Abstract
The antioxidant and antibacterial activities of phenolic compounds from cultivated and wild Tunisian Ruta chalepensis L. leaves, stems, and flowers were assessed. The leaves and the flowers exhibited high but similar total polyphenol, flavonoid, and tannin content. Moreover, two organs showed strong, although not significantly different, total antioxidant activity, 2,2-diphenyl-1-picrylhydrazyl scavenging ability, and reducing power. Investigation of the phenolic composition showed that vanillic acid and coumarin were the major compounds in the two organs, with higher percentages in the cultivated organs than in the spontaneous organs. Furthermore, R. chalepensis extracts showed marked antibacterial properties against human pathogen strains, and the activity was organ- and origin-dependent. Spontaneous stems had the strongest activity against Pseudomonas aeruginosa. From these results, it was concluded that domestication of Ruta did not significantly affect its chemical composition and consequently the possibility of using R. chalpensis organs as a potential source of natural antioxidants and as an antimicrobial agent in the food industry.
Keywords: antibacterial, antioxidant, organ, origin, polyphenol, Ruta chalepensis L
1. Introduction
Plants constitute a valuable source of natural antioxidants such as vitamins, phenolic compounds, and flavonoids [1]. Because of their potential carcinogenicity, the utilization of synthetic antioxidants is progressively restricted in the food industry. This trend is concomitant with an increasing interest in the identification and valorization of natural antioxidants of plant origin. Moreover, microbial activity is a primary mode of deterioration of many foods and is often responsible for the loss of quality and safety. Concern over pathogenic and spoilage microorganisms in foods is increasing because of the increase in outbreaks of food-borne disease. There is growing interest in using natural antibacterial compounds such as plant extracts of herbs and spices for the preservation of foods because these extracts possess a characteristic flavor and sometimes show antioxidant activity and antimicrobial activity [2].
Ruta is a genus of Rutaceae family and features primarily shrubby plants that are native to the Mediterranean region and usually grow on rocky slopes. The three most diffused species are Ruta chalepensis L., Ruta graveolens L., and Ruta montana L. [3]. It is used for culinary purposes to flavor foods and as an aperitif [4]. This plant is commonly used as a traditional medicinal plant. It is protective against various disorders such as rheumatism, fever, mental disorders, dropsy, neuralgia, menstrual problems, convulsions, and other bleeding and nervous disorders [3,5,6]. With regard to the phytochemical composition of Ruta chalepensis extracts, Aguilar-Santamaria and Tortoriello [5] have reported that its leaves and young stems contain alkaloids, flavonoids, phenols, amino acids, furocoumarins, and saponins. However, data on the bioactivities of R. chalepensis are scarce. Almog et al [7] found that the ethanol extract of the plant reduced the oxidative stress in experimental animal models by modulating the activities of various antioxidant enzymes. Moreover, Fakhfakh et al [8] reported interesting in vitro antioxidant activities of the essential oil and solvent extracts of wild R. chalepensis. Studies on the antimicrobial activity of R. chalepensis are limited; however, Al-Bakri and Afifi [9] found that dimethyl sulfoxide extracts of R. chalepensis possess promising antibacterial activity. In Tunisia, there are two R. chalepensis provenances: the cultivated R. chalepensis (i.e., in garden houses) and the spontaneous R. chalepensis (i.e., wild, usually growing in mountains). Several researchers suggested the relevance of identifying the metabolic differences between wild and cultivated specimens to assess their quality during the domestication process [10]. In some instances, the wild forms of plants may demonstrate different phytonutrient profiles from the cultivated ones. Several studies have stated that most wild species preserve higher biological capacity and richer secondary metabolites, compared with the cultivated species [11,12]. According to previous literature reports illustrating the importance of Ruta as a medicinal plant, and because of its availability in Tunisia, in this work we aimed to evaluate the phenolic composition and the antioxidant and antibacterial properties of the methanolic extracts of Tunisian R. chalepensis as function of the plant parts (i.e., leaves, stems, and flowers) and provenance (i.e., cultivated and wild Ruta). In addition, the objective of this study was to establish differences between the two origins and to evaluate which is the most interesting. The results of this work highlighted the possibility of using R. chalepensis organs as a potential source of natural antioxidants and as an antimicrobial agent in the food industry.
2. Materials and methods
2.1. Plant material
Cultivated R. chalepensis originated from an orchard in the Center of Tunisia from El Ala (Central Tunisia: latitude 35°36′5782″ (N), longitude 9°33′34″ (E), altitude 151.80 km). Spontaneous Ruta chalepensis was collected from the mountain Traza (El Ala). The two provenances were collected in May 2012 at the flowering stage, and the different organs (i.e., leaves, stems, and flowers) were air-dried at room temperature. They were then ground with a blade-carbide grinding (Type A:10; IKA-WERK).
2.2. Preparation of extracts
Extracts were obtained by stirring 1 g of dry R. chalepensis powder of the organs with 10 mL of pure methanol for 30 minutes at 120 rpm using a magnetic stirrer plate. The extracts were then maintained for 24 hours at 4°C, filtered through Whatman No. 4 filter paper, and evaporated under vacuum to dryness. They were subsequently stored at 4°C, until analyzed.
2.3. The determination of the total phenolic content
The total phenolic content was assayed using Folin–Ciocalteu reagent, following Singleton’s method, which was slightly modified by Dewanto et al [13]. An aliquot (0.125 mL) of a suitable diluted methanolic extract (0.25 mg/mL) was added to 0.5 mL of deionized water and 0.125 mL of the Folin–Ciocalteu reagent. The mixture was shaken and allowed to stand for 6 minutes before adding 1.25 mL of 7% sodium carbonate (Na2CO3) solution. The solution was then adjusted with deionized water to a final volume of 3 mL and mixed thoroughly. After incubation for 90 minutes at 23°C, the absorbance of this solution versus a prepared blank was read at 760 nm. The total phenolic content of the organ extracts (i.e., three replicates per treatment) was expressed as milligrams gallic acid equivalent (GAE) per gram of dry weight (mg GAE/g DW) through a calibration curve with gallic acid. The calibration curve range was 50–400 mg/mL (R2 = 0.99).
2.4. Determination of the total flavonoid content
The total flavonoid content was measured by a colorimetric assay, based on the method described by Dewanto et al [13]. An aliquot (250 μL) of the appropriately diluted plant extract was mixed with 75 μL sodium nitrite (NaNO2; 5%). After 6 minutes, we added 150 μL of 10% aluminum chloride. Five minutes later, 500 μL of sodium hydroxide (NaOH; 1M) was added to the mixture. The mixture was finally adjusted to 2.5 mL with distilled water. The absorbance of the mixture versus a prepared blank was read at 510 nm. The total flavonoid content of flowers (i.e., three replicates per treatment) was expressed as mg catechin equivalent (CE)/g DW through the calibration curve with catechin. The calibration curve range was 50–500 mg/mL.
2.5. The determination of the condensed tannin content
The condensed tannins (i.e., proanthocyanidins) were analyzed by the method of Sun et al [14]. An aliquot of 50 μL of the extract appropriately diluted, was added to 3 mL of 4% methanol vanillin solution and 1.5 mL of sulfuric acid (H2SO4). After 15 minutes, the absorbance was measured at 500 nm. The condensed tannin content of the extracts was expressed as mg CE/g DW through the calibration curve with catechin. The calibration curve range was 50–600 mg/mL.
2.6. Identification of phenolic compounds using reverse-phase high-performance liquid chromatography
The obtained methanolic extracts was filtered through a 0.45 μm membrane filter and injected into a high-performance liquid chromatography (HPLC) system. The phenolic compounds were analyzed by using a reverse-phase-HPLC system (RP-HPLC; Agilent Technologies 1100 Series; Agilent Technologies, Santa Clara, California, USA) that was coupled with an ultraviolet/visible spectrum multiwavelength detector. The separation was performed on a 250 mm × 4.6 mm, 4 μm Hypersil ODS C18 reversed-phase column (Thermo Fisher Scientific Inc., Waltham, MA, USA) at ambient temperature. The mobile phase consisted of acetonitrile (Solvent A) and water with 0.2% sulfuric acid (Solvent B). The flow rate was maintained at 0.5 mL/min. The gradient program was as follows: 15% A/85% B for 0–12 minutes; 40% A/60% B for 12–14 minutes; 60% A/40% B for 14–18 minutes; 80% A/20% B for 18–20 minutes; 90% A/10% B for 20–24 minutes, and 100% A 24–28 minutes.
2.7. Evaluation of the total antioxidant capacity
The assay is based on the reduction of molybdenum (VI) [Mo (VI)] to Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acid pH. An aliquot of the sample extract was combined with 1 mL of a reagent solution (0.6M sulfuric acid, 28mM sodium phosphate, and 4mM ammonium molybdate). The mixture was incubated in a thermal block at 95°C for 90 minutes. After the mixture had cooled to room temperature, the absorbance of each solution was measured at 695 nm against a blank [15]. The antioxidant capacity was expressed as mg GAE/g DW. All samples were analyzed in three replications.
2.8. Scavenging ability on the 2,2-diphenyl-1-picrylhydrazyl radical
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging ability of the organ extracts was measured by the method of Hanato et al [16]. One milliliter of the extract at known concentrations was added to 0.25 mL of 0.2 mmol/L DPPH methanolic solution. The mixture was shaken vigorously, and then allowed to stand at room temperature for 30 minutes in the dark. The absorbance was then measured at 517 nm. The antiradical activity was expressed as IC50 (μg/mL), which is the concentration required to cause a 50% inhibition. The ability to scavenge the DPPH radical was calculated using the following equation:
in which A0 is the absorbance of the control at 30 minutes, and A1 is the absorbance of the sample at 30 minutes. Butylated hydroxytoluene (BHT) was used as a positive control. The samples were analyzed in triplicate.
2.9. Iron-reducing power
The reducing power was assessed using the method of Oyaizu [17]. An aliquot of 1 mL was mixed with 2.5 mL of a 0.2M sodium phosphate buffer (pH = 6.6) and 2.5 mL of 1% potassium ferricyanide (K3Fe(CN)6), and incubated in a water bath at 50°C for 20 minutes. Thereafter, 2.5 mL of 10% trichloroacetic acid were added to the mixture, which was centrifuged at 650 g for 10 minutes. The supernatant (2.5 mL) was then mixed with 2.5 mL distilled water and 0.5 mL of 0.1% ferric chloride solution. The intensity of the blue-green color was measured at 700 nm. The EC50 value (mg/mL)—the extract concentration at which the absorbance was 0.5 for the reducing power—was calculated from the graph of absorbance at 700 nm against the extract concentration. Ascorbic acid was used as the positive control.
2.10. Screening of the antibacterial activity
The antibacterial activity of the extracts (leaves, stems, and flowers) was assessed by the agar disk diffusion assay [18] against three human pathogenic bacteria: Gram positive bacteria, which included Staphylococcus aureus (ATCC 25923) (Collection National Institut of Neurologie, Tunisia), and Gram negative bacteria, which included Escherichia coli (ATCC 35218), and Pseudomonas aeruginosa (ATCC 27853). The bacterial strains were first grown on Muller Hinton medium at 37°C for 24 hours before seeding onto the nutrient agar. One or several colonies of the indicator bacteria were transferred into active pharmaceutical ingredient (API) suspension medium (BioMérieux, Hazelwood, MO, USA) and adjusted to the 0.5 McFarland turbidity standard with a Densimat (BioMérieux). A sterile filter disc, 6 mm in diameter (Whatman paper No. 3), was placed on the infusion agar seeded with bacteria and 50 μL of several extract concentrations were dropped onto each paper disc, representing 5 mg per disc. The treated Petri dishes were incubated at 37°C for 24 hours. Antibacterial activity was assessed by measuring the zone of growth inhibition surrounding the discs. Standard discs of gentamycin (10 UI) served as positive antibiotic controls according to CASFM 2005 guidelines.
3. Results and discussion
3.1. Total polyphenol, flavonoid, and condensed tannin content
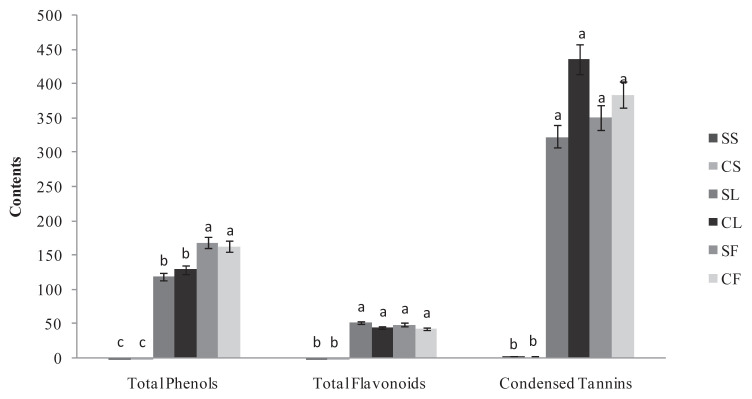
The results in Figure 1 indicated that the total phenolic content was more organ- than provenance-dependent and varied 0.2–168.91 mg of GAE/g DW. Of the two provenances, flowers were the richest organ for polyphenols. The levels were high but not significantly different between the provenances. In fact, the values were 168.91 mg CE/g DW and 163.26 mg CE/g DW for the wild and cultivated Ruta, respectively. This phenomenon was also noted for the leaves, which presented a high but not significantly different content of polyphenols at 129.69 mg CE/g DW and 119.2 mg CE/g DW for the wild and cultivated Ruta, respectively. By contrast, the stems of the two Ruta exhibited weak content. The two Ruta provenances exhibited no significant differences in the flavonoid and tannin content of their leaves and flowers s (Figure 1). In fact, the flavonoid content was high and approximately 50 mg CE/g DW. Moreover, for the two Ruta provenances, the leaves and flowers were particularly rich in condensed tannin (Figure 1) with the highest content in the cultivated Ruta leaves at 435.89 mg EC/g DW. The content was approximately 350 mg EC/g DW in the cultivated flowers and in the spontaneous leaves and flowers. For the total polyphenols, the stems of the two provenances showed weak flavonoid and tannin content. For the leaves, the polyphenol and flavonoid content in our experiment were close to the content reported by Fakhfakh et al [8] in the Tunisian wild Ruta. However, compared with other Ruta species, the Tunisian R. chalepensis seems to be a rich source of phenolics. In fact, a lower content of polyphenols was reported in R. graveolens shoots (37 mg GAE/g DW [19]) and leaves (4.3 mg GAE/g DW, [20]), and in R. montana extracts (3.13 mg GAE/g DW, [21]). Independent of the provenance, the variation of polyphenol, flavonoid, and tannin concentrations in our experiment between R. chalepensis organs suggests that phenolic production in the plant is primarily allocated to the reproductive organ (i.e., the flowers) and secondarily to the leaves. Moreover, our results demonstrated that the wild and cultivated Ruta exhibited similar phenolic contents. This finding is in accordance with Vogel et al [22]. Our results indicate that Ruta is suitable for cultivation and that its cultivation under a protected environment does not alter the production of secondary metabolites such as polyphenols, flavonoids, and condensed tannins.
Figure 1.
The total phenol (mg GAE/g DW), total flavonoid (mg CE/g DW) and condensed tannin (mg CE/g DW) contents in spontaneous and cultivated parts (i.e., stems, leaves, and flowers) from Tunisian Ruta chalepensis. The values are presented as the mean of three replicates ± the standard deviation. The data marked with different letters share significance at p < 0.05 (based on the Duncan test). CE ¼ catechin equivalent; CF ¼ cultivated flowers; CL ¼ cultivated leaves. CS: cultivated stems; DW: dry weight; GAE: gallic acid equivalent; SF: spontaneous flowers; SL: spontaneous leaves; SS: spontaneous stems.
3.2. Identification of phenolic compounds using RP-HPLC
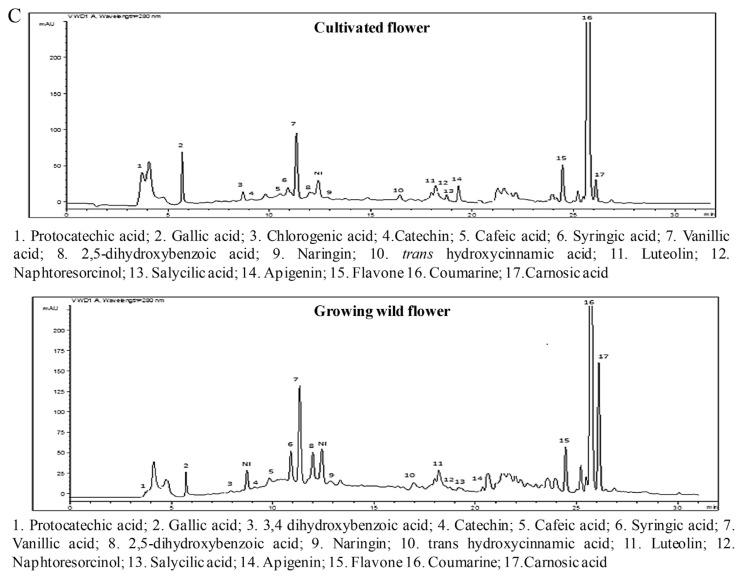
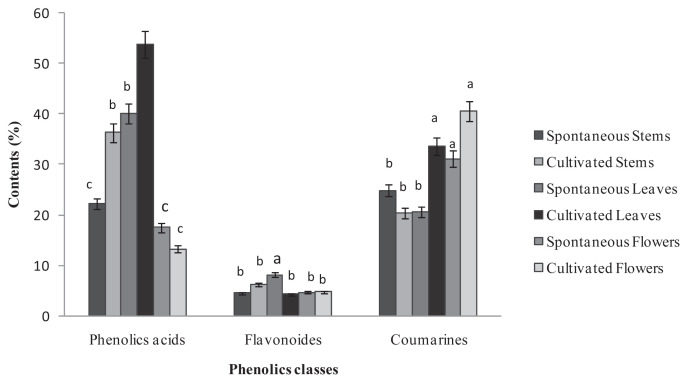
Twenty phenolic compounds were tentatively identified by HPLC analysis in the different parts of the two Ruta provenances (Figure 4) and included 12 phenolic acids with three hydroxycinnamic acids (i.e., caffeic, trans-hydroxycinnamic, and ferulic acid), and nine hydroxybenzoic acids (i.e., protocatechuic acid, 3,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, gallic acid, chlorogenic acid, syringic acid, carnosic acid, salicylic acid, and vanillic acid). In addition, seven flavonoids were identified: apigenin, catechin hydrate, flavone, luteolin, naringin, kaempferol, and naphthoresorcinol. The phenolic acids and coumarin represented the major classes of phenols in the two Ruta provenances organs, whereas the flavonoids were weakly represented with percentages that did not exceed 10% of the total phenols (Figure 2). A comparison of the two provenances revealed that stems and leaves of the cultivated Ruta exhibited significantly higher phenolic acid levels than the wild Ruta. Moreover, higher coumarin levels were encountered in the leaves and the flowers of cultivated Ruta, compared with the wild Ruta. The stems of cultivated Ruta and the leaves of cultivated and spontaneous Ruta were characterized by the predominance of vanillic acid (Table 1). Moreover, the extracts of spontaneous and cultivated Ruta stems and flowers were particularly rich in coumarin (Table 1). In accordance with our results, Al-Said et al [23] showed that the extract of the aerial parts of R. chalepensis contain a high quantity of coumarins, flavonoids, and tannins. On the other hand, our results suggested that coumarin can confer an important value to Ruta flowers and stems, which may therefore be an alternative commercial source of natural coumarins, whereas Ruta leaves can be a natural source of vanillic acid. Analysis of the phenolic composition indicated that kaempferol was detected only in cultivated Ruta stems and leaves, and naphthoresorcinol and luteolin were detected only in the stems and leaves of spontaneous Ruta (Table 1). The variation of phenolic compounds between the two Ruta provenances could be attributed to the effect of climate and location [24]. On the other hand, variations were observed between different Ruta parts; for example, ferulic acid was detected in stems and leaves, but absent in flowers; salicylic acid was detected in the stems and flowers, but absent in the leaves. The variations could be related to morphological differentiation occurring during the phenological cycle.
Figure 4.
A. Reverse-phase high-performance liquid chromatography (RP-HPLC) chromatographic profiles of the phenolic compounds in cultivated and wild-grown Ruta chalepensis stem extract monitored at 280 nm. B. The RP-HPLC chromatographic profiles of the phenolic compounds of cultivated and wild-grown R. chalepensis leaf extract monitored at 280 nm. C. The RP-HPLC chromatographic profiles of the phenolic compounds of cultivated and wild-grown R. chalepensis flower extract monitored at 280 nm.
Figure. 2.
Percentage of chemical classes of phenolic compounds in extracts of the spontaneous and cultivated parts (i.e., stems, leaves, and flowers) from Tunisian Ruta chalepensis. The values are presented the mean of three replicates ± the standard deviation. The data marked with different letters share significance at p < 0.05 (based on the Duncan test).
Table 1.
The percentages of phenolic compound of different Tunisian Ruta chalepensis parts.
| SS | CS | SL | CL | SF | CF | |
|---|---|---|---|---|---|---|
| Protocatechuic acid | — | 7.27 ± 0.75a | 0.63 ± 0.73c | 2.57 ± 0.35b | 0.88 ± 0.06c | 3.46 ± 0.53b |
| Gallic acid | 3.4 ± 0.77a | 4.27 ± 0.01a | — | 2.1 ± 0.03b | 0.82 ± 1.31c | 1.79 ± 0.11b |
| 3,4 Dihydroxybenzoic acid | 0.49 ± 0.85a | 0.19 ± 0.12b | 0.12 ± 0.99b | 0.06 ± 0.02c | 0.14 ± 1.02b | — |
| Chlorogenic acid | 5.41 ± 0.01a | 0.41 ± 0.32b | 0.71 ± 0.12b | 0.54 ± 0.56b | — | 0.5 ± 0.26b |
| Catechin hydrate | 1.09 ± 1.13c | 1.09 ± 0.01c | 5.23 ± 0.57a | 2.84 ± 0.11b | 0.09 ± 0.99d | 0.11 ± 0.45d |
| Caffeic acid | 1.52 ± 0.16b | 2.01 ± 0.02b | 0.5 ± 1.14c | 4.08 ± 0.36a | 0.52 ± 0.53c | 0.49 ± 0.28c |
| Syringic acid | 1.56 ± 0.02b | 3.76 ± 0.05a | 2.54 ± 0.20b | 4.25 ± 0.25a | 2.01 ± 0.64b | 0.95 ± 0.98c |
| Vanillic acid | 5.21 ± 0.15d | 14.25 ± 0.02c | 32.95 ± 1.23b | 38.97 ± 1.27a | 4.99 ± 0.08d | 3.57 ± 0.84d |
| Naringin | 0.8 ± 0.98c | 3.23 ± 0.65a | 1.68 ± 1.04b | 0.98 ± 0.04c | 0.43 ± 0.94c | 0.22 ± 0.63d |
| Ferulic acid | 1.69 ± 0.25a | 0.72 ± 0.65b | 0.26 ± 0.9b | 0.4 ± 0.21b | — | — |
| trans-Hydroxycinnamic acid | 1.16 ± 0.30a | 0.74 ± 0.3b | 0.25 ± 1.92b | 0.12 ± 0.02b | 0.16 ± 0.71b | 0.46 ± 0.01b |
| Luteolin | 0.67 ± 1.12b | — | 0.34 ± 0.12b | — | 1.73 ± 0.43a | 1.47 ± 0.17a |
| Kaempferol | — | 0.83 ± 1.15a | — | 0.1 ± 0.05b | — | — |
| Naphtoresorcinol | 0.63 ± 0.35a | — | 0.19 ± 0.15b | — | 0.22 ± 0.27b | 0.22 ± 0.44b |
| Apigenin | 0.29 ± 0.65b | 0.9 ± 0.06a | 0.26 ± 0.4b | 0.25 ± 0.91b | 0.14 ± 0.16b | 0.87 ± 0.02a |
| Flavone | 1.07 ± 0.01b | 0.21 ± 1.07c | 0.48 ± 0.62c | 0.13 ± 0.52c | 2.08 ± 0.04a | 1.93 ± 0.55a |
| Coumarin | 24.88 ± 0.85c | 20.38 ± 0.08d | 20.57 ± 0.11d | 33.53 ± 0.07b | 31.09 ± 0.31b | 40.54 ± 0.39a |
| Carnosic acid | 0.37 ± 0.75c | 0.71 ± 0.11c | 1.34 ± 0.01b | — | 5.88 ± 0.22a | 1.18 ± 0.22b |
| 2,5 Dihydroxybenzoic acid | 1.4 ± 0.32a | 1.78 ± 0.15a | 0.69 ± 0.02b | 0.58 ± 0.65b | 1.91 ± 0.72a | 0.43 ± 0.19b |
| Salicylic acid | — | 0.17 ± 0.09b | — | — | 0.21 ± 0.29b | 0.43 ± 1.05a |
| TPI | 51.64 | 62.92 | 68.74 | 91.5 | 53.3 | 58.62 |
CF = cultivated Ruta flowers; CL = cultivated Ruta leaves; CS = cultivated Ruta stems; SF = spontaneous Ruta flowers; SL = spontaneous Ruta leaves; SS = spontaneous Ruta stems; TPI = represent the total phenols identified. Values with different superscripts (a–d) are significantly different at p < 0.05 (means of three replicates).
3.3. Evaluation of total antioxidant capacity and scavenging ability on DPPH radical and iron-reducing power
Several methods are commonly used to measure the antioxidant capacity of extracts. Each method results in the generation of or uses a different radical that is directly involved in the oxidative process through a variety of mechanisms. No single assay can represent the total antioxidant capacity, and for this reason three different and complementary assays were used to evaluate the extract antioxidant activities: total antioxidant capacity, DPPH free radical scavenging, and the reducing power.
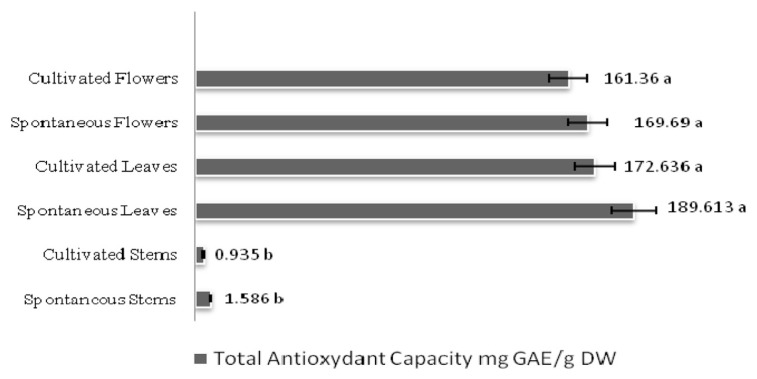
As shown in Figure 3, the total antioxidant capacity of R. chalepensis was high in the leaves and flowers of the two provenances with significantly lower levels in stems: 189.61 mg GAE/g DW and 172.63 61 mg GAE/g DW for spontaneous and cultivated Ruta leaves, respectively, and 169.6 mg GAE/g DW and 161.36 mg GAE/g DW for spontaneous and cultivated Ruta flowers, respectively. For the total antioxidant activity, spontaneous and cultivated Ruta leaf and flower extracts both had higher scavenging activity than the stem extracts (Table 2). This radical scavenging ability was high with IC50 values ranging 23.73–30.69 μg/mL. Furthermore, the scavenging ability of the spontaneous flower extract (23.73 μg/mL) was higher than that of BHT (25μg/mL).
Figure 3.
The total antioxidant capacity in spontaneous and cultivated parts (i.e., stems, leaves, and flowers) from Tunisian Ruta chalepensis. The values are presented as the mean of three replicates ± the standard deviation. The data marked with different letters share significance at p < 0.05 (based on the Duncan test).
Table 2.
The antioxidant activities of the methanolic extract from different Tunisian Ruta chalepensis parts.
| Reducing power (EC50 mg/mL) | DPPH (IC50 μg/mL) | |
|---|---|---|
| Spontaneous stems | 1.96 ± 5.492a | 79.47 ± 0.192b |
| Cultivated stems | 2.05 ± 1.786a | 80.77 ± 0.150a |
| Spontaneous leaves | 0.90 ± 6.527c | 30.69 ± 0.041c |
| Cultivated leaves | 1.71 ± 6.008b | 28.89 ± 0.171c |
| Spontaneous flowers | 1.10 ± 1.372c | 23.73 ± 0.1c |
| Cultivated flowers | 0.92 ± 0.113c | 28.48 ± 0.06c |
| BHT | — | 25 ± 0.2c |
| Ascorbic acid | 40.10−3 ± 0.13d | — |
EC50 = the effective concentration at which the absorbance is 0.5 for reducing power; IC50 = the concentration at which DPPH radicals are scavenged by 50%.
The values are presented as the mean ± the standard deviation (n = 3). Mean values with different letters within a row are significantly different (p < 0.05).
In the reducing power assay (Table 2), spontaneous Ruta leaf and cultivated Ruta leaf and flower extracts demonstrated the highest reducing power (0.90 mg/mL, 0.92 mg/mL, and 1.10 mg/mL, respectively), followed by the stem extracts (1.96 mg/mL and 2.05 mg/mL for the spontaneous and cultivated provenances, respectively). These results demonstrated that R. chalepensis extracts exhibited a variable activity to reduce iron as a function of the plant part and provenances studied.
3.4. Correlations
The results of the total phenols (TP), total flavonoids (TF), total condensed tannins (CT) analysis and the different antioxidant assays used in the present investigation were compared and correlated with each other. Correlations between the results of different assays are shown in Table 3. Significant positive correlations (R2 = 0.66–0.97) were observed between the total phenolic content and values for total antioxidant and DPPH scavenging activities of the leaves and flowers of the two Ruta origins. Moreover, total phenolics were highly correlated with the reducing power in the spontaneous flowers (R2 = 0.96) and cultivated flowers (R2 = 0.99), indicating the significant contribution of phenolics to these antioxidant assays. Furthermore, the strong correlations (R2 = 0.95–0.99) between the total antioxidant activity and DPPH scavenging activity and condensed tannin content of the spontaneous and cultivated Ruta leaves suggest that the high antioxidant capacities of these organs may in large part result in the contribution of these compounds. For the spontaneous flowers, the free radical scavenging activity was closely correlated with the flavonoid content (R2 = 0.99).
Table 3.
The correlation between antioxidant assays and total phenol, flavonoid, and tannin contents.
| SS | CS | SL | CL | SF | CF | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||||||||
| TAC | IC50 | CE50 | TAC | IC50 | CE50 | TAC | IC50 | CE50 | TAC | IC50 | CE50 | TAC | IC50 | CE50 | TAC | IC50 | CE50 | |
| TP | 0.99 | 0.54 | 0.32 | 0.75 | 0.87 | 0.53 | 0.80 | 0.77 | 0.61 | 0.97 | 0.82 | 0.43 | 0.97 | 0.98 | 0.96 | 0.87 | 0.66 | 0.99 |
| TF | 0.02 | 0.69 | 0.96 | 0.82 | 0.92 | 0.62 | 0.80 | 0.77 | 0.62 | 0.63 | 0.77 | 0.48 | 0.99 | 0.96 | 0.98 | 0.78 | 0.75 | 0.96 |
| CT | 0.62 | 0.81 | 0.10 | 0.25 | 0.97 | 0.1 | 0.99 | 0.99 | 0.97 | 0.95 | 0.99 | 0.43 | 0.67 | 0.75 | 0.69 | 0.30 | 0.25 | 0.89 |
CF = Cultivated flower; CL = Cultivated leaf; CS = Cultivated stem; CT = condensed tannins; EC50 = Reducing power; IC50 = 2,2-diphenyl-1-picrylhydrazyl scavenging activity; SF = Spontaneous flower; SL = Spontaneous leaf; SS = Spontaneous stem; TAC = Total antioxidant capacity; TF = total flavonoids; TP = total phenols.
Investigation of correlations between the percentage of coumarin, the main phenolic compound in the Ruta organs, and the different antioxidant assays indicate that samples of both spontaneous and cultivated Ruta leaves and flowers showed a linear correlation between the content of coumarin and total antioxidant activity, DPPH scavenging ability, and reducing power (R2 = 0.76–0.99). These results indicate that coumarin is the major contributor to the antioxidant activities of extracts from the leaves and the flowers of the two Ruta origins. Thus, antioxidant activity of extracts from different parts of R. chalepensis may be primarily explained by their phenolic composition. Coumarin and vanillic acid were the major components identified in R. chalepensis and are expected to quench DPPH radicals.
3.5. Screening for antibacterial activity
The emergence of multidrug resistance in human and animal pathogenic bacteria and undesirable adverse effects of certain antibiotics have triggered immense interest in the search for new antimicrobial drugs of plant origin. In the present study, methanolic extracts of different R. chalepensis parts have been tested against drug-resistant bacteria and pathogenic yeast, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. The antimicrobial activity of the extracts and their potency were quantitatively assessed by the presence or absence of an inhibition zone and zone diameter, respectively, as presented in Table 4. The results presented in Table 4 show that the extracts from the wild and cultivated Ruta organs exhibit marked antibacterial activities. Spontaneous Ruta stem extract were particularly effective against S. aureus and P. aeruginosa with diameters of inhibition of 16.3 mm and 17.7 mm, which was close to that of the standard antibiotic gentamycin. Moreover, cultivated Ruta stem extract exhibited the highest antibacterial activity against Escherichia coli.
Table 4.
Antibacterial activity of the extract from Tunisian Ruta chalepensis parts against human pathogenic bacteria.
| Inhibition zone diameter | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Gram positive bacteria | Gram negative bacteria | |||||
|
|
|
|||||
| Staphylococcus aureus | Pseudomonas aeruginosa | Escherichia coli | ||||
| Spontaneous stems | 16.3 ± 0.6b | ++ | 17.7 ± 0.4a | ++ | 13.3 ± 1.1c | + |
| Cultivated stems | 14.7 ± 1.2b | ++ | 7.7 ± 0.2c | − | 17.3 ± 0.3a | + |
| Spontaneous leaves | 15.3 ± 1.2b | ++ | 16.7 ± 0.6a | ++ | 14.3 ± 0.4c | ++ |
| Cultivated leaves | 12.3 ± 1.5b | + | 9.7 ± 1.3c | + | 16.3 ± 0.6a | ++ |
| Spontaneous flowers | 15 ± 0.6b | ++ | 16.3 ± 1.1a | ++ | 15.7 ± 0.9b | ++ |
| Cultivated flowers | 16 ± 1a | ++ | 15 ± 0.5b | ++ | 13 ± 1.2c | + |
| Gentamycin | 24–28.5 | +++ | 15.5–22.5 | +++ | 22–26.1 | +++ |
“−” = Ø < 8 mm; “+” = 9 mm < Ø < 14 mm; “++” = 15 mm < Ø < 19 mm; “+++” = Ø < 20 mm.
The data are presented as the mean ± the standard deviation.
The values are presented as the mean of three replicates ± the standard deviation. The data marked with different letters share significance at p < 0.05 (based on the Duncan test).
On the other hand, the efficiency of these extracts against the bacteria could in part be because of their phenolic composition. In fact, several studies attributed the inhibitory effect of plant extracts against bacterial pathogens to their phenolic composition [25,26]. The inhibitory effect of phenolic compounds could be explained by adsorption to cell membranes, interaction with enzymes, substrate and metal ion deprivation [27]. Moreover, comparison of the antibacterial ability between cultivated and spontaneous Ruta organs revealed that the activity was strain- and origin-dependent. Spontaneous and cultivated Ruta organs possess similar phenolic and flavonoid and tannin contents; therefore, the differences in the antibacterial activity could be because of the presence of specific antibacterial compounds, in addition to the phenolic compounds.
4. Conclusion
Our results indicated that the wild and cultivated R. chalepensis organs had similar contents of polyphenols, flavonoids, and tannins. Moreover, the organs were rich on vanillic acid and coumarin with the highest levels in the cultivated leaves and flowers, respectively. The results indicated that the leaves and flowers of the cultivated and wild-grown Ruta possess strong antioxidant and antibacterial activities with no significant differences. The cultivation of Ruta is easy; therefore, the leaves and flowers of this plant could be a prospective source of natural bioactive molecules that could replace synthetic antioxidants and serve as a potential source of antibacterial agents in the food industry.
Footnotes
Conflicts of interest
All contributing authors have no conflicts of interest to declare.
REFERENCES
- 1. El-Ghorab AH, Shibamoto T, Ozcan M. Chemical composition and antioxidant activities of buds and leaves of capers (Capparis ovata Desf. Var. Canesencene) cultivated in Turkey. J Essent Oil Res. 2007;19:72–7. [Google Scholar]
- 2.Smid EJ, Gorris LGM. Natural antimicrobials for food preservation. In: Rahman MS, editor. Handbook of food preservation. New York: Marcel Dekker; 1999. pp. 285–308. [Google Scholar]
- 3. Pollio A, De Natale A, Appetiti E, Aliotta G, Touwaide A. Continuity and change in the Mediterranean medical tradition: Ruta spp. (Rutaceae) in Hippocratic medicine and present practices. J Ethnopharmacol. 2008;116:469–82. doi: 10.1016/j.jep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 4. Pieroni A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province. Italy. J Ethnopharmacol. 2000;70:235–73. doi: 10.1016/s0378-8741(99)00207-x. [DOI] [PubMed] [Google Scholar]
- 5. Aguilar-Santamaria L, Tortoriello J. Anticonvulsant and sedative effects of crude extracts of Ternstroemia pringlei and Ruta chalepensis. Phytother Res. 1995;10:531–3. [Google Scholar]
- 6. Ali-Shtayeh MS, Abu Ghdeib SI. Antifungal activity of plant extracts against dermatophytes. Mycoses. 1990;42:665–72. doi: 10.1046/j.1439-0507.1999.00499.x. [DOI] [PubMed] [Google Scholar]
- 7. Almog S, Kurnik D, Shimoni A, Loebstein R, Hassoun E, Gopher A, Halkin H, Nagler A. Linearity and stability of intravenous busulfan pharmacokinetics and the role of glutathione in busulfan elimination. Biol Blood Marrow Transplant. 2011;17:117–23. doi: 10.1016/j.bbmt.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 8. Fakhfakh N, Zouari S, Zouari M, Loussayef C, Zouari N. Chemical composition of volatile compounds and antioxidant activities of essential oil, aqueous and ethanol extracts of wild Tunisian Ruta chalepensis L. (Rutacea) J Med Plants Res. 2012;6:593–600. [Google Scholar]
- 9. Al-Bakri AG, Afifi FU. Evaluation of antimicrobial activity of selected plant extracts by rapid XTT colorimetry and bacterial enumeration. J Microbiol Methods. 2007;68:19–25. doi: 10.1016/j.mimet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 10. Fuller DQ, Allaby R. Seed dispersal and crops domestication: shattering, germination and seasonality in evolution under cultivation. Annu Plant Rev. 2009;38:238–95. [Google Scholar]
- 11. Economal C, Demetzos C, Anastassak T, Papazoglou V, Gazouli M, Loul A, Thanosa CA, Harvala C. Volatile constituents of bracts and leaves of wild and cultivated Origonum dictamnus. Planta Med. 1999;65:189–91. doi: 10.1055/s-2006-960466. [DOI] [PubMed] [Google Scholar]
- 12. Luseba D, Letsoalo ME, Katerere DA. Comparative study of antibacterial activities of wild and cultivated plants used in ethnoveterinary medicine. Afr J Biotechnol. 2011;10:7058–62. [Google Scholar]
- 13. Dewanto V, Wu X, Adom K, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–4. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 14. Sun B, Richardo-da-Silvia JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 1998;46:4267–74. [Google Scholar]
- 15. Falleh H, Oueslati S, Guyot S, Ben Dali A, Magné C, Abdelly C, Ksouri R. LC/ESI MS/MS characterisation of procyanidins and propelargonidins responsible for the strong antioxidant activity of the edible halophyte Mesembryanthemum edule L. Food Chem. 2011;127:1732–8. [Google Scholar]
- 16. Hanato T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effect. Chem Pharmacol Bull. 1988;36:1090–7. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- 17. Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction. Japan J Nut. 1986;44:307–15. [Google Scholar]
- 18. Bagamboula M, Uyttendaele J, Debevere M. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2000;21:33–42. [Google Scholar]
- 19. Diwan R, Shinde A, Malpathak N. Phytochemical composition and antioxidant potential of Ruta graveolen L. in vitro culture lines. J Bot. 2012;17:1–6. [Google Scholar]
- 20. Proestos C, Komaitis M. Ultrasonically assisted extraction of phenolic compounds from aromatic plants: comparison with conventional extraction technics. J Food Qual. 2006;29:567–82. [Google Scholar]
- 21. Djeridane A, Yousf M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–60. [Google Scholar]
- 22. Vogel H, Jeldres P, Razmilic I, Doll U. Morphological characters, yields and active principles in wild and cultivated accessions of the Chilean medicinal plant Buddleja globosa Hope. Ind Crops Prod. 2011;34:1322–6. [Google Scholar]
- 23. Al Said M, Tariq M, Al-Yahya MA, Rafatullah S, Ginnawi OT, Ageel AM. Studies on Ruta chalepensis, an ancient medicinal herb still used in traditional medicine. J Ethnopharmacol. 1990;28:305–12. doi: 10.1016/0378-8741(90)90081-4. [DOI] [PubMed] [Google Scholar]
- 24. Ye XQ, Chen JC, Liu DH, Jiang P, Shi J, Xue S, Wua D, Xu JG, Kakuda Y. Identification of bioactive composition and antioxidant activity in young mandarin fruits. Food Chem. 2011;124:1561–6. [Google Scholar]
- 25. Baydar NG, Özkan G, Sagdiç O. Total phenolic contents and antibacterial activities of grapes (Vitis vinifera L.) extracts. Food Control. 2004;15:335–9. [Google Scholar]
- 26. Vaquero MR, Alberto MR, de Nadra MM. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007;18:93–101. [Google Scholar]
- 27. Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–83. [Google Scholar]