Abstract
The official analytical method of the Taiwan Food and Drug Administration, Ministry of Health and Welfare for testing for veterinary drug residues in foods is the multiresidue analysis of β-agonists. Samples are pretreated through liquid–liquid extraction and solid-phase extraction. This method is time consuming and requires the intensive use of solvents. To improve analytical efficiency and reduce costs, our study incorporated QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) techniques to establish a new method of multiresidue analysis of β-agonists in animal muscle and viscera. The pretreatment time was shortened and solvent usage was minimized. The modified analysis was conducted using liquid chromatography/tandem mass spectrometry (LC–MS/MS) and quantification was performed using multiple reaction monitoring. The results demonstrated that the correlation coefficients of the tissue calibration curve were higher than 0.99 and the limit of quantification (LOQ) was 1 ppb. The average recoveries in spiked samples varied from 70% to 120%, and the relative difference between duplicated analysis results was lower than 10%. On the basis of the results, the proposed method was concluded to be an appropriate procedure for determining the presence of β-agonists, and demonstrated the advantages of high recovery rates in spiked samples, high precision, reduced analysis time and solvent usage, and lower costs.
Keywords: β-agonists, LC-MS/MS, multiresidue analysis, QuEChERS
1. Introduction
In recent years, the safety of meat products has been a source of great public concern [1,2] and methods of detecting drug residues have become a focus of research worldwide. Effective determination methods are required to guarantee meat safety [3,4]. Veterinary drugs are widely used at therapeutic levels in livestock breeding systems for treating various diseases; one type of these drugs, β-agonists, was originally intended to treat asthma and preterm labor in humans [5]. However, these compounds also promote lipolysis in muscle tissue and exhibit a considerable nutrition redistribution function, resulting in higher feed efficiency and a greater muscle-to-fat ratio in livestock [6–8]. β-Agonists have been utilized in livestock such as pigs and ruminants to reduce carcass fat and increase muscle mass, while improving the growth rate and feed conversion [9–12]. Previous research indicated that the residues of six β-agonists are prone to accumulate in the retinal tissue of food-producing animals [13]. Moreover, the ingestion of β-agonists deposited in animal tissues can cause acute poisoning in humans, particularly in patients with symptoms such as muscular tremors, cardiac palpitation, nervousness, headaches, and muscular pain [14]. Tissues contaminated with β-agonists appear to be harmful and pose a potential risk to human health [15]. Although their use in the therapeutic treatment of cattle with respiratory diseases is permitted, the use of β-agonists as growth promoters in cattle is forbidden in certain countries and the European Union [16]. However, illicit usage in animal feed persists in numerous countries [17]. Sensitive and specific analytical methods are therefore required to determine the level of β-agonists in meat, in order to monitor food safety. These analytical methods are essential for assessing human exposure to β-agonists and supporting the enforcement of laws and regulations.
A review of the literature shows that the methods used to monitor β2-agonist residues include high-performance liquid chromatography [18,19], gas chromatography–mass spectrometry [20,21], and liquid chromatography–mass spectrometry or liquid chromatography–tandem mass spectrometry (LC–MS/MS) [22,23]. LC–MS/MS is currently considered the most suitable technique used to detect multiple classes of veterinary drugs in foodstuffs because it provides unambiguous identification and reliable confirmation [24]. In order for a method to be deemed confirmatory, identification must be carried out according to relative retention times, the identification points of each analyte, and the relative ion ratios of selected multiple reaction monitoring (MRM) transitions. Despite the use of selective detection techniques such as MS, appropriate sample preparation is a challenging but necessary step in analytical procedures that reduces interference and averts possible matrix effects. Techniques such as immunoaffinity chromatography [25], liquid–liquid extraction [26], solid-phase extraction (SPE) [27], and matrix SPE [28] are used for extraction and purification. Most of the current extraction methods are time consuming and tedious. Additionally, the general SPE cleanup techniques lack specificity and selectivity and can mix analytes and interferents. This leads to interference and matrix effects and reduces the reliability of determination. In addition, the large quantities of organic solvents employed in these methods may cause environmental pollution [29].
Consequently, analytical laboratories are increasingly interested in developing new analytical methods that are quicker and enable higher sample throughput. In 2003, the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method for the multiclass and multiresidue analysis of pesticides in fruits and vegetables was first published by Anastassiades et al [30]. It has received widespread attention since its development and expanded beyond its application in traditional samples to include meat products, fish, blood, and even soil. The QuEChERS method is now a widely known methodology for the extraction of several classes of drugs, including pesticides and veterinary drugs, from different matrices. This method minimizes the time required for the extraction and cleaning processes and reduces the sample size, costs, required quantities of laboratory glassware, and levels of solvent consumption [31]. Additionally, the established QuEChERS pretreatment procedure without covering is simple and economical and requires only small amounts of organic solvents. This method can reduce the sample volume, solvent consumption, and required analytical time. The adaptation of the QuEChERS method for β-agonist monitoring is therefore strongly recommended. The aim of this study was to develop a rapid and easy multiresidue analytical method involving the QuEChERS procedure to determine the levels of seven β-agonists in muscle and viscera samples.
2. Materials and methods
2.1. Samples
Samples (muscle and viscera) were purchased from local supermarkets and were confirmed to be free of the target drugs. The tissues were homogenized and stored at −20°C until analysis was begun.
2.2. Chemicals, reagents, and solutions
All chemicals were of analytical grade. Acetonitrile, methanol (high-performance liquid chromatography grade), and ammonium acetate were obtained from Merck (Darmstadt, Germany). Ultrapure water (Milli-Q, Millipore Corporation, Bedford, MA, USA) was used to prepare all aqueous solutions.
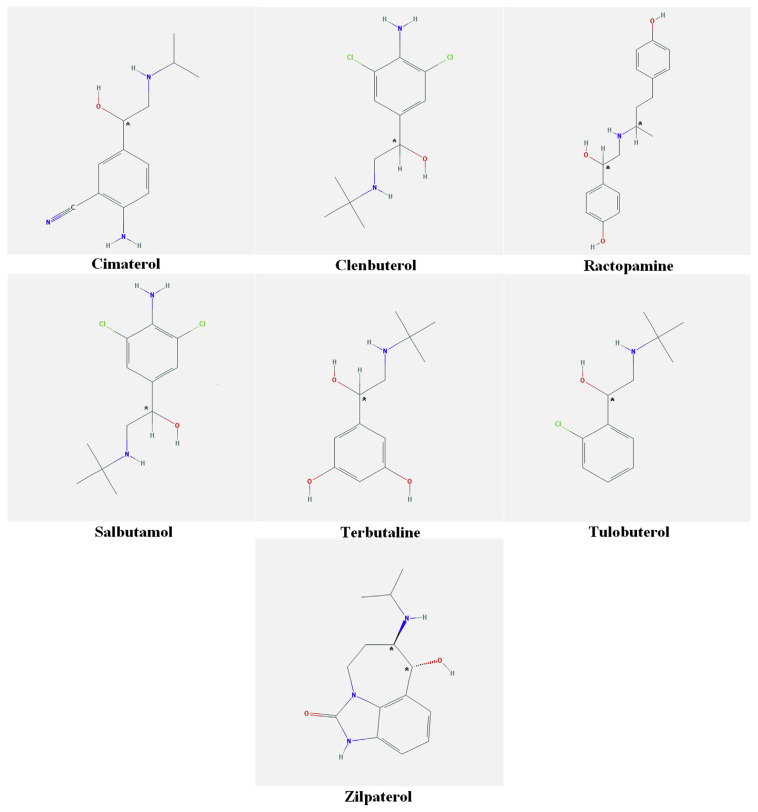
All β-agonists (cimaterol, clenbuterol, ractopamine, salbutamol, terbutaline, tulobuterol, and zilpaterol) and corresponding internal standards (cimaterol-d7, clenbuterol-d9, ractopamine-d6, clenbuterol-d9, salbutamol-d6, terbutaline-d9, and zilpaterol-d7) were purchased from Sigma (St. Louis, MO, USA). The minimum purity of all standards was 98.0%. The chemical structures of all seven β-agonists are shown in Figure 1.
Figure 1.
Chemical structures of the β-agonists in this study.
2.3. Preparation of standards
Individual stock solutions (100 μg/mL) were prepared by dissolving 1 mg of each compound in 10 mL of methanol. These were stored at −20°C in brown glass to prevent photodegradation. Mixed standard solutions at concentrations of 1 μg/mL of each standard were prepared by mixing 100 μL of additives with each stock solution and diluting the solution to 10 mL with methanol. The mixed standard solutions were stored in amber bottles at −20°C. Six mixed IS solutions were prepared and stored in the same manner. All working solutions and calibration standards were obtained through gradient dilution of the intermediate solutions in concentrations varying from 1 μg/mL to 1 ng/mL. A working standard solution of ISs at a concentration of 1 μg/mL was determined with subsequent dilutions of their stock solutions in methanol. When not in use, the working solutions were kept at −20°C and renewed weekly.
2.4. Sample preparation
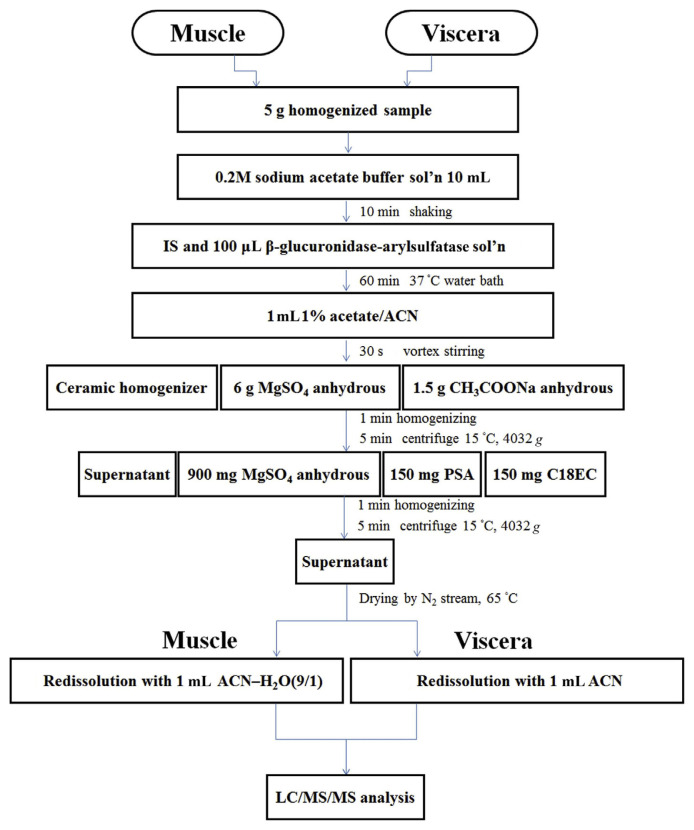
Five grams of finely chopped muscle and viscera homogenates was weighed into a 50 mL Falcon tube and spiked as appropriate with target compounds and the IS. Subsequently, 10 mL of 0.2 M sodium acetate solution was added, and the tubes were shaken for 10 minutes. Following the addition of the IS and 100 μL of β-glucuronidase-arylsulfatase, the sample was incubated in a 37°C water bath for 1 hour before extraction. One milliliter of 1% acetate–acetonitrile was added and the mixture was stirred in a shaker for 30 seconds. A ceramic homogenizer, 6 g of anhydrous magnesium sulfate, and 1.5 g of anhydrous sodium acetate were then added and the tubes were shaken vigorously for 1 minute. Following centrifugation at 4032 × g and 15°C for 5 minutes, the supernatant was transferred to a new tube containing 900 mg of anhydrous magnesium sulfate, 150 mg of primary secondary amine (PSA), and C18EC and then subjected to high-speed homogenization for 1 minute and a second round of centrifugation under the same conditions. The supernatant was evaporated to dryness by a nitrogen blowing concentrator in a water bath at 65°C. The muscle and viscera residues were redissolved with 1 mL of an acetonitrile–H2O solution (9:1, v/v) and acetonitrile, respectively. The sample extract was filtered through a 0.22-μm polytetrafluoroethylene filter into an autosampler vial for the LC–MS/MS analysis. A scheme diagram of this sample preparation is shown in Figure 2.
Figure 2.
Diagram of sample preparation.
2.5. LC–MS/MS analysis
LC was performed on a Dionex Ultimate 3000 Rs system (Sunnyvale, CA, USA) coupled to an AB SCIEX Q TRAP 5500 mass spectrometer (Framingham, MA, USA). Chromatographic separation was performed using an Agilent Zorbox SB-C18 column (150 mm × 4.6 mm, 5 μm, Palo Alto, CA, USA). Linear gradient elution was performed as shown in Table 1. The injection volume was 10 μL. Mass analysis was carried out using an electrospray ionization source in positive mode. The operation conditions were as follows: ionspray voltage, 5.5 kV; source temperature, 650°C; curtain gas, 20 psi; ion source gases 1 and 2, 65 psi. The optimal MRM parameters are summarized in Table 2.
Table 1.
Gradient program of the mobile phase for HPLC separation of the seven β-agonists in the present study (flow rate 1 mL/min).
| Time (min) | 5 mM ammonium acetate in methanol (%) |
|---|---|
| 0 | 15 |
| 1 | 15 |
| 6 | 25 |
| 14 | 70 |
| 16 | 80 |
| 17 | 80 |
| 17.5 | 95 |
| 19.5 | 95 |
| 20 | 15 |
| 25 | 15 |
| 5 mM ammonium acetate in deionized water (%) + 5 mM ammonium acetate in methanol (%) = 100 | |
HPLC = high performance liquid chromatography.
Table 2.
Parameters of MRM condition and retention times of the β-agonists.
| Compound | ESI | Retention time (min) | Precursor ion (m/z) | Product ions (m/z) | Decluster potential (V) | Entrance potential (V) | Collision energy (eV) | Collision cell exit potential (V) |
|---|---|---|---|---|---|---|---|---|
| Cimaterol | + | 4.40 | 220 | 202, 160 | 45 | 10 | 12, 10 | 13 |
| Clenbuterol | + | 7.62 | 277 | 203, 259 | 58 | 10 | 20,15 | 13 |
| Ractopamine | + | 6.34 | 320.01 | 284, 107 | 59 | 10 | 18, 33 | 13 |
| Salbutamol | + | 4.53 | 240.01 | 148, 222 | 45 | 10 | 24, 13 | 13 |
| Terbutaline | + | 4.45 | 226 | 152, 125 | 60 | 10 | 20, 32 | 13 |
| Tulobuterol | + | 8.48 | 228 | 154, 118 | 40 | 10 | 22, 33 | 13 |
| Zilpaterol | + | 4.70 | 262.2 | 244, 185 | 54 | 10 | 19, 33 | 13 |
ESI = electrospray ionization; MRM = multiple reaction monitoring.
2.6. Method validation
For the method validation, various parameters such as linearity, accuracy, precision, and limits of quantification (LOQs) were evaluated. The peak area of the most intense transition versus the concentration was used to establish the linear regression equation. The linearity of the method was evaluated on the basis of tissue calibration.
2.6.1. Linearity
Tissue calibration curves were used to quantify and test the linearity of the developed method. The blank muscle and viscera samples were fortified at five concentrations of 1–50 μg/kg of the target analytes. Three replications of each concentration were performed. Sample preparation was executed according to the aforementioned procedures. The tissue calibration curves for β-agonists were constructed by calculating the ratio of each peak area relative to the corresponding IS. The linearity of the LC–MS/MS method was evaluated using the regression coefficient measured for each analyte. The acceptance criterion was a correlation coefficient (R2) >0.99.
2.6.2. Accuracy and precision
The results for accuracy and precision were expressed as the percentage of recovery and the coefficient of variation (CV). Recovery and repeatability were assessed by spiking blank muscle and viscera samples at two concentration levels (1.5 μg/kg and 3.0 μg/kg) for target analytes in five replicates at each level.
2.6.3. LOQs
LOQs were calculated by analyzing blank samples fortified at 1.0 μg/kg and defined as the lowest concentration of an analyte for which the signal-to-noise ratio was >10.
3. Results and discussion
3.1. Optimization of LC–MS/MS parameters
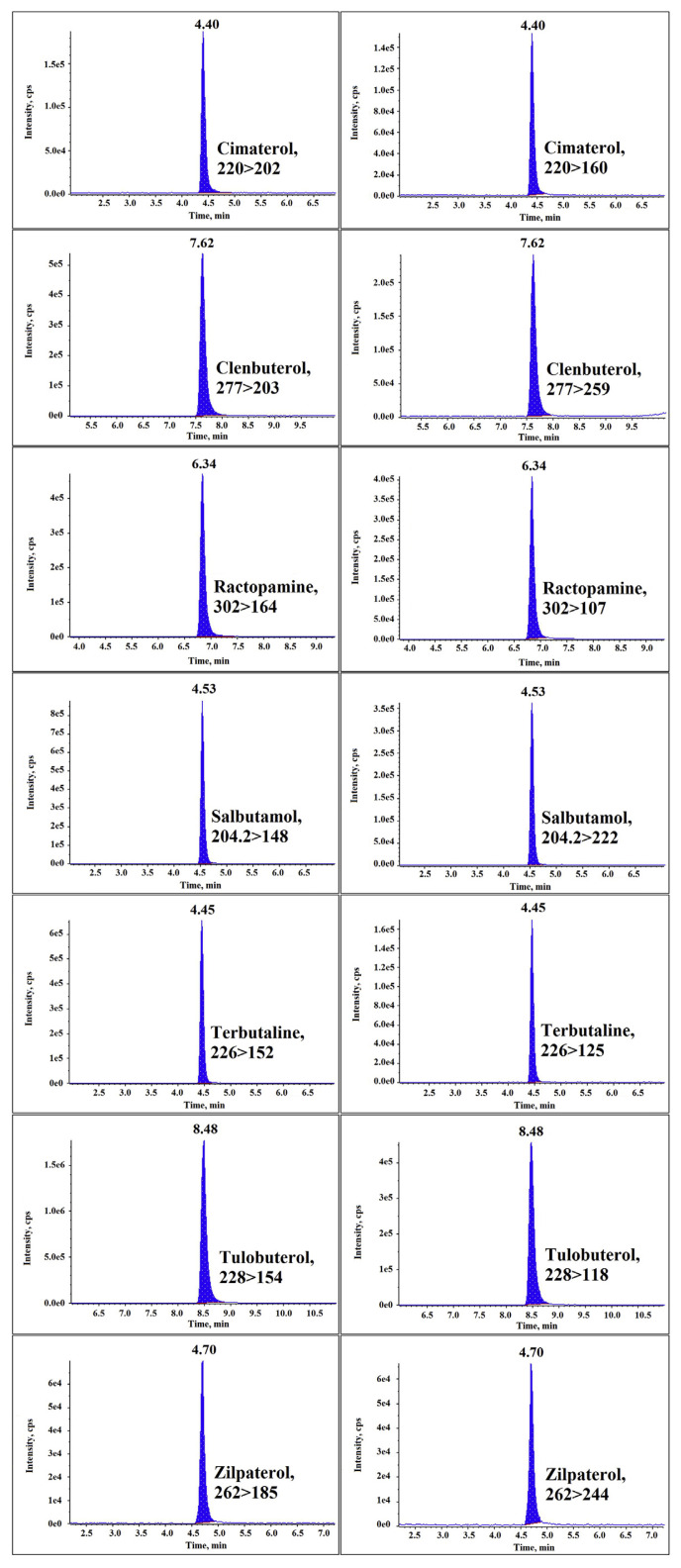
The LC–MS/MS method was developed to provide confirmatory data for the analysis of seven β-agonists in animal muscle and viscera tissue. Separation was performed in an Agilent Zorbox SB-C18 column (150 mm × 4.6 mm, 5 μm). Chromatographic parameters such as the choice of column, mobile phase composition, gradient conditions, and flow rate were tested to obtain the optimal separation of β-agonists. With reference to previous reports, a mobile phase consisting of 5 mM ammonium acetate in water and methanol was chosen [32–34]. The addition of ammonium acetate greatly improved peak shape and facilitated resolving closely eluted compounds. After optimization, the ammonium acetate concentration was set at 5 mM, and its addition to the aqueous phase further improved separation and overall peak shapes [8]. β-Agonists belong to Group A of Annex I, Council Directive 96/23/EC [35], therefore a minimum of four identification points are required, and were obtained by monitoring one parent ion (1 point) and two transitions (1.5 points each). The product ion with the stronger signal was selected as the ion for quantification, and the product ion with the weaker signal was selected as the ion for identification. The selected transitions for β-agonists and the optimal MS–MS conditions are described in Section 2.6. The gradient program in Table 1 was used for chromatographic separation. The flow rate was set at 1.0 mL/min and the analysis was completed in 25 minutes for the seven β-agonists. The MRM chromatograms of the seven β-agonists in the present study are shown in Figure 3.
Figure 3.
Extract chromatograms of the seven β-agonists spiked at 3.0 μg/kg in muscle samples.
3.2. Optimization of sample preparation
Sample preparation plays an important role in analytical methods. Various pretreatment methods have been proposed for monitoring the illegal use of β-agonists [25,26,36]. Salts and endogenous compounds cannot be fully removed due to the complexity of the biological matrices and the trace levels in real samples, leading to possible matrix effects. In addition, these techniques are time consuming, and the large quantities of organic solvents required, including acetonitrile and methanol [2,29], may cause environmental pollution. By contrast, the established QuEChERS pretreatment procedure without covering is simple and economical and requires only small amounts of organic solvents. The principle of the QuEChERS method relies on sample cleaning using various dispersive SPE sorbents, including PSA, C18, and silica, as well as magnesium sulfate for the elimination of residual water prior to analysis [37]. To improve efficiency and reduce time-consuming sample preparation, the QuEChERS method was developed for the cleaning of target analytes in tissue extracts. The procedure begins with sodium acetate buffer solution extraction and enzymatic hydrolysis. Previous studies have demonstrated that favorable recoveries were obtained for most compounds when acetonitrile acidified with 1% acetic acid was used as the extraction solvent [38]. In this work, 10 mL of acetonitrile acidified with 1% acetic acid was used as the extraction solvent. While the analytes are transferred to an organic phase, some polar matrix impurities are left in the aqueous layer. A combination of 1.5 g sodium acetate and 6 g magnesium sulfate was used as a salting-out agent to partition analyte residues into the acetonitrile layer. Several sorbents such as PSA and C18 enabled satisfactory analyte recovery. The muscle and viscera extracts were evaporated under a stream of nitrogen at 65°C and the final residue was dissolved in 1 mL of an acetonitrile–H2O solution (9:1, v/v) and acetonitrile, respectively. The optimized sample preparation protocol enabled a high percentage of recovery for the seven β-agonists.
3.3. Method validation
3.3.1. Linearity
The linearity of the analytical method was validated using the tissue calibration curves for each compound at different concentration levels to prevent matrix effects. Table 3 shows the tissue calibration parameters of the correlation coefficients. Table 3 shows R2 > 0.99 for all seven β-agonists in muscle and viscera samples, which revealed good linearity in the concentration range for each β-agonist.
Table 3.
Linearity and LOQs of the β-agonists.
| β-Agonists | Muscle | Viscera | ||
|---|---|---|---|---|
|
|
|
|||
| R 2 | LOQ (ng/g) | R 2 | LOQ (ng/g) | |
| Cimaterol | 0.998 | 1 | 0.997 | 1 |
| Clenbuterol | 1.000 | 1 | 0.999 | 1 |
| Ractopamine | 0.998 | 1 | 0.997 | 1 |
| Salbutamol | 0.998 | 1 | 0.995 | 1 |
| Terbutaline | 0.996 | 1 | 0.995 | 1 |
| Tulobuterol | 0.999 | 1 | 0.998 | 1 |
| Zilpaterol | 0.993 | 1 | 0.993 | 1 |
LOQ = limit of quantification.
3.3.2. Recovery
The rates of recovery of each compound from muscle and viscera samples were evaluated at two concentration levels (1.5 μg/kg and 3.0 μg/kg) by determining the ratios of the measured and added amounts of the target analyte. The results of the recovery test for the seven β-agonists in muscle and viscera samples are listed in Tables 4 and 5. In muscle samples, the recovery rate of the 1.5 μg/kg and 3.0 μg/kg spiking levels ranged from 90.5% to 101.2% and 87.6% to 102.5%, respectively. In viscera samples, the recovery rate of the two spiking levels varied from 90.7% to 110.3% and 91.7% to 111.5%, respectively. These results showed that the accuracy of this method was satisfactory.
Table 4.
Recovery rates and CVs of the β-agonists from muscle samples.
| β-Agonists | Spiked level (ng/g) | Recovery (%) | CV (%) |
|---|---|---|---|
| Cimaterol | 1.5 | 90.5 | 5.4 |
| 3.0 | 95.1 | 3.1 | |
| Clenbuterol | 1.5 | 99.2 | 1.7 |
| 3.0 | 87.6 | 5.8 | |
| Ractopamine | 1.5 | 98.5 | 4.3 |
| 3.0 | 100.3 | 4.6 | |
| Salbutamol | 1.5 | 101.2 | 4.2 |
| 3.0 | 90.8 | 5.8 | |
| Terbutaline | 1.5 | 96.3 | 6.3 |
| 3.0 | 100.2 | 3.5 | |
| Tulobuterol | 1.5 | 97.9 | 7.7 |
| 3.0 | 102.5 | 3.2 | |
| Zilpaterol | 1.5 | 94.4 | 3.2 |
| 3.0 | 97.4 | 6.4 |
CV = coefficient of variation.
Table 5.
Recovery rates and CVs of the β-agonists from viscera samples.
| β-Agonists | Spiked level (ng/g) | Recovery (%) | CV (%) |
|---|---|---|---|
| Cimaterol | 1.5 | 102.0 | 3.2 |
| 3.0 | 95.0 | 4.2 | |
| Clenbuterol | 1.5 | 101.1 | 2.5 |
| 3.0 | 102.1 | 2.1 | |
| Ractopamine | 1.5 | 99.6 | 5.7 |
| 3.0 | 103.6 | 3.6 | |
| Salbutamol | 1.5 | 110.3 | 4.2 |
| 3.0 | 106.9 | 3.4 | |
| Terbutaline | 1.5 | 100.8 | 4.2 |
| 3.0 | 111.5 | 2.9 | |
| Tulobuterol | 1.5 | 102.4 | 1.8 |
| 3.0 | 105.5 | 6.2 | |
| Zilpaterol | 1.5 | 90.7 | 6.6 |
| 3.0 | 91.7 | 6.9 |
CV = coefficient of variation.
3.3.3. Precision
The precision of the assay was assessed at 1.5 μg/kg and 3.0 μg/kg for each analyte in the spiked samples and expressed as the CV. The results are presented in Tables 4 and 5. The CV of the muscle samples spiked at 1.5 μg/kg and 3.0 μg/kg ranged from 1.7% to 7.7% and 3.1% to 6.4%, respectively, and the corresponding values for the viscera samples varied from 1.8% to 6.6% and 2.1% to 6.9%, respectively. Thus, all CV values were less than 10% at the two concentrations (1.5 μg/kg and 3.0 μg/kg).
3.3.4. LOQ
The LOQ was defined as the concentration at 10 times the signal intensity of noise. The LOQs of all β-agonists were 1.0 μg/kg for the spiked muscle and viscera sample. The LOQs of this method are generally more favorable than those of some traditional methods, but the LOQs obtained in the current study are equal to or less favorable than those of other methods. Nevertheless, very low LOQs were achieved for spiked muscle and viscera samples. These results clearly demonstrate the feasibility of the proposed method. The method ensured reliable preparation and precise quantification of seven β-agonists. This comparison is shown in Table 6 [8,16,18,25,38–46].
Table 6.
Comparison of LODs and LOQs in selected methods.
| Analytes | Matrix | Sample preparation | Detection | LOD (ng/g) | LOQ (ng/g) | Reference |
|---|---|---|---|---|---|---|
| β-Agonist (7) | Muscle, viscera | QuEChERS | LC–ESI(+)-MS | — | 1.00 | Our work |
| Cimateol | Milk | Modified QuEChERS approach | UPLC-QTOF-MS | 1.00 | 3.34 | [38] |
| Clenbuterol | 0.53 | 1.78 | ||||
| Ractopamine | 1.46 | 4.87 | ||||
| Clenbuterol | Butter, egg, fish, milk | SLE with ultrasonic-assisted extraction | LC-ESI(+)-MS | — | 0.02–0.17 | [25] |
| Clenbuterol | Pork | Acidic extraction, SPE/C18 clean-up | HPLC-UV | 0.02 | — | [39] |
| Ractopamine | 0.05 | — | ||||
| Salbutamol | 0.05 | — | ||||
| Clenbuterol | Bovine liver | extracted with acetonitrile and isopropanol | HPLC-UV | 0.20 | 0.42 | [18] |
| β-Agonist (5) | Feed | QuEChERS | LC-ESI(+)-MS | 15 | 50 | [40] |
| β-Agonist (7) | Feed, urine, serum, muscle, liver | SPE technology | UHPLC-Q-Orbitrap HRMS | 0.02–2.18 | 0.07–7.26 | [8] |
| β-Agonist (6) | Beef muscle, beef liver, goat muscle, goat liver | QuEChERS | LC-ESI(+)-MS | 0.30–1.00 | 0.90–3.20 | [41] |
| Clenbuterol | Liver | MSPD/MIP-SPE | LC–ESI(+)IT-MS | <0.10 | — | [42] |
| Zilpaterol | Feed | LPE/C18-SPE/derivative | GC–EI-MS | 8 | — | [43] |
| Zilpaterol | Urine, plasma, tissues, retina | hydrol/LLE/mixurea-SPE tissues: hydrolyzed/hexane defatted/Extrelut-SPE | LC–ESI(+)QqQ-MS | <0.10 | — | [16] |
| β-Agonists | Feed | LPE/Mixa-SPE or IA-SPE | LC–bioassay/Q-ToF-MS | 5–50 | — | [44] |
| β-Agonists | Retina | LPE/SPE/derivative | GC–EI-MS | 4–10 | — | [45] |
| β-Agonists | Liver | Deconjugation/HLB-SPE | SPR (screening) | 0.02–0.2 | — | [46] |
LOD = limit of detection; LOQ = limit of quantification.
4. Conclusion
In the present study, a simple, fast, and sensitive multiresidue analytical method involving LC–MS/MS was developed and validated for the simultaneous determination of seven β-agonists from two types of animal tissue (muscle and viscera). Analytes were extracted using the QuEChERS extraction procedure and analyzed using LC electrospray ionization MS/MS. This method was validated with fortified blank samples and the extraction procedure was fully optimized. Favorable values of validation parameters such as linearity, recovery, precision, and LOQs were obtained, indicating the suitability of the proposed solvent extraction method for the analysis of β-agonists. The proposed method possesses the following advantages: simplicity, speed, reliability, low cost, and low solvent consumption. QuEChERS is therefore a green technique for sample preparation.
Footnotes
Conflicts of interest
The authors certify that they have no conflicts of interest with any financial organization regarding the material discussed in this manuscript.
REFERENCES
- 1. Andrée S, Jira W, Schwind KH, Wagner H, Schwagele F. Chemical safety of meat and meat products. Meat Sci. 2010;86:38–48. doi: 10.1016/j.meatsci.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 2. Jenson I, Sumner J. Performance standards and meat safety—developments and direction. Meat Sci. 2012;92:260–6. doi: 10.1016/j.meatsci.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 3. Baert K, Van Huffel X, Jacxsens L, Berkvens D, Diricks H, Huyghebaert A, Uyttendaele M. Measuring the perceived pressure and stakeholders’ response that may impact the status of the safety of the food chain in Belgium. Food Res Int. 2012;48:257–64. [Google Scholar]
- 4. Castro-Puyana M, Herrero M. Metabolomics approaches based on mass spectrometry for food safety, quality and traceability. Trends Analyt Chem. 2013;52:74–87. [Google Scholar]
- 5.Hardman JG, Limburd LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 9th ed. New York: McGraw-Hill; 1996. [Google Scholar]
- 6. Stolker AAM, van Ginkel LA, Stephany RW, Maxwell RJ, Parks OW, Lightfield AR. Supercritical fluid extraction of methyltestosterone, nortestosterone and testosterone at low ppb levels from fortified bovine urine. J Chromatogr B Biomed Appl. 1999;726:121–31. doi: 10.1016/s0378-4347(99)00039-0. [DOI] [PubMed] [Google Scholar]
- 7. Stolker AAM, Brinkman UAT. Analytical strategies for residue analysis of veterinary drugs and growth-promoting agents in food-producing animals—a review. J Chromatogr A. 2005;1067:15–53. doi: 10.1016/j.chroma.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 8. Li T, Cao J, Li Z, Wang X, He P. Broad screening and identification of β-agonists in feed and animal body fluid and tissues using ultra-high performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry combined with spectra library search. Food Chem. 2016;192:188–96. doi: 10.1016/j.foodchem.2015.06.104. [DOI] [PubMed] [Google Scholar]
- 9. Bareille N, Faverdin P. Lipid metabolism and intake behavior of dairy cows: effects of intravenous lipid and β-adrenergic supplementation. J Dairy Sci. 1996;79:1209–20. doi: 10.3168/jds.S0022-0302(96)76474-3. [DOI] [PubMed] [Google Scholar]
- 10. Byrem TM, Beermann DH, Robinson TF. The beta-agonist cimaterol directly enhances chronic protein accretion in skeletal muscle. J Anim Sci. 1998;76:988–98. doi: 10.2527/1998.764988x. [DOI] [PubMed] [Google Scholar]
- 11. Cardoso LA, Taveira O. Effect of clenbuterol on growth, nitrogen and energy balances and endocrine status in food-restricted sheep. J S Afr Vet Assoc. 2002;73:127–30. doi: 10.4102/jsava.v73i3.574. [DOI] [PubMed] [Google Scholar]
- 12. Shook JN, VanOverbeke DL, Kinman LA, Krehbiel CR, Holland BP, Streeter MN, Yates DA, Hilton GG. Effects of zilpaterol hydrochloride and zilpaterol hydrochloride withdrawal time on beef carcass cutability, composition, and tenderness. J Anim Sci. 2009;87:3677–85. doi: 10.2527/jas.2009-1816. [DOI] [PubMed] [Google Scholar]
- 13. Gowik P, Julicher B, Ladwig M, Behrendt D. Measurement of beta-agonist residues in retinal tissue of food producing animals. Analyst. 2000;125:1103–7. doi: 10.1039/b000994f. [DOI] [PubMed] [Google Scholar]
- 14. Brambilla G, Cenci T, Franconi F, Galarini R, Macri A, Rondoni F, Strozzi M, Loizzo A. Clinical and pharmacological profile in a clenbuterol epidemic poisoning of contaminated beef meat in Italy. Toxicol Lett. 2000;114:47–53. doi: 10.1016/s0378-4274(99)00270-2. [DOI] [PubMed] [Google Scholar]
- 15. Zhang GJ, Fang BH, Liu YH, Wang XF, Xu LX, Zhang YP, He LM. Development of a multi-residue method for fast screening and confirmation of 20 prohibited veterinary drugs in feedstuffs by liquid chromatography tandem mass spectrometry. J Chromatogr B. 2013;936:10–7. doi: 10.1016/j.jchromb.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 16. Stachel CS, Radeck W, Gowik P. Zilpaterol—a new focus of concern in residue analysis. Anal Chim Acta. 2003;493:63–7. [Google Scholar]
- 17. Preželj A, Obreza A, Pečar S. Abuse of clenbuterol and its detection. Curr Med Chem. 2003;10:281–90. doi: 10.2174/0929867033368330. [DOI] [PubMed] [Google Scholar]
- 18. Morales-Trejo F, León SV-y, Escobar-Medina A, Gutiérrez-Tolentino R. Application of high-performance liquid chromatography–UV detection to quantification of clenbuterol in bovine liver samples. J Food Drug Anal. 2013;21:414–20. [Google Scholar]
- 19. Sirhan AY, Tan GH, Wong RCS. Method validation in the determination of aflatoxins in noodle samples using the QuEChERS method (Quick, Easy, Cheap, Effective, Rugged and Safe) and high performance liquid chromatography coupled to a fluorescence detector (HPLC–FLD) Food Control. 2011;22:1807–13. [Google Scholar]
- 20. Di Corcia D, Morra V, Pazzi M, Vincenti M. Simultaneous determination of β2-agonists in human urine by fast-gas chromatography/mass spectrometry: method validation and clinical application. Biomed Chromatogr. 2010;24:358–66. doi: 10.1002/bmc.1300. [DOI] [PubMed] [Google Scholar]
- 21. Gallo P, Brambilla G, Neri B, Fiori M, Testa C, Serpe L. Purification of clenbuterol-like β2-agonist drugs of new generation from bovine urine and hair by α1-acid glycoprotein affinity chromatography and determination by gas chromatography–mass spectrometry. Anal Chim Acta. 2007;587:67–74. doi: 10.1016/j.aca.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 22. Du X-D, Wu Y-L, Yang H-J, Yang T. Simultaneous determination of 10 β2-agonists in swine urine using liquid chromatography–tandem mass spectrometry and multi-walled carbon nanotubes as a reversed dispersive solid phase extraction sorbent. J Chromatogr A. 2012;1260:25–32. doi: 10.1016/j.chroma.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 23. González-Antuña A, Domínguez-Romero JC, García-Reyes JF, Rodríguez-González P, Centineo G, García Alonso JI, Molina-Díaz A. Overcoming matrix effects in electrospray: Quantification of β-agonists in complex matrices by isotope dilution liquid chromatography–mass spectrometry using singly 13C-labeled analogues. J Chromatogr A. 2013;1288:40–7. doi: 10.1016/j.chroma.2013.02.074. [DOI] [PubMed] [Google Scholar]
- 24. Dasenaki ME, Thomaidis NS. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography–tandem mass spectrometry. Anal Chim Acta. 2015;880:103–21. doi: 10.1016/j.aca.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 25. Shen J, Zhang Z, Yao Y, Shi W, Liu Y, Zhang S. Time-resolved fluoroimmunoassay for ractopamine in swine tissue. Anal Bioanal Chem. 2007;387:1561–4. doi: 10.1007/s00216-006-1063-4. [DOI] [PubMed] [Google Scholar]
- 26. Keskin S, Özer D, Temizer A. Gas chromatography-mass spectrometric analysis of clenbuterol from urine. J Pharm Biomed Anal. 1998;18:639–44. doi: 10.1016/s0731-7085(98)00284-2. [DOI] [PubMed] [Google Scholar]
- 27. Wang L, Zeng Z, Wang X, Yang J, Chen Z, He L. Multiresidue analysis of nine β-agonists in animal muscles by LC-MS/MS based on a new polymer cartridge for sample cleanup. J Sep Sci. 2013;36:1843–52. doi: 10.1002/jssc.201201088. [DOI] [PubMed] [Google Scholar]
- 28. Qiao F, Du J. Rapid screening of clenbuterol hydrochloride in chicken samples by molecularly imprinted matrix solid-phase dispersion coupled with liquid chromatography. J Chromatogr B. 2013;923–924:136–40. doi: 10.1016/j.jchromb.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 29. Shishani E, Chai SC, Jamokha S, Aznar G, Hoffman MK. Determination of ractopamine in animal tissues by liquid chromatography-fluorescence and liquid chromatography/tandem mass spectrometry. Anal Chim Acta. 2003;483:137–45. [Google Scholar]
- 30. Anastassiades M, Lehotay SJ, Tajnbaher D, Schenck FJ. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J AOAC Int. 2003;86:412–31. [PubMed] [Google Scholar]
- 31. Xu Z, Hu Y, Hu Y, Li G. Investigation of ractopamine molecularly imprinted stir bar sorptive extraction and its application for trace analysis of β2-agonists in complex samples. J Chromatogr A. 2010;1217:3612–8. doi: 10.1016/j.chroma.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 32. Nakajima T, Nagano C, Sasamoto T, Hayashi H, Kanda M, Kanai S, Takeba K, Matsushima Y, Takano I. Development and validation of rapid analysis method for multi-class veterinary drugs in livestock products by LC-MS/MS. Shokuhin Eiseigaku Zasshi. 2012;53:243–53. doi: 10.3358/shokueishi.53.243. [DOI] [PubMed] [Google Scholar]
- 33. Noguchi S, Terada H, Tamura Y. Simultaneous determination of veterinary drugs in livestock foods and seafoods using liquid chromatography/tandem mass spectrometry. Shokuhin Eiseigaku Zasshi. 2008;49:177–88. doi: 10.3358/shokueishi.49.177. [DOI] [PubMed] [Google Scholar]
- 34. Kajita H, Hatakeyama E. Simultaneous determination of residual veterinary drugs in livestock products and fish by liquid chromatography with tandem mass spectrometry. Shokuhin Eiseigaku Zasshi. 2008;49:381–9. doi: 10.3358/shokueishi.49.381. [DOI] [PubMed] [Google Scholar]
- 35.Directive 96/23/EC. Office for Official Publications of the European Communities. 1996. [4]. http://publications.europa.eu/en/publication-detail/-/publication/ebc5f19b-fd6c-4d23-b092-4f96d08d73da/language-en.
- 36. Wang X, Wang S, Cai Z. The latest developments and applications of mass spectrometry in food-safety and quality analysis. Trends Analyt Chem. 2013;52:170–85. [Google Scholar]
- 37. Boscher A, Guignard C, Pellet T, Hoffmann L, Bohn T. Development of a multi-class method for the quantification of veterinary drug residues in feedingstuffs by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2010;1217:6394–404. doi: 10.1016/j.chroma.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y, Li X, Liu X, Zhang J, Cao Y, Shi Z, Sun H. Multi-class, multi-residue analysis of trace veterinary drugs in milk by rapid screening and quantification using ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry. J Dairy Sci. 2015;98:8433–44. doi: 10.3168/jds.2015-9826. [DOI] [PubMed] [Google Scholar]
- 39. Yan K, Zhang H, Hui W, Zhu H, Li X, Zhong F, Tong Xe, Chen C. Rapid screening of toxic salbutamol, ractopamine, and clenbuterol in pork sample by high performance liquid chromatography—UV method. J Food Drug Anal. 2016;24:277–83. doi: 10.1016/j.jfda.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng L, Wu Y, Zhao Y, Li L, Ma Y. Simultaneous determination of 18 β-agonist residues in feed using QuEChERS sample preparation and high performance liquid chromatography-tandem mass spectrometry. Se Pu. 2014;32:867–73. doi: 10.3724/sp.j.1123.2014.03029. [DOI] [PubMed] [Google Scholar]
- 41. Xiong L, Gao YQ, Li WH, Yang XL, Shimo SP. Simple and sensitive monitoring of β2-agonist residues in meat by liquid chromatography–tandem mass spectrometry using a QuEChERS with preconcentration as the sample treatment. Meat Sci. 2015;105:96–107. doi: 10.1016/j.meatsci.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 42. Crescenzi C, Bayoudh S, Cormack PAG, Klein T, Ensing K. Determination of clenbuterol in bovine liver by combining matrix solid-phase dispersion and molecularly imprinted solid-phase extraction followed by liquid chromatography/electrospray ion trap multiple-stage mass spectrometry. Anal Chem. 2001;73:2171–7. doi: 10.1021/ac0014360. [DOI] [PubMed] [Google Scholar]
- 43. Bocca B, Di Mattia M, Cartoni C, Fiori M, Felli M, Neri B, Brambilla G. Extraction, clean-up and gas chromatography–mass spectrometry characterization of zilpaterol as feed additive in fattening cattle. J Chromatogr B. 2003;783:141–9. doi: 10.1016/s1570-0232(02)00528-7. [DOI] [PubMed] [Google Scholar]
- 44. Nielen MWF, Elliott CT, Boyd SA, Courtheyn D, Essers ML, Hooijerink HH, Bennekom EOv, Fuchs REM. Identification of an unknown β-agonist in feed by liquid chromatography/bioassay/quadrupole time-of-flight tandem mass spectrometry with accurate mass measurement. Rapid Commun Mass Spectrom. 2003;17:1633–41. doi: 10.1002/rcm.1099. [DOI] [PubMed] [Google Scholar]
- 45. Gowik P, Julicher B, Ladwig M, Behrendt D. Measurement of β-agonist residues in retinal tissue of food producing animals. Analyst. 2000;125:1103–7. doi: 10.1039/b000994f. [DOI] [PubMed] [Google Scholar]
- 46. Traynor IM, Crooks SRH, Bowers J, Elliott CT. Detection of multi-β-agonist residues in liver matrix by use of a surface plasma resonance biosensor. Anal Chim Acta. 2003;483:187–91. [Google Scholar]