorganisms. Crit Rev Microbiol. 1987;14:73–97. doi: 10.3109/10408418709104436. [DOI] [PubMed] [Google Scholar]
- 17.Lane G A, Christensen M J, Miles C O. Coevolution of fungal endophytes with grasses: the significance of secondary metabolites. In: White J F, Bacon C W, editors. Microbial endophytes. New York, N.Y: Marcel Dekker; 2000. pp. 341–388. [Google Scholar]
- 18.Latch G C M, Christensen M J. Artificial infection of grasses with endophytes. Ann Appl Biol. 1985;107:17–24. [Google Scholar]
- 19.Levina N N, Lew R R, Heath I B. Cytoskeletal regulation of ion channel distribution in the tip-growing organism Saprolegnia ferax. J Cell Sci. 1994;107:127–134. doi: 10.1242/jcs.107.1.127. [DOI] [PubMed] [Google Scholar]
- 20.Levina N N, Lew R R, Hyde G J, Heath I B. The roles of Ca2+ and plasma membrane ion channels in hyphal tip growth of Neurospora crassa. J Cell Sci. 1995;108:3405–3417. doi: 10.1242/jcs.108.11.3405. [DOI] [PubMed] [Google Scholar]
- 21.McGowan T. Construction of a novel fungal GUS expression plasmid, and its evaluation in Aspergillus nidulans. M.Sc. thesis. Palmerston North, New Zealand: Massey University; 1996. [Google Scholar]
- 22.Murray F R, Latch G C M, Scott D B. Surrogate transformation of perennial ryegrass, Lolium perenne, using genetically modified Acremonium endophyte. Mol Gen Genet. 1992;233:1–9. doi: 10.1007/BF00587554. [DOI] [PubMed] [Google Scholar]
- 23.Oliver R P, Roberts I N, Harling R, Kenyon L, Punt P J, Dingemanse M A, van den Hondel C A M J J. Transformation of Fulvia fulva, a fungal pathogen of tomato, to hygromycin B resistance. Curr Genet. 1987;12:231–233. [Google Scholar]
- 24.Pazoutova S, Flieger M, Sajdl P, Rehacek Z. The relationship between intensity of oxidative metabolism and predominance of agroclavine or elymoclavine in submerged Claviceps purpurea cultures. J Nat Prod. 1981;44:225–235. [Google Scholar]
- 25.Penny P, Penny D. Rapid response to phytohormones. In: Letham D S, Goodwin P B, Higgins TJV, editors. Phytohormones and related compounds: a comprehensive treatise. II. Amsterdam, The Netherlands: Elsevier/North-Holland Biomedical Press; 1978. pp. 537–597. [Google Scholar]
- 26.Perera T H, Gregory D W, Marshall D, Gow N A. Contact-sensing by hyphae of dermatophytic and saprophytic fungi. J Med Vet Mycol. 1997;35:289–293. doi: 10.1080/02681219780001301. [DOI] [PubMed] [Google Scholar]
- 27.Prosser J I. Kinetics of filamentous growth and branching. In: Gow N A R, Gadd G M, editors. The growing fungus. London, England: Chapman & Hall, Ltd.; 1995. pp. 301–318. [Google Scholar]
- 28.Richardson M D. Alkaloids of endophyte-infected grasses: defence chemicals or biological abnormalities? In: White J F, Bacon C W, editors. Microbial endophytes. New York, N.Y: Marcel Dekker; 2000. pp. 323–340. [Google Scholar]
- 29.Roberts I N, Oliver R P, Punt P J, van den Hondel C A M J J. Expression of the Escherichia coli β-glucuronidase gene in industrial and phytopathogenic filamentous fungi. Curr Genet. 1989;15:177–180. doi: 10.1007/BF00435503. [DOI] [PubMed] [Google Scholar]
- 30.Russel P J, Rodland K D, Rachlin E M, McCloskey J A. Differential DNA methylation during the vegetative life cycle of Neurospora crassa. J Bacteriol. 1987;169:2902–2905. doi: 10.1128/jb.169.6.2902-2905.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders K. Development of the β-glucuronidase reporter gene system to study Acremonium endophyte interactions with perennial ryegrass. M.Sc. thesis. Palmerston North, New Zealand: Massey University; 1997. [Google Scholar]
- 32.Schmid J, Christensen M J. Ryegrass endophyte: host-fungus interaction. In: Woodfield D, Easton S, editors. Ryegrass endophyte: an essential New Zealand symbiosis. Vol. 7. Napier, New Zealand: New Zealand Grassland Association; 1999. pp. 101–106. [Google Scholar]
- 33.Schmid J, Spiering M J, Christensen M J. Metabolic activity, distribution, and propagation of grass endophytes in planta: Investigations using the GUS reporter gene system. In: White J F, Bacon C W, editors. Microbial endophytes. New York, N.Y: Marcel Dekker; 2000. pp. 295–322. [Google Scholar]
- 34.Scott B, Schardl C. Fungal symbionts of grasses: evolutionary insights and agricultural potential. Trends Microbiol. 1993;1:196–200. doi: 10.1016/0966-842x(93)90091-5. [DOI] [PubMed] [Google Scholar]
- 35.Siegel M R, Schardl C L. Fungal endophytes of grasses: detrimental and beneficial associations. In: Andrew J H, Hirano S S, editors. Microbial ecology of leaves. Berlin, Germany: Springer Verlag; 1991. pp. 198–221. [Google Scholar]
- 36.Skory C D, Chang P K, Linz J E. Regulated expression of the nor-1 and ver-1 genes associated with aflatoxin biosynthesis. Appl Environ Microbiol. 1993;59:1642–1646. doi: 10.1128/aem.59.5.1642-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soper K, Mitchell K J. The developmental anatomy of perennial ryegrass (Lolium perenne L.) N Z J Sci Technol. 1956;37:484–504. [Google Scholar]
- 38.Spiering M. Distribution of Neotyphodium lolii-endophyte metabolic activity in ryegrass (Lolium perenne, L.) and its implications for alkaloid distribution and photosynthesis. Ph.D. thesis. Palmerston North, New Zealand: Massey University; 2000. [Google Scholar]
- 39.Spiers A G, Hopcroft D H. Black canker and leaf spot of Salix in New Zealand caused by Glomerella miyabeana (Colletotrichum gloeosporioides) Eur J Forest Pathol. 1993;23:92–102. [Google Scholar]
- 40.Trinci A P J, Collinge A J. Hyphal wall growth in Neurospora crassa and Geotrichum sandinum. J Gen Microbiol. 1975;91:355–361. doi: 10.1099/00221287-91-2-355. [DOI] [PubMed] [Google Scholar]
- 41.Venkitasubramanian T A, Gupta S K. Biosynthesis of aflatoxins. Ann Nutr Aliment. 1977;31:635–642. [PubMed] [Google Scholar]
- 42.Watts H J, Very A A, Perera T H, Davies J M, Gow N A. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology. 1998;144:689–695. doi: 10.1099/00221287-144-3-689. [DOI] [PubMed] [Google Scholar]
- 43.Werner-Washburne M, Braun E, Johnston G C, Singer R A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993;57:383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werner-Washburne M, Braun E L, Crawford M E, Peck V M. Stationary phase in Saccharomyces cerevisiae. Mol Microbiol. 1996;19:1159–1166. doi: 10.1111/j.1365-2958.1996.tb02461.x. [DOI] [PubMed] [Google Scholar]
- 45.Wessels J G H. Fungal growth and development: a molecular perspective. In: Hawksworth D L, editor. Frontiers in mycology. Regensburg, Germany: C.A.B. International; 1991. pp. 27–47. [Google Scholar]
- 46.West C P. Physiology and drought tolerance of endophyte-infected grasses. In: Bacon C W, White J F Jr, editors. Bio/technology of endophytic fungi of grasses. London, England: CRC Press; 1994. pp. 87–99. [Google Scholar]
- 47.White J F, Jr, Reddy P V, Bacon C W. Biotrophic endophytes of grasses: a systematic approach. In: White J F, Bacon C W, editors. Microbial endophytes. New York, N.Y: Marcel Dekker; 2000. pp. 49–62. [Google Scholar]
- 48.Woldringh C L, Huls P G, Vischer N O. Volume growth of daughter and parent cells during the cell cycle of Saccharomyces cerevisiae a/alpha as determined by image cytometry. J Bacteriol. 1993;175:3174–3181. doi: 10.1128/jb.175.10.3174-3181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang N, Scott V, Al-Samarrai T H, Tan Y Y, Spiering M J, McMillan L, Scott D B, Christensen M, Schmid J. Transformation of Neotyphodium lolii with plasmids containing a native promoter disturbs the symbiotic interaction with its host. In: Paul V H, Dapprich P D, editors. Proceedings of the 4th Neotyphodium/Grass Interactions Symposium. Soest, Germany: Fachbereich Agrarwirtschaft; 2001. pp. 325–331. [Google Scholar]