Abstract
One new naturally isoflavone compound, 5,7,2′,3′,4′ penta hydroxyl isoflavone-4′-O-β-glucopyranoside (1) was isolated from the aqueous methanol extract (AME) of Pulicaria undulata subsp. undulata, together with seven known compounds: kaempferol (2), kaempferol 3-O-β-glucoside (3), quercetin (4), quercetin 3-O-β-glucoside (5), quercetin 3-O-β-galactoside (6), quercetin 3,7-di OCH3 (7), and caffeic acid (8). Their structures were established through chemical (acid hydrolysis) and spectral analysis (UV, NMR, and ESIM). The AME and some isolated compounds were evaluated as protective agents. Free radical scavenging using a microscaled 2,2-diphenyl-1-picrylhydrazyl assay was used to assess the direct antioxidant properties that were evaluated by the ability to protect murine Hepa1c1c7 liver cells against damage induced by the organic peroxide tert-butyl hydroperoxide. The neutral red uptake assay (NRU) was used to record the activity. Results of the 2,2-diphenyl-1-picrylhydrazyl assay recorded differential scavenging properties in ascending order: 5,7,2′,3′,4′ penta hydroxyl isoflavone-4′-O-β-glucopyranoside > quercetin > quercetin 3-O-galactoside > caffeic acid > quercetin 3,7-di-OCH3 > kaempferol with 50% inhibitory concentrations of 3.9 μM, 7.5 μM, 11.4 μM, 12.2 μM, 78.1 μM, and 252.3 μM, respectively. The antioxidative potential reveals the potency of AME, quercetin, and quercetin 3,7-di-OCH3. The latter compound showed full protection at 100 μM (33 μg/mL) against the induced toxicant effect where the 50% effective concentration was calculated as 33.6 ± 1.7 μM (11.1 μg/mL). In addition to quercetin, which was extensively shown previously as a cytoprotective agent, AME was less potent; it was capable of protecting 75% at 100 μg/mL with 50% effective concentration of 92.3 ± 4 μg/mL. Moreover, the isolated flavonoids were found to be significantly chemosystematic markers.
Keywords: chemosystematics, DPPH, flavonoids, Pulicaria undulata, TBHP
1. Introduction
Many plant genera belonging to the Asteraceae family are used as herbal medicines and as beverage ingredients in Asian countries, especially in China [1]. Among such plants, the genus Pulicaria Gaertn. (Inuleae: Asteraceae), which consists of 100 species widely distributed from Europe to North Africa and Asia [2]. Previous phytochemical studies on Pulicaria species gave rise to the isolation of flavonoids and phenolics [3–10] mono-, di-, and sesquiterpenes [11–17], essential oils and caryophyllene derivatives [18–21]. The plants of this genus are used in traditional medicine as tonic, antispasmodic, and antihypoglycemic drugs, as well as ingredients of perfumes [17,22]. Also, they have antimicrobial [23–25], antioxidant [25,26], and anticancer [28] properties.
Pulicaria undulata (L.) C.A. Mey. is one of the most widespread desert plants growing wild in Egypt [29,30]. It is known as “Dethdath”, and the flower branches are used for preparing a powerful sneezing powder as an insect repellent and as a herbal tea [29]. Previous studies of P. undulata allowed the isolation of various flavonoids [5,8–10] and sesquiterpenes [13–16].
The present study aimed to evaluate the phenolic constituents of P. undulata (L.) C.A. Mey. in comparison with those previously isolated from P. undulata (L.) Kostel., to find out the chemosystematics relationship between them. In addition, the study aimed to investigate the potential protective activity against oxidative stress.
2. Materials and methods
2.1. General
Nuclear magnetic resonance (NMR) experiments were recorded on a Jeol EX-500 spectroscopy (JOEL Inc., Tokyo, Japan): 500 MHz (1H NMR) and 125 MHz (13C NMR). UV spectra were obtained using Shimadzu model-2401 CP spectrophotometer (Shimadzu Inc., Tokyo, Japan). Electrospray ionization mass spectrometry (ESIMS) spectra were measured on LCQ Advantage Thermo Finnigan spectrometer (Thermo Fisher Scientific Inc., Waltham, MA). Column chromatography (CC) was carried out on a Polyamide 6S (Riedel-De-Haen AG, Seelze Haen AG, Seelze Hanver, Germany) and Sephadex LH-20 (Pharmazia, Uppsala, Sweden) using methanol/water as eluent. Paper chromatography (PC, descending) Whatman No. 1 mm and 3 mm papers, was performed using solvent systems; water, 15% acetic acid (acetic acid:water, 15:85), BAW (n-butanol:-acetic acid:water, 4:1:5, upper layer) and BBWP (benzene:n-butanol:water:pyridine, 1:5:3:3, upper layer). Complete acid hydrolysis (2N HCl, 2 hours, 100°C) was carried out and followed by paper cochromatography with authentic samples to identify the aglycones and sugar moieties [31]. Authentic samples were obtained from the Department of Phytochemistry and Plant Systematics, National Research Center, Dokki, Giza, Egypt.
2.2. Plant material
The plant material was collected 73 km along the Cairo–Suez desert road in March 2010 (leg. S.R. Hussein, M.M. Marzouk, s.n. 853) and identified according to Boulos [29,30]. A voucher specimen was deposited in the herbarium of the National Research Centre, Dokki, Giza, Egypt (CAIRC).
2.3. Extraction and isolation
Fine-powdered, air-dried whole plant of P. undulata (1.2 kg) was extracted under reflux three times with 70% methanol/water, and then evaporated under reduced pressure and temperature. The extract (86 g) was subjected to a polyamide column (120 cm × 6 cm), eluted with methanol/water mixtures of decreasing polarities to yield five main fractions (I–V). Fraction I was chromatographed on a Sephadex column (70 cm × 2.5 cm) using methanol:water (1:1) for elution yielded compounds 1 (18 mg), 6 (37 mg), and 7 (14 mg). Fraction II was subjected to preparative paper chromatography (PPC) using 15% acetic acid followed by BAW as solvent systems, yielding compounds 3 (21 mg), 4 (15 mg), and 5 (21 mg). Compounds 2 (14 mg) and 8 (22 mg) were obtained from fractions (III–V) with a combination of the Sephadex column (35 cm × 2.5 cm) using methanol:water (1:1) and PPC using 15% acetic acid and BAW as eluents. The purification was achieved on a Sephadex column (35 cm × 1.5 cm) column using methanol as an eluent [31].
2.4. Cell-free antioxidant assay (anti-2,2-diphenyl-1-picrylhydrazyl)
AME and the isolated flavonoids from P. undulata were prepared in dimethyl sulfoxide (DMSO). The method used in the present study was based on a modified procedure [32] based on previously published methods [33]. The AME/compound stock solutions (20 μL/well) were pipetted in triplicate onto flat-bottomed, 96-well plates (Nunc TM, Thermo Fisher Scientific, NY, USA). The assay was started with the addition of 2,2-diphenyl-1-picrylhydrazyl (DPPH) reagent (0.004% weight/volume in methanol, 180 μL/well). Appropriate blanks were prepared using the sample vehicle only (i.e., 20 μL DMSO) in addition to the same amount of DPPH reagent to eliminate any inherent vehicle activity. Negative control (replacing DPPH with methanol) was also run simultaneously to eliminate the optical density (OD) values resulting from colored samples. The plate was immediately shaken for 30 seconds and incubated in the dark for 30 minutes at room temperature. The remaining DPPH was measured in a Fluostar Optima microplate reader (BMG LABTECH GmbH, Ortenberg, Germany) at 540 nm. The percentage of antioxidant activity was calculated using the following equation: percentage of antioxidant activity = (OD(blank) − OD(sample)/OD(blank)) × 100. To determine the 50% inhibitory concentration (IC50; concentration of sample producing 50% scavenging of the DPPH radical present in the blank), the mean percentage scavenging to control were plotted against concentrations, and the resulting plot was fitted to a nonlinear regression curve using GraphPad Prism version 5.0 software (GraphPad Software Inc., San Diego, USA).
2.5. Indirect antioxidant assay (antitert-butyl hydroperoxide cytoprotection assay)
2.5.1. Cell culture
Monolayer cultures of murine hepatoma cell line Hepa1c1c7 (generously provided by Professor M.S. Denison, University of California, CA, USA) were grown in 75-cm2 flasks at 37°C and 5% CO2 in a humidified CO2 incubator (Certomat-20S, Sartorius group, NY, USA). At 75–85% confluence, the monolayer was routinely subcultured using trypsin–versene (EDTA) solution and maintained in α-minimal essential medium supplemented with 10% fetal bovine serum, 2mM l-glutamine and triple antibiotic mixture containing 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 250 ng/mL amphotericin B. All culture reagents were purchased from Lonza, Verviers, Belgium.
2.5.2. Bioassay procedure
We followed the published antitert-butyl hydroperoxide (anti-TBHP) bioassay procedure [32] with some modifications. Hepa1c1c7 (2 × 105 cells/well) were seeded onto 24-well culture plates (Greiner Bio-one, Frickenhausen, Germany) and left to adhere for 24 hours to form semiconfluent monolayers. Monolayers were treated with DMSO (vehicle control, 0.5% volume/volume), AME, or test compounds. In the screening experiment, a single final concentration of 100 μg/mL of the extract or 100μM of the compounds was separately examined; the insufficient amount of compound 1 allowed only the examination of 11.2 μM. Quercetin dihydrate (4 μg/mL) was run in parallel as a positive control for cytoprotection. Treated plates were incubated for 24 hours prior to exposure to 125 μM TBHP as an inducer dose for oxidative cytotoxicity [34]. The neutral red uptake assay (NRU) was performed as the endpoint for cell viability measurement to monitor the cytoprotection produced by tested samples [35]. The sample that was able to protect > 50% of Hepa1c1c7 cells was further subjected to a concentration-dependent response curve to determine its 50% effective concentration (EC50; the concentration that protected 50% of the oxidative induced culture). The obtained data were analyzed using GraphPad Prism. The EC50 values were determined from the average of two independent experiments using nonlinear regression concentration–response curve fit.
3. Results and discussion
3.1. Identification of isolated compounds
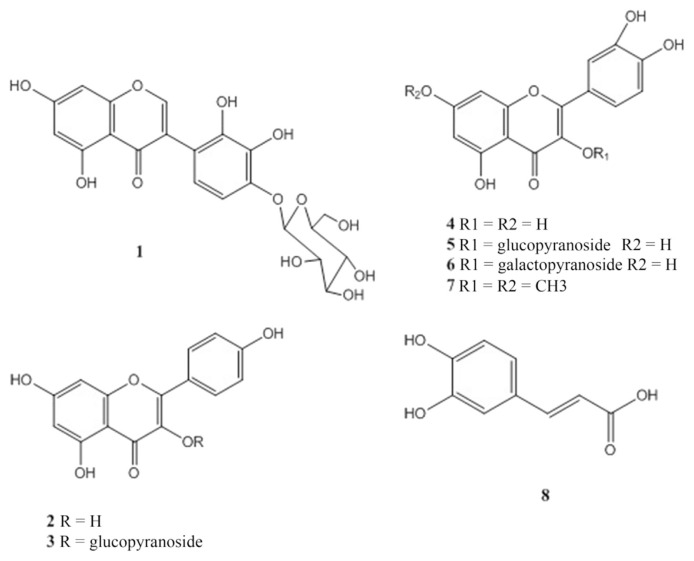
One new naturally isoflavone compound; 5,7,2′,3′,4′ pentahydroxyl isoflavone-4′-O-β-glucopyranoside (1) was isolated and identified, together with seven known compounds: kaempferol (2), kaempferol 3-O-β-glucoside (3), quercetin (4), quercetin 3-O-β-glucoside (5), quercetin 3-O-β-galactoside (6), quercetin 3,7-di OCH3 (7), and caffeic acid (8) (Figure 1). Their purity was determined using high-performance liquid chromatography by calculating the percentage of peak area in relation to the total area of peaks and ranged from 98% to 99%. Their chemical structures were established through chemical and spectral analyses. Compounds 1–3 and 6–8 were isolated for the first time from this species. The chemical structures of the known compounds were determined by complete acid hydrolysis, UV, one-dimensional NMR and mass spectrometry techniques and confirmed by comparing their spectral data with those from the literature [36,37].
Figure 1.
Chemical structure of compounds 1–8. 1 =5,7,2′,3′,4′ penta hydroxyl isoflavone-4′-O-β-glucopyranoside; 2 =kaempferol; 3 =kaempferol 3-O-β-glucoside; 4 =quercetin; 5 =quercetin 3-O-β-glucoside; 6 =quercetin 3-O-β-galactoside; 7 =quercetin 3,7-di OCH3; 8 = caffeic acid.
Compound 1 was isolated as colorless crystals. Negative ESIMS analysis showed a molecular ion peak at 463.2 m/z (C21H20O13). Complete acid hydrolysis (2N HCl, 1 hour, 100°C) revealed the presence of an isoflavones aglycone (UV, 1H NMR) and glucose as the sugar moiety [Co-PC]. The UV, λmax/nm, methanol (262,294 sh, 332 sh), 1H NMR (δ 8.59, s, for H-2) and 13C NMR (156.9 for C-2 and 125.2 for C-3) were consistent with an isoflavone skeleton. In addition, the 1H NMR spectra showed two meta-couplings (J = 1.5 Hz) of Ring A at δ 6.09 and δ 6.27 for H-6 and H-8, respectively. The aromatic protons of the B ring appeared as two doublets with ortho-coupling (J = 8.5 Hz) at δ 6.77 and δ 7.18 assigned to H-6′ and H-5′, respectively. The anomeric proton signal resonated at δ5.09 (J = 7.5 Hz) indicated the β configuration of the glucopyranose unit [36]. The heteronuclear multiple-bond correlation spectroscopy spectrum confirmed the isoflavone structure [38]; H-2 (δ 8.59) showed cross-correlations with C1′ (115.9), C4 (177.6), and C9 (159). H-6′ showed three-bond heteronuclear multiple-bond correlation spectroscopy correlations; with C-3 (156.9), C-2′ (145.2), and C4′ (148.9). Further correlation between H-1″ (δ 5.09) and C-4′ (148.9) confirmed the occupation of OH at position 4′.
3.1.1. 5,7,2′,3′,4′ pentahydroxyl isoflavone-4′-O-β-glucopyranoside (1)
Colorless crystals, Rf 0.54 (BAW). ESI-MS [M-H]−; m/z 463.2. UV/Vis λmax; methanol: 262,294 sh, 332 sh; methanol/sodium methoxide: 270,331(dec); AlCl3: 272,298 sh, 332 sh; AlCl3/HCl: 273,371; sodium acetate; 270,322 sodium acetate/boric acid: 266,294 sh, 335 sh. 1H NMR (500 MHz, DMSO-d6, δ, ppm, J/Hz): 8.59 (1H, s, H-2); 7.18 (1H, d, J = 8.5, H-5′); 6.77 (1H, d, J = 8.5, H-6′); 6.27 (1H, d, J = 1.5, H-8); 6.09 (1H, d, J = 1.5, H-6); 5.09 (1H, d, J = 7.5, H-1″).13C NMR (DMSO-d6, δ, ppm): 177.6 (C-4), 163.1. (C-7), 161.6 (C-5), 159 (C-9), 156.9 (C-3), 148.9 (C-4′), 145.2 (C-2′), 134.5 (C-3′), 125.2 (C-2), 120.7 (C-6′), 115.9 (C-1′), 110.4 (C-5′), 104.1 (C-10), 98.3 (C-6), 94.6 (C-8), 101.2 (C-1″), 77.3 (C-5″), 74.9 (C-3″), 74.2 (C-2″), 72.2 (C-4″), 61.1(C-6″).
3.2. Direct antioxidant assay (DPPH assay)
The cell-free direct antioxidant assay using DPPH revealed the potency of the tested samples to scavenge free radicals in the order of 7,2′,3′,4′ penta hydroxyl isoflavone-4′-O-β-glucopyranoside > quercetin > quercetin 3-O-galactoside > caffeic acid > quercetin 3,7-di-OCH3 > kaempferol. Their IC50 values were calculated as: 3.9μM, 7.5μM, 11.4μM, 12.2μM, 78.1μM, and 252.3μM, respectively, while the IC50 of AME was calculated to be 27.5 μg/mL (Table 1). Comparing the structure of the tested compounds (Figure 1), the anti-DPPH activity showed a chemical structural dependence where, in case of compounds 1 (5,7,2′,3′,4′ pentahydroxyl isoflavone-4′-O-β-glucopyranoside) and 4 (quercetin), both shared the presence of 5 and 7 hydroxyl groups in Ring A, along with the ortho-dihydroxyl basic structure of Ring B, which seemed essential for the displayed activity. Furthermore, either glycosylation or methylation of the 3-hydroxy group (quercetin 3-O-β-galactoside; 6 and quercetin 3,7-di OCH3; 7) decreased the radical-scavenging activities of flavonoids. These interpretations are supported by previous reports that concluded that the glycosylation and/or methylation of any hydroxyl group in flavonol dihydroxyl basic structure might condense the activity of flavonoids against free radicals [39]. Moreover, the tested phenolic acid (caffeic acid; 8) showed a significant activity with IC50 of 12.2 μM. This activity was also due to the ortho-dihydroxyl pattern, which is generally considered as important for the radical-scavenging activity of phenolic acids [40].
Table 1.
IC50 values of DPPH scavenging by AME and the isolated flavonoids of Pulicaria undulata subsp. undulata.
| IC50 values of DPPH scavenging | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| AME (μg/mL) | Isolated compounds (μM) | GA (μM) | |||||
|
| |||||||
| 1 | 2 | 4 | 6 | 7 | 8 | ||
| 27.5 | 3.9 | 252.3 | 7.5 | 11.4 | 78.1 | 12.2 | 6.9 |
1: 5,7,2′,3′,4′ penta hydroxyl isoflavone-4′-O-β-glucopyranoside, 2: kaempferol, 4: quercetin, 6: quercetin 3-O-β-galactoside, 7: quercetin 3,7-di OCH3, 8: caffeic acid.
AME = aqueous methanol extract; DPPH = 2,2-diphenyl-1-picrylhydrazyl; GA = gallic acid (positive control); IC50 = 50% inhibitory concentration.
3.3. Indirect antioxidant assay (anti-TBHP cytoprotection assay)
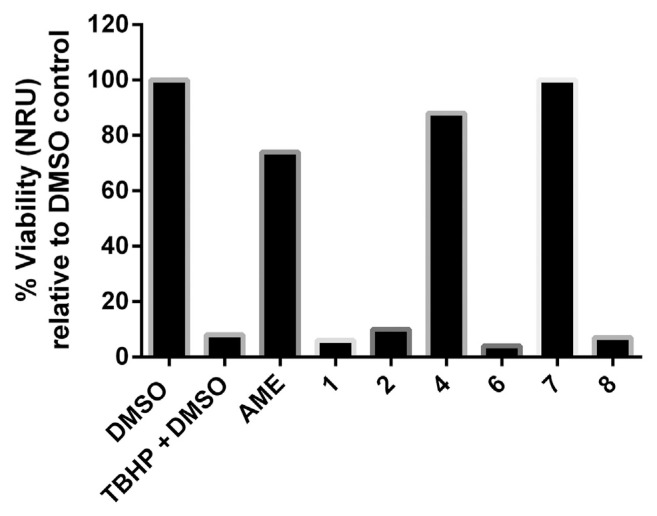
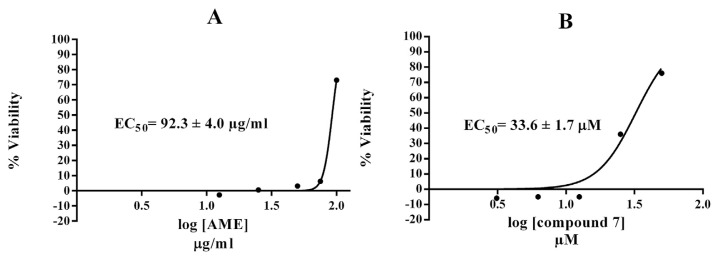
In this assay, we used the murine hepatoma Hepa1c1c7 cell line because it is well known to be inducible for antioxidant phase II genes such as quinone reductase and glutathione-S-transferase that detoxify free radicals [41,42]. The cells have functional promoter of the antioxidant response element that encodes many cytoprotective genes [43]. The results of the anti-TBHP cytoprotection screen are displayed in Figure 2. Exposure to 125 μM TBHP caused a dramatic loss (92%) of Hepa1c1c7 cell viability relative to the non-TBHP treated DMSO control. Quercetin dihydrate as a positive standard produced full cytoprotection at 4 μg/mL. The pretreatment of Hepa1c1c7 cells with 100 μg/mL AME produced 74% viability (i.e., protecting 66% of their intact cells), while quercetin (4) showed 80% protection (88% viability), which was previously demonstrated as a liver cytoprotective [44,45]. The toxicity of TBHP was completely inhibited by 100 μM quercetin 3,7-di OCH3 (7) with EC50 = 33.6 ± 1.7 μM (Figure 3). The remaining tested compounds were not able to protect Hepa1c1c7 cells against the cytotoxicity of organic peroxide. A nonpolar solvent extract from Egyptian P. undulata was recently shown to induce the activity of the Nrf2-dependent cytoprotective enzyme quinone reductase in Hepa1c1c7 cells [46]. Nevertheless, the 70% aqueous methanolic extract of the same plant was devoid of the inducer activity up to a concentration of 50 μg/mL. In the same study, however, the nonpolar extract of Pulicaria incisa strongly upregulated both the Nrf2-dependent cytoprotective enzyme quinone reductase activity and protein expression in addition to upregulation of other cytoprotective proteins including glutathione S transferase and hemoxygenase-1. These findings about the Pulicaria genus could support our results for the protective effect of P. undulata constituents against TBHP injury.
Figure 2.
Screening results of the anti-TBHP cytoprotection assay. Quercetin dihydrate was screened as positive control and possessed full protection at 4 μg/mL. 1 =5,7,2′,3′,4′ penta hydroxyl isoflavone-4′-O-β-glucopyranoside; 2 =kaempferol; 4 =quercetin; 6 =quercetin 3-O-β-galactoside; 7 =quercetin 3,7-di OCH3; 8 =caffeic acid. AME =aqueous methanol extract; DMSO =dimethyl sulfoxide; NRU =neutral red uptake; TBHP =tert-butyl hydroperoxide.
Figure 3.
Dose–response curve of calculating the EC50 of (A) AME and (B) compound 7 against TBHP-induced cytotoxicity. 7 =quercetin 3,7-di OCH3. AME = aqueous methanol extract; EC50 = 50% effective concentration; TBHP = tert-butyl hydroperoxide.
3.4. Chemosystematic significance
P. undulata is a plant species that has two different authorities: P. undulata (L.) C.A. Mey. and P. undulata (L.) Kostel.; both of which are antonyms with each other. P. undulata (L.) C.A. Mey. has many synonyms; Aster crispus Forssk., Duchesnia crispa (Forssk.) Cass., Francoeuria crispa (Forssk.) Cass., Francoeuria crispa var. discoidea Boiss., Francoeuria undulata (L.) Lack, Inula crispa (Forssk.) Pers., Inula undulata L., and Pulicaria crispa (Forssk.) Oliv. [47]. Many authors deal with the name P. undulata (L.) C.A. Mey. as an accepted name with only one subspecies: undulata. Recently, Hind and Boulos [48] recorded four new subspecies: fogensis, candidissima, tomentosa, and argyrophylla and only the subsp. undulata is distributed in Egypt.
Täckholm [49] deals with the two species morphologically as P. undulata (L.) Kostel. and F. crispa (Forssk.) Cass (= P. undulata (L.) C. A. Mey.). El-Kamali and Mahjoub [50] studied the antibacterial activity of both taxa and showed a different activity. Moreover, Liu et al [27], in their review on the phytochemical and biological activities of Pulicaria species, were transacting with the species without demonstrating the authority of each species. Furthermore, through studying the genetic variation between P. undulata and P. crispa (Forssk.) Benth and Hook (= P. undulata (L.) C. A. Mey.), the authors did not clarify the authority of P. undulata [51]. Also, Metwally et al [21], Abdel-Mogib et al [9], and Liu et al [27] characterized the chemical constituents of P. undulata without mentioning the authority.
From the flavonoids point of view, few studies have dealt with the isolation and identification of flavonoids from P. undulata (L.) C.A. Mey. and its synonyms (Table 2) [6,8–10]. Two flavonol aglycones (kaempferol and quercetin) and their methyl ether derivatives either at position 3, 7, or 6 were common. Glucopyranose is the main sugar moiety; the glucosylation of OH group at position 3 is common, while the glucosylation of that at position 7 or 6 is rare and is represented as quercetin 7-O-glucopyranoside and 6-hydroxykaempferol 3-methyl ether 6-O-glucopyranoside [6,8]. One di-O-glucopyranoside flavonol (pulicaroside) was also identified [6]. Only one study has dealt with the isolation and identification of five flavonoids from P. undulata (L.) Kostel. [10]. Moreover, the two species are completely different; the methylation of flavonols in P. undulata (L.) C.A. Mey. takes place at position 3 or 3,6, or 3,7, while in P. undulata (L.) Kostel., it occurs at position 7 or 3,7. In order to avoid this confusion over nomenclature, the authority of the scientific plant names must be used in the upcoming studies.
Table 2.
Flavonoid constituents of Pulicaria undulata (L.) Kostel and P. undulata (L.) C. A. Mey.
| Compound | P. undulata (L.) Kostel | P. undulata (L.) C.A. Mey. |
|---|---|---|
| Kaempferol | − | +b,c |
| Kaempferol 3-O-β-glucopyranoside | − | +c |
| Kaempferol 7-methyl ether | +a | − |
| Kaempferol 3-methyl ether | − | +b |
| 6-Methoxykaempferol | − | +b |
| 6-Methoxykaempferol 3-O-β-glucopyranoside | − | +b |
| 6-Hydroxykaempferol 3-methyl ether 6-O-β-glucopyranoside | − | +b |
| 6-Hydroxykaempferol 3- methyl ether- 6-O-(6″-O-β-glucopyranoside)- β-glucopyranoside (Pulicaroside) | − | +b |
| Quercetin | − | +b,c |
| Quercetin 3-O-β-glucopyranoside | +a | +b,c |
| Quercetin 3-O-β-galactopyranoside | − | +c |
| Quercetin 7-O-β-glucopyranoside | − | +b |
| Quercetin 3-methyl ether | − | +b |
| Quercetin 7-methyl ether | +a | − |
| Quercetin 3,7-dimethyl ether | +a | +c |
| Quercetin 3,6-dimethyl ether | − | +b |
| 5,7,2′,3′,4′ pentahydroxyl isoflavone-4′-O-β-glucoside | − | +c |
| Dihydrokaempferol | +a | − |
Acknowledgments
The phytochemical part of this work was financially supported by National Research Centre, Egypt, Project (10010002).
Funding Statement
The phytochemical part of this work was financially supported by National Research Centre, Egypt, Project (10010002).
REFERENCES
- 1. Zou HQ, Lu G, Liu Y, Bauer R, Tao O, Gong JT, Zhao HY, Li JH, Ren ZY, Yan YH. Is it possible to rapidly and noninvasively identify different plants from Asteraceae using electronic nose with multiple mathematical algorithms? J Food Drug Anal. 2015;23:788–94. doi: 10.1016/j.jfda.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams CA, Harborne JB, Greenham J. Geographical variation in the surface flavonoids of Pulicaria dysenteria. Biochem System Ecol. 2000;28:679–87. doi: 10.1016/s0305-1978(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 3. Melek FR, El-Ansari MA, Hassan A, Regaila A, Ahmed AA, Mabary TJ. Methoxylated flavonoid aglycones from Pulicaria arabica. Rev Latinoamer Quim. 1988;19:119–20. [Google Scholar]
- 4. Mansour RMA, Ahmed AA, Melek FR, Saleh NAM. The flavonoids of Pulicaria incisa. Fitoterapia. 1990;61:186–7. [Google Scholar]
- 5. Wollenweber E, Dörr M, Fritz H, Valant-Vetschera KM. Exudate flavonoids in several Asteroideae and Cichorioideae (Asteraceae) Z Naturforsch C. 1997;52:137–43. [Google Scholar]
- 6. Rizk AM, Hammouda FM, Ismail SI, Hussiney HA. Constituents of plants growing in Qatar XXIII. Qatar Univ Sci J. 1993;13:51–2. [Google Scholar]
- 7. El-Negoumy SI, Mansour RMA, Saleh NAM. Flavonols of Pulicaria arabica. Phytochemistry. 1982;21:953–4. [Google Scholar]
- 8. Ahmad VU, Rasool N, Abbasi MA, Rashid MA, Kousar F, Zubair M, Ejaz A, Choudhary MJ, Tareen RB. Antioxidant flavanoids from Pulicaria undulata. Polish J Chem. 2006;80:745–51. [Google Scholar]
- 9. Abdel-Mogib M, Dawidar AM, Metwally MA, Abou-Elzahab M. Flavonols of Pulicaria undulata. Pharmazie. 1989;44:801. [Google Scholar]
- 10. Bishady DW, Gomaa CS, Assaf MH. Flavonoids from Pulicaria undulata (L.) Kostel growing in Egypt. Bull Pharm Sci Assiut Univ. 1982;5:65–71. [Google Scholar]
- 11. San Feliciano A, Medarde M, Gordaliza M, Del Olmo E, Del Corral JMM. Sesquiterpenoids and phenolics of Pulicaria paludosa. Phytochemistry. 1989;28:2717–21. [Google Scholar]
- 12. Das B, Reddy MR, Ramu RN, Ravindranath HH, Ramakrishna KVS, Murthy USN. Clerodane diterpenoids from Pulicaria wightiana. Phytochemistry. 2005;66:633–8. doi: 10.1016/j.phytochem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13. Abdel-Mogib M, Jakupovic J, Dawidar AM, Metwally MA, Abou-Elzahab M. Sesquiterpene lactones and kaurane glycosides from Francoeuria crispa. Phytochemistry. 1990;29:2581–4. [Google Scholar]
- 14. Hegazy MEF, Matsuda H, Nakamura S, Yabe M, Matsumoto T, Yoshikawa M. Sesquiterpenes from an Egyptian herbal medicine, Pulicaria undulate, with inhibitory effects on nitric oxide production in RAW264. 7 macrophage cells. Chem Pharm Bull. 2012;60:363–70. doi: 10.1248/cpb.60.363. [DOI] [PubMed] [Google Scholar]
- 15. Hegazy MEF, Nakamura S, Tawfik WA, Abdel-Azim NS, Abdel-Lateff A, Matsuda H, Paré PW. Rare hydroperoxyl guaianolide sesquiterpenes from Pulicaria undulata. Phytochem Lett. 2015;12:177–81. [Google Scholar]
- 16. Dendougui H, Benayache S, Benayache F, Connoly JD. Sesquiterpene lactones from Pulicaria crispa. Fitoterapia. 2000;71:373–8. doi: 10.1016/s0367-326x(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 17. Stavri M, Mathew KT, Gordon A, Shnyder SD, Falconer RA, Gibbons S. Sesquiterpenes from Pulicaria crispa (Forssk.) Oliv. Phytochemistry. 2008;69:1915–8. doi: 10.1016/j.phytochem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 18. Nematollahi F, Rustaiyan A, Larijani K, Nadimi M, Masoudi SJ. Essential oil composition of Artemisia biennisz Willd. and Pulicaria undulata (L.) C.A. Mey., two compositae herbs growing wild in Iran. Essent Oil Res. 2006;18:339–41. [Google Scholar]
- 19. Bashi DS, Ghani A, Asili J. Essential oil composition of Pulicaria gnaphalodes (Vent.) Boiss. growing in Iran. J Essent Oil Bear Plants. 2013;16:252–6. [Google Scholar]
- 20. Al-Hajj NQM, Wang H, Gasmalla MA, Ma C, Thabit R, Rahman MRT, Tang Y. Chemical composition and antioxidant activity of the essential oil of Pulicaria inuloides. J Food Nutr Res. 2014;2:221–7. [Google Scholar]
- 21. Metwally MA, Dawidar AM, Metwally S. A new thymol derivative from Pulicaria undulata. Chem Pharm Bull. 1986;34:378–9. [Google Scholar]
- 22. Maghraby AS, Shalaby N, Abd-Alla HI, Ahmed SA, Khaled HM, Bahgat MM. Immunostimulatory effects of extract of Pulicaria crispa before and after Schistosoma mansoni infection. Acta Pol Pharm. 2010;67:75–9. [PubMed] [Google Scholar]
- 23. Elshiekh YH, Abd El Moniem MA. Phytochemical, antibacterial screening and antioxidant activity of Pulicaria crispa extracts. Pharm Innov J. 2015;3:12–5. [Google Scholar]
- 24. Ezoubeiri A, Gadhi CA, Fdil N, Benharref A, Jana M, Vanhaelen M. Isolation and antimicrobial activity of two phenolic compounds from Pulicaria odora L. J Ethnopharmacol. 2005;99:287–92. doi: 10.1016/j.jep.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 25. Foudah AI, Alam A, Soliman GA, Salkini MA, Ahmed EOI, Yusufoglu HS. Pharmacognostical, antioxidant and antimicrobial studies of aerial part of Pulicaria crispa (Family: Asteraceae) Bull Env Pharmacol Life Sci. 2015;4:19–27. [Google Scholar]
- 26. Algabr MN, Mekkiou R, Ameddah S, Menad A, Boumaza O, Seghiri R, Benayache F. Antioxidant activities from the aerial parts of Pulicaria jaubertii. Adv Nat Appl Sci. 2010;4:63–71. [Google Scholar]
- 27. Liu LL, Yang JL, Shi YP. Phytochemicals and biological activities of Pulicaria species. Chem Biodivers. 2010;7:327–49. doi: 10.1002/cbdv.200900014. [DOI] [PubMed] [Google Scholar]
- 28. Elshiekh YH, Abd El Moniem MA. Gas chromatography–mass spectrometry analysis of Pulicaria crispa (whole plant) petroleum ether extracts. Am J Res Commun. 2005;3:58–67. [Google Scholar]
- 29.Boulos L. Flora of Egyptvol. Vol. 3. Cairo: Al Hadara Publishing; 2002. [Google Scholar]
- 30.Boulos L. Flora of Egypt checklist, revised annotated edition. Cairo: Al Hadara Publishing; 2009. [Google Scholar]
- 31.Mabry TJ, Markham KR, Thomas MB. The systematic identification of flavonoids. Berlin: Springer; 1970. [Google Scholar]
- 32. Hamed A, Soltan M, Fry J, Hammouda F, Zaki A. Antioxidant and cytoprotective properties of three Egyptian Cyperus species using cell-free and cell based assays. J Pharm Crops. 2012;3:88–93. [Google Scholar]
- 33. Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379–81. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 34. Soltan MM, Hamed AR, Hetta MH, Hussein AA. Egyptian Pancratium maritimum L. flowers as a source of anti-Alzheimer’s agents. Bull Fac Pharm (Cairo Univ) 2015;53:19–22. [Google Scholar]
- 35. Repetto G, Del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protocol. 2008;3:1125–31. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 36.Markham KR, Geiger H. 1H-NMR of flavonoids and their glycosides in DMSO-d6. In: Harborne JB, editor. The flavonoids: advances in research since 1986. London: Chapman and Hall; 1994. pp. 441–97. [Google Scholar]
- 37.Agrawal PK. Carbon-13 NMR of flavonoids. New York: Elsevier; 1989. [Google Scholar]
- 38.Andersen QM, Markham KR. Flavonoids: chemistry, biochemistry and applications. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 39. Zheng CD, Li G, Li HQ, Xu XJ, Gao JM, Zhang AL. DPPH-scavenging activities and structure-activity relationships of phenolic compounds. Nat Prod Commun. 2010;5:1759–65. [PubMed] [Google Scholar]
- 40. Jing P, Zhao SJ, Jian WJ, Qian BJ, Dong Y, Pang J. Quantitative studies on structure-DPPH• scavenging activity relationships of food phenolic acids. Molecules. 2012;17:12910–24. doi: 10.3390/molecules171112910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci. 1992;89:2394–8. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. El-Sayed WM, Aboul-Fadl T, Roberts JC, Lamb JG, Franklin MR. Murine hepatoma (Hepa1c1c7) cells: A responsive in vitro system for chemoprotective enzyme induction by organoselenium compounds. Toxicol In Vitro. 2007;21:157–64. doi: 10.1016/j.tiv.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hwang YP, Choi JH, Han EH, Kim HK, Kang SK, Chung YC, Jeong HG. Protectivemechanisms of Aralia continentalis extract against tert-butyl hydroperoxide-induced hepatotoxicity: in vivo and in vitro studies. Food Chem Toxicol. 2006;46:3512–21. doi: 10.1016/j.fct.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 44. Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull. 2003;26:1398–402. doi: 10.1248/bpb.26.1398. [DOI] [PubMed] [Google Scholar]
- 45. Materska M. Quercetin and its derivatives: chemical structure and bioactivity–a review. Polish J Food Nut Sci. 2008;58:407–13. [Google Scholar]
- 46. Hamed AR, Hegazy M-EF, Higgins M, Mohamed TA, Abdel-Azim NS, Pare PW, Dinkova-Kostova AT. Potency of extracts from selected Egyptian plants as inducers of the Nrf2-dependent chemopreventive enzyme NQO1. J Nat Med. 2016;70:683–8. doi: 10.1007/s11418-016-0994-0. [DOI] [PubMed] [Google Scholar]
- 47.The Plant List: The Plant List Version 1.1. 2013. [Online] Available from: http://www.theplantlist.org/cited Sep 22, 2013.
- 48. Hind DJN, Boulos L. Four new combinations in Pulicaria (Compositae: Inuleae) Kew Bull. 2002;57:495–8. [Google Scholar]
- 49.Täckholm V. Students’ flora of Egypt. 2nd ed. Cairo: Cairo University; 1974. pp. 562–3. [Google Scholar]
- 50. El-Kamali HH, Mahjoub SAT. Antibacterial activity of Francoeuria crispa, Pulicaria undulata, Ziziphus spina-christi, and Cucurbita pepo against seven standard pathogenic bacteria. Ethnobotanical Leaflets. 2009;2009:6. [Google Scholar]
- 51. El-Kamali HH, Habeballa R, Abdalla I, Mohammed AY, Abdelkarim ND, Abbas IM, Ali SM. Genetic relationships of two Pulicaria species and identification of their putative hybrids using RAPD markers. World Appl Sci J. 2010;8:687–93. [Google Scholar]