Abstract
A xylanolytic strain of Brevibacterium lactofermentum containing the Streptomyces halstedii His-tagged xysA gene was generated. The new strain contains DNA derived from S. halstedii, expresses xylanolytic activity, and was obtained by an integrative process mediated by a conjugative plasmid targeted to a dispensable chromosomal region located downstream from the essential cell division gene ftsZ. The His-tagged Xys1 enzyme was constitutively expressed under the control of the kan promoter from Tn5 and was easily purified by use of Ni-nitrilotriacetic acid-agarose. The new strain is stable for more than 200 generations, lacks any known antibiotic resistance gene, and does not need any selective pressure to maintain the integrated gene. This strategy can be used to integrate any gene into the B. lactofermentum chromosome and to maintain it stably without the use of antibiotics for selection.
Coryneform bacteria are industrially used for amino acid production and, due to the lack of detectable extracellular hydrolytic enzymes (cellulases, glucanases, xylanases, proteases, and so forth), the production medium in the fermentation industry is mainly composed of beet molasses and protein hydrolysates. However, the use of recombinant DNA technology has led to the construction of Brevibacterium lactofermentum strains able to degrade starch (3, 23), xylan, and cellulose (1) by cloning of the corresponding genes in corynebacterial or Escherichia coli-corynebacterial plasmids. The induction of such abilities is a marked improvement for any industrial strain because of the lower cost of the substrates and the production of extracellular enzymes by these microorganisms. However, the above-mentioned strains carry plasmids and antibiotic resistance genes and thus might not be useful in the near future because of stringent regulations on genetically manipulated microorganisms, especially when the fermentation product is to be used in human or animal food (food-grade microorganisms) (5, 14).
To overcome this problem, we designed a method that can be used to integrate any gene in the genome of B. lactofermentum by double recombination. There are several descriptions of plasmids that can be used to integrate genes in the genome of corynebacteria based on the presence of a chromosomal gene in the plasmid (18, 19, 22) or very short homologous DNA segments (8 to 12 bp) in the vector and in the host DNA (15). However, all of them require the presence of antibiotics for selection. The generation of a drug-resistant recombinant strain can both reduce the in vivo applicability of the strain and preclude the use of recombinant vectors that use the same drug resistance marker.
Here we describe the construction of a suicide conjugative plasmid, pK18-3, which allows the recombination of any exogenous gene onto the B. lactofermentum chromosome. The exogenous gene integrates by double recombination in one of the three nonessential open reading frames (ORFs) located downstream from ftsZ in the genome of B. lactofermentum (8, 9), and the resulting strain lacks any exogenous drug resistance marker. The method was checked by the integration of the His-tagged xysA gene from Streptomyces halstedii JM8 (20).
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this work are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | r−m−; used for general cloning experiments | 6 |
| E. coli S17-1 | Used for conjugation experiments | 22 |
| B. lactofermentum 13869 | Wild type | American Type Culture Collection |
| B. lactofermentum R31 | 13869 derivative used as a host for transformation, electroporation, or conjugation experiments | 21 |
| B. lactofermentum X1 | XylA+ Kanr R31 derivative containing plasmid pK18-3X integrated in the chromosome by a single recombination event | This work |
| B. lactofermentum X2 | XylA+ Kans R31 derivative containing the six-His-tagged ΔxysA gene integrated in the chromosome by double recombination | This work |
| Plasmids | ||
| pK18mob | Mobilizable plasmid containing an E. coli origin of replication and kan | 22 |
| pK18-3 | pK18mob derivative carrying a 3.3-kb BamHI fragment from the B. lactofermentum chromosome containing part of ftsZ and the ORFs YFIH, ORF5, and ORF6 | This work |
| pIJ2925 | pUC18 derivative with BglII sites flanking a modified multiple cloning site | 11 |
| pXHis-Npro | pIJ2925 derivative containing the DNA fragment six-His-tagged ΔxysA (encodes the catalytic domain of xylanase Xys1 with a tag of six histidines) under the control of the promoter of the kan gene from Tn5 and flanked by the terminator of the methylenomycin resistance gene (T1) and the terminator of phage fd (T2) | |
| pK18-3X | pK18-3 derivative carrying His-tagged xylanase from plasmid pXHis-Npro | This work |
| pUL880M | Bifunctional E. coli-B. lactofermentum promoter-probe vector with bla and hyg genes as selective markers and the promoterless kan gene as a reporter gene | 13 |
| p880X2A | pUL880M derivative containing six-His-tagged Pkan-ΔxysA in the same orientation as the kan gene; the kan gene is expressed in E. coli and B. lactofermentum transformed with this plasmid |
Culture conditions and transformation procedures.
The culture media for B. lactofermentum were tryptic soy broth (TSB) (21) and S2 (16). The plasmids to be transferred by conjugation from E. coli to B. lactofermentum were introduced by transformation into donor strain E. coli S17-1 (Table 1). B. lactofermentum R31 was used as the recipient strain. Conjugation between E. coli and B. lactofermentum was performed as described by Fernández-González et al. (4). For direct selection of antibiotic-resistant transconjugants, tryptic soy agar (TSA) plates (21) were supplemented with kanamycin at a final concentration of 30 μg ml−1.
E. coli cells were grown in Luria broth medium (17) and transformed as described by Hanahan (6). Transformants were selected on Luria agar plates containing 100 μg of kanamycin ml−1.
DNA isolation, manipulation, and characterization.
Plasmid preparations were obtained from E. coli as described by Holmes and Quigley (7). Total DNA from corynebacteria was isolated using a method described for Streptomyces (10), but cells were treated with lysozyme for 3 h at 30°C. Total DNA from B. lactofermentum transconjugants was digested with different restriction enzymes and hybridized with the kan gene, with the xysA gene, or with the 3.3-kb BamHI fragment of B. lactofermentum containing the three ORFs (YFIH, ORF5, and ORF6) (probe 3) labeled with digoxigenin according to the manufacturer's instructions (Boerhinger Mannhein). The rest of the DNA manipulations were performed using standard procedures (10).
Xylanase assays.
The production of xylanase by B. lactofermentum transconjugants was assayed on TSA containing 0.3% Remazol brilliant blue R–d-xylan (Sigma) or on minimal medium or TSA supplemented with 0.4% xylan. After 2 days of growth, the plates were flooded with Congo red (0.1%) and washed with NaCl (1 M), and clear zones around the colonies due to xylan degradation were observed.
Xylanase activity in the culture broth was calculated by measuring the amount of reducing sugars released from 0.4% (wt/vol) oat spelts xylan at 60°C for 10 min as described previously (1a). Protein concentrations in the culture broth were determined as described by Bradford (2) using bovine serum albumin as a standard.
PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) of the culture broth was carried out essentially as described by Laemmli (12). Electrophoresis was performed at room temperature with a vertical slab gel (170 by 130 by 1.5 mm) and 10% (wt/vol) polyacrylamide at 100 V and 60 mA. After electrophoresis, proteins were stained with Coomassie blue or electroblotted on polyvinylidene difluoride membranes (Millipore) and immunostained with rabbit polyclonal antiserum raised against purified Xys1 from S. halstedii.
Purification of His-tagged xylanase from B. lactofermentum.
Purification of the His-tagged xylanase from the culture medium of B. lactofermentum X1 or B. lactofermentum X2 by using Ni-NTA agarose was performed according to the standard procedures of the manufacturer (Qiagen).
RESULTS AND DISCUSSION
Construction of a conjugative plasmid designed to insert any gene into the chromosome of B. lactofermentum.
A method for inserting any DNA fragment into the chromosome of B. lactofermentum has been developed. The method relies on the suicide conjugative plasmid pK18-3, a pK18mob derivative containing (i) a gene conferring resistance to kanamycin as a selectable marker, (ii) a chromosomal region necessary for its integration into the B. lactofermentum chromosome, and (iii) a unique NdeI restriction site useful for cloning. Any gene that we wish to integrate (under the control of a promoter capable of functioning in B. lactofermentum) is cloned into the unique restriction site of the plasmid, transformed into E. coli, and transferred by conjugation to B. lactofermentum.
The initial plasmid used was pK18mob, an E. coli plasmid described by Schäfer et al. (22) as a suicide vector for B. lactofermentum carrying a kanamycin resistance gene that is expressed in both E. coli and B. lactofermentum. It contains the origin of replication of ColE1, the broad-host-range transfer machinery of plasmid RP4, the lacZα gene, and a multiple cloning site. This plasmid cannot replicate in B. lactofermentum but can be transferred by conjugation from E. coli S17-1. This plasmid has been used to interrupt several genes in B. lactofermentum or Corynebacterium glutamicum (4, 8, 22).
The chromosomal region used for integration of the exogenous DNA is that corresponding to three ORFs of unknown function located downstream from ftsZ in the B. lactofermentum chromosome (9). It has previously been demonstrated that interruption of any of the three ORFs is not deleterious for B. lactofermentum growth or cell division (8).
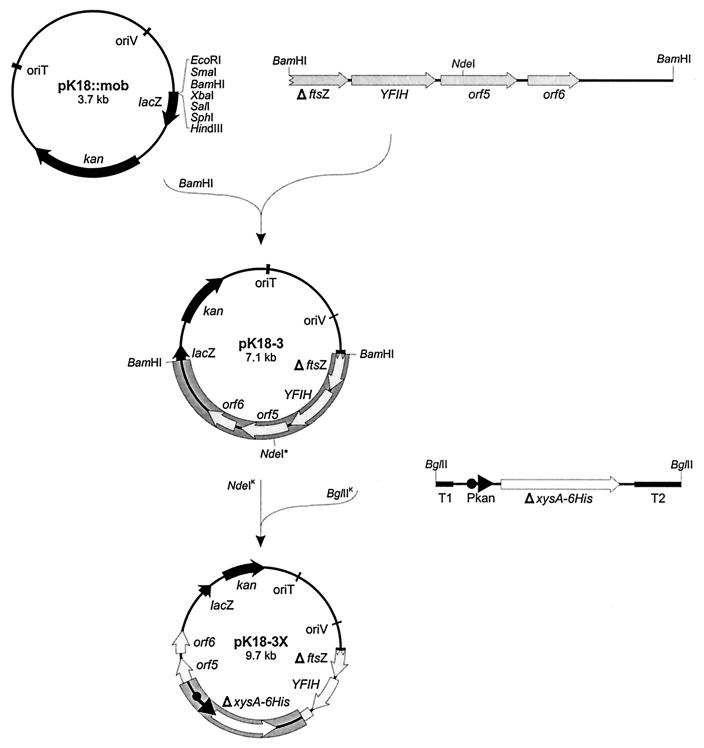
The 3.3-kb BamHI fragment containing the three ORFs was cloned into the unique BamHI restriction site of pK18mob, giving rise to plasmid pK18-3; as indicated in Fig. 1, there is a single restriction site for NdeI in pK18-3 that can be used for the cloning of any exogenous DNA fragment.
FIG. 1.
Schematic representation of the construction of the suicide plasmid pK18-3 designed to deliver any gene into the B. lactofermentum chromosome and subcloning of the six-His-tagged xylanase gene (ΔxysA-6His) into the unique NdeI site (NdeI*). For details, see the text. NdeIK and BglIIK, Klenow-filled NdeI and BglII; Pkan, promoter of the kan gene from Tn5; T1, terminator of the methylenomycin resistance gene from Streptomyces coelicolor A3 (2); T2, terminator of phage fd.
The six-His-tagged ΔxysA gene from S. halstedii under the control of the promoter of the kan gene from Tn5 and flanked by terminators was obtained from plasmid pXHis-Npro (Table 1) and subcloned as a 2.6-kb BglII fragment (Klenow filled) into the unique NdeI site (Klenow filled) of pK18-3. The ligation mixture was transformed into E. coli DH5α competent cells, and selection was made for kanamycin resistance and xylanase production. Plasmid DNA was isolated from a Kanr Xyl+ transformant and named pK18-3X. In E. coli, xylanase expression takes place from the Pkan promoter because of the presence of the terminators (1).
Integration of pK18-3X in the chromosome of B. lactofermentum by single recombination.
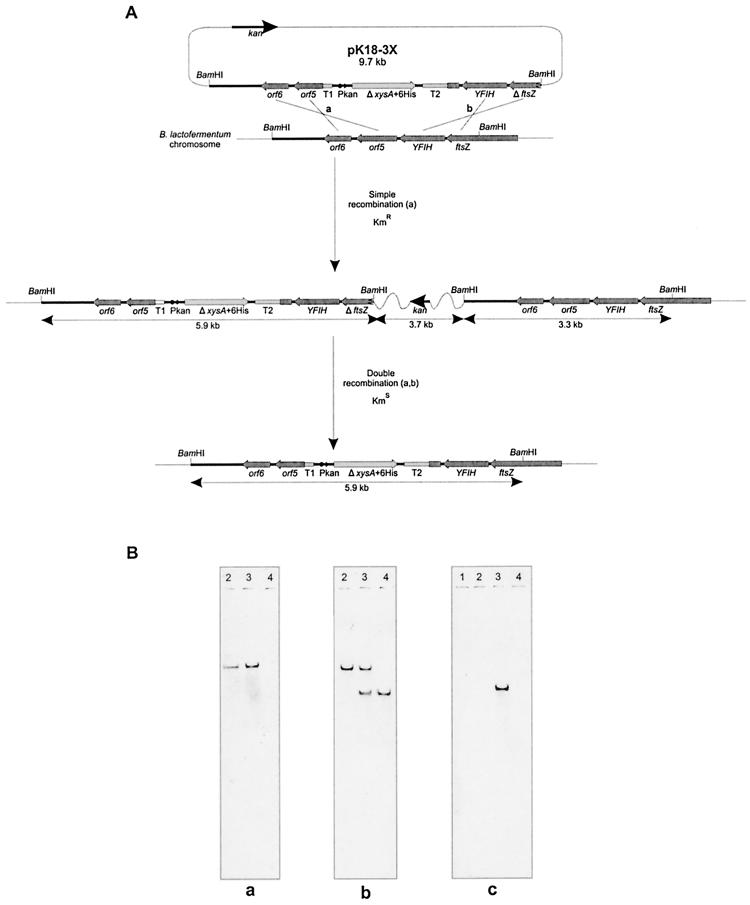
Because chromosomal integration events occur rarely, plasmids used for integration do require the concomitant integration of a drug resistance marker in order to identify colonies of recombinant cells. The conjugative suicide plasmid pK18-3X was transferred by conjugation from E. coli S17-1(pK18-3X) cells to B. lactofermentum R31, and B. lactofermentum kanamycin-resistant transconjugants were readily obtained (10−2 to 10−3 transconjugants per recipient colony). They expressed xylanolytic activity on 0.3% Remazol brilliant blue R–d-xylan plates, in contrast to the lack of xylanolytic activity of B. lactofermentum R31. Because of the relative position of the xylanase gene inside ORF5 (Fig. 2) and the presence of terminators, the xysA gene should be expressed in B. lactofermentum from the Pkan promoter. Total DNA isolated from 10 transconjugants did hybridize with the kan gene, with the xysA gene, and with probe 3 (Fig. 2). Two BamHI chromosomal DNA fragments, one corresponding to the original fragment in the chromosome (3.3 kb) and a second one (5.9 kb) corresponding to the sum of the original fragment (3.3 kb) plus six-His-tagged ΔxysA (2.6 kb), hybridized with probe 3 (Fig. 2); the 5.9-kb BamHI DNA band also hybridized with the xysA gene, as expected. The integrated plasmid is stable, and the xylanase is stably expressed without continued selection for kanamycin. One of the 10 B. lactofermentum transconjugants was named B. lactofermentum X1.
FIG. 2.
Integration of the six-His-tagged ΔxysA gene in the chromosome of B. lactofermentum. (A) Schematic representation of the relevant part of the recipient strain B. lactofermentum chromosome and interpretation of the possible integration results. (B) Southern hybridization analysis. Lambda DNA digested with HindIII (lane 1) and chromosomal DNAs of B. lactofermentum X2 (lane 2), B. lactofermentum X1 (lane 3), and B. lactofermentum R31 (lane 4) digested with BamHI were transferred to nitrocellulose membranes and hybridized separately with the xysA gene (a), with probe 3 (b), and with the kan cassette (c).
Because kanamycin-resistant strain X1 has reduced applicability in vivo, we attempted to construct a double-recombinant strain expressing the xylanase gene and lacking any exogenous drug resistance marker.
Screening for double recombination.
Since the simple recombination strain was stable, screening for a double crossover was difficult because of the lack of a positive selection marker. A single colony of B. lactofermentum X1 (XylA+ Kanr) was incubated in TSB medium without kanamycin at 30°C for 200 generations in order to allow the second recombination event, which would excise the plasmid, rendering XylA+ Kans colonies. Almost 50,000 isolated colonies were replica plated from TSA to TSA supplemented with kanamycin. Many false kanamycin-sensitive colonies were checked (probably because of the lack of inoculum in the transfer), but four of them were clearly XylA+ Kans. Therefore, the frequency of appearance was 1 out of ca. 10,000 viable colonies assayed. The second recombination event was confirmed by Southern blot hybridization, and no signal was obtained when the kan gene was used as a probe. There was a positive signal of the expected size (5.9 kb) when the xysA gene was used as a probe and with probe 3. The integrated xysA gene is stably maintained without the need for selective pressure, as confirmed after 20 successive cultures using as an inoculum 1 ml from the previous culture and assaying 20 different single colonies for xylanase activity on solid media. One of these XylA+ Kans colonies was named B. lactofermentum X2.
Gene expression and secretion of xylanase by B. lactofermentum.
The integration of foreign genes into the genome constitutes an interesting option for stably maintaining cloned genes without the need for selective markers. However, the level of expression of a given gene is expected to be lower than in a multicopy plasmid.
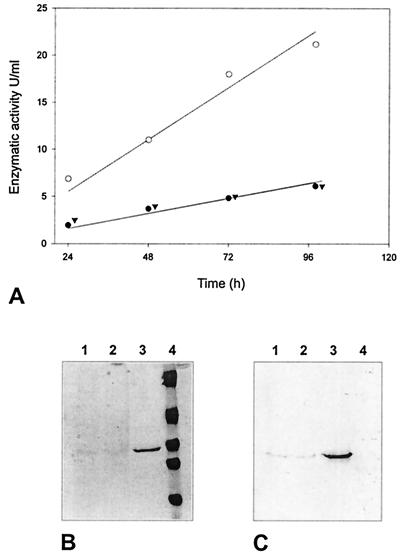
To compare xylanase production by B. lactofermentum X1 and B. lactofermentum X2 with that by B. lactofermentum R31 containing plasmid p880X2A, cells were grown in TSB medium for 24 h and then transferred to S2 medium. The growth curves of the three strains were similar, indicating that the integration of any exogenous DNA in ORF5 was not deleterious for growth and viability, as previously described (8). As indicated in Fig. 3, the production of xylanase increased during fermentation, but the production of six-His–ΔXys1 by B. lactofermentum(p880X2A) was always higher than the production of six-His–ΔXys1 by B. lactofermentum X1 or X2 in all the conditions assayed. For these strains, xylanase activity was found in the culture supernatant, could be easily purified by use of Ni-nitrilotriacetic acid, and did react with anti-Xys1 antibodies (Fig. 3).
FIG. 3.
Production of xylanase by B. lactofermentum. (A) Time course of extracellular xylanase activity in cultures of B. lactofermentum carrying p880X2A (○), B. lactofermentum X1 (●), and B. lactofermentum X2 (▾) grown in S2 medium. (B) Sodium dodecyl sulfate-PAGE of culture broth supernatants of B. lactofermentum grown in S2 medium. (C) Western blot probed with anti-Xys1 antibodies. Supernatants of cultures were taken at 72 h from B. lactofermentum X2 (lane 1), B. lactofermentum X1 (lane 2), and B. lactofermentum (p880X2A) (lane 3). Lane 4, Molecular mass markers.
Conclusions.
Several vectors have been developed to express genes and to secrete proteins in Brevibacterium. However, if these vectors are to be considered safe for humans, animals, or the environment, only DNA from organisms generally regarded as safe should be used, and no antibiotic resistance markers should remain after genetic manipulation. In this work, we report the construction of a conjugative plasmid designed to integrate any gene into the genome of B. lactofermentum and the construction of a strain intended to produce extracellular xylanase containing the xysA gene from S. halstedii and lacking antibiotic resistance genes. This new strain can be considered a food-grade microorganism for the production of extracellular enzymes.
ACKNOWLEDGMENTS
Sirin A. I. Adham and Ana B. Campelo contributed equally to this work.
This work was funded by a grant from the European Community-Ministerio de Ciencia y Tecnología (1-FD1997-1134-C03-02). Sirin A. I. Adham, Ana B. Campelo, and Angelina Ramos were recipients of fellowships from the Agencia Española de Cooperación Internacional (AECI), Excelentísima Diputación Provincial de León, and Junta de Castilla y León, respectively.
REFERENCES
- 1.Adham, S. A. I., P. Honrubia, M. Díaz, J. M. Fernández-Ábalos, R. I. Santamaría, and J. A. Gil. Expression of the genes coding for the xylanase Xys1 and the cellulase CelA1 from the straw-decomposing Streptomyces halstedii JM8 cloned into the amino-acid producer Brevibacterium lactofermentum ATCC 13869. Arch. Microbiol., in press. [DOI] [PubMed]
- 1a.Bernfeld P. Enzymes of starch degradation and synthesis. Adv Enzymol. 1951;12:379–428. doi: 10.1002/9780470122570.ch7. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantification of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Cadenas R F, Gil J A, Martín J F. Expression of Streptomyces genes encoding extracellular enzymes in Brevibacterium lactofermentum: secretion proceeds by removal of the same leader peptide as in Streptomyces lividans. Appl Microbiol Biotechnol. 1992;38:362–369. doi: 10.1007/BF00170087. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-González C, Gil J A, Mateos L M, Schwarzer A, Schäfer A, Kalinowski J, Pühler A, Martín J F. Construction of l-lysine-overproducing strains of Brevibacterium lactofermentum by targeted disruption of the hom and thrB genes. Appl Microbiol Biotechnol. 1996;46:554–558. doi: 10.1007/s002530050860. [DOI] [PubMed] [Google Scholar]
- 5.Gosalbes M J, Esteban C D, Galán J L, Pérez-Martínez G. Integrative food-grade expression system based on the lactose regulon of Lactobacillus casei. Appl Environ Microbiol. 2000;66:4822–4828. doi: 10.1128/aem.66.11.4822-4828.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 7.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 8.Honrubia M P, Ramos A, Gil J A. The cell division genes ftsQ and ftsZ, but not the three downstream open reading frames YFIH, ORF5 and ORF6, are essential for growth and viability in Brevibacterium lactofermentum ATCC 13869. Mol Gen Genet. 2001;265:1022–1030. doi: 10.1007/s004380100497. [DOI] [PubMed] [Google Scholar]
- 9.Honrubia M P, Fernández F J, Gil J A. Identification, characterization, and chromosomal organization of the ftsZ gene of Brevibacterium lactofermentum. Mol Gen Genet. 1998;259:97–104. doi: 10.1007/s004380050793. [DOI] [PubMed] [Google Scholar]
- 10.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, England: John Innes Foundation; 1985. [Google Scholar]
- 11.Janssen G R, Bibb M J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene. 1993;124:133–134. doi: 10.1016/0378-1119(93)90774-w. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–686. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Malumbres M. Cloning and characterisation of genes involved in threonine and lysine biosynthesis in Brevibacterium lactofermentum. Ph.D. thesis. León, Spain: University of León; 1993. [Google Scholar]
- 14.Martín M C, Alonso J C, Suárez J E, Álvarez M A. Generation of food-grade recombinant lactic acid bacterium strains by site-specific recombination. Appl Environ Microbiol. 2000;66:2599–2604. doi: 10.1128/aem.66.6.2599-2604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateos L M, Schafer A, Kalinowski J, Martín J F, Puhler A. Integration of narrow-host-range vectors from Escherichia coli into the genomes of amino acid-producing corynebacteria after intergeneric conjugation. J Bacteriol. 1996;178:5768–5775. doi: 10.1128/jb.178.19.5768-5775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesas J M. Characterization of asparaginase, aspartase and aspartokinase of Corynebacterium glutamicum. Ph.D. thesis. University of Le; 1986. ón, León, Spain. [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 18.Pisabarro A, Correia A, Martín J F. Characterization of the rrnB operon of the plant pathogen Rhodococcus fascians and targeted integrations of exogenous genes at rrn loci. Appl Environ Microbiol. 1998;64:1276–1282. doi: 10.1128/aem.64.4.1276-1282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes O, Guyonvarch A, Bonamy C, Salti V, David F, Leblon G. ‘Integron’-bearing vectors: a method suitable for stable chromosomal integration in highly restrictive corynebacteria. Gene. 1991;107:61–68. doi: 10.1016/0378-1119(91)90297-o. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Arribas A, Sánchez P, Calvete J J, Raida M, Fernández-Ábalos J M, Santamaría R I. Analysis of xysA, a gene from Streptomyces halstedii JM8 that encodes a 45-kilodalton modular xylanase, Xys1. Appl Environ Microbiol. 1997;63:2983–2988. doi: 10.1128/aem.63.8.2983-2988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santamaría R I, Gil J A, Martín J F. High-frequency transformation of Brevibacterium lactofermentum protoplasts by plasmid DNA. J Bacteriol. 1985;162:463–467. doi: 10.1128/jb.162.1.463-467.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schäfer A, Tauch A, Jäger W, Kalinowsski J, Thierbach G, Pühler A. Small mobilizable multi-purpose cloning vectors derived from Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 23.Smith M D, Flickinger J L, Lineberger D W, Schmidt B. Protoplast transformation in coryneform bacteria and introduction of an α-amylase gene from Bacillus amyloliquefaciens into Brevibacterium lactofermentum. Appl Environ Microbiol. 1986;51:634–639. doi: 10.1128/aem.51.3.634-639.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]