Abstract: Background
It is unclear whether vitamin D benefits inpatients with COVID-19. Objective: To examine the relationship between vitamin D and COVID-19 outcomes. Design: Cohort study. Setting: National COVID Cohort Collaborative (N3C) database. Patients: 158,835 patients with confirmed COVID-19 and a sub-cohort with severe disease (n = 81,381) hospitalized between 1 January 2020 and 31 July 2021. Methods: We identified vitamin D prescribing using codes for vitamin D and its derivatives. We created a sub-cohort defined as having severe disease as those who required mechanical ventilation or extracorporeal membrane oxygenation (ECMO), had hospitalization >5 days, or hospitalization ending in death or hospice. Using logistic regression, we adjusted for age, sex, race, BMI, Charlson Comorbidity Index, and urban/rural residence, time period, and study site. Outcomes of interest were death or transfer to hospice, longer length of stay, and mechanical ventilation/ECMO. Results: Patients treated with vitamin D were older, had more comorbidities, and higher BMI compared with patients who did not receive vitamin D. Vitamin D treatment was associated with an increased odds of death or referral for hospice (adjusted odds ratio (AOR) 1.10: 95% CI 1.05–1.14), hospital stay >5 days (AOR 1.78: 95% CI 1.74–1.83), and increased odds of mechanical ventilation/ECMO (AOR 1.49: 95% CI 1.44–1.55). In the sub-cohort of severe COVID-19, vitamin D decreased the odds of death or hospice (AOR 0.90, 95% CI 0.86–0.94), but increased the odds of hospital stay longer >5 days (AOR 2.03, 95% CI 1.87–2.21) and mechanical ventilation/ECMO (AOR 1.16, 95% CI 1.12–1.21). Limitations: Our findings could reflect more aggressive treatment due to higher severity. Conclusion: Vitamin D treatment was associated with greater odds of extended hospitalization, mechanical ventilation/ECMO, and death or hospice referral.
Keywords: COVID-19, vitamin D
1. Introduction
The COVID-19 pandemic has resulted in over 6.3 million deaths worldwide since March 2020 [1]. Although therapeutic advances are accelerating, efforts to understand the optimal management of people who are hospitalized with COVID-19 are critical to improving outcomes. Vitamin D is known to have complex effects on immunity, including activating innate immunity [2,3,4,5]. Vitamin D receptors are found on B cells, T cells, and antigen-presenting cells [6,7,8]. Vitamin D also modulates the respiratory epithelial and systemic response to viral infections [9,10]. It also reduces the overactive immune response, which may be relevant in the proinflammatory cytokine production and storm observed in severe infections [11,12,13] and acute respiratory distress syndrome (ARDS) [14]. A body of evidence from observational studies shows lower vitamin D levels in people with advanced age as well as chronic illnesses that are associated with poor COVID-19 outcomes, including chronic kidney disease and people with obesity [15,16,17]. However, this may simply be a marker of overall health and chronic disease burden. Efforts to supplement vitamin D for acutely ill patients have not demonstrated benefit in randomized trials conducted before the COVID-19 pandemic [18].
Studies examining the relationship between clinical outcomes from COVID-19 and vitamin D have produced varied results. In people with mild to moderate disease treated with high-dose vitamin D as outpatients, the time to clinical recovery was accelerated [19]. In an analysis of a large biobank in the UK, baseline vitamin D serum concentration was not associated with COVID-19 severity or mortality [20]. Among inpatients, a systematic review and meta-analysis suggested vitamin D supplementation is beneficial for reducing intensive care stays and mortality for people hospitalized with severe COVID-19 [21]. In contrast, a single high dose of vitamin D did not reduce length of stay in hospitalized COVID-19 patients with moderate to severe disease in a randomized trial [22]. Because there were no established practice guidelines for COVID-19 early in the pandemic, physicians may have prescribed vitamin D as part of a regimen aimed at the most likely mechanisms of disease progression, including cytokine storm and ARDS [12]. Inpatient physicians may also have been more inclined to prescribe vitamin D in patients experiencing more severe COVID-19 disease manifestations because of the relative safety of vitamin D, but we are unaware of any large-scale reports on vitamin D prescribing using national data.
The purpose of this analysis was to better understand the relationship between vitamin D given during hospitalization for COVID-19 and clinical outcomes in a national study according to underlying risk groups, including people with more severe disease and those at higher risk of poor outcomes.
2. Methods
We analyzed data from the National COVID Cohort Collaborative (N3C), a nationwide patient database created and managed by the National COVID Cohort Collaborative (N3C) [23]. The N3C is a partnership among NIH’s National Center for Advancing Translational Sciences (NCATS) programs including Clinical and Translational Science Awards (CTSA) Program hubs, the National Center for Data to Health (CD2H), and NIGMS-supported Institutional Development Award Networks for Clinical and Translational Research (IDeA-CTR), with overall stewardship by NCATS.
The N3C database is a centralized repository of electronic health record (EHR)-based clinical information on COVID-19 patients and controls submitted by 61 medical centers nationwide (as of 4 November 2021). Each study site provides demographic, medication, laboratory, diagnoses, and vital status data which is harmonized into the Observational Medical Outcomes Partnership (OMOP) data model. Information is available for COVID-19 encounters as well as a patient look-back data period to 1 January 2018 to provide information on pre-existing conditions and encounters. The N3C design, data collection, sampling approach, and data harmonization methods have been described previously [23,24].
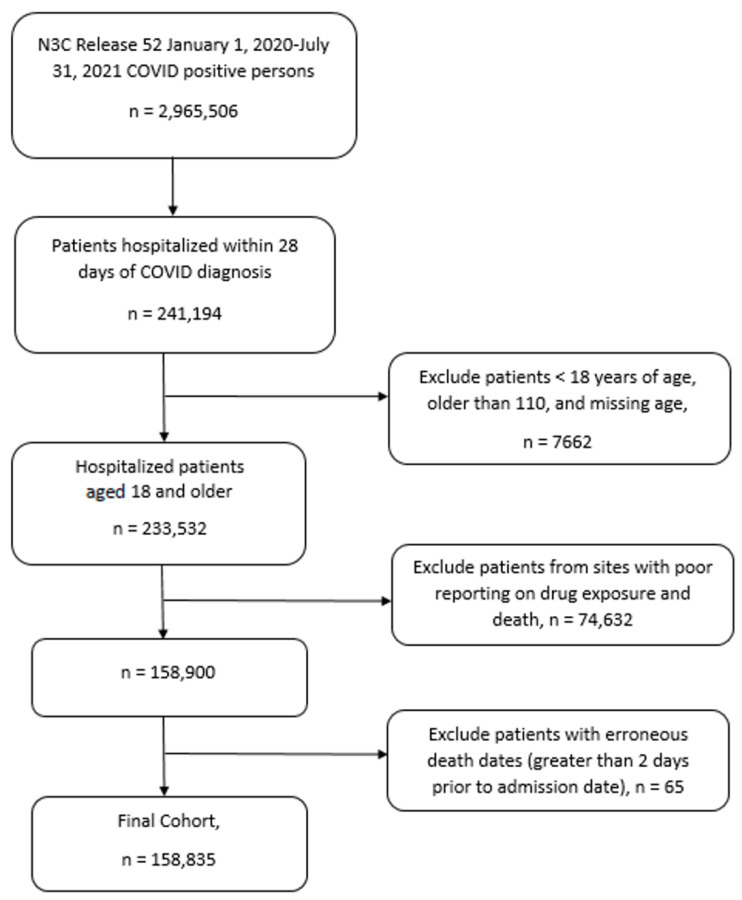
This analysis included 158,835 patients diagnosed with severe acute respiratory syndrome associated with coronavirus-2 (SARS-CoV-2) who were hospitalized within 28 days of their COVID-19 diagnosis. We included patients 18 years of age and older, diagnosed between 1 January 2020 and 31 July 2021 (Figure 1). We defined COVID-19 using positive lab measurements (either polymerase chain reaction or antigen test), positive antibodies, or positive COVID-19 diagnosis (corresponding to U07.1 ICD10-CM code). We excluded patients from data submitters with lower-than-expected reporting for death and for drug exposure information (10 sites). As a result, we retained patients from 51 study sites.
Figure 1.
N3C Cohort Selection Consort Diagram.
The study outcomes included death or referral to hospice, length of stay longer than 5 days, and mechanical ventilation or extracorporeal membrane oxygenation (ECMO). We selected longer than 5 days as a criterion because the national data show that five to six days is the median length of stay for COVID-19 [25,26], and five days was the median in our study. We examined the relationship between vitamin D treatment during hospitalization and these three major outcomes. We categorized a patient as having received vitamin D if any dose of vitamin D was recorded, regardless of preparation. We were unable to reliably determine the dose, duration, or frequency due to source system information sparsity. Additionally, we were not able to fully capture vitamin D treatment prior to hospitalization because self-reporting of over-the-counter vitamin D is not available in the database. While information on patients prescribed vitamin D prior to hospitalization was available for those patients treated within the health systems submitting data to N3C, we do not have prescription data on patients outside of those reporting health systems.
The main independent variable of interest was vitamin D treatment during hospitalization. Descriptive analyses were performed to inform poor outcomes associated with SARS-CoV-2. The data were compared by outcomes using Student’s t-tests for continuous data and Pearson’s chi square tests were used as appropriate for categorical data. Logistic regression analyses were performed to identify the factors associated with death or referral to hospice, length of stay of 5 days or longer, and mechanical ventilation or ECMO.
The potential covariates were chosen a priori based on clinical knowledge and literature review. The models were adjusted for age, sex, race, year and calendar quarter of COVID-19 diagnosis, urban/rural residence, BMI, Charlson comorbidity score [27] (0, 1, 2, or 3 or more), and study site. The covariates and concept definitions are available in Supplementary Table S7. The analyses were conducted using SQL and R (v3.5.1) in the N3C data enclave (data release v52, 4 November 2021). We used the R package MatchIt (R Foundation for Statistical Computing, Vienna, Austria, v4.1.0) [28] for propensity scoring in separate sensitivity analyses using nearest neighbor, matching on vitamin D and using all covariates.
Because of hypotheses from the literature review and findings from the first phases of analysis that patients with more severe disease were more likely to receive vitamin D, we created a sub-cohort to examine relationships between vitamin D and outcomes in patients with severe COVID-19 disease (n = 81,381). Severe disease was defined as having experienced any one of the adverse outcomes under study: death or referral to hospice, length of stay longer than 5 days, or mechanical ventilation or ECMO.
3. Results
The characteristics of the full cohort included the following: 44% were aged 65 or older, 49% female, 52% white, 50% overweight or obese, and 35% had two or more comorbidities (Table 1). Eleven percent of the patients died or were referred to hospice (n = 16,838), 48% stayed longer than 5 days in the hospital (n = 75,751), and 13% required mechanical ventilation or ECMO (n = 20,417). Patients who were treated with vitamin D were older, had a greater number of comorbidities, and were slightly more overweight or obese compared with patients who did not receive vitamin D. In the full cohort of 158,835 patients, 28,993 (18%) received vitamin D during their hospital stay, while in the sub-cohort of 81,381 with severe disease, 18,132 (22%) received vitamin D. The majority of the patients who received vitamin D were treated within 5 days of hospitalization (88% among all patients, and 80% among patients with severe COVID-19).
Table 1.
Characteristics of inpatient COVID-19 cohort according to vitamin D treatment.
| All Patients | Patients with Severe COVID-19 | |||
|---|---|---|---|---|
| No Vitamin D | Vitamin D | No Vitamin D | Vitamin D | |
| n = 129,842 (82%) 1 | n = 28,993 (18%) 1 | n = 63,249 (78%) 1 | n = 18,132 (22%) 1 | |
| Age at Diagnosis | ||||
| 18–29 | 11,153 (8.6%) | 1012 (3.5%) | 2538 (83%) | 536 (17%) |
| 30–49 | 28,434 (22%) | 4374 (15%) | 9982 (81%) | 2394 (19%) |
| 50–64 | 35,699 (27%) | 8151 (28%) | 17,884 (78%) | 4936 (22%) |
| 65–74 | 25,047 (19%) | 6944 (24%) | 14,293 (76%) | 4486 (24%) |
| 75 and older | 29,509 (23%) | 8512 (29%) | 18,552 (76%) | 5780 (24%) |
| Sex | ||||
| Female | 63,349 (49%) | 14,537 (50%) | 27,233 (76%) | 8664 (24%) |
| Male | 65,643 (51%) | 14,353 (50%) | 35,585 (79%) | 9394 (21%) |
| Unknown | 850 (0.7%) | 103 (0.4%) | 431 (85%) | 74 (15%) |
| Race | ||||
| White | 5093 (3.9%) | 1117 (3.9%) | 31,891 (75%) | 10,652 (25%) |
| Black or African American | 25,252 (19%) | 5459 (19%) | 12,797 (79%) | 3469 (21%) |
| Asian | 4083 (3.1%) | 808 (2.8%) | 2633 (78%) | 735 (22%) |
| Other | 29,377 (23%) | 4284 (15%) | 2004 (77%) | 613 (23%) |
| Unknown | 66,037 (51%) | 17,325 (60%) | 13,924 (84%) | 2663 (16%) |
| Ethnicity | ||||
| Hispanic or Latino | 28,072 (22%) | 5079 (18%) | 12,705 (80%) | 3136 (20%) |
| Not Hispanic or Latino | 86,128 (66%) | 21,546 (74%) | 42,902 (76%) | 13,510 (24%) |
| Unknown | 15,642 (12%) | 2368 (8.2%) | 7642 (84%) | 1486 (16%) |
| Quarter of Diagnosis | ||||
| 2020 Q1 | 7033 (5.4%) | 857 (3.0%) | 4591 (87%) | 699 (13%) |
| 2020 Q2 | 28,417 (22%) | 4522 (16%) | 15,689 (83%) | 3229 (17%) |
| 2020 Q3 | 14,528 (11%) | 2749 (9.5%) | 6303 (79%) | 1684 (21%) |
| 2020 Q4 | 37,193 (29%) | 9891 (34%) | 17,521 (75%) | 5963 (25%) |
| 2021 Q1 | 27,857 (21%) | 7322 (25%) | 12,822 (74%) | 4460 (26%) |
| 2021 Q2 | 11,423 (8.8%) | 2781 (9.6%) | 4776 (75%) | 1580 (25%) |
| 2021 Q3 (July only) | 3391 (2.6%) | 871 (3.0%) | 1547 (75%) | 517 (25%) |
| RUCA Category: Patient Residence | ||||
| Urban | 1812 (1.4%) | 462 (1.6%) | 41,349 (77%) | 12,072 (23%) |
| Large rural | 5996 (4.6%) | 1973 (6.8%) | 3298 (73%) | 1233 (27%) |
| Small rural | 3173 (2.4%) | 837 (2.9%) | 1802 (75%) | 586 (25%) |
| Isolated | 35,625 (27%) | 6766 (23%) | 1064 (77%) | 318 (23%) |
| Unknown | 83,236 (64%) | 18,955 (65%) | 15,736 (80%) | 3923 (20%) |
| BMI | ||||
| Obese | 17,040 (13%) | 4159 (14%) | 18,428 (75%) | 6194 (25%) |
| Overweight | 38,866 (30%) | 9673 (33%) | 11,606 (77%) | 3493 (23%) |
| Normal weight | 23,880 (18%) | 5541 (19%) | 9002 (77%) | 2756 (23%) |
| Underweight | 3069 (2.4%) | 500 (1.7%) | 1659 (83%) | 351 (17%) |
| Unknown | 46,987 (36%) | 9120 (31%) | 22,554 (81%) | 5338 (19%) |
| Charlson Index Category | ||||
| 0 | 42,385 (33%) | 6786 (23%) | 16,199 (80%) | 3941 (20%) |
| 1 | 15,617 (12%) | 3350 (12%) | 6999 (78%) | 1919 (22%) |
| 2 | 11,228 (8.6%) | 2912 (10%) | 5512 (76%) | 1773 (24%) |
| 3 or more | 30,937 (24%) | 10,137 (35%) | 17,635 (73%) | 6463 (27%) |
| Unknown | 29675 (23%) | 5808 (20%) | 16904 (81%) | 4036 (19%) |
1 Statistic presented: n (%). Percent = row percent.
We observed a higher percentage of each of the three adverse outcomes among patients receiving vitamin D in the full cohort (Table 2). In the sub-cohort of patients with severe COVID-19, a slightly lower percentage of people who received vitamin D died or were referred to hospice (20% vs. 21%). However, a higher percentage of those who received vitamin D had a length of stay greater than 5 days (96% vs. 92%), and required mechanical ventilation or ECMO (27% vs. 25%).
Table 2.
Outcomes by full cohort compared to patients with severe COVID-19 disease.
| All Patients with COVID-19 | Patients with Severe COVID-19 | |||||
|---|---|---|---|---|---|---|
| No Vitamin D 129,842 (82%) 1 |
Vitamin D 28,993 (18%) 1 |
p-Value | No Vitamin D 63,249 (78%) 1 |
Vitamin D 18,132 (22%) 1 | p-Value | |
| Death/referral to hospice 2 |
13,185 (10%) | 3653 (13%) | <0.001 | 13,185 (21%) | 3653 (20%) | 0.04 |
| Length of stay > 5 days | 58,372 (45%) | 17,379 (60%) | <0.001 | 58,372 (92%) | 17,379 (96%) | <0.001 |
| Mechanical ventilation/ECMO | 15,520 (12%) | 4897 (17%) | <0.001 | 15,520 (25%) | 4897 (27%) | <0.001 |
1 Statistic presented: n (%). Percent = column percent. 2 Death or referral to hospice during hospitalization.
Table 3 shows the results from multivariable logistic regression models for vitamin D treatment compared with not receiving vitamin D during hospitalization for all three outcomes in the full cohort and those with severe disease. In the full cohort, vitamin D treatment during hospitalization was associated with an increased odds of death or referral for hospice (adjusted odds ratio (AOR) 1.10: 95% confidence interval (CI) 1.05–1.14), requiring a hospital stay longer than 5 days (AOR 1.78: 95% CI 1.74–1.83), and requiring mechanical ventilation or ECMO (AOR 1.49: 95% CI 1.44, 1.55). For patients with severe COVID-19, we observed a decreased odds of death or referral to hospice (AOR 0.90, 95% CI 0.86–0.94) and an increased odds of having a stay 5 days or longer (AOR 2.03, 95% CI 1.87–2.21) or requiring mechanical ventilation or ECMO (AOR 1.16, 95% CI 1.12–1.21) in those treated with vitamin D.
Table 3.
Multivariable logistic regression models for outcomes associated with vitamin D receipt.
| All Patients with COVID-19 | Patients with Severe COVID-19 | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusted OR * (95% CI) |
Unadjusted OR (95% CI) |
Adjusted OR * (95% CI) |
|
| Death/hospice | 1.27 (1.23–1.33) | 1.10 (1.05–1.14) | 0.96 (0.92–1.00) | 0.90 (0.86–0.94) |
| Length of stay > 5 days | 1.83 (1.78–1.88) | 1.78 (1.74–1.83) | 1.93 (1.78–2.09) | 2.02 (1.87–2.20) |
| Mechanical ventilation/ECMO | 1.50 (1.45–1.55) | 1.49 (1.44–1.55) | 1.14 (1.10–1.18) | 1.16 (1.12–1.21) |
* Adjusted for age category, sex, race, time, (year/quarter), data partner, Charlson Comorbidity Index, vitamin D treatment, BMI, and RUCA code.
As a sensitivity analysis, we also conducted a propensity score analysis to assess the relationships between clinical factors and vitamin D prescribing. Our findings were not materially different using this approach.
We observed a marked variation in the use of vitamin D during hospitalization across the 51 study sites, from 3% to 40% (with one outlier, at 58%). Additional details from descriptive analyses and multivariable models are available in Supplementary Tables S1–S6.
4. Discussion
In this national cohort sample of people hospitalized with COVID-19, we observed an association between vitamin D administration and an increased risk of adverse outcomes including death, length of stay of more than 5 days, and the need for mechanical ventilation. However, among patients with more severe COVID-19, the receipt of vitamin D was associated with a lower risk of death or referral to hospice, despite the higher risk of requiring mechanical ventilation and longer length of stay. We also observed that 18% of inpatients received vitamin D during their hospital stay, although this varied substantially by reporting site, during a period when there was little evidence for its effect on COVID-19 outcomes.
Our findings are similar to another large cohort study showing no association between vitamin D status and length of stay or prognosis among patients with moderate to severe COVID-19 in adjusted analyses [29]. In contrast, a population-based cohort in Spain showed a modestly lower risk of severe disease and death in COVID-19 patients taking vitamin D [30]. Recent meta-analyses and systematic reviews of both randomized trials and non-randomized intervention studies showed a reduced risk of both the need for intensive care and mortality in people receiving supplementation in the hospital [21,31].
There are several possible explanations for our conflicting findings. Because vitamin D is associated with improved cellular immune function [2,32], the receipt of vitamin D may improve outcomes through improvements in the immune response. Additionally, or alternatively, because vitamin D may reduce cytokine storm [33], it may reduce the complications of COVID-19 in people with more severe disease [12]. It is possible that the most vulnerable and higher-risk patients with COVID-19 had lower vitamin D status at baseline, particularly since many are older and have comorbidities associated with poor health, and that vitamin D was beneficial in reducing death because of those relationships in the severely ill sub-cohort [34,35]. Our findings could also reflect the more aggressive treatment of people who were most ill from COVID-19, with vitamin D simply being part of a complex treatment plan. Although we adjusted for known risk factors for poor outcomes (e.g., age, obesity, and other comorbidities), residual confounding is always possible in cohort studies, and is, in fact, likely. Other clinical factors may have been driving prescribing practices, and although we use a propensity-matched approach for a sensitivity analysis, we have incomplete ascertainment of all clinical factors in this observational study.
This study had several limitations. We were unable to determine the dose, duration, or frequency of vitamin D, or the preparation for most patients, and therefore used “any” vitamin D receipt during the hospitalization as the exposure of interest. It is possible that the receipt of a specific dose or duration during a specific time window of the disease course could have been more effective for improving disease outcomes in selected patients with COVID-19. The baseline vitamin D status was not known, and it is also possible that people with poor outcomes also had suboptimal vitamin D status at baseline, therefore benefitting from vitamin D administration during hospitalization, while those with adequate vitamin D before hospitalization did not benefit from supplementation. As noted above, since this was an observational study, there may be unknown confounders or residual confounding even in the adjusted analyses.
In summary, we found a positive association between receiving vitamin D during hospitalization with COVID-19 and poor outcomes (death/hospice, extended length of stay, and mechanical ventilation or ECMO). Among patients with severe COVID-19 disease, we observed similar results except for death/hospice, which was inversely associated with vitamin D treatment. Large-scale clinical trials, already underway, are the best way to answer this question definitively, particularly with regard to nuanced differences in underlying vitamin D status, dose, timing, and duration. This work will inform additional randomized clinical trials and possibly clinical care pending those studies. In the interim, it may be prudent to only administer vitamin D to those with demonstrated deficiency.
Acknowledgments
The project described was supported by the National Institute of General Medical Sciences, U54GM104942-05S2, U54GM115458, U54GM104940, U54GM104938, U54GM115516, U54GM115677, U54GM115428, and U54GM104941. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The analyses described in publication were conducted with data or tools accessed through the NCATS N3C Data Enclave (https://covid.cdh2.org/, accessed on 21 June 2022) and supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organizations (https://ncats.nih.gov/n3c/resources/data-contribution/data-transfer-agreement-signatories, accessed on 21 June 2022) and scientists who have contributed to the ongoing development of this community resource (https://doi.org/10.1093/jamia/ocaa196, accessed on 21 June 2022). We gratefully acknowledge contributions from the following N3C core teams: (Asterisks indicate leads) • Principal Investigators: Melissa A. Haendel*, Christopher G. Chute*, Kenneth R. Gersing, Anita Walden. • Workstream, subgroup, and administrative leaders: Melissa A. Haendel*, Tellen D. Bennett, Christopher G. Chute, David A. Eichmann, Justin Guinney, Warren A. Kibbe, Hongfang Liu, Philip R.O. Payne, Emily R. Pfaff, Peter N. Robinson, Joel H. Saltz, Heidi Spratt, Justin Starren, Christine Suver, Adam B. Wilcox, Andrew E. Williams, Chunlei Wu. • Key liaisons at data partner sites. • Regulatory staff at data partner sites.• Individuals at the sites who are responsible for creating the datasets and submitting data to N3C • Data Ingest and Harmonization Team: Christopher G. Chute*, Emily R. Pfaff*, Davera Gabriel, Stephanie S. Hong, Kristin Kostka, Harold P. Lehmann, Richard A. Moffitt, Michele Morris, Matvey B. Palchuk, Xiaohan Tanner Zhang, Richard L. Zhu.• Phenotype Team (Individuals who create the scripts that the sites use to submit their data, based on the COVID and Long COVID definitions): Emily R. Pfaff*, Benjamin Amor, Mark M. Bissell, Marshall Clark, Andrew T. Girvin, Stephanie S. Hong, Kristin Kostka, Adam M. Lee, Robert T. Miller, Michele Morris, Matvey B. Palchuk, Kellie M. Walters. • Project Management and Operations Team: Anita Walden*, Yooree Chae, Connor Cook, Alexandra Dest, Racquel R. Dietz, Thomas Dillon, Patricia A. Francis, Rafael Fuentes, Alexis Graves, Julie A. McMurry, Andrew J. Neumann, Shawn T. O’Neil, Usman Sheikh, Andréa M. Volz, Elizabeth Zampino. • Partners from NIH and other federal agencies: Christopher P. Austin*, Kenneth R. Gersing*, Samuel Bozzette, Mariam Deacy, Nicole Garbarini, Michael G. Kurilla, Sam G. Michael, Joni L. Rutter, Meredith Temple-O’Connor. • Analytics Team (Individuals who build the Enclave infrastructure, help create codesets, variables, and help Domain Teams and project teams with their datasets): Benjamin Amor*, Mark M. Bissell, Katie Rebecca Bradwell, Andrew T. Girvin, Amin Manna, Nabeel Qureshi. • Publication Committee Management Team: Mary Morrison Saltz*, Christine Suver*, Christopher G. Chute, Melissa A. Haendel, Julie A. McMurry, Andréa M. Volz, Anita Walden. • Publication Committee Review Team: Carolyn Bramante, Jeremy Richard Harper, Wenndy Hernandez, Farrukh M Koraishy, Federico Mariona, Amit Saha, Satyanarayana Vedula. Data partners with released data: Stony Brook University—U24TR002306 • University of Oklahoma Health Sciences Center—U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • West Virginia University—U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • University of Mississippi Medical Center—U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center—U54GM115458: Great Plains IDeA-Clinical & Translational Research • Maine Medical Center—U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Wake Forest University Health Sciences—UL1TR001420: Wake Forest Clinical and Translational Science Institute • Northwestern University at Chicago—UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • University of Cincinnati—UL1TR001425: Center for Clinical and Translational Science and Training • The University of Texas Medical Branch at Galveston—UL1TR001439: The Institute for Translational Sciences • Medical University of South Carolina—UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • University of Massachusetts Medical School Worcester—UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Southern California—UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • Columbia University Irving Medical Center—UL1TR001873: Irving Institute for Clinical and Translational Research • George Washington Children’s Research Institute—UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • University of Kentucky—UL1TR001998: UK Center for Clinical and Translational Science • University of Rochester—UL1TR002001: UR Clinical & Translational Science Institute • University of Illinois at Chicago—UL1TR002003: UIC Center for Clinical and Translational Science • Penn State Health Milton S. Hershey Medical Center—UL1TR002014: Penn State Clinical and Translational Science Institute • The University of Michigan at Ann Arbor—UL1TR002240: Michigan Institute for Clinical and Health Research • Vanderbilt University Medical Center—UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • University of Washington—UL1TR002319: Institute of Translational Health Sciences • Washington University in St. Louis—UL1TR002345: Institute of Clinical and Translational Sciences • Oregon Health & Science University—UL1TR002369: Oregon Clinical and Translational Research Institute • University of Wisconsin-Madison—UL1TR002373: UW Institute for Clinical and Translational Research • Rush University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Chicago—UL1TR002389: The Institute for Translational Medicine (ITM) • University of North Carolina at Chapel Hill—UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Minnesota—UL1TR002494: Clinical and Translational Science Institute • Children’s Hospital Colorado—UL1TR002535: Colorado Clinical and Translational Sciences Institute • The University of Iowa—UL1TR002537: Institute for Clinical and Translational Science • The University of Utah— UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center—UL1TR002544: Tufts Clinical and Translational Science Institute • Duke University—UL1TR002553: Duke Clinical and Translational Science Institute • Virginia Commonwealth University—UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • The Ohio State University—UL1TR002733: Center for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine—UL1TR002736: University of Miami Clinical and Translational Science Institute • University of Virginia—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Carilion Clinic—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Alabama at Birmingham—UL1TR003096: Center for Clinical and Translational Science • Johns Hopkins University—UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • Nemours—U54GM104941: Delaware CTR ACCEL Program • University Medical Center New Orleans—U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Colorado Denver, Anschutz Medical Campus—UL1TR002535: Colorado Clinical and Translational Sciences Institute • Mayo Clinic Rochester—UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Tulane University—UL1TR003096: Center for Clinical and Translational Science • Loyola University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM) • Advocate Health Care Network—UL1TR002389: The Institute for Translational Medicine (ITM) • OCHIN—INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks. Additional Data Partners Who Have Signed Data and Data Release Pending. The Rockefeller University—UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute—UL1TR002550: Scripps Research Translational Institute • University of Texas Health Science Center at San Antonio—UL1TR002645: Institute for Integration of Medicine and Science • The University of Texas Health Science Center at Houston—UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • NorthShore University HealthSystem—UL1TR002389: The Institute for Translational Medicine (ITM) • Yale New Haven Hospital—UL1TR001863: Yale Center for Clinical Investigation • Emory University—UL1TR002378: Georgia Clinical and Translational Science Alliance • Weill Medical College of Cornell University—UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • Montefiore Medical Center—UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Medical College of Wisconsin—UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • University of New Mexico Health Sciences Center—UL1TR001449: University of New Mexico Clinical and Translational Science Center • George Washington University—UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Stanford University—UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • Regenstrief Institute—UL1TR002529: Indiana Clinical and Translational Science Institute • Cincinnati Children’s Hospital Medical Center—UL1TR001425: Center for Clinical and Translational Science and Training • Boston University Medical Campus—UL1TR001430: Boston University Clinical and Translational Science Institute • The State University of New York at Buffalo—UL1TR001412: Clinical and Translational Science Institute • Aurora Health Care—UL1TR002373: Wisconsin Network For Health Research • Brown University—U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Rutgers, The State University of New Jersey—UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Loyola University Chicago—UL1TR002389: The Institute for Translational Medicine (ITM) • #N/A—UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • University of Kansas Medical Center—UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Icahn School of Medicine at Mount Sinai—UL1TR001433: ConduITS Institute for Translational Sciences • Ochsner Medical Center—U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • HonorHealth—None (Voluntary) • University of California, Irvine—UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, San Diego—UL1TR001442: Altman Clinical and Translational Research Institute • University of California, Davis—UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, San Francisco—UL1TR001872: UCSF Clinical and Translational Science Institute • University of California, Los Angeles—UL1TR001881: UCLA Clinical Translational Science Institute • University of Vermont—U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Arkansas Children’s Hospital—UL1TR003107: UAMS Translational Research Institute.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14153073/s1, Table S1: Detail of Characteristics of Patients Associated with Key Outcomes in Hospitalized Patients with COVID-19; Table S2: Detail of Characteristics of Patients Associated with Key Outcomes in Hospitalized Patients with Severe COVID-19; Table S3: Full Detail of Characteristics of Patients Associated with Key Outcomes in Hospitalized Patients with COVID-19 (n = 158,835); Table S4: Full Detail of Characteristics Associated with Key Outcomes in Hospitalized Patients with Severe COVID-19 (n = 81,381); Table S5: Multivariable Models for Vitamin D Receipt and Key Outcomes: Full Cohort; Table S6: Multivariable Models for Vitamin D and Key Outcomes: Patients with Severe COVID; Table S7. Key variables and concept definitions.
Author Contributions
Conceptualization, K.M.F., C.J.R., S.L.H. and K.A.M.; methodology, K.M.F., K.A.M., W.B. and C.J.R.; validation, J.H., W.B., A.J.A. and N3C Consortium; formal analysis, K.A.M., M.K., A.J.A. and K.M.F.; investigation, C.J.R. and S.L.H.; resources, S.S.; writing—original draft preparation, K.M.F. and K.A.M.; writing—review and editing, C.J.R. and S.S.; project administration, K.A.M.; funding acquisition, C.J.R., S.L.H. and S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol # IRB00249128 or individual site agreements with NIH. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board above.
Informed Consent Statement
Patient consent was waived due to the fact this is a retrospective observational study using data from electronic medical records, and obtaining consent was not possible.
Data Availability Statement
All diagnostic, medication, procedure, and laboratory concepts used in this study are available in Supplementary Table S7. Raw code (R, SQL) is available upon request. N3C is a public resource maintained by NCATS to support COVID-19 research. To access patient-level data from the N3C consortium, institutions must have a signed Data Use Agreement executed with NCATS and investigators must complete mandatory training along with submitting a Data Use Request to N3C. Investigators can request access to the Enclave here (https://covid.cd2h.org/onboarding, accessed on 21 June 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The project described was supported by the National Institute of General Medical Sciences, 5U54GM104942-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Authorship was determined using ICMJE recommendations.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 10 June 2022)]. Available online: https://covid19.who.int.
- 2.Adams J.S., Hewison M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher J., Bishop E.L., Harrison S.R., Swift A., Cooper S.C., Dimeloe S.K., Raza K., Hewison M. Autoimmune disease and interconnections with vitamin D. Endocr. Connect. 2022;11:e210554. doi: 10.1530/EC-21-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop E.L., Ismailova A., Dimeloe S.K., Hewison M., White J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus. 2021;5:e10405. doi: 10.1002/jbm4.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Provvedini D.M., Tsoukas C.D., Deftos L.J., Manolagas S.C. 1,25-Dihydroxyvitamin D3 Receptors in Human Leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 7.Provvedini D.M., Tsoukas C.D., Deftos L.J., Manolagas S.C. 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: Effects on immunoglobulin production. J. Immunol. 1986;136:2734–2740. [PubMed] [Google Scholar]
- 8.Bhalla A.K., Amento E.P., Clemens T.L., Holick M.F., Krane S.M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in t lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 9.Greiller C.L., Martineau A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin, D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White J.H. Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients. 2022;14:284. doi: 10.3390/nu14020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Leung D.Y.M., Richers B.N., Liu Y., Remigio L.K., Riches D.W., Goleva E. Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., Madhavan M.V., Nair N., Babalyan V., Hutchings N., et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183:R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gönen M.S., Alaylıoğlu M., Durcan E., Özdemir Y., Şahin S., Konukoğlu D., Nohut O.K., Ürkmez S., Küçükece B., Balkan I.I., et al. Rapid and Effective Vitamin D Supplementation May Present Better Clinical Outcomes in COVID-19 (SARS-CoV-2) Patients by Altering Serum INOS1, IL1B, IFNg, Cathelicidin-LL37, and ICAM1. Nutrients. 2021;13:4047. doi: 10.3390/nu13114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dancer R.C.A., Parekh D., Lax S., D’Souza V., Zheng S., Bassford C.R., Park D., Bartis D.G., Mahida R., Turner A.M., et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Looker A.C., Dawson-Hughes B., Calvo M.S., Gunter E.W., Sahyoun N.R. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/S8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 16.Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 17.Schleicher R.L., Sternberg M.R., Lacher D.A., Sempos C.T., Looker A.C., Durazo-Arvizu R.A., Yetley E.A., Chaudhary-Webb M., Maw K.L., Pfeiffer C.M., et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am. J. Clin. Nutr. 2016;104:454–461. doi: 10.3945/ajcn.115.127985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langlois P.L., Szwec C., D’Aragon F., Heyland D.K., Manzanares W. Vitamin D supplementation in the critically ill: A systematic review and meta-analysis. Clin. Nutr. 2018;37:1238–1246. doi: 10.1016/j.clnu.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Sabico S., Enani M.A., Sheshah E., Aljohani N.J., Aldisi D.A., Alotaibi N.H., Alshingetti N., Alomar S.Y., Alnaami A.M., Amer O.E., et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. Nutrients. 2021;13:2170. doi: 10.3390/nu13072170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastie C.E., Pell J.P., Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2021;60:545–548. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseini B., El Abd A., Ducharme F.M. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients. 2022;14:2134. doi: 10.3390/nu14102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., Silva C.B.R., Franco A.S., Macedo M.B., Dalmolin H.H.H., et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haendel M.A., Chute C.G., Bennett T.D., Eichmann D., Guinney J., Kibbe W.A., Payne P.R.O., Pfaff E.R., Robinson P.N., Saltz J.H., et al. The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment. J. Am. Med. Inform. Assoc. 2021;28:427–443. doi: 10.1093/jamia/ocaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett T.D., Moffitt R.A., Hajagos J.G., Amor B., Anand A., Bissell M.M., Bradwell K.R., Bremer C., Byrd J.B., Denham A., et al. Clinical Characterization and Prediction of Clinical Severity of SARS-CoV-2 Infection Among US Adults Using Data From the US National COVID Cohort Collaborative. JAMA Netw. Open. 2021;4:e2116901. doi: 10.1001/jamanetworkopen.2021.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan X., Johnson B.H., Johnston S.S., Elangovanraaj N., Kothari P., Spira A., Coplan P., Khanna R. Monthly trend in mortality and length of stay among coronavirus disease 2019 (COVID-19) patients: Analysis of a nationwide multihospital US database. Infect. Control Hosp. Epidemiol. 2021;42:1132–1135. doi: 10.1017/ice.2021.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen N.T., Chinn J., Nahmias J., Yuen S., Kirby K.A., Hohmann S., Amin A. Outcomes and Mortality Among Adults Hospitalized with COVID-19 at US Medical Centers. JAMA Netw. Open. 2021;4:e210417. doi: 10.1001/jamanetworkopen.2021.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 28.Ho D., Imai K., King G., Stuart E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 29.Reis B.Z., Fernandes A.L., Sales L.P., Santos M.D., dos Santos C.C., Pinto A.J., Goessler K.F., Franco A.S., Duran C.S.C., Silva C.B.R., et al. Influence of vitamin D status on hospital length of stay and prognosis in hospitalized patients with moderate to severe COVID-19: A multicenter prospective cohort study. Am. J. Clin. Nutr. 2021;114:598–604. doi: 10.1093/ajcn/nqab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oristrell J., Oliva J.C., Casado E., Subirana I., Domínguez D., Toloba A., Balado A., Grau M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022;45:167–179. doi: 10.1007/s40618-021-01639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah K., Varna V.P., Sharma U., Mavalankar D. Does vitamin D supplementation reduce COVID-19 severity?—A systematic review. QJM. 2022 doi: 10.1093/qjmed/hcac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomaszewska A., Rustecka A., Lipińska-Opałka A., Piprek R.P., Kloc M., Kalicki B., Kubiak J.Z. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front. Pharmacol. 2022;13:836738. doi: 10.3389/fphar.2022.836738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weir E.K., Thenappan T., Bhargava M., Chen Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. 2020;20:e107–e108. doi: 10.7861/clinmed.2020-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zajic P., Amrein K. Vitamin D deficiency in the ICU: A systematic review. Minerva Endocrinol. 2014;39:275–287. [PubMed] [Google Scholar]
- 35.Amrein K., Schnedl C., Holl A., Riedl R., Christopher K.B., Pachler C., Purkart T.U., Waltensdorfer A., Münch A., Warnkross H., et al. Effect of High-Dose Vitamin D3 on Hospital Length of Stay in Critically Ill Patients with Vitamin D Deficiency: The VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All diagnostic, medication, procedure, and laboratory concepts used in this study are available in Supplementary Table S7. Raw code (R, SQL) is available upon request. N3C is a public resource maintained by NCATS to support COVID-19 research. To access patient-level data from the N3C consortium, institutions must have a signed Data Use Agreement executed with NCATS and investigators must complete mandatory training along with submitting a Data Use Request to N3C. Investigators can request access to the Enclave here (https://covid.cd2h.org/onboarding, accessed on 21 June 2022).