Abstract
The cholesterol biosynthesis represents a crucial metabolic pathway for cellular homeostasis. The end products of this pathway are sterols, such as cholesterol, which are essential components of cell membranes, precursors of steroid hormones, bile acids, and other molecules such as ubiquinone. Furthermore, some intermediates of this metabolic system perform biological activity in specific cellular compartments, such as isoprenoid molecules that can modulate different signal proteins through the prenylation process. The defects of prenylation represent one of the main causes that promote the activation of inflammation. In particular, this mechanism, in association with oxidative stress, induces a dysfunction of the mitochondrial activity. The purpose of this review is to describe the pleiotropic role of prenylation in neuroinflammation and to highlight the consequence of the defects of prenylation.
Keywords: prenylation, cholesterol, neuroinflammation, oxidative stress
1. Introduction
The biosynthetic pathway of mevalonic acid or mevalonate is essential in both eukaryotic and prokaryotic organisms because it leads to the formation of organic compounds of enormous physiological importance, involved in many cellular processes. It is in fact an anabolic pathway that, starting from acetyl-CoA, leads to the synthesis of a family of molecules both of steroid nature, including cholesterol, and of non-steroidal nature, the isoprenoids or terpenes. Isoprenoids constitute a heterogeneous class of lipophilic molecules, being the widest family of natural molecules. They have both functional and structural properties in diverse biological processes, which range from cell membranes’ organization, gene expression regulation, post-translational modification of proteins, control of signal transduction, involvement in photosynthesis and electron transport chain, synthesis of cholesterol and its derivatives, pheromones, reproductive hormones in mammals, vitamins, and even defense against infections in plants [1,2]. Long-chain isoprenoids include ubiquinone and heme A, important for mitochondrial electron transport; the dolichol, necessary for the glycosylation of proteins; the isopentenyl group of t-RNAs; and carotenoids, which are part of the photosynthetic system of phototrophic organisms. Short-chain isoprenoids are farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which mediate one of the most important post-translational modifications of proteins, namely prenylation. This type of ubiquitous and irreversible modification, also known as lipidation, involves the post-translational addition of hydrophobic isoprenoids to proteins and represents a crucial step as it ensures correct localization and functionality of numerous proteins essential for cellular activity. Among the prenylated proteins, there are the small proteins belonging to the family of GTP-ases, such as Ras, Rac, and Rho, as well as the nuclear laminae [3]. This modification is essential for numerous biological functions, such as cell targeting, the processes of cellular life and death (growth, differentiation, movement, autophagy), the localization of proteins in the anchoring phase to the membrane, and the regulation of their activity (protein–protein/protein–membrane interactions). As cells need a constant supply of isoprenoid compounds, they must finely tune the mevalonate pathway while avoiding excessive build-up of potentially toxic molecules, such as cholesterol itself [4]. Through numerous experimental and clinical studies, it seems that isoprenoids, essential for cell growth and differentiation, may be potential therapeutic targets in many research fields, including tumors, autoimmune diseases, atherosclerosis, and Alzheimer′s disease [5]. Moreover, an altered flux through the mevalonate pathway is involved in the pathophysiology of the Hyperimmunoglobulin D syndrome (HIDS) and Mevalonic Aciduria (MA), autoinflammatory disorders together known as mevalonate kinase deficiency (MKD) disorders, which are precisely due to a hereditary deficiency of the Mevalonate Kinase (MVK), one of the first enzymes of the mevalonate pathway [6].
2. The Mevalonate Pathway
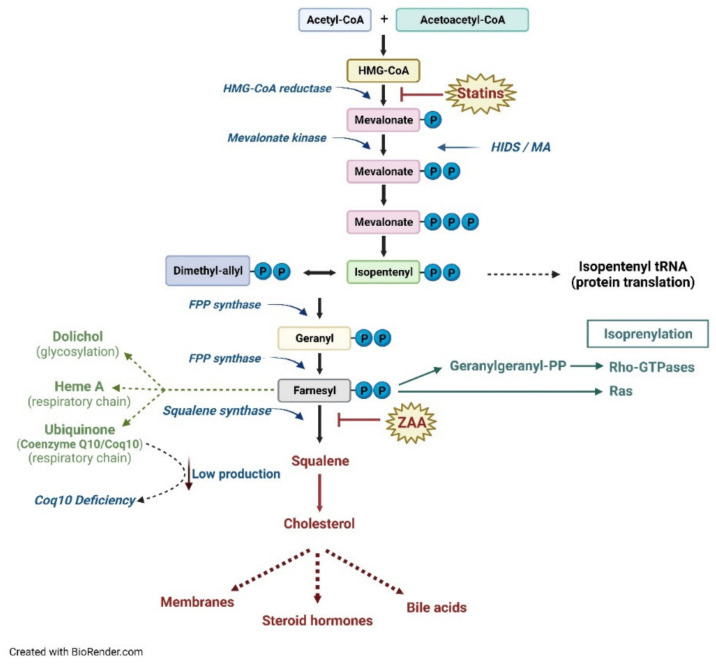
The biosynthesis of the different products of the mevalonate pathway begins in the cytosol with the condensation by the thiolase of two molecules of acetyl-CoA into acetocetyl-CoA, which reacts with another acetyl-CoA molecule to form, by HMG-CoA synthase (HMGS), 3-hydroxy-3-methylglutaryl-CoA, or HMG-CoA. HMG-CoA is then reduced to mevalonic acid thanks to the action of HMG-CoA reductase (HMGR), an oxidoreductase localized in the smooth endoplasmic reticulum (ER) that uses NADPH as a cofactor (Figure 1).
Figure 1.
Schematic representation of the metabolic pathway of cholesterol.
Subsequently, the synthesis of mevalonate-5-phosphate by MVK occurs in the cytosol followed by the decarboxylation and transformation into a compound with five carbon atoms (C5), Δ3-isopentenyl-5-pyrophosphate (IPP), which is the basic isoprene unit for the synthesis of all other isoprenoids, such as geranyl pyrophosphate (GPP, C10), FPP (C15), and GGPP (C20), through a series of head-to-tail condensations of isoprene units catalyzed by the prenyltransferases [4]. The FPP represents the link between the synthetic pathways of non-sterols/isoprenoids and sterols. In the isoprenoid pathway, the addition of another unit of IPP to the FPP leads to the formation of GGPP. The elongation process, by incorporating further portions of IPP, generates longer isoprenoids, which have a key biological relevance, such as dolichol (essential for proteins N-glycosylation), ubiquinone (Coenzyme Q10), and heme A [7,8,9].
In particular, Coenzyme Q10 is placed in the membranes of the endoplasmic reticulum, peroxisomes and lysosomes, in the vesicles and within the membrane of the mitochondria, where it plays an important role in the electron transport chain.
The enzyme farnesyl pyrophosphate synthase (FPPS) instead catalyzes the addition of dimethylallyl pyrophosphate (DMAPP) and geranyl diphosphate to IPP to form the isomer E of FPP [10]. In the sterol branch of the metabolic pathway, in the ER, the condensation of two FPP moieties, catalyzed by the enzyme squalene synthase (SQS), produces a molecule of squalene (a molecule with 30 carbon atoms). The last stages of cholesterol biosynthesis involve the cyclization of squalene to lanosterol, which already contains the four characteristic rings of cholesterol. From lanosterol, through a series of other reactions (demethylations and isomerizations), first desmosterol and then cholesterol (at 27 carbon atoms) are formed [11] (Figure 1).
3. Critical Points to Regulate the Metabolic Pathway of Cholesterol
In mammalian cells, most of the mevalonate is converted into cholesterol, while the remaining mevalonate is transformed into isoprenoids; therefore, the regulation of the whole pathway is fundamental. Under physiological conditions, the levels of cholesterol and its main metabolites depend on the amount of cholesterol introduced with the diet in a homeostatic balance between processes of synthesis, absorption, transport, catabolism, and excretion. Alterations in cholesterol homeostasis, due to genetic and/or environmental factors, are thus involved in various diseases such as obesity [12], atherogenesis and cardiovascular disorders [13,14], gallstones, and some inherited neuro-metabolic diseases. In humans, the brain is the organ that presents the highest percentage of cholesterol in the whole organism, and it is located in the myelin sheath. The presence of the blood brain barrier prevents the exchange of lipoproteins and free cholesterol between plasma and cerebrospinal fluid, so that brain tissue regulates cholesterol homeostasis autonomously [15]. Consequently, cerebral cholesterol constitutes a cholesterol pool independently regulated with respect to those present in all other parts of the body [16].
The regulation of cholesterol levels, and consequently of all of the other products that derive from this biosynthetic pathway, results from the control of the HMGR enzyme, the most finely regulated enzyme of the pathway, and the rate-limiting one This mechanism is irreversible, and it represents the main system for regulating the process [17]. When HMGR sterol-sensing domain (SSD) perceives a high cholesterol content inside the cell, its conformation changes, causing enzyme proteolysis. It has been reported that several polymorphisms in the HMGR gene determine a failure of this mechanism. Such polymorphisms have been associated with statins’ efficacy, obesity, lipid metabolism, Parkinson disease, cardiovascular adverse events, and other pathologies [18,19]. Several ER proteins are able to sense cholesterol levels including HMGR, sterol regulatory-element-binding protein (SREBP), and squalene epoxidase (SQLE). A recent paper pointed to the role of mitochondrial dysfunction on the mevalonate pathway, through the reduction of pathway intermediates and downregulation of the expression of the gene pathway in an SREBP2 dependent mechanism [20]. Moreover, MVK plays an essential regulatory role in the pathway; indeed, in the two MKD pathologies, the loss of its activity causes both the accumulation of mevalonic acid and, consequently, a deficiency of the isoprenoid products downstream, demonstrating the peculiar role of this enzyme throughout the pathway [21].
The MVK enzyme is regulated at the transcriptional level in the same way as HMGR; in fact, the MVK promoter contains a sterol-regulated element (SRE) capable of inducing gene transcription following a deficit of the downstream products of the pathway (positive feedback) through SREBP2 [22]. In addition, the MVK enzyme is also subject to post-translational regulation with negative feedback by the isoprenoids GPP, FPP, and GGPP. This inhibition is of the competitive type and occurs at the binding site of the enzyme for ATP [23]. It has been recently observed that NF-E2-related factor 3 (NRF3), a transcription factor that binds ER and is involved in lipid metabolism, upregulates the expression of GGPP synthase in an SREBP2-dependent manner [24].
The study of pathologies that involve these regulatory enzymes and similarly have a serious impact on the central nervous system, such as Mevalonic Aciduria (a severe form of MKD) (OMIM#610377) [25], Smith–Lemli–Opitz syndrome (due to deficiency of 7-dehydrocholesterol-Δ7 reductase) (OMIM#270400) [26,27], Niemann–Pick syndrome (characterized by altered cholesterol trafficking and accumulation) (OMIM#257220; #607625) [28], C oenzyme Q10 deficiency (OMIM#607426) [29,30,31], made it possible to better understand the mechanisms and the regulatory systems at the basis of this metabolic pathway [32,33,34].
4. The Prenylation Process
Prenylation, catalyzed by a prenyltransferase, involves the addition of FPP or GGPP (with 20 carbon atoms), with the formation of a thioether covalent bond, with a thiol residue of cysteine at the C-terminal end of target proteins [35]. The bound lipid is necessary for the correct functioning of the protein itself, as it is responsible for both membrane attachment and peculiar protein–protein interactions.
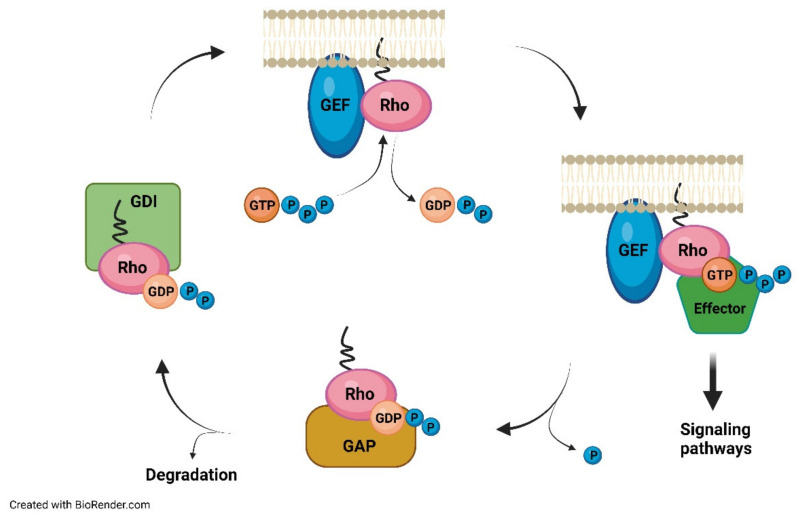
In all tissues, there are three intracellular cytosolic prenyltransferases: farnesyltransferase (FTase), geranylgeranyltransferase-I, and geranylgeranyltransferase-II (GGTase-I and II). FTase and GGTase-I are metallo-enzymes that contain a zinc atom, with 30% identity, especially in the central portion. It is unknown what the real function of zinc is in the process; perhaps it participates in catalysis, making the cysteine of the target protein more nucleophilic, or perhaps it has only a structural role [36]. FTase and GGTase-I recognize a sequence made by four amino acids, the CAAX motif in which C is a cysteine residue, A is usually an aliphatic residue, and X is specific for each enzyme. Indeed, FTase has a preference for Cys, Ala, Gln, Met, or Ser as the X residue, while GGTase-I prefers Leu, Ile, or Phe [37,38]. Genetic screening in yeast has highlighted a longer sequence target for FTase, that is, C(x)3X, expanding the list of possible human proteins that contain this motif and could thus be farnesylated [39]. GGTase-II, on the other hand, recognizes C-terminal motifs such as CC, CXC, CCX, CCXX, and CCXXX, and generally transfers GGPP to both the Cys amino acids in such sequences [40]. Prenylation increases hydrophobicity in the C-terminal domain and facilitates binding to the membrane of the ER, where the –AAX motif is cut [41]. The farnesylated and geranyl-geranylated proteins, in fact, usually are subject to a proteolytic step, catalyzed by proteases, for example, the CAAX endopeptidase 1 (RCE1), which removes the residues of –AAX downstream of the prenylated Cys [42]. The modified cysteine is then methylated by a methyl transferase, such as isoprenylcysteine carboxymethyl transferase (ICMT), to produce a protein containing a C-term farnesyl cysteine methyl ester [43]. The farnesyl group confers a weak affinity for the membrane, so other modifications are necessary for the correct localization of the proteins [44]. For example, several proteins are subject to further lipid modifications following prenylation, such as palmitoylation (palmitic acid transfer on the Cys residue with thioester bond formation) [45], useful for traffic control and anchoring to the membrane through electrostatic interactions with the anionic phospholipids positioned on the inner side of the membrane [46]. The typical targets of FTase and GGTase-I are members of the Ras superfamily, which includes a wide variety of proteins, such as Ras, Rho, and Rab, which show great functional diversification in the context of a preserved structural framework and a characteristic binding domain to GTP (Figure 2) [47].
Figure 2.
GTPase rho act as molecular switches between active and inactive GTP.
GGTase-II, on the other hand, has a rigorous specificity for the protein substrate compared with the other two prenyltransferases; in fact, it binds with great affinity to the C-terminal residues of a complex, which also includes the Rab protein, its effective substrate [48]. All small GTPases that belong to these families must bind to membranes to activate the downstream signaling pathway, and this is possible through prenylation (or other lipid modifications) [49]. It has been recently observed in Caenorhabditis elegans that intracellular lipid homeostasis depends on the sequestration of the nuclear hormone receptor NHR-49 into endosomes through a specific interaction with geranylgeranylated Rab11.1. Lipid depletion, and thus a reduced flux through the mevalonate pathway, reducing Rab11.1 geranylgeranylation, induces NHR-49 translocation to the nucleus and the activation of a transcription program that leads to increased nutrient absorption [50].
5. Consequences Caused by Prenylation Defects
To date, it is known that prenylation defects in key enzymes of the mevalonate pathway are the basis of the pathogenesis of multiple diseases, and the two enzymes most involved in these mechanisms are FPPS and MVK [51].
FPPS is a key enzyme in the regulation of the flow of carbon atoms from the pathway of mevalonate and is responsible, among other things, for the prenylation of proteins involved in cell cycle regulation [52,53].
Among the effects of reduced FPPS activity, there is the lack of prenylation of lamin B, a protein involved in maintaining the integrity of the nuclear membrane, and the localization of the same protein in the cytoplasm. Experimental evidence suggests that G0/G1 cell cycle phase arrest is likely induced by the reduction of isoprenoid derivatives linked to the nuclear membrane protein [54].
In vitro and in vivo studies indicate that inhibition of the mevalonate pathway shows effects on the growth and progression of prostate cancer (PC) [55]. The expression of FPPS appears, in fact, increased in patients with PC and nitrogen-containing bisphosphonates, inhibitors of FPPS, represent the elective treatment for bone metastases of this carcinoma. In vitro studies on PC cells have found that bisphosphonates affect tumor invasion and angiogenesis, and that zoledronic acid, a drug belonging to this group, inhibits the survival and proliferation of cancer cells with effects that appear to be the result of a lack of prenylation of small GTPases [56].
GTPases, such as Ras, among the most frequent oncoproteins mutated in human tumors, also need to be prenylated and are localized on the inner surface of the cell membrane so that proliferation cell pathways, such as those of PI3K/Akt and Raf/Mek/ERK, can be activated [57]. In particular, in the central nervous system (CNS), the Ras farnesylation plays a crucial role in regulating synaptic plasticity and determining synapse identity, while Rho GTPase carries out neuroprotection activity [58,59].
Impaired activity of MVK leads to a reduction in the production of isoprenoid molecules and to a defective protein prenylation, with consequent cytosolic accumulation of non-prenylated proteins [60].
It has been proposed that the excessive production of IL1β observed in patients with MKD, suffering from the congenital deficiency of the enzymatic activity of MVK [61], could be caused by the loss of protein prenylation, in particular when GGPP is missing; this event favors an overactivation of the inflammasome, thus triggering the systemic inflammatory peaks characteristic of the pathology [62,63].
In in vitro experiments, MKD models were obtained by treating cells with statins (HMGR inhibitors), bisphosphonates (FPPS inhibitors), or specific GGTase inhibitors to mimic protein prenylation blocking [64]. Recently, studies conducted by Skinner et al., have clearly shown that the loss of prenylation of some GTPases, such as Rac1 or RhoA, leads to the activation of inflammasome and thus of caspase 1, with increased production of IL1β; this has been observed in both human monocytic cells treated pharmacologically with statins, and directly in cells of patients suffering from MKD, upon stimulation with LPS [65].
Other studies have shown that reduced RhoA prenylation may be the basis for the excessive production of IL1β observed in MKD [66]. In cases of isoprenoid deficiency, there is increased RhoA activity, which leads to a further increase in the gene expression of pro-IL1β, as well as the activation of Rac1, which induces pro-caspase-1 in the inflammasome [67]. Similarly, the lack of isoprenoids also impairs mitochondrial function and stability, as well as autophagic clearance of damaged mitochondria, further promoting hypersecretion of IL1β [67].
Similar mitochondrial disorders and pro-inflammatory cell death have also been observed in statin-treated neuronal cells, suggesting that these events may contribute to neurological damage observed in patients severely affected by MA [67].
The results obtained from these studies are especially important from the clinical point of view, as they could help to overcome the deficit associated with the mevalonate pathway, restoring the normal prenylation of proteins that play a fundamental role in the activation of inflammation.
6. Neuroinflammation, Oxidative Stress, and Fever as a Consequence of Altered Mevalonate Pathway Flux
It is well known that the process of inflammation is mediated by the cells of the immune system and by specific chemical factors such as pro-inflammatory molecules; once the damaging agent is recognized, leukocytes and proteins are called back through chemical mediators from the bloodstream to the damaged site, where, once activated, they intervene in different ways [68]. Initially, monocytes/macrophages are the first cell population that releases pro-inflammatory cytokines and chemokines and induce phagocytosis [69]. Neuroinflammation plays a fundamental role in the CNS, exerting both possible beneficial and harmful effects on nervous tissue: a mild and rapid inflammatory state has a neuroprotective action, while the presence of a chronic inflammatory process could lead to negative effects [70]. As in the case of classical inflammation, at the nervous system level, it is also possible to make a distinction between acute neuroinflammation, which is basically a defensive response of the body to a harmful insult, resulting in repair of the damaged site, and chronic neuroinflammation, characterized by persistent damaging stimuli, which can result in neurodegeneration [71].
The neuroimmune system plays a particularly important role because it is involved in normal functioning, development, and aging, and intervenes in the case of CNS lesions. The homeostasis of this anatomical area is based on the good functioning of the blood–brain barrier and the presence of a large variety of cells: neurons, astrocytes, oligodendrocytes, pericytes, and microglia cells interact with each other, and their activity is essential for a multitude of brain functions [72,73].
Microglia cells represent 5–12% of all cells present in the CNS and play the fundamental role of the first line of defense of the CNS [70,74]. The essential role attributed to microglia cells is the sentinel function, which is the ability to constantly detect changes in their environment; the cleaning function, which promotes neuronal well-being; and the aforementioned defense function, providing neuroprotection [75]. Microglia cells are involved in maintaining CNS homeostasis; controlling synaptic density, connectivity, and plasticity; eliminating myelin debris and apoptotic cells; and affecting germination, migration, anastomosis, and improvement of the increasing vascularization of the CNS [76].
In addition, these cells, as all the other macrophages present in the body, perform phagocytosis, activate cytotoxicity mechanisms, and contribute to the inflammatory response through the production of signal molecules [77]. However, prolonged activation of microglia involves the acquisition of a harmful phenotype, with the release of inflammatory mediators that promote protein aggregation and neuronal damage [78].
An imbalance of these microglial functions may trigger the onset or exacerbation of neurodegeneration, a severe and debilitating neuroinflammatory disease that may occur as a result of specific and persistent stimuli, with progressive degeneration and death of neurons. All of this is because of microglia cells, which could also damage and kill neurons, depending on the type of inflammatory response, resulting in psychomotor damage, which characterizes the phenotype of neurodegenerative diseases [79]. Noteworthy, MKD syndromes are characterized, among the other clinical features, by recurrent episodes of fever, one of the clinical signs that unites autoinflammatory diseases with other inflammatory symptoms and neurological involvement especially in the most severe forms (mental and psychomotor retardation, progressive cerebellar ataxia, visual impairment, epilepsy) [6]. Fever represents an adaptive, temporary, and reversible reaction, implemented systematically by the body in response to an inflammatory stimulus that can be caused by substances, called pyrogens, of both the exogenous kind, such as viruses, bacterial agents, and their products, or the endogenous kind, such as various cytokines and pro-inflammatory molecules [80].
From a physiopathological point of view, fever is the result of the action of prostaglandin E2 (PGE2), a metabolite of arachidonic acid, which acts on the thermoregulatory center of the hypothalamus [81]. As a result of the phlogistic insult, pyrogen cytokines, such as IL1β, IL6, and TNFα, are produced by macrophages; they interact indirectly on the thermoregulatory neurons of the hypothalamus, because they stimulate the endothelial cells of the hypothalamic vessels to produce PGE2, which in turn acts on the neurons. Finally, the concentration of cyclic AMP (cAMP) at the hypothalamic level increases and the body temperature increases above the threshold [82].
In general, oxidative stress is a condition in which there is an imbalance between the production of reactive oxygen species (ROS) and the action of antioxidant defenses [83]. Overproduction of ROS leads to progressive damage to cellular molecules, such as DNA, and a mitochondrial dysfunction that in turn generates a further increase in ROS production, compromising cell integrity and viability. Brain cells, in particular, are very sensitive to the effects of oxidative stress and, in such conditions, microglia and astrocytes are stimulated to release inflammatory mediators such as iNOS and to trigger cyclooxygenase 2 (COX-2), causing a neuroinflammatory response [84,85,86].
In particular, oxidative stress in neuroinflammation is a process characterized by the activation of the glia, which underlies a continuous cycle of inflammatory events with the release of cytokines and other neurotoxic mediators [84].
To understand the role of oxidative stress in the biogenesis of neuroinflammation, it should be considered that the inflammation at the molecular level sees the involvement of inflammasome, a multi-protein complex to which NLRP1, NLRP3, NLRP6, and NLRPC4 belong. These proteins are part of the super-family of cytoplasmic receptors called NOD-like receptors (NLR) and are activated in the presence of molecular patterns associated with pathogens (PAMPs) or stress/cell damage (DAMPs) [87,88]. One of the best known components is the NLRP3-inflammasome, which is able to recruit and activate the pro-inflammatory caspase 1, belonging to a family of molecules responsible for the apoptotic cell process. Activated caspase-1, in turn, allows the activation of three pro-inflammatory cytokines: IL-1β, IL-18, and IL-33.
NLRP3-inflammosoma also induces the activation of nuclear factor kB (NF-kB), the main orchestrator of gene transcription during the inflammatory process, the resolution phase of which occurs through a particular form of apoptosis, called pyroptosis [89]. The delicate balance between these two forms of programmed death is fundamental in sustaining inflammation, both systemic and nervous, through the pathway dependent on caspase-9, further confirming the mitochondrial involvement in these processes [90].
In this regard, several literature data have shown that an impaired mitochondrial function is associated with the release of ROS or nitric oxide (NO), which in turn determines the activation of inflammasome [91,92,93].
Finally, in the pathogenesis of neurodegenerative diseases, NLRP3-inflammasome plays a crucial role, also thanks to the fact that the literature data indicate that it is expressed in the cells of the immune system and in the CNS [94,95].
Mitochondrial damage represents a pivotal event of apoptosis in response to various conditions of intracellular stress (DNA damage, cytotoxic damage, oxidative stress, and infections). These stimuli act by inhibiting or activating members of the Bcl-2 family, such as Bak, Bax, Bad, Bcl-xl, and Bim [96]. These pro-apoptotic proteins play a fundamental role because, in the presence of overproduction of ROS, they induce the formation of channels in the mitochondria, as reported in Coenzyme Q10 deficiency [97,98]. Recent landmark works have demonstrated that both a limited as well as a permanently increased flux through the mevalonate pathway trigger alarms and lead to distinct inflammatory and immune responses [99,100]. Increased levels of brain isoprenoids FPP and GGPP have been detected in hyperglycemia, where RhoC is induced in the liver by proinflammatory cytokines and in male Alzheimer patients [101,102]. These sex-dependent alterations in the mevalonate flux have been reported in both the liver and brain, where marked differences in regions involved in memory and learning functions could be at the basis of clinically relevant differences among males and females in both neurodevelopmental and neurodegenerative diseases [103]. Interestingly, FPP has been recently reported to act as a danger signal in the brain, inducing neuronal death in a mouse stroke model through the activation of transient receptor potential melastatin 2 [104]. In diseases associated with prenylation defects such as MKD, the typical neurodegeneration has been reported to be linked to both caspase-9/3-dependent apoptosis, triggered by mitochondrial damage, and to pyroptosis mediated by caspase-1, which in turn activates cytokines and pro-inflammatory chemokines, playing a crucial role in neuroinflammatory mechanisms [90,105,106].
The overproduction of IL-1β in MKD syndromes, linked to neuroinflammation, fever, and oxidative stress, is thus considered a causal factor and is the reason anti-IL-1 therapeutic approaches (anakinra, canakinumab) have been approved for MKD treatment and have been reported to be at least partially effective in some patients [25,61,107,108].
7. Coenzyme Q10: The Fine Regulation of Its Antioxidant Properties
Coenzyme Q10 (Coq10), also improperly called vitamin Q, is a lipid-soluble molecule with powerful antioxidant properties, identified for the first time only in 1957, and produced naturally by the human organism in which it has an ubiquitous distribution, that is, it is present in all its cells [109]. Coenzyme Q10 is located in particular in cell membranes and mitochondria, highly differentiated structures present in the cytoplasm of plant and animal cells with aerobic metabolism. It plays a decisive role in the synthesis of ATP at the level of the electron transport chain in the mitochondria, allowing the production of ATP, and thus of energy; without the key role of Coq10, the chain would be interrupted by preventing the production of ATP.
As mitochondria are present in greater numbers in tissues characterized by a particularly active oxidative metabolism, such as the heart, brain, liver, pancreas, skeletal muscles, and brown adipose tissue, the role of Coq10 is particularly crucial in these anatomical districts. Moreover, Coq10 protects LDL (low-density lipoprotein), sometimes called “bad” cholesterol, from oxidation, and oxidized LDL is particularly harmful because it triggers inflammatory processes in the blood vessels, contributing to the creation of atherosclerotic plaques [110,111,112].
Thanks to these properties, supplements of Coenzyme Q10 are proposed in the case of deficiency or from a prevention perspective, used as nutraceutical, in heart disease, hypertension, neurodegenerative pathologies, cellular aging, and photo-aging. In addition, the integration of Coq10 has also been proposed as a support to drug therapies, and as protection from oxidative stress in the case of intense exercise, for the reduction of fatigue, and improvement of sports performance.
Coq10 supplementation is often associated with the pharmacological treatment of statins. Statins, in fact, are generally very well tolerated, but can induce some kind of muscle toxicity, characterized by various clinical manifestations, and the main reason is associated with the biochemical function of statins as a hypocholesterifying that cause a reduction in the physiological level of Coq10. Statin myopathy includes muscle weakness or pain (myalgia), hypersensitivity and muscle stiffness, cramps, and arthralgia, and it is detected by plasma creatine kinase levels [113,114]. The prevalence of this complication in patients treated with statin varies between 7% and 29%; this muscle toxicity is caused by an accumulation of statin in myocytes and can be caused by defects in the metabolism of the statin, as well as by muscle factors such as mitochondrial damage and production of ROS.
Coq10 has been shown to be very useful in counteracting these effects, as it has a myo-protective action and promotes muscle well-being. In addition, it helps to reduce the sense of fatigue and it is essential to maintain a good physical efficiency and a proper cellular metabolism [115,116].
Symptomatology resulting from a Coq10 synthesis is an induced and transient phenomenon that must be distinguished from rare genetic neurometabolic disease, referred to as Coq10 deficiency (OMIM #607426, #614652). It is an autosomal recessive transmission disease and the damage caused by this condition can affect all organs; however, depending on the form of the pathology, the organs more affected are the kidneys, cerebellum, skeletal, and heart muscles [117].
When the kidneys are involved, nephrotic syndrome is established, which can lead to renal failure [118,119]. If the cerebellum is mainly affected, the disease manifests itself with difficulty in walking and coordinating movements or convulsions [120,121], while the third form affects the muscles and manifests itself with muscle weakness [117,122]. Typically, symptoms appear during childhood, but may also occur later. To date, the best pharmacological treatment for this pathology is represented by the oral administration of Coq 10.
The study of the wide-ranging effects of this deficit has made possible the understanding of the antioxidant and protective role played by Coq10.
8. Conclusions
The manipulation of the metabolic pathway of cholesterol by inhibitors is aimed at regulating the synthesis of the final product. The blocking of activity of the HMG-CoA reductase and the FPP by inhibitors is focused on acting specifically at different levels of the pathway to counteract the overproduction of cholesterol. The mechanisms implemented by these compounds, which are the basis of the pharmacological active principles commonly used in the treatment of hypercholesterolemia such as statins and bisphosphonates, have highlighted transduction pathways′ signals triggered by the block of the metabolic pathway. One of the most important regulatory mechanisms is undoubtedly the reduction of regulatory proteins that bind GTP through the blocking of the production of farnesyl-pyrophosphate. The main substrates of post-translational prenylation modifications are represented by G proteins such as Rho and Rac, which function as molecular switches and, through the transduction of extracellular signals, act on cell survival, growth, and programmed cell death. These inhibitors also act at the endothelial level through the upregulation of nitric oxide synthase, and this system is certainly the basic mechanism to counteract the formation of atherosclerotic plaque. The prenylation defects are considered responsible for the inflammatory process owing to the deficiency of the intermediates of the metabolic pathway such as isoprenoids. This condition is the basis of the pathogenesis of rare pediatric diseases such as MKD, the most severe form of which experiences a significant state of neuroinflammation and clinically manifests itself with a psychomotor delay in patients. The neuroinflammatory response is characterized by a series of changes that mainly involve the role of microglial cells in the maintenance of cerebral homeostasis. An imbalance at this level involves activation, by specific brain mediators, of microglia that is in turn responsible for amplifying and maintaining the inflammatory state. Moreover, oxidative stress has been shown to be one of the main causes of mitochondrial dysfunction, resulting in alteration of ATP production, as in Coq10 deficiency.
The effects of prenylation deregulation and oxidative stress converge to determine specific morphological changes, especially on the mitochondrial compartment, and the deepening of the molecular mechanisms underlying these modifications is at the heart of considerable scientific interest as the potential spillovers of such knowledge are aimed at identifying transversally innovative therapeutic targets.
Author Contributions
Conceptualization, S.P., M.B. and A.M.; methodology, E.R., E.M. and R.M.; validation, S.P. and A.M.; formal analysis, E.P. and E.Z.; investigation, E.P. and E.Z.; resources, M.B., R.M. and A.M.; data curation, S.P., R.M. and M.B.; writing—original draft preparation, S.P., E.R., E.M. and A.M.; writing—review and editing, S.P. E.R., E.M., R.M. and A.M.; supervision, M.B.; project administration, R.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The financial support of Telethon—Italy (Grant no. GEP14111) is gratefully acknowledged.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jeong A., Suazo K.F., Wood W.G., Distefano M.K., Li L. Isoprenoids and Protein Prenylation: Implications in the Pathogenesis and Therapeutic Intervention of Alzheimer’s Disease. Crit. Rev. Biochem. Mol. Biol. 2018;53:279–310. doi: 10.1080/10409238.2018.1458070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desmet S., Morreel K., Dauwe R. Origin and Function of Structural Diversity in the Plant Specialized Metabolome. Plants. 2021;10:2393. doi: 10.3390/plants10112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamphear C.L., Zverina E.A., Hougland J.L., Fierke C.A. Global Identification of Protein Prenyltransferase Substrates: Defining the Prenylated Proteome. Enzymes. 2011;29:207–234. [Google Scholar]
- 4.Goldstein J.L., Brown M.S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 5.Buhaescu I., Izzedine H. Mevalonate pathway: A review of clinical and therapeutical implications. Clin. Biochem. 2007;40:575–584. doi: 10.1016/j.clinbiochem.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Rigante D. Phenotype variability of autoinflammatory disorders in the pediatric patient: A pictorial overview. J. Evid.-Based Med. 2020;13:227–245. doi: 10.1111/jebm.12406. [DOI] [PubMed] [Google Scholar]
- 7.Kawamukai M. Biosynthesis and applications of prenylquinones. Biosci. Biotechnol. Biochem. 2018;82:963–977. doi: 10.1080/09168451.2018.1433020. [DOI] [PubMed] [Google Scholar]
- 8.Rivett E.D., Heo L., Feig M., Hegg E.L. Biosynthesis and trafficking of heme o and heme a: New structural insights and their implications for reaction mechanisms and prenylated heme transfer. Crit. Rev. Biochem. Mol. Biol. 2021;56:640–668. doi: 10.1080/10409238.2021.1957668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierrel F., Burgardt A., Lee J.H., Pelosi L., Wendisch V.F. Recent advances in the metabolic pathways and microbial production of coenzyme Q. World J. Microbiol. Biotechnol. 2022;38:58. doi: 10.1007/s11274-022-03242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciaglia E., Laezza C., Abate M., Pisanti S., Ranieri R., D’alessandro A., Picardi P., Gazzerro P., Bifulco M. Recognition by natural killer cells of 6-isopentenyladenosine-treated human glioma cell lines. Int. J. Cancer. 2018;142:176–190. doi: 10.1002/ijc.31036. [DOI] [PubMed] [Google Scholar]
- 11.Mitsche M.A., McDonald J.G., Hobbs H.H., Cohen J.C. Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways. eLife. 2015;4:e07999. doi: 10.7554/eLife.07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchernof A., Després J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 13.Tall A.R., Yvan-Charvet L., Westerterp M., Murphy A.J. Cholesterol efflux: A novel regulator of myelopoiesis and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2012;32:2547–2552. doi: 10.1161/ATVBAHA.112.300134. [DOI] [PubMed] [Google Scholar]
- 14.Yvan-Charvet L., Bonacina F., Guinamard R.R., Norata G.D. Immunometabolic function of cholesterol in cardiovascular disease and beyond. Cardiovasc. Res. 2019;115:1393–1407. doi: 10.1093/cvr/cvz127. [DOI] [PubMed] [Google Scholar]
- 15.Lütjohann D., Björkhem I., Diczfalusy U., Ståhle L., Ahlborg G., Wahren J. Cholesterol homeostasis in human brain: Turnover of 24(S)-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J. Lipid Res. 1998;39:1594–1600. [PubMed] [Google Scholar]
- 16.Dietschy J.M., Turley S.D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Schumacher M.M., DeBose-Boyd R.A. Posttranslational Regulation of HMG CoA Reductase, the Rate-Limiting Enzyme in Synthesis of Cholesterol. Annu. Rev. Biochem. 2021;90:659–679. doi: 10.1146/annurev-biochem-081820-101010. [DOI] [PubMed] [Google Scholar]
- 18.Pierzchlińska A., Droździk M., Białecka M. A Possible Role for HMG-CoA Reductase Inhibitors and Its Association with HMGCR Genetic Variation in Parkinson’s Disease. Int. J. Mol. Sci. 2021;22:12198. doi: 10.3390/ijms222212198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon S.J., Hong K.W., Choi S., Hong J.S., Kim J.W., Kim J.W., Lee H.J., Jang H.B., Yum K.S. Association of 3-hydroxy-3-methylglutaryl-coenzyme A reductase gene polymorphism with obesity and lipid metabolism in children and adolescents with autism spectrum disorder. Metab. Brain Dis. 2022;37:319–328. doi: 10.1007/s11011-021-00877-3. [DOI] [PubMed] [Google Scholar]
- 20.Wall C.T.J., Lefebvre G., Metairon S., Descombes P., Wiederkehr A., Santo-Domingo J. Mitochondrial respiratory chain dysfunction alters ER sterol sensing and mevalonate pathway activity. J. Biol. Chem. 2022;298:101652. doi: 10.1016/j.jbc.2022.101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S. Natural history of mevalonate kinase deficiency: A literature review. Pediatr. Rheumatol. Online J. 2016;14:30. doi: 10.1186/s12969-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue L., Qi H., Zhang H., Ding L., Huang Q., Zhao D., Wu B.J., Li X. Targeting SREBP-2-Regulated Mevalonate Metabolism for Cancer Therapy. Front. Oncol. 2020;10:1510. doi: 10.3389/fonc.2020.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinson D.D., Chambliss K.L., Toth M.J., Tanaka R.D., Gibson K.M. Post-translational regulation of mevalonate kinase by intermediates of the cholesterol and nonsterol isoprene biosynthetic pathways. J. Lipid Res. 1997;38:2216–2223. doi: 10.1016/S0022-2275(20)34935-X. [DOI] [PubMed] [Google Scholar]
- 24.Waku T., Hagiwara T., Tamura N., Atsumi Y., Urano Y., Suzuki M., Iwami T., Sato K., Yamamoto M., Noguchi N., et al. NRF3 upregulates gene expression in SREBP2-dependent mevalonate pathway with cholesterol uptake and lipogenesis inhibition. iScience. 2021;24:103180. doi: 10.1016/j.isci.2021.103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elhani I., Hentgen V., Grateau G., Georgin-Lavialle S. Neurological manifestations in mevalonate kinase deficiency: A systematic review. Mol. Genet. Metab. 2022;136:85–93. doi: 10.1016/j.ymgme.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Caligiuri G., Norata G.D. Fuel for thought: Immunometabolism is a paradigm shift in understanding immunity in cardiovascular disease. Cardiovasc. Res. 2019;115:1383–1384. doi: 10.1093/cvr/cvz155. [DOI] [PubMed] [Google Scholar]
- 27.Nowaczyk M.J., Irons M.B. Smith-Lemli-Opitz syndrome: Phenotype, natural history, and epidemiology. Am. J. Med. Genet. C Semin. Med. Genet. 2012;160C:250–262. doi: 10.1002/ajmg.c.31343. [DOI] [PubMed] [Google Scholar]
- 28.Maresca G., Formica C., Nocito V., Latella D., Leonardi S., De Cola M.C., Triglia G., Bramanti P., Corallo F. Neuropsychological assessment in Niemann-Pick disease type C: A systematic review. Neurol. Sci. 2021;42:3167–3175. doi: 10.1007/s10072-021-05337-5. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves I., Heaton R.A., Mantle D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020;21:6695. doi: 10.3390/ijms21186695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinzii C.M., DiMauro S., Hirano M. Human coenzyme Q10 deficiency. Neurochem. Res. 2007;32:723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manzar H., Abdulhussein D., Yap T.E., Cordeiro M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020;21:9299. doi: 10.3390/ijms21239299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genaro-Mattos T.C., Anderson A., Allen L.B., Korade Z., Mirnics K. Cholesterol Biosynthesis and Uptake in Developing Neurons. ACS Chem. Neurosci. 2019;10:3671–3681. doi: 10.1021/acschemneuro.9b00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vance J.E. Dysregulation of cholesterol balance in the brain: Contribution to neurodegenerative diseases. Dis. Model. Mech. 2012;5:746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas D., Kelley R.I., Hoffmann G.F. Inherited disorders of cholesterol biosynthesis. Neuropediatrics. 2001;32:113–122. doi: 10.1055/s-2001-16618. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F.L., Casey P.J. Protein prenylation: Molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 36.Casey P.J., Seabra M.C. Protein Prenyltransferases. J. Biol. Chem. 1996;71:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 37.Reiss Y., Stradley S.J., Gierasch L.M., Brown M.S., Goldstein J.L. Sequence Requirement for Peptide Recognition by the Rat Brain p21 Protein Farnesyltransferase. Proc. Natl. Acad. Sci. USA. 1991;88:732–736. doi: 10.1073/pnas.88.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama K., Goodwin G.W., Ghomashchi F., Glomset J.A., Gelb M.H. A protein geranylgeranyltransferase from bovine brain: Implications for protein prenylation specificity. Proc. Natl. Acad. Sci. USA. 1991;88:5302–5306. doi: 10.1073/pnas.88.12.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanden M.J., Suazo K.F., Hildebrandt E.R., Hardgrove D.S., Patel M., Saunders W.P., Distefano M.D., Schmidt W.K., Hougland J.L. Efficient farnesylation of an extended C-terminal C(x)3X sequence motif expands the scope of the prenylated proteome. J. Biol. Chem. 2018;293:2770–2785. doi: 10.1074/jbc.M117.805770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiemer A.J., Wiemer D.F., Hohl R.J. Geranylgeranyl diphosphate synthase: An emerging therapeutic target. Clin. Pharmacol. Ther. 2011;90:804–812. doi: 10.1038/clpt.2011.215. [DOI] [PubMed] [Google Scholar]
- 41.Noguera-Salvà M.A., Guardiola-Serrano F., Martin M.L., Marcilla-Etxenike A., Bergo M.O., Busquets X., Escribá P.V. Role of the C-terminal basic amino acids and the lipid anchor of the Gγ2 protein in membrane interactions and cell localization. Biochim. Biophys. Acta Biomembr. 2017;859:1536–1547. doi: 10.1016/j.bbamem.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Hampton S.E., Dore T.M., Schmidt W.K. Rce1: Mechanism and inhibition. Crit. Rev. Biochem. Mol. Biol. 2018;53:157–174. doi: 10.1080/10409238.2018.1431606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borini Etichetti C.M., Arel Zalazar E., Cocordano N., Girardini J. Beyond the Mevalonate Pathway: Control of Post-Prenylation Processing by Mutant p53. Front. Oncol. 2020;10:595034. doi: 10.3389/fonc.2020.595034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jun J.E., Rubio I., Roose J.P. Regulation of Ras Exchange Factors and cellular localization of Ras activation by lipid messengers in T cells. Front. Immunol. 2013;4:239. doi: 10.3389/fimmu.2013.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bifulco M. Role of the isoprenoid pathway in Ras transforming activity, cytoskeleton organization, cell proliferation and apoptosis. Life Sci. 2005;77:1740–1749. doi: 10.1016/j.lfs.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 46.Prior I.A., Hancock J.F. Compartmentalization of Ras proteins. J. Cell Sci. 2001;114:1603–1608. doi: 10.1242/jcs.114.9.1603. [DOI] [PubMed] [Google Scholar]
- 47.Lane K.T., Beese L.S. Thematic review series: Lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J. Lipid Res. 2006;47:681–699. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Sindhu M., Saini V., Piplani S., Kumar A. Molecular Dynamics of Rab7::REP1::GGTase-II Ternary Complex and Identification of Their Putative Drug Binding Sites. Indian J. Pharm. Sci. 2013;75:23–30. doi: 10.4103/0250-474X.113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchwicka A., Kamińska D., Monirialamdari M., Błażewska K.M., Gendaszewska-Darmach E. Protein Prenyltransferases and Their Inhibitors: Structural and Functional Characterization. Int. J. Mol. Sci. 2022;23:5424. doi: 10.3390/ijms23105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watterson A., Tatge L., Wajahat N., Arneaud S.L.B., Solano Fonseca R., Beheshti S.T., Metang P., Mihelakis M., Zuurbier K.R., Corley C.D., et al. Intracellular lipid surveillance by small G protein geranylgeranylation. Nature. 2022;605:736–740. doi: 10.1038/s41586-022-04729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manaswiyoungkul P., de Araujo E.D., Gunning P.T. Targeting prenylation inhibition through the mevalonate pathway. RSC Med. Chem. 2019;11:51–71. doi: 10.1039/C9MD00442D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Y., Wu T.-Y., Zhao M.-F., Li C.-J. The balance of protein farnesylation and geranylgeranylation during the progression of nonalcoholic fatty liver disease. J. Biol. Chem. 2020;295:5152–5162. doi: 10.1074/jbc.REV119.008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waller D.D., Park J., Tsantrizos Y.S. Inhibition of farnesyl pyrophosphate (FPP) and/or geranylgeranyl pyrophosphate (GGPP) biosynthesis and its implication in the treatment of cancers. Crit. Rev. Biochem. Mol. Biol. 2019;54:41–60. doi: 10.1080/10409238.2019.1568964. [DOI] [PubMed] [Google Scholar]
- 54.Laezza C., Notarnicola M., Caruso M.G., Messa C., Macchia M., Bertini S., Minutolo F., Portella G., Fiorentino L., Stingo S., et al. N6-isopentenyladenosine arrests tumor cell proliferation by inhibiting farnesyl diphosphate synthase and protein prenylation. FASEB J. 2006;20:412–418. doi: 10.1096/fj.05-4044lsf. [DOI] [PubMed] [Google Scholar]
- 55.Roy M., Kung H.J., Ghosh P.M. Statins and prostate cancer: Role of cholesterol inhibition vs. prevention of small GTP-binding proteins. Am. J. Cancer Res. 2011;1:542–561. [PMC free article] [PubMed] [Google Scholar]
- 56.Todenhöfer T., Hennenlotter J., Kühs U., Gerber V., Gakis G., Vogel U., Aufderklamm S., Merseburger A., Knapp J., Stenzl A., et al. Altered expression of farnesyl pyrophosphate synthase in prostate cancer: Evidence for a role of the mevalonate pathway in disease progression? World J. Urol. 2013;31:345–350. doi: 10.1007/s00345-012-0844-y. [DOI] [PubMed] [Google Scholar]
- 57.Reddy J.M., Raut N.G.R., Seifert J.L., Hynds D.L. Regulation of Small GTPase Prenylation in the Nervous System. Mol. Neurobiol. 2020;57:2220–2231. doi: 10.1007/s12035-020-01870-0. [DOI] [PubMed] [Google Scholar]
- 58.Hottman D., Li L. Protein prenylation and synaptic plasticity: Implications for Alzheimer’s disease. Mol. Neurobiol. 2014;50:177–185. doi: 10.1007/s12035-013-8627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu J., Selzer M.E. RhoA as a target to promote neuronal survival and axon regeneration. Neural Regen. Res. 2017;12:525–528. doi: 10.4103/1673-5374.205080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munoz M.A., Jurczyluk J., Simon A., Hissaria P., Arts R.J.W., Coman D., Boros C., Mehr S., Rogers M.J. Defective Protein Prenylation in a Spectrum of Patients with Mevalonate Kinase Deficiency. Front. Immunol. 2019;10:1900. doi: 10.3389/fimmu.2019.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeyaratnam J., Frenkel J. Management of Mevalonate Kinase Deficiency: A Pediatric Perspective. Front. Immunol. 2020;11:1150. doi: 10.3389/fimmu.2020.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Politiek F.A., Waterham H.R. Compromised Protein Prenylation as Pathogenic Mechanism in Mevalonate Kinase Deficiency. Front. Immunol. 2021;12:724991. doi: 10.3389/fimmu.2021.724991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munoz M.A., Jurczyluk J., Mehr S., Chai R.C., Arts R.J., Sheu A., McMahon C., Center J.R., Singh-Grewal D., Chaitow J., et al. Defective protein prenylation is a diagnostic biomarker of mevalonate kinase deficiency. J. Allergy Clin. Immunol. 2017;140:873–875.e6. doi: 10.1016/j.jaci.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 64.Jurczyluk J., Munoz M.A., Skinner O.P., Chai R.C., Ali N., Palendira U., Quinn J.M., Preston A., Tangye S.G., Brown A.J., et al. Mevalonate kinase deficiency leads to decreased prenylation of Rab GTPases. Immunol. Cell Biol. 2016;94:994–999. doi: 10.1038/icb.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skinner O.P., Jurczyluk J., Baker P.J., Masters S.L., Rios Wilks A.G., Clearwater M.S., Robertson A.A.B., Schroder K., Mehr S., Munoz M.A., et al. Lack of protein prenylation promotes NLRP3 inflammasome assembly in human monocytes. J. Allergy Clin. Immunol. 2019;143:2315–2317. doi: 10.1016/j.jaci.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 66.van der Burgh R., Pervolaraki K., Turkenburg M., Waterham H.R., Frenkel J., Boes M. Unprenylated RhoA contributes to IL-1β hypersecretion in mevalonate kinase deficiency model through stimulation of Rac1 activity. J. Biol. Chem. 2014;289:27757–27765. doi: 10.1074/jbc.M114.571810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Favier L.A., Schulert G.S. Mevalonate kinase deficiency: Current perspectives. Appl. Clin. Genet. 2016;9:101–110. doi: 10.2147/TACG.S93933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoogland I.C., Houbolt C., van Westerloo D.J., van Gool W.A., van de Beek D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L., Dai L., Deyuan L. Mitophagy in neurological disorders. J. Neuroinflamm. 2021;18:297. doi: 10.1186/s12974-021-02334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaur N., Chugh H., Sakharkar M.K., Dhawan U., Chidambaram S.B., Chandra R. Neuroinflammation Mechanisms and Phytotherapeutic Intervention: A Systematic Review. ACS Chem. Neurosci. 2020;11:3707–3731. doi: 10.1021/acschemneuro.0c00427. [DOI] [PubMed] [Google Scholar]
- 71.Stolp H.B., Dziegielewska K.M. Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2009;35:132–146. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 72.Umpierre A.D., Wu L.J. How microglia sense and regulate neuronal activity. Glia. 2021;69:1637–1653. doi: 10.1002/glia.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liddelow S.A., Marsh S.E., Stevens B. Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol. 2020;41:820–835. doi: 10.1016/j.it.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Rodríguez A.M., Rodríguez J., Giambartolomei G.H. Microglia at the Crossroads of Pathogen-Induced Neuroinflammation. ASN Neuro. 2022;14:17590914221104566. doi: 10.1177/17590914221104566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hickman S., Izzy S., Sen P., Morsett L., El Khoury J. Microglia in neurodegeneration. Nat. Neurosci. 2018;21:1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borst K., Schwabenland M., Prinz M. Microglia metabolism in health and disease. Neurochem. Int. 2019;130:104331. doi: 10.1016/j.neuint.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Colonna M., Butovsky O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uccelli A., Gattorno M. Neurological manifestations in autoinflammatory diseases. Clin. Exp. Rheumatol. 2018;36:61–67. [PubMed] [Google Scholar]
- 79.Wendimu M.Y., Hooks S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells. 2022;11:2091. doi: 10.3390/cells11132091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hasday J.D., Singh I.S. Fever and the heat shock response: Distinct, partially overlapping processes. Cell Stress Chaperones. 2000;5:471–480. doi: 10.1379/1466-1268(2000)005<0471:FATHSR>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blatteis C.M., Li S., Li Z., Feleder C., Perlik V. Cytokines, PGE2 and endotoxic fever: A re-assessment. Prostaglandins Other Lipid Mediat. 2005;76:1–18. doi: 10.1016/j.prostaglandins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Garami A., Steiner A.A., Romanovsky A.A. Fever and hypothermia in systemic inflammation. Handb. Clin. Neurol. 2018;157:565–597. doi: 10.1016/B978-0-444-64074-1.00034-3. [DOI] [PubMed] [Google Scholar]
- 83.Szarka A., Lőrincz T., Hajdinák P. Friend or Foe: The Relativity of (Anti)oxidative Agents and Pathways. Int. J. Mol. Sci. 2022;23:5188. doi: 10.3390/ijms23095188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patergnani S., Morciano G., Carinci M., Leo S., Pinton P., Rimessi A. The “mitochondrial stress responses”: The “Dr. Jekyll and Mr. Hyde” of neuronal disorders. Neural Regen. Res. 2022;17:2563–2575. doi: 10.4103/1673-5374.339473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vitória J.J.M., Trigo D., da Cruz E., Silva O.A.B. Revisiting APP secretases: An overview on the holistic effects of retinoic acid receptor stimulation in APP processing. Cell. Mol. Life Sci. 2022;79:101. doi: 10.1007/s00018-021-04090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zindler E., Zipp F. Neuronal injury in chronic CNS inflammation. Best Pract. Res. Clin. Anaesthesiol. 2010;24:551–562. doi: 10.1016/j.bpa.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 88.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 89.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tricarico P.M., Kleiner G., Valencic E., Campisciano G., Girardelli M., Crovella S., Knowles A., Marcuzzi A. Block of the mevalonate pathway triggers oxidative and inflammatory molecular mechanisms modulated by exogenous isoprenoid compounds. Int. J. Mol. Sci. 2014;15:6843–6856. doi: 10.3390/ijms15046843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 92.Misawa T., Takahama M., Kozaki T., Lee H., Zou J., Saitoh T., Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 93.Lawlor K.E., Vince J.E. Ambiguities in NLRP3 inflammasome regulation: Is there a role for mitochondria? Biochim. Biophys. Acta. 2014;1840:1433–1440. doi: 10.1016/j.bbagen.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 94.Yin Y., Yan Y., Jiang X., Mai J., Chen N.C., Wang H., Yang X.-F. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int. J. Immunopathol. Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V.L., El-Deiry W.S., Fulda S., et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simon H.U., Haj-Yehia A., Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- 98.Santos-Ocaña C., Cascajo M.V., Alcázar-Fabra M., Staiano C., López-Lluch G., Brea-Calvo G., Navas P. Cellular Models for Primary CoQ Deficiency Pathogenesis Study. Int. J. Mol. Sci. 2021;22:10211. doi: 10.3390/ijms221910211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akula M.K., Shi M., Jiang Z., Foster C.E., Miao D., Li A.S., Zhang X., Gavin R.M., Forde S.D., Germain G., et al. Control of the innate immune response by the mevalonate pathway. Nat. Immunol. 2016;17:922–929. doi: 10.1038/ni.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masumoto J., Zhou W., Morikawa S., Hosokawa S., Taguchi H., Yamamoto T., Kurata M., Kaneko N. Molecular biology of autoinflammatory diseases. Inflamm. Regen. 2021;41:33. doi: 10.1186/s41232-021-00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okin D., Medzhitov R. The Effect of Sustained Inflammation on Hepatic Mevalonate Pathway Results in Hyperglycemia. Cell. 2016;165:343–356. doi: 10.1016/j.cell.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eckert G.P., Hooff G.P., Strandjord D.M., Igbavboa U., Volmer D.A., Müller W.E., Wood W.G. Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol. Dis. 2009;35:251–257. doi: 10.1016/j.nbd.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tonini C., Segatto M., Pallottini V. Impact of Sex and Age on the Mevalonate Pathway in the Brain: A Focus on Effects Induced by Maternal Exposure to Exogenous Compounds. Metabolites. 2020;10:304. doi: 10.3390/metabo10080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen J., Zhang X., Li L., Ma X., Yang C., Liu Z., Li C., Fernandez-Cabezudo M.J., Al-Ramadi B.K., Wu C., et al. Farnesyl pyrophosphate is a new danger signal inducing acute cell death. PLoS Biol. 2021;19:e3001134. doi: 10.1371/journal.pbio.3001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marcuzzi A., Zanin V., Piscianz E., Tricarico P.M., Vuch J., Girardelli M., Monasta L., Bianco A.M., Crovella S. Lovastatin-induced apoptosis is modulated by geranylgeraniol in a neuroblastoma cell line. Int. J. Dev. Neurosci. 2012;30:451–456. doi: 10.1016/j.ijdevneu.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Tricarico P.M., Marcuzzi A., Piscianz E., Monasta L., Crovella S., Kleiner G. Mevalonate kinase deficiency and neuroinflammation: Balance between apoptosis and pyroptosis. Int. J. Mol. Sci. 2013;14:23274–23288. doi: 10.3390/ijms141223274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Benedetti F., Gattorno M., Anton J., Ben-Chetrit E., Frenkel J., Hoffman H.M., Koné-Paut I., Lachmann H., Ozen S., Simon A., et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N. Engl. J. Med. 2018;378:1908–1919. doi: 10.1056/NEJMoa1706314. [DOI] [PubMed] [Google Scholar]
- 108.Grom A.A., Horne A., De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 2016;12:259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Szkopińska A. Ubiquinone. Biosynthesis of quinone ring and its isoprenoid side chain. Intracellular localization. Acta Biochim. Pol. 2000;47:469–480. doi: 10.18388/abp.2000_4027. [DOI] [PubMed] [Google Scholar]
- 110.Rabanal-Ruiz Y., Llanos-González E., Alcain F.J. The Use of Coenzyme Q10 in Cardiovascular Diseases. Antioxidants. 2021;10:755. doi: 10.3390/antiox10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suárez-Rivero J.M., Pastor-Maldonado C.J., de la Mata M., Villanueva-Paz M., Povea-Cabello S., Álvarez-Córdoba M., Villalón-García I., Suárez-Carrillo A., Talaverón-Rey M., Munuera M., et al. Atherosclerosis and Coenzyme Q10. Int. J. Mol. Sci. 2019;20:5195. doi: 10.3390/ijms20205195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmelzer C., Niklowitz P., Okun J.G., Haas D., Menke T., Döring F. Ubiquinol-induced gene expression signatures are translated into altered parameters of erythropoiesis and reduced low density lipoprotein cholesterol levels in humans. IUBMB Life. 2011;63:42–48. doi: 10.1002/iub.413. [DOI] [PubMed] [Google Scholar]
- 113.Somagutta M.K.R., Shama N., Pormento M.K.L., Jagani R.P., Ngardig N.N., Ghazarian K., Mahmutaj G., El-Faramawy K., Mahadevaiah A., Jain M.S. Statin-induced necrotizing autoimmune myopathy: A systematic review. Reumatologia. 2022;60:63–69. doi: 10.5114/reum.2022.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel K.K., Sehgal V.S., Kashfi K. Molecular targets of statins and their potential side effects: Not all the glitter is gold. Eur. J. Pharmacol. 2022;922:174906. doi: 10.1016/j.ejphar.2022.174906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aaseth J., Alexander J., Alehagen U. Coenzyme Q10 supplementation—In ageing and disease. Mech. Ageing Dev. 2021;197:111521. doi: 10.1016/j.mad.2021.111521. [DOI] [PubMed] [Google Scholar]
- 116.Raizner A.E., Quiñones M.A. Coenzyme Q10 for Patients with Cardiovascular Disease: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021;77:609–619. doi: 10.1016/j.jacc.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 117.Alcázar-Fabra M., Trevisson E., Brea-Calvo G. Clinical syndromes associated with Coenzyme Q10 deficiency. Essays Biochem. 2018;62:377–398. doi: 10.1042/EBC20170107. [DOI] [PubMed] [Google Scholar]
- 118.Tan W., Airik R. Primary coenzyme Q10 nephropathy, a potentially treatable form of steroid-resistant nephrotic syndrome. Pediatr. Nephrol. 2021;36:3515–3527. doi: 10.1007/s00467-020-04914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schijvens A.M., van de Kar N.C., Bootsma-Robroeks C., Cornelissen E.A., van den Heuvel L.P., Schreuder M.F. Mitochondrial Disease and the Kidney with a Special Focus o.on CoQ10 Deficiency. Kidney Int. Rep. 2020;5:2146–2159. doi: 10.1016/j.ekir.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caglayan A.O., Gumus H., Sandford E., Kubisiak T.L., Ma Q., Ozel A.B., Per H., Li J.Z., Shakkottai V.G., Burmeister M. COQ4 Mutation Leads to Childhood-Onset Ataxia Improved by CoQ10 Administration. Cerebellum. 2019;18:665–669. doi: 10.1007/s12311-019-01011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stefely J.A., Licitra F., Laredj L., Reidenbach A.G., Kemmerer Z.A., Grangeray A., Jaeg-Ehret T., Minogue C.E., Ulbrich A., Hutchins P.D., et al. Cerebellar Ataxia and Coenzyme Q Deficiency through Loss of Unorthodox Kinase Activity. Mol. Cell. 2016;63:608–620. doi: 10.1016/j.molcel.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baschiera E., Sorrentino U., Calderan C., Desbats M.A., Salviati L. The multiple roles of coenzyme Q in cellular homeostasis and their relevance for the pathogenesis of coenzyme Q deficiency. Free Radic. Biol. Med. 2021;166:277–286. doi: 10.1016/j.freeradbiomed.2021.02.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.