Abstract
The genus Curcuma, composed of 93 species mainly originating from Asia, Australia, and South America, has been used for medicinal purposes, aromatic, and nutritional values as well as cosmetic. It plays a vital role in flavoring and coloring as well as exhibiting therapeutic agents against different diseases. Nepalese farmers are unaware of the essential oil compositions of Curcuma species, viz. C. aeruginosa, C. zedoaria, and C. longa. The investigation of these three essential oils provides insight into their potential as cash crops and earns a reasonable return from their production. The essential oils were obtained from the rhizomes of each plant by hydrodistillation and subjected to Gas Chromatography/Mass Spectrometry (GC–MS) analysis to identify its volatile chemical constituents as well as chiral GC-MS to identify the enantiomeric distribution of chiral terpenoids. The order of extraction yields were C. longa (0.89%) > C. zedoaria (0.74%) > C. aeruginosa (0.37%). In total, the presence of 65, 98, and 84 compounds were identified in C. longa, C. zedoaria, and C. aeruginosa, representing 95.82%, 81.55%, and 92.59% of the total oil, respectively. The most abundant compounds in C. longa essential oils were ar-turmerone (25.5%), α-turmerone (24.4%), β-turmerone (14.0%), terpinolene (7.2%), β-sesquiphellandrene (5.1%), α-zingiberene (4.8%), β-caryophyllene (2.9%), ar-curcumene (1.6%) and 1,8-cineole (1.3%). The most dominant compounds in C. zedoaria were curzerenone (21.5%), 1,8-cineole (19.6%), curzerene (6.2%), trans-β-Elemene (5.1%), camphor (2.6%), and germacrone (2.3%). The major components in C. aeruginosa were curzerenone (59.6%), germacrone (5.3%), curzerene (4.7%), camphor (3.6%), trans-β-Elemene (2.6%), and β-eudesmol (1.6%). C. zedoaria, and C. aeruginosa essential oil from Nepal for the very first time. This study reports for the first time chiral terpenoids from C. aeruginosa, C. zedoaria, and C. longa essential oil. A chemical blueprint of these essential oils could also be used as a tool for identification and quality assessment.
Keywords: α-turmerone, β-turmerone, ar-turmerone, curzerenone, enantiomeric distribution
1. Introduction
The genus Curcuma (Zingiberaceae) is composed of 93 species that mainly originate from Asia, Australia, and South America, and are now cultivated worldwide. Members of this genus have a long history for their medicinal purposes and nutritional values as well as in cosmetics industries [1,2,3]. The major pharmacologically active constituents of Curcuma species are curcuminoids and essential oils. Curcuminoids, a mixture of three phenolic compounds namely curcumin, demethoxycurcumin, and bis-demethoxycurcumin, have been proven to posses significant health benefits along with the potential to prevent various diseases [4,5,6]. Essential oils of Curcuma species are relatively complex with hundreds of components including terpenes and oxygenated terpenoids. Fragrances and characteristic aromas of Curcuma essential oils are due to either a large number of monocyclic bisabolane derivatives or guaiane type sesquiterpenes or germacranes type sesquiterpenes [7]. Different Curcuma species essential oils were used in food applications (flavoring, coloring, and preservatives) [8], personal goods (cosmetics and perfumes) [9], and to cope and deal with a variety of ailments (inflammatory conditions of various organs, digestive tract problems, neurodegenerative diseases, wound healing, cancer, viral diseases, and diabetes) [10,11,12,13,14].
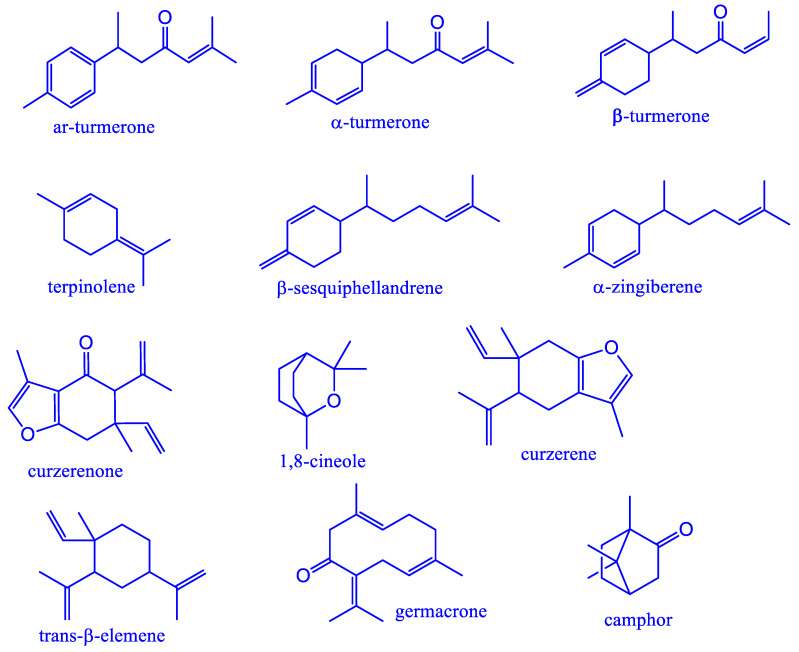
The medicinal and culinary properties of the Curcuma species rhizome are well-known. Among them, Curcuma longa, and Curcuma zedoaria were investigated widely around the world due to their high commercial value [15]. On other hand, Curcuma aeruginosa essential oil data are less common in the scientific world. C. longa is known as yellow turmeric and is most common in the world as a culinary item to enhance flavoring and coloring as well as a good source of antioxidant agents [3]. C. longa essential oil had demonstrated good therapeutic properties in diabetes [16], inflammation [17], neuroprotective [18], antimicrobial [19], antioxidant [20] and so on. Most representative components in its essential oil are α-turmerone, ar-turmerone, β-turmerone, β-sesquiphellandrene, α-zingiberene, 1,8-cineole, germacrone, ar-curcumene, and α-phellandrene [8,21]. C. zedoaria rhizome is also called white turmeric and its essential oil is generally composed of 1,8-cineole, curzerenone, camphor, β-caryophyllene, α-elemol, germacrone, curzerene, and β-elemene [22]. The essential oil showed promising activity against cancer [23], diabetes [24], anti-angiogenic [25], and antioxidant [26]. C. aeruginosa is known as blue curcuma and its essential oil dominated by curzerenone, 1,8-cineole, camphor, β-pinene, iso-borneol, germacrone, curzerene, and curcumenol [15]. The essential oil demonstrated good antimicrobial activity [27], and help in prevention of hair loss [9]. The major compounds of these species are presented in Figure 1. The demand for these three Curcuma species essential oils is steadily increasing as a result of a plethora of scientific articles on the health benefits. However, differences in genotype, edaphic variables, pedo-climatic conditions, harvest time, extraction procedure, maturity of rhizome, and analytical procedures all contributed to variations in the chemical composition of Curcuma species essential oils [10].
Figure 1.
Major compounds present in C. longa, C. zedoaria, and C. aeruginosa.
Curcuma species can be grown in a variety of soil types as well as in warm and humid climates. Nepal has an extreme altitudinal range with heterogeneous topography with distinct climatic variation and is favorable for the growth of C. longa, C. zedoaria, and C. aeruginosa rhizome. The essential oil of these species has huge demand in the international market. However, Nepalese farmers are still struggling to earn a reasonable return from their production due to lack of sophisticated processing unit and still unknown about the essential oil composition. Hence, there is a dire need to investigate the chemical compositions of rhizome essential oil among Curcuma species, viz. C. aeruginosa, C. zedoaria, and C. longa. This is the first research paper from Nepal and includes an in-depth analysis of the Curcuma essential oil compositions. Furthermore, the chemical profiles of Curcuma species essential oils from Nepal could provide additional chemical fingerprints for identification and quality assessment.
2. Results and Discussion
2.1. Isolation of Essential Oil and Yields
The fresh mature mother rhizomes of C. zedoaria, C. longa, and C. aeruginosa with light yellow, yellow and blue flesh respectively, were hydrodistilled creating colorless essential oil. The highest extraction yield was observed in C. longa (0.89%) followed by C. zedoaria (0.74%) and C. aeruginosa (0.37%). The extraction yield was quite low, but in close agreement with hydrodistillation yields previously reported [1,22]. In the case of C. longa, there were differences in extraction yields; cured had the highest yield, followed by fresh, and then dry rhizome [28]. To the best of our knowledge, maximum yield on hydrodistillation of C. longa, C. zedoaria, and C. aeruginosa had been reported upto 5.5% [29], 1.6% [25], and 0.63% [30], respectively. Genetic variation, harvesting time, the extraction process, and rhizome maturity are all factors that influence extraction yield.
2.2. Chemical Composition of Essential Oils
The chemical composition of C. longa rhizome essential oils is shown in Table 1. The total number of identified compounds in C. longa essential oil is 65 and accounted for 95.82%. The dominant volatile constituents in the C. longa essential oil was ar-turmerone (25.5%), α-turmerone (24.4%), β-turmerone (14.0%), terpinolene (7.2%), β-sesquiphellandrene (5.1%), α-zingiberene (4.8%), β-caryophyllene (2.9%), ar-curcumene (1.6%) and 1,8-cineole (1.3%), and this is comparable to C. longa essential oil compositions grown in North Alabama and India. Furthermore, there was little variation observed between dry and fresh as well as lateral and mother rhizomes [21,28]. Prior research on the essential oils of C. longa rhizomes from various geographic locations identified four distinct clusters based on the relative concentrations of turmerones. The first class of clusters is dominated by turmerones, but with relatively large concentrations of other constituents as well such as β-sesquiphellandrene, α-zingiberene, ar-curcumene, and 1,8-cineole. The second class of clusters is dominated by turmerones, especially ar-turmerone, the third class is dominated by turmerones, especially α-turmerone, and the fourth is dominated by very high concentrations of ar-turmerone [15]. So, C. longa rhizome essential oils from Nepal fall under the category of the first cluster, i.e., turmerones were dominant, but there were other significant constituents as well. Interestingly, an essential oil from Brazil had ar-turmerone, α-zingiberene, β-sesquiphellandrene, humulene epoxide II, cis-α-trans-bergamotol, and β-turmerone [31]. A sample from India was dominated by 1,8-cineole, β-caryophyllene, α-turmerone, β-turmerone, ar-turmerone, and α-phellandrene [32]. All of this suggests that turmerone concentrations, whether high or low, should be desirable in C. longa essential oil.
Table 1.
Individual constituents of Curcuma longa essential oil.
| RIcalc | RIdb | Compound Name | Area% of Constituents in Curcuma longa |
|---|---|---|---|
| 925 | 924 | α-Thujene | t |
| 933 | 931 | α-Pinene | 0.1 |
| 972 | 972 | Sabinene | t |
| 978 | 978 | β-Pinene | t |
| 989 | 989 | Myrcene | 0.1 |
| 1001 | 1000 | δ-2-Carene | t |
| 1007 | 1008 | α-Phellandrene | 0.2 |
| 1009 | 1009 | δ-3-Carene | 0.1 |
| 1017 | 1016 | α-Terpinene | 0.4 |
| 1020 | 1024 | p-Cymene | 0.1 |
| 1024 | 1028 | Limonene | 0.1 |
| 1025 | 1029 | β-Phellandrene | t |
| 1033 | 1031 | 1,8-Cineole | 1.3 |
| 1035 | 1034 | (Z)-β-Ocimene | t |
| 1045 | 1044 | (E)-β-Ocimene | t |
| 1057 | 1058 | γ-Terpinene | t |
| 1069 | 1071 | cis-Sabinene hydrate | t |
| 1085 | 1086 | Terpinolene | 7.2 |
| 1090 | 1091 | p-Cymenene | 0.1 |
| 1108 | 1111 | 1,3,8-p-Menthatriene | t |
| 1130 | 1132 | 1(7),3,8-o-Menthatriene | t |
| 1138 | 1143 | Epoxyterpinolene | t |
| 1146 | 1145 | Myrcenone | t |
| 1180 | 1180 | Terpinen-4-ol | t |
| 1182 | 1184 | Anethofuran | t |
| 1187 | 1187 | p-Cymen-8-ol | 0.2 |
| 1195 | 1195 | α-Terpineol | t |
| 1234 | 1237 | Turmeric dione | t |
| 1291 | 1292 | 2-Undecanone | t |
| 1365 | 1368 | (Z,Z)-Megastigma-4,6,8-triene | t |
| 1390 | 1389 | 7-epi-Sesquithujene | t |
| 1402 | 1405 | Sesquithujene | t |
| 1412 | 1413 | cis-α-Bergamotene | t |
| 1418 | 1418 | β-Caryophyllene | 2.9 |
| 1451 | 1452 | (E)-β-Farnesene | 0.4 |
| 1455 | 1454 | α-Humulene | 0.5 |
| 1476 | 1464 | β-Acoradiene | t |
| 1479 | 1477 | γ-Curcumene | t |
| 1480 | 1483 | ar-Curcumene | 1.6 |
| 1484 | 1485 | trans-β-Bergamotene | t |
| 1496 | 1495 | α-Zingiberene | 4.8 |
| 1508 | 1507 | β-Bisabolene | 0.7 |
| 1514 | 1509 | β-Curcumene | 0.1 |
| 1522 | 1525 | β-Sesquiphellandrene | 5.1 |
| 1528 | 1528 | (E)-γ-Bisabolene | 0.2 |
| 1542 | 1547 | cis-Sesquisabinene hydrate | 0.2 |
| 1560 | 1561 | (E)-Nerolidol | 0.5 |
| 1578 | 1575 | ar-Turmerol | 0.3 |
| 1582 | 1578 | Caryophyllene oxide | 0.2 |
| 1583 | 1580 | trans-Sesquisabinene hydrate | 0.3 |
| 1595 | 1599 | 2,4,4,6-Tetramethyl-6-phenyl-1-heptene | 0.3 |
| 1611 | 1612 | Humulene epoxide II | 0.2 |
| 1615 | 1615 | Zingiberenol | 0.7 |
| 1629 | 1629 | 7-epi-cis-Sesquisabinene hydrate | 0.6 |
| 1630 | 1632 | α-Tumerone | 0.1 |
| 1634 | 1632 | Biotol isomer | 0.9 |
| 1659 | 1655 | β-Eudesmol | 0.1 |
| 1664 | 1662 | α-Turmerone | 24.4 |
| 1667 | 1664 | β-Turmerone | 14.0 |
| 1668 | 1665 | ar-Turmerone | 25.5 |
| 1681 | 1684 | 7-epi-trans-Sesquisabinene hydrate | 0.3 |
| 1688 | 1685 | α-Bisabolol | t |
| 1714 | 1711 | Curcuphenol | 0.2 |
| 1743 | 1741 | 6S,7R-Bisabolone | 0.8 |
| 1771 | 1771 | trans-α-Atlantone | 0.3 |
The chemical composition of C. aeruginosa, and C. zedoaria compositions are presented in Table 2. In the case of C. zedoaria essential oil, the total number of identified compounds was 98 and accounted for 81.55%. The most representative compounds werecurzerenone (21.5%), 1,8-cineole (19.6%), curzerene (6.2%), trans-β-elemene (5.1%), camphor (2.6%), and germacrone (2.3%). Previous research on the essential oils of C. zedoaria rhizomes from India and Nepal revealed two distinct clusters; the first cluster rich in curzerenone/epi-curzerenone followed by camphor, germacrone, 1,8-cineole, and α-copaene and second cluster represented by a single sample with a high concentration of 1,8-cineole [15]. So, C. zedoaria essential oils from Nepal fall under the curzerenone/epi-curzerenone chemotype.
Table 2.
Individual constituents of Curcuma aeruginosa and Curcuma zedoaria essential oil.
| RIcalc | RIdb | Compound Name | Area % of Constituents in | |
|---|---|---|---|---|
| Curcuma zedoaria | Curcuma aeruginosa | |||
| 882 | 885 | 3-Hepten-6-ol | 0.1 | - |
| 889 | 888 | 2-Heptanone | t | - |
| 894 | 894 | 2-Heptanol | 0.3 | 0.2 |
| 918 | 921 | Tricyclene | t | t |
| 925 | 924 | α-Thujene | t | t |
| 933 | 931 | α-Pinene | 0.8 | 0.2 |
| 945 | 945 | α-Fenchene | t | - |
| 949 | 948 | Camphene | 1.2 | 0.8 |
| 953 | 954 | Thuja-2,4(10)-diene | t | - |
| 972 | 972 | Sabinene | 0.2 | t |
| 978 | 978 | β-Pinene | 1.7 | 1.3 |
| 989 | 989 | Myrcene | 0.3 | 0.1 |
| 998 | 994 | 2-Octanol | t | t |
| 1007 | 1008 | α-Phellandrene | t | t |
| 1017 | 1016 | α-Terpinene | t | t |
| 1020 | 1024 | p-Cymene | t | t |
| 1024 | 1028 | Limonene | 1.1 | 0.2 |
| 1033 | 1031 | 1,8-Cineole | 19.6 | 1.2 |
| 1035 | 1034 | (Z)-β-Ocimene | t | - |
| 1045 | 1044 | (E)-β-Ocimene | t | - |
| 1057 | 1058 | γ-Terpinene | 0.1 | t |
| 1069 | 1071 | cis-Sabinene hydrate | t | - |
| 1085 | 1086 | Terpinolene | t | t |
| 1087 | 1090 | 2-Nonanone | 0.6 | t |
| 1090 | 1090 | (3Z)-Hexenyl methyl carbonate | t | - |
| 1099 | 1099 | Linalool | 0.2 | 0.3 |
| 1105 | 1103 | 2-Nonanol | 0.6 | 0.4 |
| 1112 | 1118 | trans-Thujone | - | t |
| 1119 | 1122 | trans-p-Mentha-2,8-dien-1-ol | t | - |
| 1122 | 1124 | cis-p-Menth-2-en-1-ol | t | - |
| 1133 | 1137 | cis-p-Mentha-2,8-dien-1-ol | t | - |
| 1135 | 1140 | trans-Pinocarveol | 0.1 | - |
| 1141 | 1145 | Camphor | 2.59 | 3.6 |
| 1159 | 1156 | trans-β-Terpineol | t | t |
| 1164 | 1161 | iso-Borneol | - | 1.2 |
| 1165 | 1167 | exo-Acetoxy camphene | t | - |
| 1167 | 1168 | epi-Borneol | 0.7 | - |
| 1170 | 1170 | δ-Terpineol | 0.1 | - |
| 1173 | 1171 | Borneol | 1.2 | 0.2 |
| 1175 | 1174 | cis-Pinocamphone | - | t |
| 1180 | 1180 | Terpinen-4-ol | 0.4 | 0.1 |
| 1187 | 1187 | p-Cymen-8-ol | t | - |
| 1191 | 1190 | 2-Decanone | t | t |
| 1195 | 1195 | α-Terpineol | 1.1 | 0.1 |
| 1195 | 1197 | cis-Piperitol | t | - |
| 1198 | 1198 | 2-Decanol | t | - |
| 1206 | 1206 | Verbenone | t | t |
| 1215 | 1218 | trans-Carveol | t | - |
| 1226 | 1232 | cis-Carveol | 0.1 | t |
| 1243 | 1242 | Carvone | - | t |
| 1258 | 1252 | trans-Myrtanol | t | - |
| 1274 | 1274 | Cyclooctyl acetate | t | - |
| 1283 | 1282 | Bornyl acetate | 0.1 | t |
| 1287 | 1288 | iso-Bornyl acetate | 1.7 | t |
| 1291 | 1292 | 2-Undecanone | 0.3 | t |
| 1298 | 1294 | trans-Pinocarvyl acetate | t | - |
| 1302 | 1299 | Perillyl alcohol | t | - |
| 1310 | 1307 | 2-Undecanol | t | t |
| 1331 | 1333 | Bicycloelemene | t | t |
| 1334 | 1334 | δ-Elemene | 0.7 | 0.3 |
| 1346 | 1346 | α-Terpinyl acetate | t | - |
| 1381 | 1381 | cis-β-Elemene | 0.3 | 0.1 |
| 1390 | 1391 | trans-β-Elemene | 5.1 | 2.6 |
| 1393 | 1394 | trans-Sativene | t | t |
| 1418 | 1418 | β-Caryophyllene | 0.6 | 0.7 |
| 1428 | 1430 | γ-Elemene | 0.3 | 0.2 |
| 1440 | 1443 | 6,9-Guaiadiene | t | t |
| 1447 | 1443 | iso-Germacrene D | 0.2 | - |
| 1451 | 1451 | trans-Muurola-3,5-diene | - | 0.1 |
| 1451 | 1452 | (E)-β-Farnesene | t | - |
| 1455 | 1454 | α-Humulene | 0.1 | 0.1 |
| 1475 | 1473 | trans-Cadina-1(6),4-diene | t | t |
| 1476 | 1475 | Selina-4,11-diene | 0.1 | t |
| 1476 | 1478 | γ-Gurjunene | - | t |
| 1478 | 1479 | α-Amorphene | - | t |
| 1480 | 1484 | Germacrene D | 1.9 | 0.8 |
| 1483 | 1487 | Guaia-1(10),11-diene | t | - |
| 1496 | 1488 | δ-Selinene | t | t |
| 1488 | 1489 | β-Selinene | 0.5 | 0.3 |
| 1494 | 1493 | Curzerene | 6.2 | 4.7 |
| 1496 | 1495 | Aciphyllene | 0.1 | - |
| 1498 | 1498 | α-Selinene | t | 0.2 |
| 1504 | 1502 | trans-β-Guaiene | 0.2 | 0.1 |
| 1504 | 1503 | β-Dihydroagarofuran | - | 0.1 |
| 1508 | 1505 | Germacrene A | 0.1 | t |
| 1513 | 1514 | γ-Cadinene | - | t |
| 1517 | 1516 | Cubebol | - | t |
| 1518 | 1519 | δ-Cadinene | 0.1 | 0.1 |
| 1540 | 1540 | Occidentalol | t | t |
| 1549 | 1549 | α-Elemol | 0.1 | 0.1 |
| 1558 | 1560 | Germacrene B | 0.7 | 0.5 |
| 1560 | 1561 | (E)-Nerolidol | t | - |
| 1582 | 1578 | Caryophyllene oxide | 0.1 | 0.2 |
| 1590 | 1584 | Globulol | 0.7 | 0.4 |
| 1595 | 1592 | Viridiflorol | - | 0.4 |
| 1601 | 1594 | (E)-β-Elemenone | 0.3 | 0.5 |
| 1605 | 1600 | Curzerenone | 21.5 | 59.6 |
| 1607 | 1607 | 5-epi-7-epi-α-Eudesmol | - | 0.2 |
| 1610 | 1610 | iso-Curzerenone | - | 0.6 |
| 1614 | 1616 | Curcumenol | 1.3 | - |
| 1622 | 1623 | 10-epi-γ-Eudesmol | - | 0.2 |
| 1630 | 1627 | iso-Spathulenol | 0.6 | 0.7 |
| 1630 | 1631 | γ-Eudesmol | t | 0.5 |
| 1646 | 1637 | Agarospirol | - | 0.2 |
| 1646 | 1640 | epi-α-Cadinol | 0.1 | 0.2 |
| 1659 | 1655 | β-Eudesmol | - | 1.6 |
| 1652 | 1657 | α-Eudesmol | 0.5 | - |
| 1658 | 1659 | Selin-11-en-4α-ol | 0.6 | 0.4 |
| 1660 | 1660 | Selin-11-en-4β-ol | 0.1 | - |
| 1670 | 1663 | Bulnesol | - | 0.2 |
| 1693 | 1692 | Germacrone | 2.3 | 5.3 |
| 1705 | 1708 | Aromadendrane-4,10-diol A | - | t |
| 1710 | 1708 | Thujopsenal | - | 0.1 |
| 1777 | 1775 | Curzerenone A | - | 0.5 |
| 1790 | 1795 | Curzerenone B | - | t |
| 1828 | 1832 | iso-Germacrone C | t | t |
| 1843 | 1834 | Curcumenone | 0.5 | - |
| 1989 | 1992 | 4-Methoxystilbene | t | - |
| 2366 | 2376 | Butyl stearate | t | - |
The total identified compounds in C. aeruginosa essential oil were 86 and accounted for 92.59%. The dominant compounds were curzerenone (59.6%), germacrone (5.3%), curzerene (4.7%), camphor (3.6%), trans-β-elemene (2.6%), and β-eudesmol (1.6%). There are only few data on C. aeruginosa essential oil. Previous research on the essential oils of C. aeruginosa rhizomes from Malaysia, Thailand, and India revealed three distinct clusters. The first cluster is represented by a camphor/germacrone-rich cluster with large concentrations of iso-borneol, curzerene, and germacrone. The second cluster was a curcumenol/β-pinene rich cluster. The third cluster was a curzerenone/1,8-cineole cluster [15]. C. aeruginosa essential oil from Nepal fall into the curzerenone/1,8-cineole rich chemotype.
Essential oils from the curcuma species exhibit impressive biological activities. However, the variations in volatile constituents depending on the geographical location may/may not have same biological activity. Curcuma essential oil displayed remarkable antioxidant activity that may be used to minimize the food spoilage in industry. Reactive oxygen species (ROS) in our body initiates the cascade of reaction that leads to different diseases. Antioxidants secondary metabolites remove ROS in body to terminate the oxidative response by free radical [35]. Curcuma species essential oil is rich in antioxidant secondary metabolites. C. longa essential oil rich in turmerone is thought to be responsible for inhibiting brain-edema formation, inhibiting the key enzymes of diabetes. Besides these C. longa essential oil acts as anti-inflammatory, anticancer, antibacterial, and so on. C. zedoaria essential oil showed cytotoxicity against different cell line, antidiabetic, antimicrobial as well as larvicidal activity. C. aeruginosa essential oil acts as antibacterial, hair re-growth stimulant, and anti-inflammatory. So, these essential oil has been used to treat life threatening diseases, minimizing the food spoilage, cosmetics’, as well as in aromatherapy [10].
2.3. Enantiomeric Composition of Essential Oils
In total, 9, 10, and 15 chiral compounds were identified in C. longa, C. zedoaria, and C. aeruginosa, respectively. Relative percentages of the levorotatory (–) and dextrorotatory (+) compounds of Curcuma species essential oil are listed in Table 3. The majority of chiral compounds in C. aeruginosa were levorotatory. In C. aeruginosa essential oil chiral terpenoids such as camphene, β-pinene, linalool, camphor, borneol, and germacrene D exist in dextrorotatory form. On the other hand, sabinene, limonene, bornyl acetate, terpinen-4-ol, β-elemene, and β-caryophyllene exist only in the levorotatory state. In C. zedoaria essential oil camphene, linalool, camphor, and germacrene D exists in dextrorotatory form and other detected chiral terpenoids in levorotatory form. Additionally, bornyl acetate, β-caryophyllene, and β-elemene exist in pure levorotatory form. However, germacrene D exists in only dextrorotatory form. α-Pinene, α-phellandrene, δ-3-carene, β-phellandrene, α-terpineol, and β-bisabolene dominated in the dextrorotatory form in C. longa essential oil. α-Phellandrene, and δ-3-carene in absolute dextrorotatory form whereas, (E)-nerolidol and β-caryophyllene in absolute levorotatory form. Interestingly, fourteen chiral compounds had been detected from Vietnamese C. longa cultivated in North Alabama. However, α-terpineol and α-pinene show contrasting types of enantiomeric distribution as compared to Nepalese essential oil [1]. On the other hand, β-phellandrene and (E)-nerolidol were reported for the very first time from Nepalese C. longa essential oil. To the best of our knowledge, we have reported chiral terpenoids from C. longa, C. zedoaria, and C. aeruginosa essential oil from Nepal for the very first time which may be a blueprint for identification and authentication.
Table 3.
Enantiomeric distributions of chiral terpenoids of Curcuma aeruginosa, Curcuma zedoaria, and Curcuma longa essential oil.
| Chiral Terpenoid Compound | Curcuma aeruginosa | Curcuma zedoaria | Curcuma longa |
|---|---|---|---|
| α-Pinene | (+)24.7: (–)75.3 | (+)43.5: (–)56.5 | (+)70.6: (–)29.4 |
| Camphene | (+)90.7: (–)9.3 | (+)92.3: (–)7.7 | - |
| β-Pinene | (+)59.1: (–)40.9 | (+)47.8: (–)52.2 | - |
| Sabinene | (+)0: (–)100 | - | - |
| α-Phellandrene | - | - | (+)100: (–)0 |
| δ-3-Carene | - | - | (+)100: (–)0 |
| Limonene | (+)0: (–)100 | - | (+)45.6: (–)54.4 |
| β-Phellandrene | - | - | (+)91.4: (–)8.6 |
| Linalool | (+)62.4: (–)37.6 | (+)55.3: (–)44.7 | - |
| Camphor | (+)99.8: (–)0.2 | (+)92.3: (–)7.7 | - |
| Bornyl acetate | (+)0: (–)100 | (+)0: (–)100 | - |
| Terpinen-4-ol | (+)0: (–)100 | - | - |
| δ-Elemene | (+)45.0: (–)55.0 | (+)33.7: (–)66.3 | - |
| α-Terpineol | (+)29.1: (–)70.9 | (+)51.5: (–)48.5 | |
| Borneol | (+)100: (–)0 | - | |
| β-Elemene | (+)0: (–)100 | (+)0: (–)100 | - |
| β-Caryophyllene | (+)0: (–)100 | (+)0: (–)100 | (+)0: (–)100 |
| Germacrene D | (+)100: (–)0 | (+)100: (–)0 | - |
| β-Bisabolene | - | - | (+)92.9: (–)7.1 |
| (E)-Nerolidol | - | - | (+)0: (–)100 |
3. Materials and Methods
3.1. Plant Material and Isolation of Essential Oils
The Fresh cultivated, mature mother rhizomes of Curcuma species (C. aeruginosa, C. zedoaria, and C. longa) were collected in March, 2020 from Kirtipur (27°40’28.8” N 85°15’48.1” E), an elevation of 1348 m. The plants were identified by taxonomists from Central Department of Botany, Tribhuvan University, Kirtipur. The fresh rhizome of each sample (300 g) was cleaned with tap water, and cut into small pieces and was extracted by hydrodistillation in Clevenger apparatus as previously described [36]. The extracted essential oil was dried with anhydrous sodium sulfate and was stored in bottles under 4 °C until use for further studies.
3.2. Chemical Composition Analysis by Gas Chromatography/Mass Spectrometry (GC-MS)
Analysis of the chemical constituents in the Curcuma species (C. aeruginosa, C. zedoaria, and C. longa) essential oils was carried out using Shimadzu GCMS-QP2010 Ultra under the following condition: mass selective detector (MSD), operated in the EI mode (electron energy = 70 eV), with scan range = 40–400 m/z, and scan rate = 3.0 scans/s. The GC column was a ZB-5MS fused silica capillary with a (5% phenyl)-polydimethylsiloxane stationary phase, a film thickness of 0.25 μm, a length of 60 m, and an internal diameter of 0.25 mm. The carrier gas was helium with a column head pressure of 552 kPa and a flow rate of 1.37 mL/min. The injector temperature was 260 °C, and the detector temperature was 280 °C. The column temperature was set at 50 °C for 2 min and then increased at 2 °C/min to the temperature of 260 °C. For each essential oil sample, 1:10 v/v solution in dichloromethane (DCM) was prepared, and 0.3 μL was injected using a split ratio of 1:30. Identification of the individual components of the essential oils was determined by comparison of the retention indices determine by reference to a homologous series of n-alkanes and comparison of the mass spectral fragmentation patterns (over 80% similarity match) with those reported in the literature [33] and our own in-house library [34] using the LabSolutions GC-MS solution software version 4.45 (Shimadzu Scientific Instruments, Columbia, MD, USA). The individual components of C. longa essential oil are presented in Table 1 and Table 2 for C. aeruginosa and C. zedoaria essential oil.
3.3. Enantiomeric Analysis by Chiral Gas Chromatography-Mass Spectrometry (CGC-MS)
Chiral GC-MS was carried out as previously reported [37]. Shimadzu GCMS-QP2010S with EI mode (70 eV) and B-Dex 325 chiral capillary GC column was used to perform enantiomeric analysis of Curcuma species (C. aeruginosa, C. zedoaria, and C. longa) essential oil. Scans in the 40–400 m/z range at a scan rate of 3.0 scan/s. The column temperature was set at 50 °C, at first increased by 1.5 °C/min to 120 °C and then 2 °C/min to 200 °C. The final temperature of the column was 200 °C and was kept constant. The carrier gas was helium with a constant flow rate of 1.8 mL/min. For each essential oil sample, 3% w/v solution in DCM was prepared, and 0.1 μL was injected using a split ratio of 1:45. The enantiomer percentages were determined from the peak areas. A comparison of retention times and mass spectral fragmentation patterns with authentic samples obtained from Sigma-Aldrich (Milwaukee, WI, USA) was used to identify the enantiomers. Table 3 shows the enantiomeric distribution of chiral terpenoids from C. aeruginosa, C. zedoaria, and C. longa essential oils.
4. Conclusions
In this study, the volatile chemical composition and enantiomeric distribution of C. aeruginosa, C.zedoaria, and C. longa essential oil analyzed by GC-MS and by chiral GC-MS respectively, reported for the first time from Nepal. The extraction yield and chemical composition were comparable to those growing in tropical and subtropical regions of the world, suggesting that these varieties are suitable for commercialization in the international market. These plants volatile constituents’ are not representative of the entire Nepal. Nepal has an extreme altitudinal range with heterogeneous topography with distinct climatic variation that contributes to variations in the chemical compositions of essential oil. However, farmers should be eyeing on systematic production and harvesting as well as investment in sophisticated processing units to create contaminants essential oil as well as increase the quality of Curcuma essential oil. The research will surely be useful to encourage farmers to earn a reasonable return from C. aeruginosa, C. zedoaria, and C. longa rhizome production. Additionally, the results of this study can be utilized to provide a baseline for quality assessments of these Curcuma species.
Acknowledgments
The authors are thankful to the APRC and ARC for GC-MS and chiral GC-MS analysis. We acknowledge Sunita Satyal, Sujan Timsina and Ambika Satyal for their constructive suggestions and support.
Author Contributions
Conceptualization, D.K.P. and P.S.; methodology, P.S.; validation, P.S.; formal analysis, D.K.P.; investigation, D.K.P., P.K.O., A.R. and R.S.; data curation, P.S.; writing—original draft preparation, D.K.P.; writing—review and editing, D.K.P. and W.N.S.; supervision, P.S.; project administration, P.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data are available in the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Duong L., Mentreddy S.R., Satyal R., Satyal P., Setzer W.N. Essential Oil Chemotypes of Four Vietnamese Curcuma Species Cultivated in North Alabama. Horticulturae. 2022;8:360. doi: 10.3390/horticulturae8050360. [DOI] [Google Scholar]
- 2.Fuloria S., Mehta J., Chandel A., Sekar M., Rani N.N.I.M., Begum M.Y., Subramaniyan V., Chidambaram K., Thangavelu L., Nordin R., et al. A Comprehensive Review on the Therapeutic Potential of Curcuma Longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022;13:820806. doi: 10.3389/fphar.2022.820806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi-Rad J., Rayess Y.E., Rizk A.A., Sadaka C., Zgheib R., Zam W., Sestito S., Rapposelli S., Neffe-Skocińska K., Zielińska D., et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020;11:01021. doi: 10.3389/fphar.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amalraj A., Pius A., Gopi S., Gopi S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2016;7:205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaliyadasa E., Samarasinghe B.A. A Review on Golden Species of Zingiberaceae Family around the World: Genus Curcuma. AJAR. 2019;14:519–531. doi: 10.5897/AJAR2018.13755. [DOI] [Google Scholar]
- 6.Sohn S.-I., Priya A., Balasubramaniam B., Muthuramalingam P., Sivasankar C., Selvaraj A., Valliammai A., Jothi R., Pandian S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics. 2021;13:2102. doi: 10.3390/pharmaceutics13122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob J.N. Comparative studies in relation to the structure and biochemical properties of the active compounds in the volatile and nonvolatile fractions of turmeric (C. Longa) and ginger (Z. Officinale) In: Attaur R., editor. Studies in Natural Products Chemistry. Volume 48. Elsevier; Amsterdam, The Netherlands: 2016. pp. 101–135. [Google Scholar]
- 8.Ibáñez M.D., Blázquez M.A. Curcuma Longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants. 2021;10:44. doi: 10.3390/plants10010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivilai J., Phimnuan P., Jaisabai J., Luangtoomma N., Waranuch N., Khorana N., Wisuitiprot W., Scholfield C.N., Champachaisri K., Ingkaninan K. Curcuma Aeruginosa Roxb. Essential Oil Slows Hair-Growth and Lightens Skin in Axillae; a Randomised, Double Blinded Trial. Phytomedicine. 2017;25:29–38. doi: 10.1016/j.phymed.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Dosoky N.S., Setzer W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients. 2018;10:1196. doi: 10.3390/nu10091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobo R., Prabhu K.S., Shirwaikar A., Shirwaikar A. Curcuma Zedoaria Rosc. (White Turmeric): A Review of Its Chemical, Pharmacological and Ethnomedicinal Properties. J. Pharm. Pharmacol. 2010;61:13–21. doi: 10.1211/jpp.61.01.0003. [DOI] [PubMed] [Google Scholar]
- 12.Peng Y., Ao M., Dong B., Jiang Y., Yu L., Chen Z., Hu C., Xu R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021;15:4503–4525. doi: 10.2147/DDDT.S327378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahaman M.M., Rakib A., Mitra S., Tareq A.M., Emran T.B., Shahid-Ud-Daula A.F.M., Amin M.N., Simal-Gandara J. The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives. Plants. 2021;10:63. doi: 10.3390/plants10010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopal K., Varakumar P., Baliwada A., Byran G. Activity of Phytochemical Constituents of Curcuma Longa (Turmeric) and Andrographis Paniculata against Coronavirus (COVID-19): An in Silico Approach. Futur. J. Pharm. Sci. 2020;6:104. doi: 10.1186/s43094-020-00126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dosoky N.S., Satyal P., Setzer W.N. Variations in the Volatile Compositions of Curcuma Species. Foods. 2019;8:53. doi: 10.3390/foods8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lekshmi P.C., Arimboor R., Indulekha P.S., Nirmala Menon A. Turmeric (Curcuma Longa L.) Volatile Oil Inhibits Key Enzymes Linked to Type 2 Diabetes. Int. J. Food Sci. Nutr. 2012;63:832–834. doi: 10.3109/09637486.2011.607156. [DOI] [PubMed] [Google Scholar]
- 17.Funk J.L., Frye J.B., Oyarzo J.N., Zhang H., Timmermann B.N. Anti-Arthritic Effects and Toxicity of the Essential Oils of Turmeric (Curcuma Longa L.) J. Agric. Food Chem. 2010;58:842–849. doi: 10.1021/jf9027206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohare P., Garg P., Sharma U., Jagannathan N., Ray M. Neuroprotective Efficacy and Therapeutic Window of Curcuma Oil: In Rat Embolic Stroke Model. BMC Complement. Altern. Med. 2008;8:55. doi: 10.1186/1472-6882-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y., Zhang J., Kong W., Zhao G., Yang M. Mechanisms of Antifungal and Anti-Aflatoxigenic Properties of Essential Oil Derived from Turmeric (Curcuma Longa L.) on Aspergillus Flavus. Food Chem. 2017;220:1–8. doi: 10.1016/j.foodchem.2016.09.179. [DOI] [PubMed] [Google Scholar]
- 20.Singh G., Kapoor I.P.S., Singh P., de Heluani C.S., de Lampasona M.P., Catalan C.A.N. Comparative Study of Chemical Composition and Antioxidant Activity of Fresh and Dry Rhizomes of Turmeric (Curcuma Longa Linn.) Food Chem. Toxicol. 2010;48:1026–1031. doi: 10.1016/j.fct.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Setzer W.N., Duong L., Poudel A., Mentreddy S.R. Variation in the Chemical Composition of Five Varieties of Curcuma Longa Rhizome Essential Oils Cultivated in North Alabama. Foods. 2021;10:212. doi: 10.3390/foods10020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angel G.R., Menon N., Vimala B., Nambisan B. Essential Oil Composition of Eight Starchy Curcuma Species. Ind. Crops Prod. 2014;60:233–238. doi: 10.1016/j.indcrop.2014.06.028. [DOI] [Google Scholar]
- 23.Chen C., Chen Y., Hsi Y.-T., Chang C.-S., Huang L.-F., Ho C.-T., Way T.-D., Kao J.-Y. Chemical Constituents and Anticancer Activity of Curcuma Zedoaria Roscoe Essential Oil against Non-Small Cell Lung Carcinoma Cells in vitro and in vivo. J. Agric. Food Chem. 2013;61:11418–11427. doi: 10.1021/jf4026184. [DOI] [PubMed] [Google Scholar]
- 24.Handajani J., Narissi D.H. The Effects of Curcuma Zedoaria Oil on High Blood Sugar Level and Gingivitis. Dent. J. (Majalah Kedokteran Gigi) 2015;48:69. doi: 10.20473/j.djmkg.v48.i2.p69-73. [DOI] [Google Scholar]
- 25.Chen W., Lu Y., Gao M., Wu J., Wang A., Shi R. Anti-Angiogenesis Effect of Essential Oil from Curcuma Zedoaria in vitro and in vivo. J. Ethnopharmacol. 2011;133:220–226. doi: 10.1016/j.jep.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Jena S., Ray A., Sahoo A., Panda P.C., Nayak S. Deeper Insight into the Volatile Profile of Essential Oil of Two Curcuma Species and Their Antioxidant and Antimicrobial Activities. Ind. Crops Prod. 2020;155:112830. doi: 10.1016/j.indcrop.2020.112830. [DOI] [Google Scholar]
- 27.Akarchariya N., Sirilun S., Julsrigival J., Chansakaowa S. Chemical Profiling and Antimicrobial Activity of Essential Oil from Curcuma Aeruginosa Roxb., Curcuma Glans K. Larsen & J. Mood and Curcuma Cf. Xanthorrhiza Roxb. Collected in Thailand. Asian Pac. J. Trop. Biomed. 2017;7:881–885. doi: 10.1016/j.apjtb.2017.09.009. [DOI] [Google Scholar]
- 28.Kutti Gounder D., Lingamallu J. Comparison of Chemical Composition and Antioxidant Potential of Volatile Oil from Fresh, Dried and Cured Turmeric (Curcuma Longa) Rhizomes. Ind. Crops Prod. 2012;38:124–131. doi: 10.1016/j.indcrop.2012.01.014. [DOI] [Google Scholar]
- 29.Sharma R.K., Misra B.P., Sarma T.C., Bordoloi A.K., Pathak M.G., Leclercq P.A. Essential Oils of Curcuma Longa L. from Bhutan. J. Essent. Oil Res. 1997;9:589–592. doi: 10.1080/10412905.1997.9700783. [DOI] [Google Scholar]
- 30.Abdul Aziz J., Saidi N.B., Ridzuan R., Mohammed A.K.S., Abdul Aziz M., Abdul Kadir M., Abdullah N.A.P., Hussein S., Yusoff H. Chemical Profiling of Curcuma Aeruginosa Roxb. Essential Oil and Their Antimicrobial Activity against Pathogenic Microbes. J. Essent. Oil Bear. Plants. 2021;24:1059–1071. doi: 10.1080/0972060X.2021.1971570. [DOI] [Google Scholar]
- 31.Quemel F.d.S., Dantas A.P., Sanches L., Viana A.C.G.A., Silva E.S., Monteiro E.R., Gazim Z.C., Gonçalves J.E., Lopes A.D. Chemotypes of Turmeric (Curcuma Longa L.) Essential Oil from Four Different States of Brazil. Aust. J. Crop Sci. 2021:1035–1042. doi: 10.21475/ajcs.21.15.07.p3146. [DOI] [Google Scholar]
- 32.Raina V.K., Srivastava S.K., Jain N., Ahmad A., Syamasundar K.V., Aggarwal K.K. Essential Oil Composition of Curcuma Longa L. Cv. Roma from the Plains of Northern India. Flavour Fragr. J. 2002;17:99–102. doi: 10.1002/ffj.1053. [DOI] [Google Scholar]
- 33.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 34.Satyal P. Ph.D. Thesis. University of Alabama in Huntsville; Huntsville, AL, USA: 2015. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. [Google Scholar]
- 35.Tirzitis G., Bartosz G. Determination of Antiradical and Antioxidant Activity: Basic Principles and New Insights. Acta Biochim. Pol. 2010;57 doi: 10.18388/abp.2010_2386. [DOI] [PubMed] [Google Scholar]
- 36.Ojha P.K., Poudel D.K., Dangol S., Rokaya A., Timsina S., Satyal P., Setzer W.N. Volatile Constituent Analysis of Wintergreen Essential Oil and Comparison with Synthetic Methyl Salicylate for Authentication. Plants. 2022;11:1090. doi: 10.3390/plants11081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poudel D.K., Rokaya A., Ojha P.K., Timsina S., Satyal R., Dosoky N.S., Satyal P., Setzer W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum Camphora L. and Their Antimicrobial Activities. Molecules. 2021;26:5132. doi: 10.3390/molecules26175132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the article.