Abstract
Chemical and enzymatic barriers in the gastrointestinal (GI) tract hamper the oral delivery of many labile drugs. The GI epithelium also contributes to poor permeability for numerous drugs. Drugs with poor aqueous solubility have difficulty dissolving in the GI tract, resulting in low bioavailability. Nanomedicine provides an opportunity to improve the delivery efficiency of orally administered drugs. Solid lipid nanoparticles (SLNs) are categorized as a new generation of lipid nanoparticles consisting of a complete solid lipid matrix. SLNs used for oral administration offer several benefits over conventional formulations, including increased solubility, enhanced stability, improved epithelium permeability and bioavailability, prolonged half-life, tissue targeting, and minimal side effects. The nontoxic excipients and sophisticated material engineering of SLNs tailor the controllable physicochemical properties of the nanoparticles for GI penetration via mucosal or lymphatic transport. In this review, we highlight the recent progress in the development of SLNs for disease treatment. Recent application of oral SLNs includes therapies for cancers, central nervous system-related disorders, cardiovascular-related diseases, infection, diabetes, and osteoporosis. In addition to drugs that may be active cargos in SLNs, some natural compounds with pharmacological activity are also suitable for SLN encapsulation to enhance oral bioavailability. In this article, we systematically introduce the concepts and amelioration mechanisms of the nanomedical techniques for drug- and natural compound-loaded SLNs.
Keywords: drug, gastrointestinal tract, natural compound, oral delivery, solid lipid nanoparticles
1. Introduction
Oral delivery is the most accepted drug administration route among the various delivery pathways because of its advantages: painlessness, easy self-administration, high patient compliance, and feasibility for outpatients [1]. Nevertheless, chemical and enzymatic barriers in the gastrointestinal (GI) tract hinder the effectiveness of oral drug delivery. The epithelial cell monolayer in the GI membrane also contributes to poor permeability for numerous drugs [2]. Some poorly soluble drug molecules are difficult to dissolve in the GI tract, resulting in low bioavailability. Novel and sophisticated drug delivery systems are necessary to conquer these limitations. By optimizing the formulations, the delivery efficiency and bioavailability can be ameliorated to promote the therapeutic potency with reduced side effects. The oral delivery improvement using nanocarrier systems has gained more attention recently [3]. Nanoparticles are defined as particles ranging in size from 1 nm to several hundred nanometers that can load drugs for efficient delivery. The drugs or actives can either be integrated in the core or matrix or attached to the surface of nanoparticles that have a high surface/volume ratio [4]. With respect to pharmacokinetics, the drugs in nanocarriers generally revealed prolonged circulation time, increased half-life, reduced clearance, and increased mean residence time (MRT) [5].
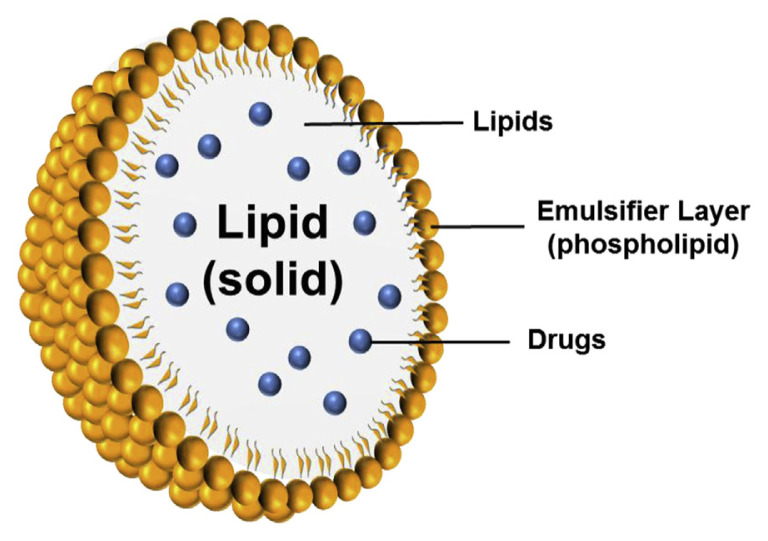
Among the different types of nanocarriers, solid lipid nanoparticles (SLNs) are at the forefront of the potential application in oral drug delivery systems [6]. SLNs are nano-colloids developed at the beginning of the 1990s by Schwarz et al [7]. They are used as alternative carriers to conventional colloids such as emulsions, liposomes, and polymeric micelles. Basically, SLNs are made of a solid lipid core with a monolayer phospholipid shell. The lipophilic moiety of phospholipids is embedded in the lipid matrix (Figure 1). Many drugs or diagnostics can be entrapped by SLNs, especially lipophilic ingredients [8]. The use of SLNs for oral administration is a promising approach for enhancing and controlling drug delivery. The solid state of the nanoparticulate matrix provides protection to chemically labile drugs and prolongation of drug release. SLNs show low cytotoxicity to mammalian cells, demonstrating an acceptable tolerance to the body. SLNs can be orally administered as aqueous dispersions or in the dosage forms of capsules, tablets, and pellets [9]. With the evolution of nanomedicine, the application of SLNs is expected to change the landscape of oral delivery. In this review, we highlight recent advances in the application of SLNs for oral delivery of drugs and bioactive natural compounds. We focus on the reports of SLN development during the past 5 years of orally administered drugs for therapy against cancers, central nervous system (CNS) diseases, cardiovascular (CV) diseases, bacterial/viral infection, and inflammation. The promising perspective in this emerging application is also discussed.
Figure 1.
Structures of solid lipid nanoparticles (SLNs).
2. Nanocarriers for the oral route
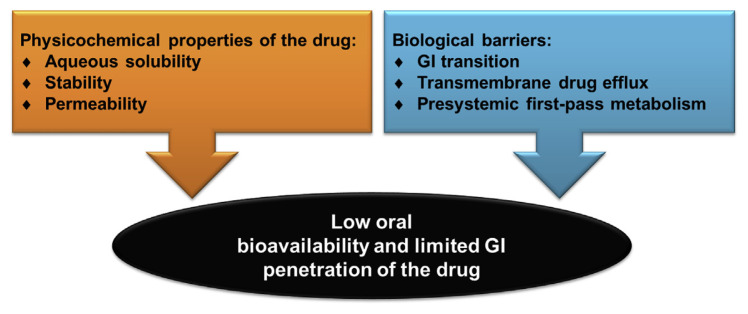
The drugs should go through the stomach, intestinal lumen, the mucus membrane coating the intestinal epithelium, and finally the epithelium itself after oral administration. The inside of the stomach is composed of four layers; from the innermost layer to the outermost layer, these are the mucosa, submucosa, muscularis externa, and the serosa. The stomach is lined by a mucous membrane that contains glands (with chief cells) that secrete gastric fluid. The intestinal epithelium is made up of villi that vastly increase the surface region available for drug absorption [10]. Absorptive enterocyte cells and mucus-secreting goblet cells cover the villi, which are interspersed with the follicle-associated epithelium. The physiology of the GI tract can lead to poor absorption and availability of the drugs or actives because of the low mucosa permeability and drug degradation prior to absorption [11]. The multidrug efflux proteins such as P-glycoprotein rich in the epithelial cell membrane are another barrier for orally administered drugs. Some drugs or active ingredients show low aqueous solubility, and a high hepatic first-pass effect also limits GI absorption. The additional oral permeation challenges are chemical instability, a short half-life, and the effect of food [12]. Figure 2 summarizes the barriers and challenges for efficient oral delivery for administering drugs.
Figure 2.
Schematic diagram of the challenges for oral drug delivery. GI = gastrointestinal.
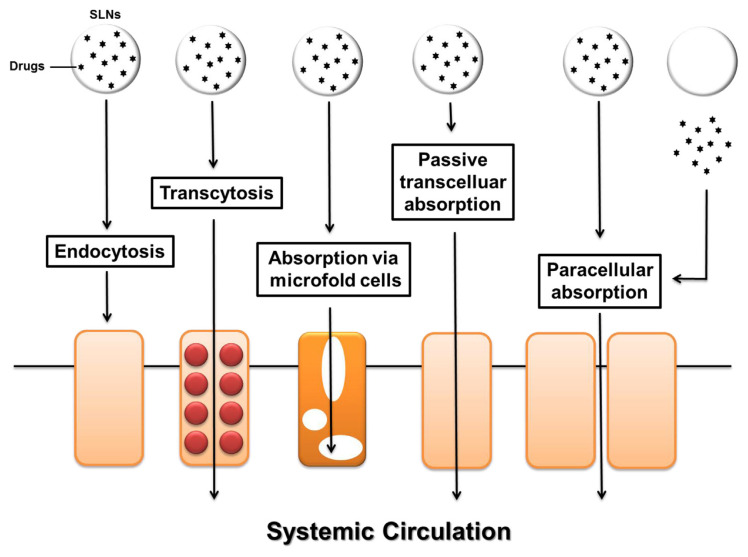
Nanomedicine offers improvement of oral delivery by bioavailability enhancement, adverse-effect minimization, and food-effect mitigation [13]. The nanocarriers can increase the dissolution rate of poorly soluble molecules in the GI tract. The poor stability in the GI tract can be overcome by encapsulating the drugs in nanocarriers, thus rendering a protective impact toward chemical and enzymatic attacks in the tract. The designed nanocarrier systems provide intimate contact with the GI epithelium, prolonging residence time and revealing permeation enhancement for drug delivery [14]. The nanoparticles can at least to some extent transport into the mucus layer, releasing the payload. A presystemic metabolism functioning between the delivery system and the absorption membrane can thus be excluded. The nanocarriers are designed to modulate dimension, size, surface charge, surface property, and relative lipophilicity for preferentially penetrating across the GI membrane. Figure 3 illustrates nanocarrier strategies for ameliorating drug absorption across the GI tract.
Figure 3.
Schematic diagram of various routes for oral delivery of solid lipid nanoparticles (SLNs).
Lymphatic drug delivery in the intestine is an alternative approach to bypassing first-pass metabolism. The lymphatic system forms a channel network throughout the body similar to blood circulation, but it is a one-way passage. The predominant categories of conduits in the lymphatic system are the capillaries, collecting vessels, lymph nodes, and ducts [15]. The total lymph flow amount is about 120 mL/h. The lymphoid system is found to be an efficient route for oral administration of lipids across the intestinal membrane. The lipid-based nanoparticles are considered to favorably transfer into the lymph [16]. The nanoparticles reach the lymphatic system via microfold cells (M cells). The enhanced lymphatic delivery decreases the first-pass effect and improves oral bioavailability owing to the lymph vessels draining directly into the thoracic duct and farther into the venous blood [17].
3. Structure of SLNs
SLNs are colloidal systems made up of a solid lipid core matrix, which is stabilized in aqueous solution by emulsifiers. This nanosystem provides advantages as a drug-delivery carrier, including stability, controlled release kinetics, tolerability, and protection of labile drugs [18]. The solid lipids commonly used in SLNs are glyceride mixtures, highly purified glycerides, or waxes that do not melt at body temperature. The safety of oral nanoformulations is a serious concern for their application [19,20]. A prerequisite for a new drug or formulation discovery is the confirmation of its safety when administered to the body. A balanced efficacy and safety is necessary when developing the nanomedicine for therapeutic application. The SLNs are developed by solid lipids and emulsifiers generally recognized as safe with respect to biocompatibility and nontoxicity. The lipid matrices are the natural or synthetic lipids that can be degraded, including triglycerides (trimyristin, tripalmitin, and tristearin), glyceryl monostearate (Imwitor), glyceryl behenate (Compritol 888 ATO), glyceryl palmitostearate (Precirol ATO 5), hard fat types (Witepsol), fatty acids (decanoic acid, palmitic acid, and stearic acid), waxes (cetyl palmitate, beeswax, and carnauba wax), and steroids (cholesterol) [8,21]. The drugs can be embedded in the voids of solid lipid matrix crystals. SLNs can encapsulate both lipophilic and hydrophilic drugs. Drug entrapment in the matrix depends on the types of solid lipids, drug solubility in the lipids, processing techniques, and polymorphic change in lipid crystals [22]. The emulsifiers or surfactants usually used for stabilizing the interface between the SLN shell and the aqueous medium are phospholipids (egg lecithin and soy lecithin), nonionic surfactants (Poloxamer, Pluronic, Brij, Tween, and Span), ionic surfactants (sodium cholate, sodium lauryl sulfate, stearylamine and trimethylammonium bromide), polyethylene glycol (PEG), and polyvinyl alcohol [23,24]. The lipids and surfactants are the two main materials used in SLNs. The materials cited in this section are basically safe for application.
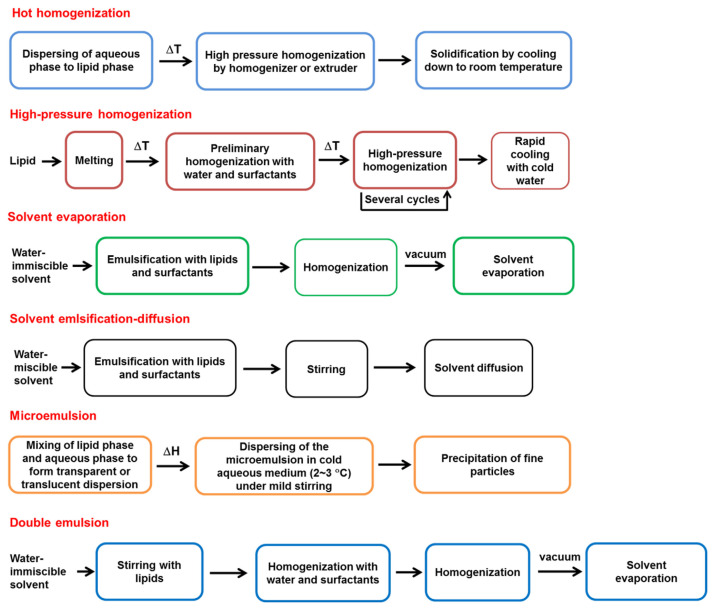
Most of the materials for preparing SLNs are low cost with ease of scale-up for industrial production [25]. The solid lipid should be melted during the preparation process and then recrystallized at room temperature. There are several preparation methods for SLN production from the laboratory scale to the industrial scale. These include hot homogenization, high-pressure homogenization, ultrasonication, solvent evaporation, solvent emulsification–diffusion, microemulsion, double emulsion, supercritical fluid technique, and spray drying [26]. Figure 4 shows the preparation procedures of some methods. The physicochemical characterization of SLNs after preparation is a critical step for quality control and prediction of stability, drug release, and therapeutic efficacy. Photon correlation spectroscopy can be used to determine the particulate diameter, polydispersity, and zeta potential of SLNs [27]. The imaging systems such as scanning electron microscopy (SEM), transmission electron microscopy, and atomic force microscopy are the tools used for observing the particulate size, surface morphology, and particle shape. The crystalline confirmation of the lipids inside SLNs significantly affects the drug loading and stability. The polymorphism of the lipids is detected by X-ray diffraction, wide-angle X-ray scattering, and X-ray photoelectron spectroscopy [28]. Differential scanning calorimetry and Fourier transform infrared spectroscopy are used to identify the drug–matrix interaction.
Figure 4.
The general methods for preparation of solid lipid nano particles (SLNs).
4. Application of SLNs as oral delivery systems
The drugs or actives should be solubilized to be absorbed by the GI system. In the lipid-based SLNs, the drugs are absorbed from the nanoformulations in the solid state [18]. Digestion of oral nanoparticles containing glycerides starts in the stomach by gastric lipase. The mechanical mixing of gastric fluid with amphiphilic products of lipid digestion results in the formation of crude emulsion. The SLNs are further digested in the intestinal fluid. The degradation products of lipids such as monoglycerides and fatty acids are able to promote intestinal drug transport by the production of mixed micelles with bile acids and subsequent uptake into the enterocytes [29]. The enormous effective surface area by virtue of the nano-sized SLNs can lead to an increased absorption rate. The tiny size of SLNs permits them to adhere to the GI mucus and also to enter the intervillar space, thus increasing the residence period for improved bioavailability. The surface-active capability of the emulsifiers can augment the drug absorption via the GI tract because of the altered GI membrane fluidity [30]. Cationic SLNs composed of cationic surfactants or lipids can be designed to ameliorate bioadhesion mediated by electrostatic interaction between the positively charged nanoparticles and the negatively charged mucosal surface [31]. SLNs have unique characteristics that make them promising candidates for lymphatic delivery of orally administered drugs [32]. The effect of emulsifiers on the preferential uptake of SLNs by Peyer’s patch also improves the bioavailability of the oral drugs because of avoidance of first-pass metabolism.
SLNs can be digested in the GI tract. Thus, no translocation of intact nanoparticles to the circulation and other organs can be detected. Cai et al [32] demonstrated that no evidence supports absorption of integral SLNs via oral delivery. Contrary to this result, the in vitro experiment of SLN transcytosis into Caco-2 cell monolayers had suggested the transport of intact SLNs to the basolateral membrane side [33]. The internalization of SLNs is mediated via macropinocytosis, clathrin, and caveolae pathways. Li et al [34] reported that oral SLNs can be absorbed as intact nanoparticles through Peyer’s patch and M cells in lymphoid follicles, especially in the ileum and colon.
5. Oral delivery of SLNs for treating various diseases
Recent applications of oral SLNs include therapies for cancers, CNS-related disorders, CV-related diseases, infection, diabetes, and osteoporosis. The use of SLNs provides enhanced bioavailability and controlled drug release. Such ubiquitous properties arise from the specific features of surface modification, increased GI permeation, and resistance to degradation [35]. The following describes the different therapeutic approaches of oral SLNs for drug and pharmacologically active ingredient therapy. The pharmacokinetics, tissue distribution, and pharmacodynamics of the nanoparticles are the main evaluation platforms used to define the therapeutic effect for our description.
5.1. Cancers
Nanomedicine is an efficient approach to promote the therapeutic benefit of anticancer drugs [36]. Intravenous drug administration for cancer therapy is inconvenient and painful for patients, leading to the requirement of regular hospital visits and low patient compliance. Oral chemotherapy would be preferable to intravenous delivery because it offers the benefits of improved compliance, low cost, and increased quality of life. Unfortunately, most anticancer drugs are not readily bioavailable via the oral route because of the harsh environment and permeation barrier of the GI tract [37]. For example, the oral bioavailability of paclitaxel has been found to be <1% [38]. Paclitaxel shows effective activity against ovarian, breast, and lung cancers as well as Kaposi’s sarcoma. The oral availability of this drug is limited by cytochrome P450 activity and P-glycoprotein in the gut wall and liver [39]. SLNs as oral drug delivery systems are promising to solve the problems of oral anticancer drugs such as poor stability, healthy tissue toxicity, and high incidence of drug-resistant tumors [21].
Materials to improve the therapeutic advantages after oral administration can be applied to the surface of SLNs. Baek et al [40] had developed the surface-modified paclitaxel-loaded SLNs with hydroxypropyl-β-cyclodextrin (HPCD). This dextrin is known to solubilize the drugs and prevent oxidation of lipids [41]. The HPCD-coated SLNs showed a paclitaxel encapsulation percentage of 71% with a mean size of 251 nm. The Caco-2 cell uptake of paclitaxel from SLNs was 5.3-fold greater than the Taxol formulation. The Cmax and lymph node concentrations of SLNs (1.44 μg/mL and 11.12 ng/mg, respectively) were higher than those of the control solution (0.73 μg/mL and 0.89 ng/mg, respectively) after oral paclitaxel application at 25 mg/kg. Pooja et al [42] prepared wheat germ agglutinin (WGA)-coated SLNs to improve oral paclitaxel delivery. WGA can bind to N-acetyl-d-glucosamine and sialic acid presenting on the cell surface throughout the intestine, leading to prolonged residence time [43]. The in vitro anti-cancer activity against A549 lung cancer cells showed a half-maximal inhibitory concentration (IC50) of 41 ng/mL and 165 ng/mL for WGA-coated SLNs and free paclitaxel, respectively. After oral administration at 25 mg/kg in rats, the area under the curve (AUC) of plasma concentration–time profiles was higher for WGA-coated SLNs (30 μg h/mL) than conventional SLNs (16 μg h/mL) and the free control (8 μg h/mL). The inclusion of paclitaxel into WGA-coated SLNs led to doubling of MRT in contrast to the control solution.
Docetaxel is a second-generation taxane extensively used to treat breast, lung, prostate, and head/neck cancers. The development of an oral docetaxel formulation is hindered because of poor bioavailability. SLNs coated with Tween 80 or d-α-tocopheryl poly(ethylene glycol) succinate (TPGS) were prepared to improve the oral delivery of this drug [44]. The SLNs exhibited a sustained docetaxel release compared with Taxotere. The AUC of Tween 80 SLNs, TPGS SLNs, and Taxotere after oral administration (20 mg/kg) was 7.0 μg min/mL, 12.9 μg min/mL, and 3.9 μg min/mL, respectively. The superior bioavailability of TPGS compared to Tween 80 nanocarriers is attributable to the better inhibition of drug efflux by TPGS along with lymphatic uptake. No evidence of intestinal damage was visualized after SLN administration. Doxorubicin is an anthracycline antibiotic that can inhibit various malignant tumors. The major limitations of doxorubicin are its serious cardiotoxicity and hepatotoxicity [45]. Patro et al [46] investigated the oral bioavailability and toxicity of doxorubicin-loaded SLNs with an emulsifier system of soy lecithin and poloxamer 188. The SLNs exhibited increased the Cmax (6.7 μg/mL vs. 2.4 μg/mL) and reduced clearance (36 mL/h kg vs. 619 mL/h kg) when compared to free doxorubicin. The mean survival time of breast cancer-bearing rats increased from 34 days (positive control) to 47 days and 88 days in the free doxorubicin and SLN groups. Cardiac toxicity measured by the lactic dehydrogenase level was less in the SLN-treated group compared to the free control. Doxorubicin was included in PEGylated SLNs as mucus-penetrating nanoparticles for oral delivery across the GI mucus [47]. Modification of nanoparticles by PEG–stearic acid increased the hydrophilicity of nanoparticles with a rapid mucus-penetrating transport property [48]. The permeation ability of SLNs across the Caco-2/HT29 coculture cell monolayer increased after coating with PEG-stearic acid. The in vitro everted rat gut sac technique demonstrated that PEGylated SLNs could facilely penetrate mucus secretions. The pharmacokinetic data showed that the oral bioavailability of PEGylated nanosystems was 2.0- and 7.5-fold greater than conventional SLNs and free doxorubicin, respectively.
Vorinostat is a histone deacetylase inhibitor that efficiently induces cell cycle arrest and apoptosis for the treatment of cutaneous T cell lymphoma [49]. Tran et al [50] examined whether SLNs containing vorinostat could enhance multidrug-resistant cancer cells and oral delivery. The nanocarriers were spherical with a narrowly distributed size of about 100 nm, and were physically stable for at least 3 months. The SLNs were more cytotoxic than the free vorinostat in both sensitive (MCF-7 and A549) and resistant (MDA-MB-231) cells. The Cmax and AUC values of SLNs were 1.6- and 2.5-fold higher than those of the free drug by oral delivery at 30 mg/kg in rats. Hashem et al [51] evaluated the cytotoxicity and oral bioavailability of SLNs encapsulating tamoxifen, the drug for breast cancer management. The SLNs prepared using glyceryl monostearate and stabilized with poloxamer 188 showed an entrapment percentage of 90% for tamoxifen. The in vitro release profiles revealed an initial burst effect followed by a sustained drug release. The tamoxifen bioavailability could be increased to 1.6-fold by SLN administration.
Recent research indicates the usefulness of the compounds from natural resources for cancer prevention and treatment [52]. The antioxidant, anti-inflammatory, anti-apoptotic, vasodilating, and antimicrobial potentials of natural products are responsible for their anticancer effects [53]. γ-Tocotrienol is a natural form of vitamin E with a potential anticancer effect in breast malignancy. However, the effective oral delivery of γ-tocotrienol is very low (9%) [54]. SLNs were used as the formulations for γ-tocotrienol to improve the intestinal permeation [55]. The in situ rat intestine perfusion study demonstrated a 10-fold increase of γ-tocotrienol permeation compared to the control. The cellular endocytosis is the major contribution of γ-tocotrienol uptake from SLNs. Following the oral administration of 10 mg/kg γ-tocotrienol, SLNs increased bioavailability by 3-fold compared to the control group. Cantharidin is an anticancer active present in mylabris. It can inhibit tumor growth by interfering with the metabolism of nucleic acids and proteins. Cantharidin is shown to be poorly water soluble with low oral bioavailability in the beagle dog breed [56]. Dang and Zhu [57] used SLNs as the vehicles for oral cantharidin delivery. The mean diameter of the nanoparticles was 121 nm with a high cantharidin encapsulation of 94%. After a single oral dose of cantharidin at 0.1 mg/kg in rats, the relative bioavailability of SLNs to the free compound was 251%.
Ferulic acid is one of the most abundant antioxidants found in wheat bran. It shows anticancer potential in breast, skin, colon, and liver cancers [58]. The poor aqueous solubility of ferulic acid has led to unsatisfactory oral bioavailability. The limited bioavailability of ferulic acid can be overcome by SLNs. Zhang et al [59] prepared ferulic acid-loaded SLNs made with glyceryl behenate. The pharmacokinetic parameters showed a higher Cmax and half-life of SLNs (18.7 μg/mL and 4.6 hours, respectively) compared with those of aqueous dispersion (10.0 μg/mL and 2.1 hours, respectively), suggesting a sustained release from the lipid matrix. The in vivo imaging system also revealed a longer retention of SLNs in the small intestine than in the free control. Thakkar et al [60] combined ferulic acid and aspirin to be loaded in chitosan-coated SLNs for pancreatic cancer therapy. Chitosan-coated SLNs exhibited good stability in an acidic environment with the formation of a thick layer around the lipid core. This biopolymer coating also improved the mucoadhesion of SLNs [61]. The combination of low doses of free ferulic acid (200μM) and aspirin (1mM) inhibited pancreatic cancer cells MIA PaCa-2 and Panc-1 by 45% and 60%, respectively. A 5- and 40-fold dose decrease of ferulic acid (40μM) and aspirin (25μM) was found to show comparable cytotoxicity after SLN entrapment. In the in vivo pancreatic tumor xenograft mouse study, oral application of combined ferulic acid (75 mg/kg) and aspirin (25 mg/kg) in SLNs significantly suppressed tumor growth by 45% as compared to the control. Table 1 summarizes the profiles for anticancer therapy assisted by oral SLNs.
Table 1.
Formulations of orally administered SLNs loaded with drugs or natural compounds against cancers and their benefits.
| Active ingredient | Lipid type | Surface decoration | Average size (nm) | Outcomes offered by SLNs | Reference |
|---|---|---|---|---|---|
| Paclitaxel | Stearic acid | HPCD | 251 | Improved AUC in plasma and lymph node | Baek et al [40] |
| Paclitaxel | Monoglycerides, triglycerides, and stearic acid | WGA | 150–198 | Increased AUC and MRT | Pooja et al [42] |
| Docetaxel | Tristearin | Tween 80 and TPGS | 189–215 | Improved sustained release and AUC | Cho et al [44] |
| Doxorubicin | Precirol ATO 5 | soy lecithin and poloxamer 188 | 217 | Increased AUC and reduced cardiotoxicity | Patro et al [46] |
| Doxorubicin | Monostearin | PEG-stearic acid | 153–160 | Improved bioavailability and prolonged circulation time | Yuan et al [47] |
| Vorinostat | Compritol 888 ATO | None | ~100 | Enhanced Cmax and AUC | Tran et al [50] |
| Tamoxifen | Monostearin and stearic acid | Tween 80 and poloxamer 188 | 130–244 | Improved bioavailability | Hashem et al [51] |
| γ-Tocotrienol | Compritol 888 ATO | None | 105 | Increased intestine permeation and AUC | Abuasal et al [55] |
| Cantharidin | Monostearin | None | 121 | Improved bioavailability | Dang and Zhu [57] |
| Ferulic acid | Compritol 888 ATO | None | 86 | Increased Cmax and half-life | Zhang et al [59] |
| Ferulic acid | Stearic acid | Chitosan | 183–229 | Tumor growth suppression | Thakkar et al [60] |
AUC = area under the curve; HPCD = hydroxypropyl-β-cyclodextrin; MRT = mean residence time; PEG = polyethylene glycol; TPGS = d-α-tocopheryl poly(ethylene glycol) succinate; WGA = wheat germ agglutinin.
5.2. CNS-related disorders
CNS-related illnesses are a leading cause of disability and morbidity worldwide. However, the orally dosed drug treatments for depression, anxiety, and schizophrenia are usually limited by low aqueous solubility, food effect, first-pass effect, and a short half-life [62]. Medication for psychotic diseases often needs repeated daily dose administration. This can lead to less patient compliance, the need for hospitalization, and high cost [63]. As such, the application of nanomedicine to CNS-related diseases is a viable option for maximizing treatment efficacy. Apomorphine is a mixed dopamine D1/D2 agonist for the treatment of Parkinson’s disease approved by the U.S. Food and Drug Administration. The rapid degradation of apomorphine in the GI tract and the first-pass effect have resulted in a bioavailability of only 1.7% [64]. Tsai et al [65] developed SLNs for improving oral bioavailability and brain distribution of apomorphine. SLNs showed a bioavailability 13-fold higher than the reference solution at an oral dose of 26 mg/kg. Apomorphine distribution in the striatum, the major site of therapeutic action, also increased when using SLNs. In the rat model of Parkinson’s disease induced by 6-hydroxydopamine, the contralateral rotation number increased from 20 to 115 after oral SLN administration.
Migraines are a debilitating headache disorder with symptoms of nausea, photophobia, and cognitive function impairment. Sumatriptan is the first drug approved by the U.S. Food and Drug Administration for acute migraine treatment. This drug undergoes incomplete absorption and first-pass metabolism, leading to a low oral bioavailability of 15% [66]. Chitosan-coated SLNs were used to encapsulate sumatriptan by using a solvent injection technique [67]. The nanoformulations showed a brain sumatriptan deposition of 5.28 μg/g in the pharmacokinetic study. The AUCbrain/AUCplasma of the drug was 4.5, indicating a targeting to the brain rather than plasma. The mouse model of photophobia demonstrated that the time spent in the lit chamber of a light/dark box was significantly increased by SLNs when compared to the free control. Rizatriptan is the second-generation antimigraine drug exerting selective vasoconstriction of the intracranial and extracerebral vessels. Girotra and Singh [68] investigated the brain targeting of SLNs for rizatriptan delivery. The in vivo study in rats exhibited an 18-fold increase of brain rizatriptan uptake after SLN delivery compared to the free drug at a dose of 5 mg/kg. The light/dark box study showed the improvement of photophobia by loading rizatriptan into SLNs.
Sulpiride is a selective antagonist of the central dopamine receptor used as an antipsychotic agent. In order to ameliorate the limited intestinal permeation, SLNs composed of stearic acid and Dynasan 118 were prepared for sulpiride delivery [69]. The drug permeability across the everted rat gut sac was 6.6 μg/cm2 after 2 hours. The permeability was enhanced to 11.7 μg/cm2 by SLNs. This can be attributed to the enhancement of the surface area, leading to a higher rate of dissolution and diffusion. Quetiapine is an antipsychotic drug with poor oral bioavailability (9%) because of the extensive first-pass effect [70]. An attempt to improve the oral bioavailability of quetiapine was performed by using SLNs [71]. The stable SLNs with a mean diameter of 175 nm and an encapsulation capacity of 92% were developed. The Cmax was 1.9 μg/mL for SLNs, whereas the Cmax for the aqueous suspension was 0.1 μg/mL. The oral bioavailability of SLNs increased 3.7 times compared with the suspension. Venlafaxine is the first-line antidepressant for the treatment of major depressive disorders and anxiety. This drug is a substrate of P-glycoprotein that reduces penetration across the GI and blood–brain barrier [72]. Zhou et al [73] explored whether the venlafaxine efflux of P-glycoprotein could be reversed by SLN application. Mice were orally administered with SLNs containing venlafaxine (22 mg/kg) or free venlafaxine with verapamil. Verapamil is a P-glycoprotein inhibitor. The AUC of venlafaxine from the SLNs and verapamil-containing solution was 1.5- and 1.2-fold greater than that of venlafaxine alone, respectively. The AUC in the brain from SLNs was 1.5-fold higher compared to that in the venlafaxine solution.
Alzheimer’s disease is a devastating and progressive neurodegenerative disorder characterized by amyloid-β (Aβ) accumulation and intraneuronal neurofibrillary tangles. Numerous natural compounds and plant extracts are effective against chronic inflammation in Alzheimer’s disease [74,75]. Chrysin is one of the natural compounds inhibiting the neuroinflammation caused by Alzheimer’s disease [76]. The therapeutic efficiency of chrysin is limited because of its compromised oral delivery. The lipid peroxidation and increased acetylcholinesterase in Aβ-injected rats could be restored by chrysin-loaded SLNs (5 mg/kg and 10 mg/kg) and the free compound (50 mg/kg and 100 mg/kg) [77]. The poor memory retention examined by radial arm maze training was recovered by treatment using SLNs at the lower dose compared to the free control.
Grape skins, mulberries, and peanuts are rich in resveratrol, a polyphenol reported to possess a wide variety of health benefits. Resveratrol is especially effective in the treatment of neurodegenerative illnesses such as Alzheimer’s disease, Parkinson’s disease, and brain ischemia [78]. As with most of the polyphenols, low aqueous solubility and poor GI absorption have limited resveratrol’s therapeutic application. Pandita et al [79] entrapped resveratrol into SLNs to improve the oral bioavailability. SLNs prolonged resveratrol release up to 120 hours and followed Highchi kinetics. Resveratrol is sensitive when exposed to light. Resveratrol content in SLNs (86%) was higher than that in solution (36%) after a 6-hour ultraviolet light irradiation. The lipid formulation produced an 8-fold increase in oral resveratrol bioavailability in rats. The half-life was found to be 2.4 hours and 11.5 hours for resveratrol suspension and SLNs, respectively. N-Trimethyl chitosan was coated on the surface of SLNs to increase the retention and penetration of SLNs in the GI tract [80]. The in vitro release study of chitosan-coated SLNs revealed negligible resveratrol release in simulated gastric fluid and sustained release in simulated intestinal fluid. After oral dosing at 25 mg/kg, the bioavailability of resveratrol increased 3.8-fold compared to that in the aqueous suspension.
Curcumin is a lipophilic polyphenol derived from the spice turmeric from the ground rhizome of Curcuma longa. It has been reported that curcumin exhibits antioxidant, anti-inflammatory, antimicrobial, antiamyloid, and anticancer effects [81]. Of significant interest is curcumin’s role in treating neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease, and malignancy [82]. The oral bioavailability of curcumin is less than 1% because this compound has poor aqueous solubility and rapid metabolism [83]. Kakkar and Kaur [84] evaluated the effect of curcumin-loaded SLNs on Alzheimer’s disease using the mouse model with treatment using AlCl3, a neurotoxicant. Oral treatment of free curcumin (50 mg/kg) showed 15% recovery in lipid peroxidation and 22% recovery in acetylcholinestease with respect to the AlCl3 treatment group. The SLNs demonstrated better results (97% and 73% recovery in lipid peroxidation and acetylcholinestease) at a dose of 50 mg/kg. Improvement in learning ability in the Morris water maze test was achieved by free curcumin and SLNs at the oral dose of 50 mg/kg and 1 mg/kg, respectively. Kakkar et al [85] further examined the brain distribution of curcumin with oral delivery of SLNs. Confocal microscopy was used to visualize the yellow fluorescence of curcumin. The presence of fluorescent nanoparticles in the plasma and brain indicated effective penetration of oral SLNs across the gut wall and the blood–brain barrier. The AUC for SLNs was 8.1 times higher than that of free curcumin. A 30-fold increase in brain curcumin accumulation was detected for SLNs compared to the free control. Ji et al [86] decorated curcumin-loaded SLNs with Brij 78 and TPGS to inhibit the P-glycoprotein efflux pump. In the in situ single-pass intestinal perfusion test, the effective permeability coefficient of SLNs + Brij and SLNs + Brij + TPGS was 1.3 times and 1.4 times greater than conventional SLNs in the jejunum. The in vivo pharmacokinetic experiment in rats showed the AUC for SLNs as 12.3-fold higher than in suspension.
Chitosan as a mucoadhesive polymer was coated on the surface of SLNs to improve oral intake [87]. The oral absorption of curcumin suspension (50 mg/kg) showed a Cmax of 0.28 μg/mL. This value could be increased to 1.04 μg/mL by SLN administration. The bioavailability of curcumin was increased by a factor of 6.9 after SLN delivery compared to suspension. Methylation of chitosan to N-trimethyl chitosan increases acid resistance and the mucoadhesive property [88]. N-Trimethyl chitosan-coated SLNs were developed to further ameliorate oral absorption [89]. Curcumin in chitosan-coated SLNs resulted in the release of 81% and 48% in simulated gastric fluid and simulated intestinal fluid, respectively. Release of curcumin from quarternized chitosan-coated SLNs was negligible (9%) in simulated gastric fluid and moderate (42%) in simulated intestinal fluid. The oral AUC of N-trimethyl chitosan-coated SLNs was 12.8 μg h/mL, which was significantly higher than free curcumin (0.3 μg h/mL), non-coated SLNs (6.0 μg h/mL), and chitosan-coated SLNs (6.9 μg h/mL). Another activity of curcumin on CNS-related disorders is the inhibition of cerebral ischemia [90]. Kakkar et al [91] assessed the effect of curcumin-loaded SLNs on the experimental platform of cerebral ischemic reperfusion injury in rats. γ-Scintigraphic study exhibited a 16-fold increase in brain AUC upon oral delivery of SLNs compared to the control solution. There was an improvement of 90% in cognition and 52% inhibition of acetylcholinesterase versus the nontreatment group. Table 2 depicts the oral delivery assisted by SLNs with the aim of CNS-related disorder treatment.
Table 2.
Formulations of orally administered SLNs loaded with drugs or natural compounds against CNS-related disorders and their benefits.
| Active ingredient | Indication | Lipid type | Average size (nm) | Outcomes offered by SLNs | Reference |
|---|---|---|---|---|---|
| Apomorphine | Parkinson’s disease | Tripalmitin | 63–155 | Increased bioavailability and brain distribution | Tsai et al [65] |
| Sumatriptan | Migraine | Tripalmitin | 192–301 | Improved AUCbrain/AUCplasma ratio and photophobia | Hansraj et al [67] |
| Rizatriptan | Migraine | Precirol ATO 5 | 220 | Improved brain uptake and photophobia | Girotra and Singh [68] |
| Sulpiride | Psychosis | Stearic acid and Dynasan 118 | 256 | Increased gut permeability | Ibrahim et al [69] |
| Quetiapine | Psychosis | Dynasan 118 | 175 | Enhanced Cmax and bioavailability | Narala and Veerabrahma [71] |
| Venlafaxine | Major depressive disorder and anxiety | Monostearin | 186 | Increased AUC in both plasma and brain | Zhou et al [73] |
| Chrysin | Alzheimer’s disease | Stearic acid | 240 | Improved memory loss | Vedagiri and Thangarajan [77] |
| Resveratrol | Neurodegenerative disorders | Stearic acid | 134 | Increased bioavailability and half-life | Pandita et al [79] |
| Resveratrol | Neurodegenerative disorders | Precirol ATO 5 | 258 | Increased Cmax and AUC | Ramalingam and Ko [80] |
| Curcumin | Alzheimer’s disease | Compritol 888 ATO | 135 | Reduced neuroinflammation | Kakkar and Kaur [84] |
| Curcumin | Alzheimer’s disease | Compritol 888 ATO | 135 | Improved AUC and brain distribution | Kakkar et al [85] |
| Curcumin | Alzheimer’s disease | Monostearin | 135 | Increased jejunum permeability and bioavailability | Ji et al [86] |
| Curcumin | Alzheimer’s disease | Monostearin | 452 | Increased Cmax and AUC | Ramalingam et al [87] |
| Curcumin | Alzheimer’s disease | Palmitic acid | 412 | Increased AUC and half-life | Ramalingam and Ko [89] |
| Curcumin | Cerebral ischemia | Compritol 888 ATO | 135 | Increased AUC in brain and cognition | Kakkar et al [91] |
AUC = area under the curve; CNS = central nervous system; SLN = solid lipid nanoparticle.
5.3. CV-related diseases
SLNs have been used orally, aiming at enhancing drug plasma concentration and prolonging circulation duration. These features are especially important for accomplishing better therapeutic benefits in treating CV-related diseases. Nimodipine is a calcium channel blocker used in the treatment of hypertension and stroke. Clinical studies demonstrate a low bioavailability of 4–13% for oral nimodipine [92]. Nimodipine-loaded SLNs were prepared with palmitic acid, poloxamer 188, and soy lecithin [93]. An accelerated stability test showed no significant change in size and polydispersity of the SLNs for 3 months. The bioavailability of oral SLNs containing nimodipine (8 mg/kg) was 2-fold greater than that of the solution in rats. Nisoldipine is another antihypertensive drug with low oral bioavailability. Dudhipala and Veerabrahma [94] developed optimal formulations of nisoldipine-loaded SLNs for improved delivery. The optimized SLNs were stable under refrigeration and at room temperature for 3 months. The pharmacokinetic study displayed a 2.2-fold increase in oral bioavailability by SLNs as compared with nisoldipine suspension. The pharmacodynamic study showed that SLNs could significantly reduce systolic blood pressure for a sustained period of 36 hours.
Candesartan cilexetil is a prodrug of candesartan, an angiotensin II type 1 receptor antagonist, used for hypertension and heart failure remission. This prodrug has poor aqueous solubility and low oral absorption. After inclusion of candesartan cilexetil (10 mg/kg) into SLNs using Dynasan as the solid lipid, oral bioavailability was enhanced more than 2.8-fold compared to the free control [95]. An extension of systolic blood pressure reduction was observed after SLN administration in hypertensive rats for 48 hours, whereas the period of blood pressure reduction was only 2 hours for the drug suspension. Zhang et al [96] elucidated the absorption mechanisms of candesartan cilexetil from SLNs. The pharmacokinetic study in rats indicated that the oral bioavailability and Cmax increased more than 12- and 27-fold after SLN incorporation, respectively. In the Caco-2 cell monolayer uptake, SLNs were distributed in the lysosomes and endoplasmic reticulum after internalization. The authors had suggested that SLNs could be internalized into the enterocytes and then diffused into the circulation via portal circulation and the lymphatic pathway. Carvedilol is an antihypertensive drug with a low oral bioavailability of 20% because of the first-pass effect. To improve the drug’s oral delivery, SLNs were developed with N-carboxymethyl chitosan coating for protecting carvedilol in an acidic environment [97]. The polymer-coated SLNs had shown 5.2% of drug release in simulated gastric fluid for 24 hours. In simulated intestinal fluid (pH 7.4), the N-carboxymethyl chitosan on the SLN surface dissolved and the controlled release of carvedilol was detected. In the in vivo pharmacokinetics, the MRT was found to be 13.9 hours and 9.6 hours for polymer-coated SLNs and aqueous suspension, respectively. The AUC of polymer-coated SLNs was 6.3 μg h/mL, which was significantly higher than that of noncoated SLNs (2.9 μg h/mL) and the free control (2.0 μg h/mL).
Use of an iron supplement is an efficient way to treat the iron deficiency in anemia. However, the commercially available iron tablets often cause adverse effects on the GI system, resulting in constipation and blood in the stool [98]. Another concern is the high intersubject variability in iron absorption. This has led to the application of SLNs for oral iron delivery. Zariwala et al [99] prepared chitosan-coated SLNs for ferrous sulfate loading to test the Caco-2 absorption. The cell viability after SLN treatment was >80% of the control cells. The Caco-2 iron absorption from chitosan-coated SLNs (643 ng/mg cell protein) was significantly higher than from noncoated SLNs (584 ng/mg cell protein) and the reference control (515 ng/mg cell protein). Hosny et al [100] assessed the in vivo pharmacokinetics of ferrous sulfate in SLNs. The iron-loaded SLNs exerted a biphasic release behavior, with a burst release within 30 minutes followed by sustained release. A 4-fold increase in oral iron bioavailability was achieved after oral administration of SLNs (10 mg/kg) in rabbits.
5.4. Infection
Infection can cause host tissues to react to organisms and the toxins they produce. Nanocarriers can be effective drug delivery systems for treating infections [101]. Among the different types of nanosystems, SLNs were widely applicable for carrying anti-infection drugs to treat bacterial, fungal, viral, and parasitic infection. Isoniazid is an antitubercular drug recommended by the World Health Organization for management of all forms of tuberculosis. A short half-life of 1–4 hours suggests the need for repetitive dosing that may result in hepatotoxicity and neurotoxicity [102]. Isoniazid-incorporated SLNs were developed to attain prolonged circulation retention and improved bioavailability [103]. The in vitro isoniazid release displayed a triphasic pattern comprising an initial fast release, followed by a hump and finally a delayed release. A significant amelioration of bioavailability in rat plasma (6 times) and the brain (4 times) was found after oral SLN administration compared to free isoniazid (25 mg/kg). The SLNs showed a 3-fold higher median lethal dose (LD50) compared to the free drug in an acute toxicity study. Miconazole is a broad-spectrum antifungal drug with a low aqueous solubility of <1 μg/mL. Encapsulation of miconazole in SLNs showed a biphasic drug release with an initial burst and a following delayed release [104]. The SLNs showed better Candida albicans killing in the diffusion disk test, with the maximum inhibition diameter of 22 mm that was longer than the marketed capsule (14 mm). The in vivo pharmacokinetics in rabbits exhibited a 2.5-fold enhancement of oral bioavailability.
Lopinavir is a human immunodeficiency virus (HIV) protease inhibitor used in antiretroviral therapy. SLNs can act as a feasible carrier for lopinavir because of P-glycoprotein efflux and first-pass metabolism. The lopinavir-loaded SLNs composed of stearic acid were stable at 4°C for 4 months based on particulate size and the release profile [105]. Higher oral bioavailability was obtained for SLNs (2.5-fold) in comparison with lopinavir solution because of higher lymphatic delivery. In another study, Compritol 888 ATO was used as the solid lipid for preparing lopinavir-loaded SLNs [106]. The drug release showed a delayed pattern both in 0.1N HCl (pH 1.2) and phosphate buffer (pH 6.8). The SLNs could bypass P-glycoprotein efflux to reach systemic circulation, leading to a 3.6- and 4.9-fold increase in bioavailability and Cmax compared to solution. Efavirenz is a nonnucleoside reverse transcriptase inhibitor as the first-line antiretroviral drug to eradicate HIV infection. Gaur et al [107] investigated the enhanced oral bioavailability of efavirenz by SLNs. The optimized nanoformulations had shown good stability at 40°C for about 6 months. SLNs revealed a 5.3-fold increase in Cmax and an 11.0-fold increase in AUC compared to drug suspension after oral administration in rats. Makwana et al [108] further explored the absorption pathways of SLNs for delivering efavirenz. The profiles of lymphatic absorption and tissue biodistribution in rats indicated that a large amount of nanoparticulate efavirenz had bypassed the portal system and recovered in the lymph via chylomicron uptake. Reduction of hepatic uptake by SLNs (44.7%) demonstrated liver bypass and thereby the oral bioavailability enhancement. A great amount of efavirenz was observed in the spleen, a principal lymphatic organ.
Malaria continues to be a vast health and economic problem in tropical regions. Primaquine is the only available drug for combating the relapsing form of malaria. The oral absorption of this drug is low because of first-pass metabolism and excretion. The high-dose use of primaquine usually produces hematological- and GI-related toxicity [109]. Primaquine entrapment in SLNs exhibited a sustained drug release over 72 hours [110]. When the Plasmodium berghei-infected mouse was orally administered with SLNs at a drug dose of 2 mg/kg/d for 4 days, the suppression of 94% was achieved by SLNs, whereas only 72% suppression was found for the free control. Arteether is an artemisinin analog for treatment of multidrug-resistant malaria. SLNs were used to resolve the low stability in the gastric environment and the short half-life of arteether [111]. The cell viability remained >90% for SLNs against macrophages, indicating the safety of their application. The half-life of arteether SLNs was found to be 4.5 hours, whereas arteether in ground nut oil and water as the vehicles showed a half-life of 3.3 hours and 2.0 hours. SLNs could increase oral bioavailability compared to ground nut oil by 1.7-fold. Praziquantel is currently used to treat schistosomiasis, a parasitic disease caused by Schistosoma. Because of its limited effect on schistosomiasis, de Souza et al [112] incorporated praziquantel into SLNs to check the possible promotion of its therapeutic activity. In the experiment focused on Schistosoma mansoni inhibition, SLNs were more effective than the free drug, leading to parasite killing in less time. Contrary to the cases of enhanced intestinal permeation by SLNs, the designed SLNs for praziquantel revealed less permeation across the duodenal segment than the free drug in the everted gut sac transport study. This may be attributed to a reduced availability of SLNs in mucosal bulk. This suggested a reservoir role of SLNs for praziquantel to kill the parasites located in the mesenteric vein of the intestine. The parasite Trypanosoma cruzi causes Chagas disease, which remains a serious health problem. Carneiro et al [113] evaluated the in vitro and in vivo activity of 5-hydroxy-3-methyl-5-phenyl-pyrazoline-1-(S-benzyl dithiocarbazate) (H2bdtc) as a free active or incorporated into SLNs. H2bdtc-loaded SLNs efficiently reduced parasitemia in mice at a concentration 100 times lower than benznidazole, the currently available drug for treating Chagas disease. In the in vivo study, oral administration of H2bdtc (4 μmol/kg) in SLNs eradicated 70% of the parasites in circulation, whereas free H2bdtc and benznidazole killed only 48% and 15% of the parasites. SLNs also diminished inflammation and lesions produced by the free drug in the liver and heart (Table 3).
Table 3.
Formulations of orally administered SLNs loaded with drugs or natural compounds against infection and their benefits.
| Active ingredient | Indication | Lipid type | Average size (nm) | Outcomes offered by SLNs | Reference |
|---|---|---|---|---|---|
| Isoniazid | Tuberculosis | Compritol 888 ATO | 48 | Increased bioavailability and less acute toxicity | Bhandari and Kaur [103] |
| Miconazole | Fungi | Precirol ATO 5 | 23 | Enhanced antifungal activity and bioavailability | Aljaeid and Hosny [104] |
| Lopinavir | Retrovirus | Stearic acid | 181 | Increased bioavailability | Negi et al [105] |
| Lopinavir | Retrovirus | Compritol 888 ATO | 215 | Increased Cmax and bioavailability | Negi et al [106] |
| Efavirenz | Retrovirus | Monostearin | 125 | Increased Cmax and AUC | Gaur et al [107] |
| Efavirenz | Retrovirus | Compritol 888 ATO | 168 | Increased lymphatic uptake and bioavailability | Makwana et al [108] |
| Primaquine | Malaria | Stearic acid | 236 | Enhanced antimalarial efficacy | Omwoyo et al [110] |
| Arteether | Multidrug-resistant malaria | Monostearin | 100 | Increased bioavailability and half-life | Dwivedi et al [111] |
| Praziquantel | Schistosomiasis | Stearic acid | 506 | Enhanced parasite killing | de Souza et al [112] |
| H2bdtc | Chagas disease | Stearic acid | 127 | Enhanced parasite killing and diminished toxicity | Carneiro et al [113] |
AUC = area under the curve; H2bdtc = 5-hydroxy-3-methyl-5-phenyl-pyrazoline-1-(S-benzyl dithiocarbazate); SLNs = solid lipid nanoparticles.
5.5. Diabetes
Diabetes mellitus is one of the most common metabolic diseases worldwide. Hyperglycemia caused by diabetes is a serious pathologic condition producing neurological and CV damage. Diabetes is divided into two types: type 1 diabetes and type 2 diabetes. Type 1 diabetes occurs when the pancreas fails to produce sufficient insulin, giving rise to hyperglycemia. The patient requires a routine administration of insulin. Insulin absorption via the oral route is difficult because of acidic gastric pH, digestive enzymes, and the epithelial cells of the GI tract [114]. Researchers focus considerable attention on SLNs as the carriers to protect peptides and proteins known for their sensitivity to various environmental factors such as pH, temperature, and ionic strength [115]. Zhang et al [116] designed SLNs coated with stearic acid-octaarginine as carriers for insulin. Octaarginine is a cell-penetrating peptide that can facilitate cellular uptake of some drugs [117]. The size and insulin encapsulation of the octaarginine-coated SLNs were 162 nm and 77%, respectively. Octaarginine-coated and noncoated SLNs increased Caco-2 cell uptake by 2.3 times and 18.4 times, respectively. The SLNs containing octaarginine showed a significantly higher hypoglycemic effect (3-fold) in rats compared to noncoated SLNs. Ansari et al [118] developed SLNs composed of Dynasan 114 as the solid lipid matrix for oral insulin delivery. The permeability of insulin measured by the everted sac method showed a higher level of SLNs (14.8 μg/cm2) than the insulin solution (8.0 μg/cm2). SLNs provided better insulin protection from the GI environment as evident in the 5-fold higher bioavailability compared to the solution.
About 90% of diabetic patients are affected by noninsulin-dependent type 2. Glibenclamide is a second-generation sulfonylurea for type 2 diabetes treatment. The very low aqueous solubility is responsible for its limited oral bioavailability [119]. Gonçalves et al [120] developed SLNs coated with PEG to increase glibenclamide stability in gastric solution. The insulin release from SLNs obeyed a typical biphasic kinetic, where a complete release was achieved after 24 hours. The oral administration of free glibenclamide (5 mg/kg) in diabetic rats reduced the blood glucose concentration after 4 hours, and it then climbed quickly to the initially high level. Oral SLNs not only created a rapid onset of glucose lowering but also maintained the reduction for 8 hours after administration. Berberine is an isoquinoline alkaloid derived from Coptis chinensis. It is reported that berberine has the capability to treat diabetes by glycolysis stimulation and insulin secretion promotion [121]. Xue et al [122] encapsulated berberine by SLNs with a particulate size and entrapment efficiency of 77 nm and 58%, respectively. A single oral dose (50 mg/kg) in rats achieved a significant improvement of AUC by SLNs (179 μg h/L) compared to solution (86 μg h/L). Morphological analysis of the db/db mice indicated that SLNs potentially enhanced islet function and protected the islet from regeneration. Andrographis paniculata and its bioactive compound andrographolide have been reported to have antidiabetic and hypolipidemic activities [123]. Andrographolide is rapidly metabolized in the duodenum and jejunum to form sulfate conjugates [124]. The SLNs were prepared to load andrographolide with a high encapsulation percentage of 91% [125]. The oral bioavailability and hypolipidemic activity of andrographolide was improved by SLNs because of the increase of solubility and stability in the intestine and the change of transport mode in Caco-2 cells. The bioavailability was increased 2.4-fold after andrographolide inclusion in SLNs.
5.6. Osteoporosis
Osteoporosis is a disease in which decreased bone strength increases the risk of a broken bone. It is the most common reason for a broken bone among the elderly and postmenopausal women. Oral raloxifen is approved for prevention and treatment of postmenopausal osteoporosis. Although 60% of raloxifen is absorbed orally, the absolute bioavailability is only 2% because of poor aqueous solubility and glucuronide conjugation [126]. SLNs for raloxifen delivery were prepared using Compritol 888 ATO as the lipid and poloxamer 188 as the emulsifier [127]. The release of free raloxifen was complete within 4 hours, whereas SLNs prolonged drug release up to 24 hours. The rats were orally administered raloxifen at a dose of 30 mg/kg. The bioavailability of SLNs was nearly 5 times that of free raloxifen. Tran et al [128] also prepared raloxifen-loaded SLNs to examine oral bioavailability and safety. The optimized SLNs showed an average size of 140 nm with ease of transport into the lymphatic system. Cmax and AUC were increased by 3.1- and 2.7-fold by SLN formulation compared to the free drug. The cytotoxicity against NIH-3T3 cells exhibited nontoxicity of the SLNs. Alendronate is a nitrogen-containing biphosphonate used for osteoporosis management. The challenge associated with oral alendronate is its low bioavailability (0.6 %) because of the difficulty of crossing the GI membrane [129]. The enteric-coated SLNs were designed to conquer this challenge to prevent alendronate contact with gastric mucosa [130]. The alendronate release percentage in 0.1N HCl was 5% and 85% for SLNs and commercial tablets, demonstrating the gastric resistance of the enteric-coated carrier. The drug release from SLNs occurred only at an alkaline pH. The pharmacokinetic determination of oral SLNs revealed that alendronate bioavailability increased 7.4-fold in rabbits.
Salmon calcitonin is a calcium-regulating peptide hormone secreted from the ultimobranchial gland of salmon. It is widely used in the treatment of postmenopausal osteoporosis. The oral bioavailability of calcitonin is <0.1% in rats and dogs [131]. SLNs encapsulating salmon calcitonin could increase Caco-2 cell uptake by 4 times compared to the free control [132]. The in vivo hypocalcemic effect in rats was better with prepared SLNs than with free calcitonin (17.4% vs. 2.0% reduction). The SLNs prepared with stearic acid and tripalmitin increased oral calcitonin bioavailability from 2% to 13%. Fan et al [133] prepared salmon calcitonin-loaded SLNs by coupling with peptide ligand CSKSSDYQC, which shows affinity with goblet cells on the epithelium, or IRQRRRR, which is a cell-penetrating peptide. The protective efficacy of SLNs on calcitonin against pancreatin was examined. Most of the free calcitonin was degraded within 15 minutes. A much slower degradation rate was found for SLNs. The permeability across the Caco-2 cell monolayer for nonconjugated, CSKSSDYQC and IRQRRRR SLNs was 2.1-, 5.9-, and 4.7-fold higher than that of the free control. The absolute bioavailability of CSKSSDYQC (12.4%) and IRQRRRR SLNs (10.1%) was greater than that of unmodified SLNs (5.1%), suggesting the effectiveness of peptide conjugation for the enhancement of oral protein delivery.
6. Conclusions and perspectives
SLNs are the colloidal systems for the delivery of labile drugs with controlled release kinetics. SLN-based carriers are compatible with the oral administration route because of nontoxic excipients and increased bioavailability. This review represents the research advancements that had been undertaken to establish the oral SLNs with respect to their unique properties. In the past 5 years, the oral SLNs have gained some advances in the therapy of cancers and CNS-related diseases, which are the increasing health threats around the world. For instance, SLNs could enhance the bioavailability of docetaxel by a 3-fold compared to the commercially available Taxotere. The PEGylated SLNs provided a 7.5-fold increase of oral bioavailability than free doxorubicin. With respect to the treatment of Parkinson’s disease, a 13-fold increase of oral apomorphine bioavailability was achieved by SLNs compared with the free drug. The introduction of SLNs for improved oral delivery also promotes the possibility of natural compound application for disease management because the oral bioavailability and half-life of natural products are always low in the body.
Despite the advantages of oral SLNs for drug delivery, several challenges remain to be resolved for better application in the future. The particle growing and unpredictable gelation tendency are the storage problems in some cases. The burst drug release for some oral SLNs may cause toxicity concerns. However, the very slow drug release can lead to inefficient activity in treating the diseases. The development of the formulation design of novel SLNs to provide a feasible release profile is important. This depends on the cargo drugs selected in SLNs, because different drugs show different physicochemical characters, and on the interaction with the nanoparticles. Prolonged drug circulation is a common phenomenon for oral delivery of SLNs. However, the negative side of prolonged circulation is the slow tissue accumulation of the nanoparticles, including the targeted tissue. Again, the optimization of SLN formulations is necessary.
The SLNs as drug nanocarriers have the potential to achieve the broad objectives for treating various diseases. A wider collection of the lipid materials may be illustrated for SLNs in the future. The lipids from natural sources can be a major origin of the SLN lipid matrix. More patented dosage forms of SLNs can be expected in the near future. SLNs based on the optimized formulations should have a place in modern pharmaceutical products because of the capability of enhancing drug therapy. An innovative formulation of a drug can extend the life of patients. A novel delivery system for old actives would result in the reduction of side effects and more-effective treatment achievement. Although many oral SLNs have been developed for testing in cell-based and animal studies, clinical trials for drug delivery application are still limited. This may be because of the high cost of clinical trials and the unknown side effects that should be identified and explored first. At least a 5-fold-improved oral bioavailability needs to be achieved in most cases to justify the use of nanocarriers from the commercial point of view, unless other convincing benefits can be gained. A better understanding of the full potential of SLNs and the progress from laboratory bench to large-scale commercialization is required. The introduction and description of the SLNs for oral delivery outlined in this review may give relevant information to investigators involved in designing feasible and efficient delivery systems for the treatment of various diseases.
Acknowledgments
The authors are grateful to the financial support by Chang Gung Memorial Hospital (CMRPD1B0332) and Chang Gung University of Science and Technology (EZRPF3G0171 and EZRPF3G0181).
Funding Statement
The authors are grateful to the financial support by Chang Gung Memorial Hospital (CMRPD1B0332) and Chang Gung University of Science and Technology (EZRPF3G0171 and EZRPF3G0181).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Choonara BF, Choonara YE, Kumar P, Bijukumar D, du Toit LC, Pillay V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol Adv. 2014;32:1269–82. doi: 10.1016/j.biotechadv.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 2. Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–70. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alai MS, Lin WJ, Pingale SS. Application of polymeric nanoparticles and micelles in insulin oral delivery. J Food Drug Anal. 2015;23:351–8. doi: 10.1016/j.jfda.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jabir NR, Tabrez S, Ashraf GM, Shakil S, Damanhouri GA, Kamal MA. Nanotechnology-based approaches in anticancer research. Int J Nanomed. 2012;7:4391–408. doi: 10.2147/IJN.S33838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 6. Rostami E, Kashanian S, Azandaryani AH, Faramarzi H, Dolatabadi JE, Omidfar K. Drug targeting using solid lipid nanoparticles. Chem Phys Lipids. 2014;181:56–61. doi: 10.1016/j.chemphyslip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 7. Schwarz C, Mehnert M, Lucks JS, Müller RH. Solid lipid nanoparticles (SLN) for controlled drug delivery: I. Production, characterization and sterilization. J Control Rel. 1994;30:83–96. [Google Scholar]
- 8. Geszke-Moritz M, Moritz M. Solid lipid nanoparticles as attractive drug vehicles: composition, properties and therapeutic strategies. Mater Sci Eng C Mater Biol Appl. 2016;68:982–94. doi: 10.1016/j.msec.2016.05.119. [DOI] [PubMed] [Google Scholar]
- 9. Ezzati Nazhad Dolatabadi J, Valizadeh H, Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharm Bull. 2015;5:151–9. doi: 10.15171/apb.2015.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Clin Gastroenterol. 2008;22:391–409. doi: 10.1016/j.bpg.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 11. Sant S, Tao SL, Fisher OZ, Xu Q, Peppas NA, Khademhosseini A. Microfabrication technologies for oral drug delivery. Adv Drug Deliv Rev. 2012;64:496–507. doi: 10.1016/j.addr.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bak A, Leung D, Barrett SE, Forster S, Minnihan EC, Leithead AW, Cunningham J, Toussaint N, Crocker LS. Physicochemical and formulation developability assessment for therapeutic peptide delivery—a primer. AAPS J. 2015;17:144–55. doi: 10.1208/s12248-014-9688-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu B, Liu X, Zhang C, Zeng X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J Food Drug Anal. 2017;25:3–15. doi: 10.1016/j.jfda.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernkop-Schnürch A. Nanocarrier systems for oral drug delivery: do we really need them? Eur J Pharm Sci. 2013;49:272–7. doi: 10.1016/j.ejps.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 15. Saraf S, Ghosh A, Kaur CD, Saraf S. Novel modified nanosystem based lymphatic targeting. Res J Nanosci Nanotechnol. 2011;1:60–74. [Google Scholar]
- 16. Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 2014;22:871–82. doi: 10.3109/1061186X.2014.950664. [DOI] [PubMed] [Google Scholar]
- 17. Ali Khan A, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomed. 2013;8:2733–44. doi: 10.2147/IJN.S41521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar S, Randhawa JK. High melting lipid based approach for drug delivery: solid lipid nanoparticles. Mater Sci Eng C Mater Biol Appl. 2013;33:1842–52. doi: 10.1016/j.msec.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 19. Hunter AC, Elsom J, Wibroe PP, Moghimi SM. Polymeric particulate technologies for oral drug delivery and targeting: a pathophysiological perspective. Nanomed Nanotechnol Biol Med. 2012;8(Suppl 1):S5–20. doi: 10.1016/j.nano.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 20. Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yadav P, Soni G, Mahor A, Alok S, Singh PP, Verma A. Solid lipid nanoparticles: an effective and promising drug delivery system—a review. Int J Pharm Sci Res. 2014;5:1152–62. [Google Scholar]
- 22. Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomed. 2007;2:289–300. [PMC free article] [PubMed] [Google Scholar]
- 23. Helgason T, Awad TS, Kristbergsson K, McClements DJ, Weiss J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN) J Colloid Interface Sci. 2009;334:75–81. doi: 10.1016/j.jcis.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 24. Botto C, Mauro N, Amore E, Martorana E, Giammona G, Bondì ML. Surfactant effect on the physicochemical characteristics of cationic solid lipid nanoparticles. Int J Pharm. 2016;516:334–41. doi: 10.1016/j.ijpharm.2016.11.052. [DOI] [PubMed] [Google Scholar]
- 25. Harde H, Das M, Jain S. Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Expert Opin Drug Deliv. 2011;8:1407–24. doi: 10.1517/17425247.2011.604311. [DOI] [PubMed] [Google Scholar]
- 26. Battaglia L, Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Deliv. 2012;9:497–508. doi: 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- 27. Hwang TL, Aljuffali IA, Hung CF, Chen CH, Fang JY. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs) Chem Biol Interact. 2015;235:106–14. doi: 10.1016/j.cbi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 28. Ingham B. X-ray scattering characterization of nanoparticles. Crystallogr Rev. 2015;21:229–303. [Google Scholar]
- 29. Muchow M, Maincent P, Müller RH. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev Ind Pharm. 2008;34:1394–405. doi: 10.1080/03639040802130061. [DOI] [PubMed] [Google Scholar]
- 30. Dahan A, Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release. 2008;129:1–10. doi: 10.1016/j.jconrel.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 31. Doktorovová S, Santos DL, Costa I, Andreani T, Souto EB, Silva AM. Cationic solid lipid nanoparticles interfere with the activity of antioxidant enzymes in hepatocellular carcinoma cells. Int J Pharm. 2014;471:18–27. doi: 10.1016/j.ijpharm.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 32. Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv Drug Deliv Rev. 2011;63:901–8. doi: 10.1016/j.addr.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chai GH, Xu Y, Chen SQ, Cheng B, Hu FQ, You J, Du YZ, Yuan H. Transport mechanisms of solid lipid nanoparticles across Caco-2 cell monolayers and their related cytotoxicology. ACS Appl Mater Interfaces. 2016;8:5929–40. doi: 10.1021/acsami.6b00821. [DOI] [PubMed] [Google Scholar]
- 34. Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133:238–44. doi: 10.1016/j.jconrel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 35. Ahmad J, Amin S, Rahman M, Rub RA, Singhal M, Ahmad MZ, Rahman Z, Addo RT, Ahmad FJ, Mushtaq G, Kamal MA, Akhter S. Solid matrix based lipidic nanoparticles in oral cancer chemotherapy: applications and pharmacokinetics. Curr Drug Metab. 2015;16:633–44. doi: 10.2174/1389200216666150812122128. [DOI] [PubMed] [Google Scholar]
- 36. Fan Z, Fu PP, Yu H, Ray PC. Theranostic nanomedicine for cancer detection and treatment. J Food Drug Anal. 2014;22:3–17. doi: 10.1016/j.jfda.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mei L, Zhang Z, Zhao L, Huang L, Yang XL, Tang J, Feng SS. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv Drug Deliv Rev. 2013;65:880–90. doi: 10.1016/j.addr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 38. ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–85. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 39. Hendrikx JJ, Lagas JS, Rosing H, Schellens JH, Beijnen JH, Schinkel AH. P-glycoprotein and cytochrome P450 3A act together in restricting the oral bioavailability of paclitaxel. Int J Cancer. 2013;132:2439–47. doi: 10.1002/ijc.27912. [DOI] [PubMed] [Google Scholar]
- 40. Baek JS, So JW, Shin SC, Cho CW. Solid lipid nanoparticles of paclitaxel strengthened by hydroxypropyl-β-cyclodextrin as an oral delivery system. Int J Mol Med. 2012;30:953–9. doi: 10.3892/ijmm.2012.1086. [DOI] [PubMed] [Google Scholar]
- 41. Zafar N, Fessi H, Elaissari A. Cyclodextrin containing biodegradable particles: from preparation to drug delivery applications. Int J Pharm. 2014;461:351–66. doi: 10.1016/j.ijpharm.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 42. Pooja D, Kulhari H, Kuncha M, Rachamalla SS, Adams DJ, Bansal V, Sistla R. Improving efficacy, oral bioavailability, and delivery of paclitaxel using protein-grafted solid lipid nanoparticles. Mol Pharm. 2016;13:3903–12. doi: 10.1021/acs.molpharmaceut.6b00691. [DOI] [PubMed] [Google Scholar]
- 43. Liu Y, Wang P, Sun C, Zhao J, Du Y, Shi F, Feng N. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. Int J Pharm. 2011;419:260–5. doi: 10.1016/j.ijpharm.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 44. Cho HJ, Park JW, Yoon IS, Kim DD. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: enhanced intestinal absorption and lymphatic uptake. Int J Nanomed. 2014;9:495–504. doi: 10.2147/IJN.S56648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp. 2009;57:435–45. doi: 10.1007/s00005-009-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Patro NM, Devi K, Pai RS, Suresh S. Evaluation of bioavailability, efficacy, and safety profile of doxorubicin-loaded solid lipid nanoparticles. J Nanopart Res. 2013;15:2124. [Google Scholar]
- 47. Yuan H, Chen CY, Chai GH, Du YZ, Hu FQ. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol Pharm. 2013;10:1865–73. doi: 10.1021/mp300649z. [DOI] [PubMed] [Google Scholar]
- 48. Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed Engl. 2008;47:9726–9. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ozaki K, Kishikawa F, Tanaka M, Sakamoto T, Tanimura S, Kohno M. Histone deacetylase inhibitors enhance the chemosensitivity of tumor cells with cross-resistance to a wide range of DNA-damaging drugs. Cancer Sci. 2008;99:376–84. doi: 10.1111/j.1349-7006.2007.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tran TH, Ramasamy T, Truong DH, Shin BS, Choi HG, Yong CS, Kim JO. Development of vorinostat-loaded solid lipid nanoparticles to enhance pharmacokinetics and efficacy against multidrug-resistant cancer cells. Pharm Res. 2014;31:1978–88. doi: 10.1007/s11095-014-1300-z. [DOI] [PubMed] [Google Scholar]
- 51. Hashem FM, Nasr M, Khairy A. In vitro cytotoxicity and bioavailability of solid lipid nanoparticles containing tamoxifen citrate. Pharm Dev Technol. 2014;19:824–32. doi: 10.3109/10837450.2013.836218. [DOI] [PubMed] [Google Scholar]
- 52. Aljuffali IA, Fang CL, Chen CH, Fang JY. Nanomedicine as a strategy for natural compound delivery to prevent and treat cancers. Curr Pharm Design. 2016;22:4219–31. doi: 10.2174/1381612822666160620072539. [DOI] [PubMed] [Google Scholar]
- 53. Iriti M, Faoro F. Bioactivity of grape chemicals for human health. Nat Prod Commun. 2009;4:611–34. [PubMed] [Google Scholar]
- 54. Yap SP, Yuen KH, Lim AB. Influence of route of administration on the absorption and disposition of alpha-, gamma- and delta-tocotrienols in rats. J Pharm Pharmacol. 2003;55:53–8. doi: 10.1111/j.2042-7158.2003.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 55. Abuasal BS, Lucas C, Peyton B, Alayoubi A, Nazzal S, Sylvester PW, Kaddoumi A. Enhancement of intestinal permeability utilizing solid lipid nanoparticles increases γ-tocotrienol oral bioavailability. Lipids. 2012;47:461–9. doi: 10.1007/s11745-012-3655-4. [DOI] [PubMed] [Google Scholar]
- 56. Dang YJ, Zhu CY. Determination of trace cantharidin in plasma and pharmacokinetic study in beagle dogs using gas chromatography-mass spectrometry. J Anal Toxicol. 2009;33:384–8. doi: 10.1093/jat/33.7.384. [DOI] [PubMed] [Google Scholar]
- 57. Dang YJ, Zhu CY. Oral bioavailability of cantharidin-loaded solid lipid nanoparticles. Chin Med. 2013;8:1. doi: 10.1186/1749-8546-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Henderson AJ, Ollila CA, Kumar A, Borresen EC, Raina K, Agarwal R, Ryan EP. Chemopreventive properties of dietary rice bran: current status and future prospects. Adv Nutr. 2012;3:643–53. doi: 10.3945/an.112.002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y, Li Z, Zhang K, Yang G, Wang Z, Zhao J, Hu R, Feng N. Ethyl oleate-containing nanostructured lipid carriers improve oral bioavailability of trans-ferulic acid as compared with conventional solid lipid nanoparticles. Int J Pharm. 2016;511:57–64. doi: 10.1016/j.ijpharm.2016.06.131. [DOI] [PubMed] [Google Scholar]
- 60. Thakkar A, Chenreddy S, Wang J, Prabhu S. Ferulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticles. Cell Biosci. 2015;5:46. doi: 10.1186/s13578-015-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Luo Y, Teng Z, Li Y, Wang Q. Solid lipid nanoparticles for oral drug delivery: chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr Polym. 2015;122:221–9. doi: 10.1016/j.carbpol.2014.12.084. [DOI] [PubMed] [Google Scholar]
- 62. Dening TJ, Rao S, Thomas N, Prestidge CA. Oral nanomedicine approaches for the treatment of psychiatric illnesses. J Control Rel. 2016;223:137–56. doi: 10.1016/j.jconrel.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 63. Silva AC, Kumar A, Wild W, Ferreira D, Santos D, Forbes B. Long-term stability, biocompatibility and oral delivery potential of risperidone-loaded solid lipid nanoparticles. Int J Pharm. 2012;436:798–805. doi: 10.1016/j.ijpharm.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 64. Subramony JA. Apomorphine in dopaminergic therapy. Mol Pharm. 2006;3:380–5. doi: 10.1021/mp060012c. [DOI] [PubMed] [Google Scholar]
- 65. Tsai MJ, Huang YB, Wu PC, Fu YS, Kao YR, Fang JY, Tsai YH. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: pharmacokinetic and behavioral evaluations. J Pharm Sci. 2011;100:547–57. doi: 10.1002/jps.22285. [DOI] [PubMed] [Google Scholar]
- 66. Jagdale SC, Pawar CR. Application of design of experiment for polyox and xanthan gum coated floating pulsatile delivery of sumatriptan succinate in migraine treatment. Biomed Res Int. 2014;2014:547212. doi: 10.1155/2014/547212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hansraj GP, Singh SK, Kumar P. Sumatriptan succinate loaded chitosan solid lipid nanoparticles for enhanced anti-migraine potential. Int J Biol Macromol. 2015;81:467–76. doi: 10.1016/j.ijbiomac.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 68. Girotra P, Singh SK. Multivariate optimization of rizatriptan benzoate-loaded solid lipid nanoparticles for brain targeting and migraine management. AAPS PharmSciTech. 2016 doi: 10.1208/s12249-016-0532-0. [DOI] [PubMed] [Google Scholar]
- 69. Ibrahim WM, AlOmrani AH, Yassin AE. Novel sulpiride-loaded solid lipid nanoparticles with enhanced intestinal permeability. Int J Nanomed. 2014;9:129–44. doi: 10.2147/IJN.S54413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Raggi MA, Mandrioli R, Sabbioni C, Pucci V. Atypical antipsychotics: pharmacokinetics, therapeutic drug monitoring and pharmacological interactions. Curr Med Chem. 2004;11:279–96. doi: 10.2174/0929867043456089. [DOI] [PubMed] [Google Scholar]
- 71. Narala A, Veerabrahma K. Preparation, characterization and evaluation of quetiapine fumarate solid lipid nanoparticles to improve the oral bioavailability. J Pharm. 2013;2013:265741. doi: 10.1155/2013/265741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Uhr M, Grauer MT, Holsboer F. Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-glycoprotein gene disruption. Biol Psychiatry. 2003;54:840–6. doi: 10.1016/s0006-3223(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 73. Zhou Y, Zhang G, Rao Z, Yang Y, Zhou Q, Qin H, Wei Y, Wu X. Increased brain uptake of venlafaxine loaded solid lipid nanoparticles by overcoming the efflux function and expression of P-gp. Arch Pharm Res. 2015;38:1325–35. doi: 10.1007/s12272-014-0539-6. [DOI] [PubMed] [Google Scholar]
- 74. Apetz N, Munch G, Govindaraghavan S, Gyengesi E. Natural compounds and plant extracts as therapeutics against chronic inflammation in Alzheimer’s disease—a translational perspective. CNS Neurol Disord Drug Targets. 2014;13:1175–91. doi: 10.2174/1871527313666140917110635. [DOI] [PubMed] [Google Scholar]
- 75. Tsai CH, Yen YH, Yang JPW. Finding of polysaccharide–peptide complexes in Cordyceps militaris and evaluation of its acetylcholinesterase inhibition activity. J Food Drug Anal. 2014;23:63–70. doi: 10.1016/j.jfda.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Spilsbury A, Vauzour D, Spencer JP, Rattray M. Regulation of NF-κB activity in astrocytes: effects of flavonoids at dietary-relevant concentrations. Biochem Biophys Res Commun. 2012;418:578–83. doi: 10.1016/j.bbrc.2012.01.081. [DOI] [PubMed] [Google Scholar]
- 77. Vedagiri A, Thangarajan S. Mitigating effect of chrysin loaded solid lipid nanoparticles against amyloid β25–35 induced oxidative stress in rat hippocampal region: an efficient formulation approach for Alzheimer’s disease. Neuropeptides. 2016;58:111–25. doi: 10.1016/j.npep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 78. Diaz-Gerevini GT, Repossi G, Dain A, Tarres MC, Das UN, Eynard AR. Beneficial action of resveratrol: how and why? Nutrition. 2016;32:174–8. doi: 10.1016/j.nut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 79. Pandita D, Kumar S, Poonia N, Lather V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res Int. 2014;62:1165–74. [Google Scholar]
- 80. Ramalingam P, Ko YT. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2016;139:52–61. doi: 10.1016/j.colsurfb.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 81. Minassi A, Sánchez-Duffhues G, Collado JA, Muñoz E, Appendino G. Dissecting the pharmacophore of curcumin. Which structural element is critical for which action? J Nat Prod. 2013;76:1105–12. doi: 10.1021/np400148e. [DOI] [PubMed] [Google Scholar]
- 82. Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol. 2013;11:338–78. doi: 10.2174/1570159X11311040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kakkar V, Kaur IP. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem Toxicol. 2011;49:2906–13. doi: 10.1016/j.fct.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 85. Kakkar V, Mishra AK, Chuttani K, Kaur IP. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. Int J Pharm. 2013;448:354–9. doi: 10.1016/j.ijpharm.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 86. Ji H, Tang J, Li M, Ren J, Zheng N, Wu L. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Deliv. 2016;23:459–70. doi: 10.3109/10717544.2014.918677. [DOI] [PubMed] [Google Scholar]
- 87. Ramalingam P, Yoo SW, Ko YT. Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res Int. 2016;84:113–9. [Google Scholar]
- 88. Mansourpour M, Mahjub R, Amini M, Ostad SN, Shamsa ES, Rafiee-Tehrani M, Dorkoosh FA. Development of acid-resistant alginate/trimethyl chitosan nanoparticles containing cationic β-cyclodextrin polymers for insulin oral delivery. AAPS PharmSciTech. 2015;16:952–62. doi: 10.1208/s12249-014-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ramalingam P, Ko YT. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm Res. 2015;32:389–402. doi: 10.1007/s11095-014-1469-1. [DOI] [PubMed] [Google Scholar]
- 90. Shah FA, Gim SA, Sung JH, Jeon SJ, Kim MO, Koh PO. Identification of proteins regulated by curcumin in cerebral ischemia. J Surg Res. 2016;201:141–8. doi: 10.1016/j.jss.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 91. Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm. 2013;85:339–45. doi: 10.1016/j.ejpb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 92. Hernández-Hernández R, Coll T, Rachitzky P, Armas-Hernández MJ, Armas-Padilla MC, Velasco M, Rizzo A. Comparison of two nimodipine formulations in healthy volunteers. J Hum Hypertens. 2002;16:S142–4. doi: 10.1038/sj.jhh.1001361. [DOI] [PubMed] [Google Scholar]
- 93. Chalikwar SS, Belgamwar VS, Talele VR, Surana SJ, Patil MU. Formulation and evaluation of Nimodipine-loaded solid lipid nanoparticles delivered via lymphatic transport system. Colloids Surf B Biointerfaces. 2012;97:109–16. doi: 10.1016/j.colsurfb.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 94. Dudhipala N, Veerabrahma K. Pharmacokinetic and pharmacodynamic studies of nisoldipine-loaded solid lipid nanoparticles developed by central composite design. Drug Dev Ind Pharm. 2015;41:1968–77. doi: 10.3109/03639045.2015.1024685. [DOI] [PubMed] [Google Scholar]
- 95. Dudhipala N, Veerabrahma K. Candesartan cilexetil loaded solid lipid nanoparticles for oral delivery: characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Deliv. 2016;23:395–404. doi: 10.3109/10717544.2014.914986. [DOI] [PubMed] [Google Scholar]
- 96. Zhang Z, Gao F, Bu H, Xiao J, Li Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: in vitro characteristics and absorption mechanism in rats. Nanomed Nanotechnol Biol Med. 2012;8:740–7. doi: 10.1016/j.nano.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 97. Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R, Diwan PV. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloids Surf B Biointerfaces. 2012;95:1–9. doi: 10.1016/j.colsurfb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 98. Muñoz M, García-Erce JA, Remacha ÁF. Disorders of iron metabolism: Part II. Iron deficiency and iron overload. J Clin Pathol. 2011;64:287–96. doi: 10.1136/jcp.2010.086991. [DOI] [PubMed] [Google Scholar]
- 99. Zariwala MG, Elsaid N, Jackson TL, Corral López F, Farnaud S, Somavarapu S, Renshaw D. A novel approach to oral iron delivery using ferrous sulphate loaded solid lipid nanoparticles. Int J Pharm. 2013;456:400–7. doi: 10.1016/j.ijpharm.2013.08.070. [DOI] [PubMed] [Google Scholar]
- 100. Hosny KM, Banjar ZM, Hariri AH, Hassan AH. Solid lipid nanoparticles loaded with iron to overcome barriers for treatment of iron deficiency anemia. Drug Des Devel Ther. 2015;9:313–20. doi: 10.2147/DDDT.S77702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Aljuffali IA, Huang CH, Fang JY. Nanomedical strategies for targeting skin microbiomes. Curr Drug Metab. 2015;16:255–71. doi: 10.2174/1389200216666150812124923. [DOI] [PubMed] [Google Scholar]
- 102. Preziosi P. Isoniazid: metabolic aspects and toxicological correlates. Curr Drug Metab. 2007;8:839–51. doi: 10.2174/138920007782798216. [DOI] [PubMed] [Google Scholar]
- 103. Bhandari R, Kaur IP. Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid lipid nanoparticles. Int J Pharm. 2013;441:202–12. doi: 10.1016/j.ijpharm.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 104. Aljaeid BM, Hosny KM. Miconazole-loaded solid lipid nanoparticles: formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int J Nanomed. 2016;11:441–7. doi: 10.2147/IJN.S100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Negi JS, Chattopadhyay P, Sharma AK, Ram V. Development of solid lipid nanoparticles (SLNs) of lopinavir using hot self nano-emulsification (SNE) technique. Eur J Pharm Sci. 2013;48:231–9. doi: 10.1016/j.ejps.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 106. Negi JS, Chattopadhyay P, Sharma AK, Ram V. Development and evaluation of glyceryl behenate based solid lipid nanoparticles (SLNs) using hot self-nanoemulsification (SNE) technique. Arch Pharm Res. 2014;37:361–70. doi: 10.1007/s12272-013-0154-y. [DOI] [PubMed] [Google Scholar]
- 107. Gaur PK, Mishra S, Bajpai M, Mishra A. Enhanced oral bioavailability of efavirenz by solid lipid nanoparticles: in vitro drug release and pharmacokinetics studies. Biomed Res Int. 2014;2014:363404. doi: 10.1155/2014/363404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495:439–46. doi: 10.1016/j.ijpharm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 109. Baird JK. Primaquine toxicity forestalls effective therapeutic management of the endemic malarias. Int J Parasitol. 2012;42:1049–54. doi: 10.1016/j.ijpara.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 110. Omwoyo WN, Ogutu B, Oloo F, Swai H, Kalombo L, Melariri P, Mahanga GM, Gathirwa JW. Preparation, characterization, and optimization of primaquine-loaded solid lipid nanoparticles. Int J Nanomed. 2014;9:3865–74. doi: 10.2147/IJN.S62630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dwivedi P, Khatik R, Khandelwal K, Taneja I, Raju KS, Wahajuddin, Paliwal SK, Dwivedi AK, Mishra PR. Pharmacokinetics study of arteether loaded solid lipid nanoparticles: an improved oral bioavailability in rats. Int J Pharm. 2014;466:321–7. doi: 10.1016/j.ijpharm.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 112. de Souza AL, Andreani T, de Oliveira RN, Kiill CP, dos Santos FK, Allegretti SM, Chaud MV, Souto EB, Silva AM, Gremião MP. In vitro evaluation of permeation, toxicity and effect of praziquantel-loaded solid lipid nanoparticles against Schistosoma mansoni as a strategy to improve efficacy of the schistosomiasis treatment. Int J Pharm. 2014;463:31–7. doi: 10.1016/j.ijpharm.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 113. Carneiro ZA, Maia PI, Sesti-Costa R, Lopes CD, Pereira TA, Milanezi CM, da Silva MA, Lopez RF, Silva JS, Deflon VM. In vitro and in vivo trypanocidal activity of H2bdtc-loaded solid lipid nanoparticles. PLoS Negl Trop Dis. 2014;8:e2847. doi: 10.1371/journal.pntd.0002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gundogdu E, Yurdasiper A. Drug transport mechanism of oral antidiabetic nanomedicines. Int J Endocrinol Metab. 2014;12:e8984. doi: 10.5812/ijem.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–90. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 116. Zhang ZH, Zhang YL, Zhou JP, Lv HX. Solid lipid nanoparticles modified with stearic acid-octaarginine for oral administration of insulin. Int J Nanomed. 2012;7:3333–9. doi: 10.2147/IJN.S31711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Golan M, Feinshtein V, David A. Conjugates of HA2 with octaarginine-grafted HPMA copolymer offer effective siRNA delivery and gene silencing in cancer cells. Eur J Pharm Biopharm. 2016;109:103–12. doi: 10.1016/j.ejpb.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 118. Ansari MJ, Anwer MK, Jamil S, Al-Shdefat R, Ali BE, Ahmad MM, Ansari MN. Enhanced oral bioavailability of insulin-loaded solid lipid nanoparticles: pharmacokinetic bioavailability of insulin-loaded solid lipid nanoparticles in diabetic rats. Drug Deliv. 2016;23:1972–9. doi: 10.3109/10717544.2015.1039666. [DOI] [PubMed] [Google Scholar]
- 119. Neugebauer G, Betzien G, Hrstka V, Kaufmann B, von Mollendorff E, Abshagen U. Absolute bioavailability and bioequivalence of glibenclamide (Semi-Euglucon N) Int J Clin Pharmacol Ther Toxicol. 1985;23:453–60. [PubMed] [Google Scholar]
- 120. Gonçalves LM, Maestrelli F, Di Cesare Mannelli L, Ghelardini C, Almeida AJ, Mura P. Development of solid lipid nanoparticles as carriers for improving oral bioavailability of glibenclamide. Eur J Pharm Biopharm. 2016;102:41–50. doi: 10.1016/j.ejpb.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 121. Pirillo A, Catapano AL. Berberine, a plant alkaloid with lipid-and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis. 2015;243:449–61. doi: 10.1016/j.atherosclerosis.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 122. Xue M, Yang MX, Zhang W, Li XM, Gao DH, Ou ZM, Li ZP, Liu SH, Li XJ, Yang SY. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int J Nanomed. 2013;8:4677–87. doi: 10.2147/IJN.S51262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yang T, Shi HX, Wang ZT, Wang CH. Hypolipidemic effects of andrographolide and neoandrographolide in mice and rats. Phytother Res. 2013;27:618–23. doi: 10.1002/ptr.4771. [DOI] [PubMed] [Google Scholar]
- 124. Ye L, Wang T, Tang L, Liu W, Yang Z, Zhou J, Zheng Z, Cai Z, Hu M, Liu Z. Poor oral bioavailability of a promising anticancer agent andrographolide is due to extensive metabolism and efflux by P-glycoprotein. J Pharm Sci. 2011;100:5007–17. doi: 10.1002/jps.22693. [DOI] [PubMed] [Google Scholar]
- 125. Yang T, Sheng HH, Feng NP, Wei H, Wang ZT, Wang CH. Preparation of andrographolide-loaded solid lipid nanoparticles and their in vitro and in vivo evaluations: characteristics, release, absorption, transports, pharmacokinetics, and antihyperlipidemic activity. J Pharm Sci. 2013;102:4414–25. doi: 10.1002/jps.23758. [DOI] [PubMed] [Google Scholar]
- 126. Gluck O, Maricic M. Skeletal and nonskeletal effects of raloxifene. Curr Osteoporos Rep. 2003;1:123–8. doi: 10.1007/s11914-996-0007-4. [DOI] [PubMed] [Google Scholar]
- 127. Kushwaha AK, Vuddanda PR, Karunanidhi P, Singh SK, Singh S. Development and evaluation of solid lipid nanoparticles of raloxifene hydrochloride for enhanced bioavailability. Biomed Res Int. 2013;2013:584549. doi: 10.1155/2013/584549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tran TH, Ramasamy T, Cho HJ, Kim YI, Poudel BK, Choi HG, Yong CS, Kim JO. Formulation and optimization of raloxifene-loaded solid lipid nanoparticles to enhance oral bioavailability. J Nanosci Nanotechnol. 2014;14:4820–31. doi: 10.1166/jnn.2014.8722. [DOI] [PubMed] [Google Scholar]
- 129. Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M, Kiel D, Felsenberg D, Recker RR, Tonino RP, Roux C, Pinchera A, Foldes AJ, Greenspan SL, Levine MA, Emkey R, Santora AC, 2nd, Kaur A, Thompson DE, Yates J, Orloff JJ. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging. 2000;12:1–12. [PubMed] [Google Scholar]
- 130. Hosny KM. Alendronate sodium as enteric coated solid lipid nanoparticles; preparation, optimization, and in vivo evaluation to enhance its oral bioavailability. PLoS One. 2016;11:e0154926. doi: 10.1371/journal.pone.0154926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lee YH, Sinko PJ. Oral delivery of salmon calcitonin. Adv Drug Deliv Rev. 2000;42:225–38. doi: 10.1016/s0169-409x(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 132. Chen C, Fan T, Jin Y, Zhou Z, Yang Y, Zhu X, Zhang ZR, Zhang Q, Huang Y. Orally delivered salmon calcitonin-loaded solid lipid nanoparticles prepared by micelle-double emulsion method via the combined use of different solid lipids. Nanomedicine. 2013;8:1085–100. doi: 10.2217/nnm.12.141. [DOI] [PubMed] [Google Scholar]
- 133. Fan T, Chen C, Guo H, Xu J, Zhang J, Zhu X, Yang Y, Zhou Z, Li L, Huang Y. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur J Pharm Biopharm. 2014;88:518–28. doi: 10.1016/j.ejpb.2014.06.011. [DOI] [PubMed] [Google Scholar]