Abstract
Hyperglycemia-mediated oxidative stress plays a major role in the development of diabetic complications. Averrhoa bilimbi Linn. (Oxalidaceae) is a medicinal plant with fruits reported to possess antidiabetic activity. This study evaluated the beneficial effects of the ethyl acetate fraction of A. bilimbi fruit (ABAEE) on the antioxidant/oxidant status in diabetes mellitus. Diabetic rats were treated orally with the ethyl acetate fraction of A. bilimbi fruits at a dose of 25 mg/kg body weight for 60 days. Serum glucose, glycated hemoglobin, plasma insulin, hepatic toxicity markers, antioxidant enzymes, lipid peroxidation products, and liver histopathology were assayed checked after 60 days of extract treatment. Diabetic rats administered ABAEE showed a significant decline in serum glucose, glycated hemoglobin, and also significantly increases the level of plasma insulin, as well as a notable attenuation in thiobarbituric acid-reactive substances, conjugated dienes, and hydroperoxides. ABAEE also modulated hepatic antioxidant potential by significantly increasing the activities of catalase, glutathione peroxidase, glutathione reductase, superoxide dismutase, and reducing glutathione content. The results associated with ABAEE were more significant than those observed following treatment with the standard drug metformin. Histopathological observations showed that ABAEE effectively rescued hepatocytes from oxidative damage without affecting cellular function and structural integrity. High-performance liquid chromatography analysis of ABAEE indicated the presence of phenolic compound, quercetin, indicating that the anti-diabetic effect of the extract might be related to quercetin. These results demonstrated the potential beneficial effect of ABAEE on streptozotocin-induced diabetes in rats.
Keywords: Averrhoa bilimbi Linn., diabetes mellitus, quercetin
1. Introduction
Diabetes mellitus, a pervasive and multifactorial metabolic syndrome, is characterized by defects in insulin secretion and insulin receptor or postreceptor events, with derangement in carbohydrate, protein, and lipid metabolism resulting in chronic hyperglycemia, a clinical hallmark of diabetes [1]. In 2011, there were 366 million people with diabetes, with this number expecting to rise to 552 million by 2030 [2]. Hyperglycemia describes a stage of increased blood glucose levels in circulating blood and generates reactive oxygen species (ROS) through various pathways, including dysregulation of redox equilibrium, augmentation of advanced glycation products, activation of protein kinase C, or overproduction of mitochondrial superoxides that eventually leads to oxidative stress in various tissues [3].
Management of diabetes without side effects remains a challenge. Many synthetic drugs are used in the treatment of diabetes; however, plant-based drugs are often considered less toxic and free of side effects. Indian traditional medicines belong to one of the richest medicinal systems in the world. Therefore, there is a need to search for phytocomponents that normalize hyperglycemia and ameliorate oxidative stress for the prevention or minimization of diabetes-associated complications.
Averrhoa bilimbi Linn. (Oxalidaceae), commonly known as cucumber tree or tree sorrel, is a widely cultivated plant in India, Indonesia, Sri Lanka, Bangladesh, Myanmar, Malaysia, and Central and South America. The entire plant is used for treating coughs, colds, itches, rheumatism, whooping cough, and hypertension [4,5]. Traditionally A. bilimbi fruits are employed for curing diabetes, although no scientific data are available concerning the antidiabetic properties of the fruits.
Previous studies reported that A. bilimbi-leaf extract regulates blood glucose and lipids in streptozotocin (STZ)-induced diabetic rats [6]. Studies on A. bilimbi fruits in rats showed that the fruit (125 mg/kg), as well as its water extract (50 mg/kg), was effective in lowering lipids in rats fed a high-fat diet, and can be used as a dietary ingredient to prevent and treat hyperlipidemia [7]. Studies showed that the semipurified fractions of A. bilimbi leaves exhibited antidiabetic activity in STZ-induced diabetic rats fed a high-fat diet [8]. Our previous studies revealed the beneficial effect of aqueous extracts of A. bilimbi fruit in controlling blood glucose levels, improving lipid metabolism, and preventing diabetic complications from lipid peroxidation in experimental diabetic rats [9]. In-vitro antioxidant studies of different solvent fractions (petroleum ether, ethyl acetate, butanol, and water) of A. bilimbi fruits revealed the superior effect elicited by the ethyl acetate fraction.
However, no reports are available on the protective effect of the ethyl acetate fraction of A. bilimbi fruits (ABAEE) on hyperglycemia-mediated oxidative stress in STZ-induced diabetic rats. Therefore, the present study investigated the ameliorative potential of the ethyl acetate fraction of A. bilimbi in STZ-induced diabetic rats, and the efficacy of the ABAEE was compared with that of metformin, a standard oral antidiabetic drug.
2. Materials and methods
2.1. Chemicals
Chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA), Merck Chemical Company (Darmstadt, Germany), and Sisco Research Laboratories (Mumbai, India).
2.2. Extraction of A. bilimbi fruits
Fresh fruits of A. bilimbi were obtained from Thiruvananthapuram, Kerala, India, during the fruiting season (July–December 2014) and identified by Dr. Valsala Devi, Department of Botany, University of Kerala (Voucher number: KUBH 5865). Care was taken that the fruits, which were whitish-green in color and ~5–7.0 cm in size, were not overripe, spoiled, or damaged. Fruits (5 kg) were shade dried at a temperature of 28°C and coarsely powdered.
The crude aqueous extract was defatted with petroleum ether and the remaining defatted extract was fractionated with ethyl acetate (1:1 volume/volume) to obtain the ethyl acetate fraction (ABAEE-yield: 5%), which was concentrated and used for the experimental study.
2.3. Experimental animals
Two-month-old male Sprague Dawley rats (200–220 g body weight; 35 animals in total) bred in our departmental animal house were used for the study. Animals were housed in polypropylene cages and maintained under standard conditions [12-hour light/dark cycles, (25 ± 10°C)]. Animal care was performed per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals and the experimental protocol approved by the Institutional Animals Ethics Committee [IAEC-KU- 20/2013-14-BC-SM (22)].
2.4. Induction of experimental diabetes
Rats were rendered diabetic by a single intraperitoneal injection of freshly prepared STZ at a dose of 40 mg/kg body weight in 0.1M citrate buffer (pH 4.5) [10]. The animals were administered a 5% glucose solution by dissolving in drinking water overnight to overcome drug-induced hypoglycemia. Following STZ injection (48 hours), blood glucose levels were measured, and rats with blood glucose levels between 200 mg/dL and 400 mg/dL were considered diabetic and used for the experiment.
2.5. Experimental design
Dose-dependent toxicity studies were previously performed in our laboratory using three different doses of ABAEE (5 mg/kg body weight, 10 mg/kg body weight, and 25 mg/kg body weight), and the extract at an optimal dose of 25 mg/kg body weight was found to be effective and safe compared with the other two doses (5 mg/kg body weight and 10 mg/kg body weight). Therefore, the dose of 25 mg/kg body weight was selected for the present study, and the results were compared with a standard antidiabetic drug metformin.
The experimental animals were divided into five groups, with each group comprising of seven rats. ABAEE and metformin were administered orally using an intragastric tube once daily in the morning after food delivery for 60 days. Metformin is generally recommended as a first-line treatment for diabetes.
Group I: normal control rats.
Group II: normal rats treated with ABAEE (25 mg/kg body weight).
Group III: diabetic control rats.
Group IV: diabetic rats treated with ABAEE (25 mg/kg body weight).
Group V: diabetic rats treated with metformin (100 mg/kg body weight).
During the experimental period, body weight, blood glucose, and physical examinations were determined at regular intervals. The dosage was adjusted weekly according to changes in body weight in order to maintain similar doses per kg body weight in rats over the entire study period for each group. After 60 days, the rats were sacrificed by sodium pentothal injection, and blood and hepatic tissue were collected for various experimental analyses.
2.6. Biochemical parameters
Serum glucose (Agappe Diagnostics, Kerala, India) and glycated hemoglobin (HbA1c) were measured based on the ion-exchange method [11]. Insulin was measured using an enzyme-linked immunosorbent assay kit (DRG Diagnostics, Marburg, Germany). Liver-toxicity markers alanine transaminase (ALT) and aspartate transaminase (AST) were measured using commercially available assay kits (Agappe Diagnostics). The activities of antioxidant enzymes superoxide dismutase (SOD) [12], catalase (CAT) [13], glutathione peroxidase (GPx) [14], and glutathione reductase (GRd) [15] were assayed. Reduced glutathione (GSH) content [16] in the liver were also measured. Thiobarbituric acid-reactive substances (TBARS), hydroperoxides (HP), and conjugated dienes (CD) [17] were also assessed.
2.7. Histopathological analysis
The whole liver from each animal was collected in 10% formalin solution and immediately processed using the paraffin technique. Thin sections (5 μm) were cut and stained with hematoxylin and eosin. The tissue samples were examined and photographed under a light microscope for observation of structural abnormality [18].
2.8. High-performance liquid chromatography analysis
High-performance liquid chromatography (HPLC) analysis was performed using the HPLC Waters 2695 system (Waters, Milford, MA, USA), and the separation of ABAEE was completed by isocratic gradient elution using a C18 column (4.6 mm internal diameter × 250 mm; 5 μ particle size). The mobile phase consisted of methanol (eluent A) and acetonitrile (eluent B) at a 1:1 ratio, the total flow rate was 1.0 mL/min, and the analysis time was 15 minutes. The detector wavelength was set at 275 nm. The injection volume was 20 μL, and the temperature of the column was 40°C. The chromatographic peaks of the analytes were confirmed and quantified by comparing their retention time and area using quercetin as the standard.
2.9. Statistical analysis
Values were expressed as mean ± standard error of the mean. Statistical analyses were performed by one-way analysis of variance using SPSS version 17 (SPSS, Inc., Chicago, IL, USA). Duncan’s post hoc multiple-comparison tests were used to determine significant differences among groups. p < 0.05 was considered to be significant.
3. Results
3.1. Serum glucose, HbA1c, and plasma insulin
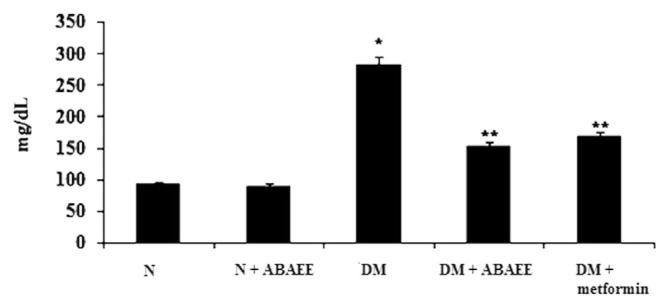
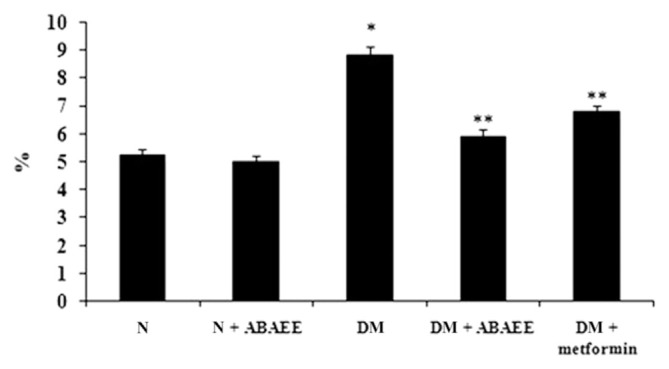
We measured levels of serum glucose, HbA1c, and plasma insulin in the different experimental groups (Figs. 1–3). We observed a significant elevation in serum glucose and HbA1c in the diabetic group as compared with normal control rats. However, oral administration of ABAEE and metformin to diabetic rats significantly reduced (p < 0.05) the levels of serum glucose and HbA1c as compared with those observed in diabetic control rats. No significant deviation was observed in normal rats treated with ABAEE. Serum glucose and HbA1c levels were comparable in diabetic rats treated with ABAEE and metformin.
Figure 1.
Serum glucose. Values are expressed as mean ± standard error of the mean of seven rats in each group. Significance determined at p < 0.05. * Denotes statistical significance as compared with the normal group. ** Denotes statistical significance as compared with the diabetic group. ABAEE = ethyl acetate fraction of Averrhoa bilimbi Linn fruits; DM = diabetes mellitus; DM + ABAEE = STZ-induced diabetic rats treated with the ethyl acetate fraction of Averrhoa bilimbi fruits at a dose of 25 mg/kg body weight/day; DM + metformin = STZ-induced diabetic rats treated with metformin at a dose of 100 mg/kg body weight/day; N = normal control rats; N + ABAEE = normal rats treated with the ethyl acetate fraction of Averrhoa bilimbi fruits; STZ = streptozotocin.
Figure 2.
Glycated hemoglobin. Values are expressed as mean ± standard error of the mean of seven rats in each group. Significance determined at p < 0.05. * Denotes statistical significance as compared with the normal group. ** Statistical significance as compared with the diabetic group. ABAEE = ethyl acetate fraction of Averrhoa bilimbi fruits; DM = diabetes mellitus; DM + ABAEE = STZ-induced diabetic rats treated with the ethyl acetate fraction of Averrhoa bilimbi fruits at a dose of 25 mg/kg body weight/day; DM + metformin = STZ-induced diabetic rats treated with metformin at a dose of 100 mg/kg body weight/day; N = normal control rats; N + ABAEE = normal rats treated with the ethyl acetate fraction of Averrhoa bilimbi fruits; STZ = streptozotocin.
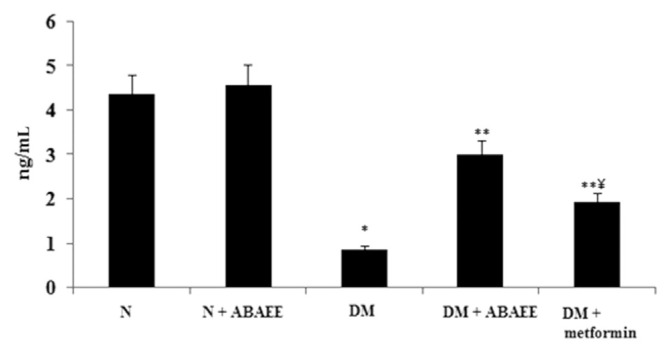
Figure 3.
Plasma insulin. Values are expressed as mean ± standard error of the mean of seven rats in each group. Significance determined at p < 0.05. * Denotes statistical significance as compared with the normal group. ** Statistical significance as compared with the diabetic group. *** statistical significance as compared with the ABAEE treated diabetic group. ABAEE = ethyl acetate fraction of Averrhoa bilimbi Linn fruits; DM = diabetes mellitus; DM + ABAEE = STZ-induced diabetic rats treated with the ethyl acetate fraction of Averrhoa bilimbi fruits at a dose of 25 mg/kg body weight/day; DM + metformin = STZ-induced diabetic rats treated with metformin at a dose of 100 mg/kg body weight/day; N = normal control rats; N + ABAEE = normal rats treated with the ethyl acetate fraction of Averrhoa bilimbi fruits; STZ = streptozotocin.
Plasma insulin levels decreased significantly in the diabetic group as compared with other groups. Administration of ABAEE and metformin to diabetic rats significantly increased (p < 0.05) the level of plasma insulin as compared with levels measured in diabetic control rats.
3.2. Hepatic toxicity markers
Table 1 shows the activities of hepatic toxicity markers (ALT and AST) in the normal and experimental groups. The level of hepatic toxicity markers in normal rats treated with ABAEE did not show any statistical difference as compared with levels observed in normal control rats. In contrast, these pathophysiological indices in diabetic rats were significantly (p < 0.05) elevated (87.75 ± 3.44 U/L and 96.50 ± 3.82 U/L, respectively) as compared with those measured in normal control rats (40.95 ± 1.60 U/L and 54.60 ± 2.14 U/L, respectively). Oral administration of ABAEE and metformin to diabetic rats significantly (p < 0.05) decreased the altered levels of ALT and AST as compared with levels in diabetic rats. However, the levels of hepatic toxicity markers decreased significantly in ABAEE-treated diabetic rats as compared with metformin-treated diabetic rats.
Table 1.
Hepatic toxicity markers.
| Groups | ALT (U/L) | AST (U/L) |
|---|---|---|
| N | 40.95 ± 1.60 | 54.60 ± 2.14 |
| N + ABAEE | 39.00 ± 1.53 | 53.50 ± 2.29 |
| DM | 87.75 ± 3.44* | 96.50 ± 3.82* |
| DM + ABAEE | 59.13 ± 2.20** | 72.15 ± 2.83** |
| DM + metformin | 72.15 ± 2.83**, *** | 85.80 ± 3.36**,*** |
Values are expressed as mean ± standard error of the mean of seven rats in each group. Significance determined at p < 0.05.
Denotes statistical significance as compared with the normal group.
Statistical significance as compared with the diabetic group.
Statistical significance as compared with the ABAEE-treated diabetic group.
ABAEE = ethyl acetate fraction of Averrhoa bilimbi Linn fruits; ALT = alanine transaminase; AST = aspartate transaminase; DM = diabetes mellitus; DM + ABAEE = streptozotocin-induced diabetic rats treated with the ethyl acetate fraction of A. bilimbi fruits at a dose of 25 mg/kg body weight/day; DM + metformin = streptozotocin-induced diabetic rats treated with metformin at a dose of 100 mg/kg body weight/day; N = normal control rats; N + ABAEE = normal rats treated with the ethyl acetate fraction of A. bilimbi fruits.
3.3. Hepatic antioxidant enzymes
The activities of the antioxidant enzymes CAT, GPx, GRd, and SOD decreased significantly (p < 0.05) in STZ-induced diabetic rats as compared with levels observed in the normal control group. However, oral administration of ABAEE significantly (p < 0.05) increased the activities of antioxidant enzymes in the diabetic group, and ABAEE-treated diabetic rats showed a significant increase in the activities of antioxidant enzymes relative to those observed in metformin-treated diabetic rats (Table 2).
Table 2.
Hepatic antioxidant enzymes.
| Groups | CAT (×10−3 U/mg protein) | GPx (U/mg protein) | GRd (U/mg protein) | SOD (U/mg protein) |
|---|---|---|---|---|
| N | 6.84 ± 0.25 | 26.71 ± 0.99 | 138.86 ± 5.17 | 1.35 ± 0.05 |
| N + ABAEE | 7.1 ± 0.27 | 27.8 ± 1.07 | 141.29 ± 5.26 | 1.58 ± 0.06 |
| DM | 2.94 ± 0.11* | 14.25 ± 0.53* | 96.49 ± 3.60* | 0.40 ± 0.02* |
| DM + ABAEE | 4.84 ± 0.18** | 19.41 ± 0.73** | 121.09 ± 4.51** | 0.88 ± 0.03* |
| DM + metformin | 4.00 ± 0.15**,*** | 16.61 ± 0.62*** | 104.55 ± 3.82 *** | 0.67 ± 0.02**,*** |
Values are expressed as mean ± standard error of the mean of seven rats in each group. Significance determined at p < 0.05.
Denotes statistical significance as compared with the normal group.
Statistical significance as compared with the diabetic group.
Statistical significance as compared with the ABAEE-treated diabetic group.
ABAEE = ethyl acetate fraction of Averrhoa bilimbi Linn fruits; CAT = catalase; GPX = glutathione peroxidase, GRd = glutathione reductase; SOD = superoxide dismutase; DM = diabetes mellitus; DM + ABAEE = STZ-induced diabetic rats treated with the ethyl acetate fraction of A. bilimbi fruits at a dose of 25 mg/kg body weight/day; DM + metformin = STZ-induced diabetic rats treated with metformin at a dose of 100 mg/kg body weight/day; N = normal control rats; N + ABAEE = normal rats treated with the ethyl acetate fraction of A. bilimbi fruits; STZ = streptozotocin.
3.4. Lipid peroxidation products and reduced GSH content in liver
Table 3 illustrates the levels of TBARS, HP, and CD in the hepatic tissues of control and experimental rats. Administration of ABAEE to normal rats did not result in statistically different levels of lipid peroxides as compared with levels observed in normal control rats. The significant (p < 0.05) elevation observed in the levels of TBARS, HP and CD in the hepatic tissues of diabetic rats declined (p < 0.05) significantly to near normal levels following ABAEE or metformin treatment, although the effect was more significant in ABAEE-treated diabetic rats as compared with those observed in metformin-treated diabetic rats.
Table 3.
Lipid peroxidation products and reduced glutathione content (GSH) in liver.
| Groups | TBARS (mM/100 g tissue) | CD (mM/100 g tissue) | HP (mM/100 g tissue) | GSH content (mM/100 g tissue) |
|---|---|---|---|---|
| N | 0.40 ± 0.02 | 3.60 ± 0.13 | 7.81 ± 0.29 | 62.85 ± 2.37 |
| N + ABAEE | 0.38 ± 0.02 | 3.51 ± 0.13 | 7.65 ± 0.28 | 66.45 ± 2.47 |
| DM | 0.84 ± 0.03* | 8.62 ± 0.32* | 23.75 ± 0.88* | 41.18 ± 1.54* |
| DM + ABAEE | 0.63 ± 0.02** | 5.09 ± 0.19** | 16.61 ± 0.61** | 49.31 ± 1.84** |
| DM + Metformin | 0.76 ± 0.03**,*** | 7.80 ± 0.31**,*** | 20.00 ± 0.76**,*** | 43.84 ± 1.75*** |
Values are expressed as mean ± standard error of the mean of seven rats in each group. Significance determined at p < 0.05.
Denotes statistical significance as compared with the normal group.
Statistical significance as compared with the diabetic group.
Statistical significance as compared with the ABAEE-treated diabetic group.
ABAEE = Averrhoa bilimbi Linn fruits; TBARS = thiobarbituric acid-reactive substances; CD = conjugated dienes; HP = hydroperoxides; GSH = reduced glutathione content; DM = diabetes mellitus; DM + ABAEE = STZ-induced diabetic rats treated with the ethyl acetate fraction of A. bilimbi fruits at a dose of 25 mg/kg body weight/day; DM + metformin = STZ-induced diabetic rats treated with metformin at a dose of 100 mg/kg body weight/day; N = normal control rats; N + ABAEE = normal rats treated with the ethyl acetate fraction of A. bilimbi fruits; STZ = streptozotocin.
The level of the hepatic non-enzymatic antioxidant GSH is shown in Table 3. No significant statistical deviation was observed in the levels of hepatic GSH in normal rats treated with ABAEE; however, diabetic rats showed a significant (p < 0.05) reduction in GSH level (41.18 ± 1.54 mM/100 g tissue) as compared with that observed in normal control rats (62.85 ± 2.36 mM/100 g tissue). Conversely, diabetic rats treated with ABAEE exhibited significantly (p < 0.05) increased GSH levels as compared with those measured in diabetic control rats. Additionally, ABAEE-treated diabetic rats showed a significant increase in GSH levels relative to those observed in metformin-treated diabetic rats.
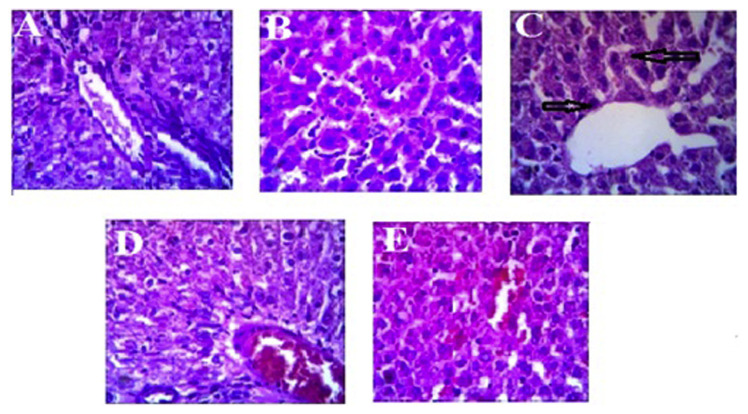
3.5. Histopathological analysis of the liver
Histopathological analysis of the liver from normal control and normal rats treated with ABAEE indicated normal cell morphology with hexagonal lobular architecture (Figures 4A and B). While the STZ-induced diabetic group (Figure 4C) exhibited hepatocellular damage in the form of mild sinusoidal dilation and focal inflammatory hepatic cell infiltration, diabetic rats treated with ABAEE or metformin exhibited less inflammation, with restored cell morphology to almost normal levels (Figures 4D and E).
Figure 4.
Photomicrographs of hematoxylineosin staining of hepatic tissue from control and experimental rat groups. Diabetes was induced by single intraperitoneal injection of streptozotocin (STZ; 40 mg/kg body weight). Averrhoa bilimbi Linn fruits (ABAEE; 25 mg/kg body weight/day) was orally administered daily for 60 days. At the end of the experimental period, rats were sacrificed, and the hepatic tissues were sectioned for histological study. (A) Normal control rats; (B) normal rats treated with the ethyl acetate fraction of A. bilimbi fruits; (C) STZ-induced diabetic rats; (D) STZ-induced diabetic rats treated with the ethyl acetate fraction of A. bilimbi fruits at a dose of 25 mg/kg body weight/day; (E) STZ-induced diabetic rats treated with metformin at a dose of 100 mg/kg body weight/day.
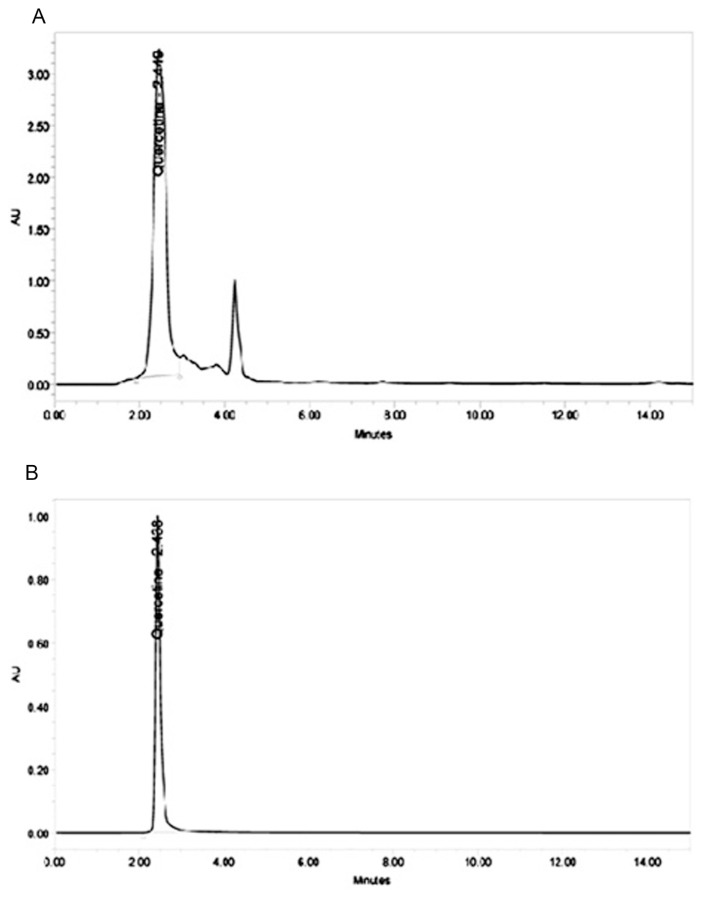
3.6. HPLC profiling of ABAEE
HPLC profiling of ABAEE (Figure 5A) showed that it contains quercetin [6% (weight/weight)] as a major compound compared with a corresponding reference standard. The presence of quercetin was confirmed by comparing the retention time of ABAEE (2.449 minutes) with that of the quercetin standard (2.438 minutes). Therefore, the activity associated with ABAEE may be attributed to the presence of the phenolic compound quercetin.
Figure 5.
High-performance liquid chromatography (HPLC): (A) HPLC profiling of ethyl acetate fraction of Averrhoa bilimbi fruits; (B) HPLC chromatogram of standard quercetin.
4. Discussion
Diabetes is characterized by hyperglycemia with a marked reduction in insulin secretion and insulin function [19]. Over the long term, hyperglycemia generates excess ROS, which induces oxidative stress. Under physiological conditions, antioxidant enzymes protect cells against harmful free radicals. A number of plant-derived products possess hypoglycemic, hypolipidemic, and antioxidant properties [20].
Low doses of STZ (40 mg/kg body weight) destroy populations of pancreatic β-cells in rats, leading to insufficient insulin secretion and resulting in moderate and stable hyperglycemia and glucose intolerance that contributes to a number of features similar to type 2 diabetes mellitus [21]. Therefore, STZ-induced diabetes in rats was chosen as the animal model for the present investigation.
Our results confirmed the antihyperglycemic and antioxidant effects of the ethyl acetate fraction of ABAEE in STZ-induced diabetic rats. HPLC analysis of ABAEE revealed the presence of quercetin, and studies reported that quercetin exerts antidiabetic properties by enhancing pancreatic secretion of insulin from β-cells in STZ-induced models [22].
Experimentally-induced diabetic rats showed severe hyperglycemia interconnected with reduced insulin secretion and release. Here, fasting blood glucose was significantly elevated in diabetic control rats, which might be due to pancreatic β-cell damage. Administration of ABAEE or metformin significantly reduced elevated blood glucose levels, possibly through increased release of insulin from existing and/or regenerated pancreatic β-cells.
Nonenzymatic and auto-oxidative glycosylation are mechanisms linking hyperglycemia and vascular complications associated with diabetes mellitus. Increased levels of HbA1c indicate that erythrocytes are more prone to oxidative stress in diabetes patients. High levels of HbA1c are found in diabetes patients, and this increase in HbA1c is directly proportional to fasting blood glucose levels [23,24]. In agreement with previous reports, our results show significantly increased HbA1c levels in the diabetic group; however, a significant decrease in HbA1c levels was observed in ABAEE- and metformin-treated groups. Decreased levels of HbA1c in ABAEE-treated diabetic rats indicated the beneficial effects of ABAEE in preventing the pathogenesis of diabetic complications caused by impaired glucose metabolism.
Diabetes is often associated with elevated activities of liver-marker enzymes, such as AST and ALT, in serum, which might be due to leakage of these enzymes from the liver into the bloodstream [25] and an indication of the toxic effects of STZ treatment. Consistent with the previous reports, ALT and AST activities increased significantly in STZ-induced diabetic rats; however, ABAEE or metformin administration reduced ALT and AST activities in diabetic rats, with a greater reduction observed following ABAEE treatment. The alterations in biochemical activity were supported by histological profiles. Decreases in ALT and AST activity in treated diabetic rats suggested that ABAEE exhibits therapeutic effects in hepatic disorders, in addition to its insulin secretagogue and anti-hyperglycemic activities. Additionally, the activities associated with liver-toxicity markers were not altered in the ABAEE-treated group as compared with the normal control group. These results indicated that ABAEE treatment was safe and exerted no significant toxicity.
Hyperglycemia causes an increased production of ROS along with reduced antioxidant status, increased lipid peroxidation, and impaired glucose metabolism in biological systems [26]. The liver plays a major role in the regulation of glucose metabolism, and various studies observed induction of severe oxidative stress in the liver of STZ-induced diabetic rats [27]. The activities of the principal enzymatic and nonenzymatic antioxidant systems decrease during oxidative stress [28]. In agreement with previous reports, our results showed that the activities of the antioxidant enzymes CAT, GPx, GRd, and SOD were significantly decreased in the diabetic control group; however, oral administration of ABAEE or metformin increased the antioxidant potential of the liver by increasing CAT, GPx, GRd, and SOD activity in diabetic rats [29]. Our findings showed maximum antioxidant activity in liver tissues from ABAEE-treated diabetic rats, which was slightly greater than that observed in metformin-treated diabetic rats.
GSH is an intracellular tripeptide that constitutes the major reducing capacity of the cytoplasm [30]. GSH protects cells against the toxic effects of lipid peroxidation [31], and exhaustion of GSH results in enhanced lipid peroxidation, which can cause increased GSH consumption correlated with increased levels of oxidized GSH [32]. In agreement with previous reports, we observed significant decreases in GSH levels in diabetic rats; however, supplementation with ABAEE or metformin elevated GSH levels, enabling the protection of the cell membrane against oxidative damage by regulating the redox status of membrane proteins [33]. Levels of tissue GSH increased to a greater extent in ABAEE-treated diabetic rats relative to the increased levels observed in metformin-treated diabetic rats. Our results suggested that ABAEE provided antioxidant potential and protected diabetic rats from secondary complications due to increased oxidative stress.
Lipid peroxides are released as a result of the toxic effects associated with ROS produced during lipid peroxidation in diabetes [34], and measurement of TBARS is considered the most reliable marker to assess the extent of lipid peroxidation [35]. Lipid peroxidation is characterized by enhanced concentrations of TBARS, HP, and CD in diabetic rats [36]. Oral administration of ABAEE or metformin restored levels of liver TBARS, HP, and CD to near normal ranges in diabetic rats, indicating the cytoprotective and free radical-scavenging properties of both treatments. We observed greater reductions in peroxidative markers in the tissues of ABAEE-treated diabetic rats relative to reductions observed in metformin-treated diabetic rats.
Previous studies showed that the liver was necrotized in STZ-induced diabetic rats [37,38]. Consistent with these reports, we observed marked hepatocellular damage in the form of mild sinusoidal dilation and focal inflammatory cell infiltration in STZ-induced diabetic rats. In comparison with the diabetic group, the administration of ABAEE or metformin significantly improved the histological architecture of the liver. Additionally, serum ALT and AST levels increased significantly in diabetic rats, but maintained near normal levels following treatment with ABAEE. These findings suggested that ABAEE administration was capable of reducing STZ-induced oxidative stress and hepatic damage.
HPLC analysis of ABAEE revealed the presence of quercetin (6%) as a major compound. Quantification indicated that 100 g of dried ABAEE material possessed 6 g of quercetin. Previous studies showed that quercetin supplementation improves glucose tolerance in STZ-induced diabetic rats [39]. Quercetin also protects STZ-induced diabetic rats from oxidative damage and preserves pancreatic β-cell integrity [40,41]. The potent antioxidant activity of ABAEE may be attributed to the presence of quercetin.
5. Conclusion
The results of this study indicate that administration of ABAEE at a dose of 25 mg/kg body weight significantly reduce hyperglycemia and oxidative stress in STZ-induced diabetic rats. This may be mediated by the activity of quercetin present in ABAEE. Further detailed studies are in progress to elucidate the exact mechanism by which the ethyl acetate fraction of ABAEE elicits its modulatory effects.
Acknowledgments
This work was financially supported by Promotion of University Research and Scientific Excellence program, Department of Science and Technology, Government of India.
Funding Statement
This work was financially supported by Promotion of University Research and Scientific Excellence program, Department of Science and Technology, Government of India.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Hepatic changes in the acute phase of streptozotocin (SZ)-induced diabetes. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. David RW, Leonor G, Clara W, Jonathan S. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3. Brownlee M. A radical explanation for glucose-induced beta cell dysfunction. J Clin Invest. 2003;112:1788–90. doi: 10.1172/JCI20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goh SH, Chuah CH, Mok JSL, Soepadmo E. Malaysian medicinal plants for the treatment of cardiovascular diseases. Kuala Lampur, Malaysia: Pelanduk Publications; 1995. [Google Scholar]
- 5. Mackeen MM, Ali AM, El Sharkawy SH, Manap MY, Salleh KM, Lajis NH, Kawazu K. Antimicrobial and cytotoxic properties of some Malaysian traditional vegetables (ulam) Int J Pharmacogn. 1997;35:174–8. [Google Scholar]
- 6.Pushparaj PN. Evaluation of the antidiabetic properties of Averrhoa bilimbi in animals with experimental diabetes mellitus. Singapore: National University of Singapore; 2004. [Google Scholar]
- 7. Ambili S, Subramoniam A, Nagarajan NS. Studies on the antihyperlipidemic properties of Averrhoa bilimbi fruit in rats. Planta Med. 2009;75:55–8. doi: 10.1055/s-0028-1088361. [DOI] [PubMed] [Google Scholar]
- 8. Tan BK, Pushparaj N, Tan CH. The mechanism of hypoglycemic action of the semi-purified fractions of Averrhoa bilimbi in streptozotocin-diabetic rats. Life Sci. 2001;70:535–47. doi: 10.1016/s0024-3205(01)01423-0. [DOI] [PubMed] [Google Scholar]
- 9. Kurup SB, Mini S. Attenuation of hyperglycemia and oxidative stress in streptozotocin induced diabetic rats by aqueous extract of Averrhoa bilimbi Linn fruits. Int J Pharm Sci Res. 2014;5:4979–86. [Google Scholar]
- 10. Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9:562–6. doi: 10.1089/jmf.2006.9.562. [DOI] [PubMed] [Google Scholar]
- 11. Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341–6. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 12. Kakkar P, Dos B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 13.Maehly AC, Chance B. The assay of catalases and peroxidases. In: Glick D, editor. Methods of biochemical analysis. Vol. 1. New York: Inter Science; 1954. pp. 357–424. [DOI] [PubMed] [Google Scholar]
- 14. Rotruck JT, Pope AL, Ganther HE. Selenium: biochemical roles as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 15.David M, Richard JS. Glutathione reductase. In: Bermeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1983. pp. 258–65. [Google Scholar]
- 16.Patterson JW, Lazarow A. Determination of glutathione. In: Glick D, editor. Methods of biochemical analysis. New York: Inter Science; 1955. p. 259. [DOI] [PubMed] [Google Scholar]
- 17. Okhawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Disbrey BD, Rack JH, editors. Book of histological laboratory methods. London: Harcourt Brace/Churchill Livingstone; 1970. [Google Scholar]
- 19. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vasu VT, Modi H, Thaikoottathil JV. Hypolipidemic and antioxidant effect of Enicostemma littorale Blume aqueous extract in cholesterol fed rats. J Ethnophamacol. 1975;101:277–82. doi: 10.1016/j.jep.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 21. Irudayaraj SS, Sunil C, Duraipandiyan V, Ignacimuthu S. Antidiabetic and antioxidant activities of Toddalia asiatica (L.) Lam. leaves in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2012;143:515–23. doi: 10.1016/j.jep.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 22. Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum) in normal and streptozotocin-induced diabetic rats. Phytomed. 2006;13:624–9. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 23. Jackson RL, Hess RL, England JD. Hemoglobin Aic values in children with overt diabetes maintained in varying degree of control. Diabetes Care. 1979;2:391–5. doi: 10.2337/diacare.2.5.391. [DOI] [PubMed] [Google Scholar]
- 24. Al-Yassin D, Ibrahim K. A minor hemoglobin fraction and the level of fasting blood glucose. J Facul Med Univ Bagh. 1981;23:373–80. [Google Scholar]
- 25. Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., in STZ induced diabetic rats. Chem Biol Interact. 2009;182:67–72. doi: 10.1016/j.cbi.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 26. Balasubashini MS, Rukkumani R, Viswanathan P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytotherapy Res. 2004;18:310–4. doi: 10.1002/ptr.1440. [DOI] [PubMed] [Google Scholar]
- 27. Ohaeri OC. Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats. Biosci Rep. 2001;21:19–24. doi: 10.1023/a:1010425932561. [DOI] [PubMed] [Google Scholar]
- 28. Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 29. Sabu MC, Kuttan Ramadasan. Antidiabetic and antioxidant activity of Terminalia belerica. Roxb. Ind J Exp Biol. 2009;47:270–5. [PubMed] [Google Scholar]
- 30. Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–83. [PubMed] [Google Scholar]
- 31. Nicotera P, Orrenius S. Role of thiols in protection against biological reactive intermediates. Adv Exp Med Biol. 1986;197:41–9. doi: 10.1007/978-1-4684-5134-4_4. [DOI] [PubMed] [Google Scholar]
- 32. Pattabiraman K, Muthukumaran P. Antidiabetic and antioxidant activity of Morinda tinctoria roxb fruits extract in streptozotocin-induced diabetic rats. Asian J Pharm Tech. 2011;1:34–9. [Google Scholar]
- 33. Inove M, Saito Y, Hirato E, Nagase S. Regulation of redox status of plasma proteins by mechanism and transport of glutathione and related compounds. J Protein Chem. 1987;36:169–73. [Google Scholar]
- 34. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 35. Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;411:1819–28. [PubMed] [Google Scholar]
- 36. Stanely Mainzen Prince P, Menon VP. Antioxidant action of Tinospora cordifolia root extract in alloxan diabetic rats. Phytother Res. 2001;15:213–8. doi: 10.1002/ptr.707. [DOI] [PubMed] [Google Scholar]
- 37. Ohaeri OC. Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats. Biosci Rep. 2001;21:19–24. doi: 10.1023/a:1010425932561. [DOI] [PubMed] [Google Scholar]
- 38. Gopalsamy RG, Savarimuthu I, Michael GP. Solanum torvum Swartz. fruit containing phenolic compounds shows antidiabetic and antioxidant effects in streptozotocin induced diabetic rats. Food Chem Toxicol. 2001;49:2725–33. doi: 10.1016/j.fct.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 39. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135:357–64. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 40. Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–23. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 41. Lekshmi RK, Divya BT, Mini S. Cissus quadrangularis extract attenuates hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2014;19:214–20. doi: 10.1179/1351000214Y.0000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]