Abstract
Recent developments in nanoscience and nanotechnology intend novel and innovative applications in the food sector, which is rather recent compared with their use in biomedical and pharmaceutical applications. Nanostructured materials are having applications in various sectors of the food science comprising nanosensors, new packaging materials, and encapsulated food components. Nanostructured systems in food include polymeric nanoparticles, liposomes, nanoemulsions, and microemulsions. These materials enhance solubility, improve bioavailability, facilitate controlled release, and protect bioactive components during manufacture and storage. This review highlights the applications of nanostructured materials for their antimicrobial activity and possible mechanism of action against bacteria, including reactive oxygen species, membrane damage, and release of metal ions. In addition, an overview of nanostructured materials, and their current applications and future perspectives in food science are also presented.
Keywords: food packaging, food science, nanocomposites, nanoencapsulation, nanostructures
1. Introduction
Applications of nanotechnology have been emphasized on the characterization, fabrication, and manipulation of nanostructures or nanomaterials. Nanostructured materials are those with at least one dimension falling in a nanometer scale and consist of nanoparticles (NPs), nanorods nanowires, thin films, and bulk materials made of nanoscale building blocks or consisting of nanoscale structures. Based on the dimension of their structural elements, nanostructured materials can be categorized into zero dimensional (e.g., NPs: nanoclusters, quantum dots, and fullerenes), one-dimensional (e.g., nanorods or nanotubes), two-dimensional (e.g., thin films), and three-dimensional (e.g., nanocomposites and den-drimers) nanomaterials.
In this review, we follow the general definition that nanotechnology is the technology dealing with both NPs and nanostructures.
Many technologies have been explored to fabricate nanostructures and nanomaterials. A nanostructure is of intermediate size between a nanodimension and a microdimension that can be developed as various forms. It is basically “structured” using soft/hard templates to form microlevel structures. In recent years, the definition of nanostructured materials has been limited to materials that show entirely new physical and chemical properties, and therefore differ considerably from macroscaled materials with the same chemical structure [1]. Nanostructure materials are basically seen in the form of layered (lamellar) films, atomic clusters, and wire structures. Additionally, nanostructured material properties are completely dependent on the size and nature of their microstructure. The building blocks of the nanostructured materials are basically NPs, which are of the simplest form; nanocomposites are made of more complicated elementary structures.
Applications of nanotechnology in food science are going to impact the vital aspects of food and related industries from food safety to the molecular synthesis of new food products and ingredients [2]. The unique properties of these nanostructures and nanomaterials including physical, chemical, and biological properties are considerably different from their bulk counterparts alter the understanding of biological and physical occurrence in food systems. Several recent reports and reviews have identified potential applications of nanotechnology for the food sector to improve food safety, to enhance packaging and lead to improved processing and nutrition [3–10].
Increasing the shelf life of the food (preservation), food safety, coloring, flavoring, and nutritional additives, and using the antimicrobial ingredients for food packaging are some of the important applications of nanotechnology in the food industry [5,11]. Nanotechnology has major advantages in its usage for packaging in comparison with the conventional ways using polymers, which may include merits such as enhanced barrier, and mechanical and heat-resistant properties, along with biodegradability [12]. In addition to enhanced antimicrobial effects, nanomaterials can be used for detection of food spoilage through nanosensors [13].
Although nanotechnology has a great potential to fabricate innovative products and processes in the food sector, there are many challenges to overcome in food science and technology. The major challenges are to produce edible delivery systems using economic processing operations with effective formulation for human consumption and safety [14]. In this context, nanostructures are produced from food-grade ingredients using simple and economic approaches (such as the layer-by-layer technique).
This review updates current applications of nanotechnology to the food industry, presenting the use of different nanostructures and related manufacturing technologies used to build functional food systems. Moreover, this article focuses on applications particularly toward current and emerging technologies that may be used for food formulations, processing, and storage. This review also highlights the applications for antimicrobial activity and their possible mechanism of action against bacteria, including reactive oxygen species (ROS), membrane damage, and release of metal ions.
2. Methods used in nanofabrication
Top-down and bottom-up are the two approaches to the synthesis of nanomaterials and fabrication of nanostructures. The top-down approach is to break bulk materials using milling to reduce the size to nanoscale. It involves mechanical grinding of bulk materials and subsequent stabilization of the resulting nanosized particles by the addition of colloidal protecting agents. However, the major problem with the top-down method is the slow production rate, which makes it unsuitable for mass production [15,16]. The other disadvantages include surface defects (i.e., imperfections), contaminations, and introduction of internal stress. This process also involves material wastage and is limited by the resolution of the tools employed.
The bottom-up approach is fundamentally different from the top-down approach, involving the building of nanomaterials from individual atoms that have the ability to self-assemble in a natural and self-regulating manner [17]. The bottom-up approach has a better chance of producing nanostructures with less defects, more homogenous chemical composition, and better short- and long-range ordering.
3. Various processes for the structuring of food materials
The specific processes of NP preparation through the top-down approach are dry milling, high-pressure homogenization or micofluidization, and ultrasound emulsification [18]. Milling is a conventional method, where mechanical energy is applied to physically break down materials to coarse particles (size reduction to nanoscale). For example, dry milling technology can be used to obtain wheat flour of fine size that has a high water-binding capacity [19] and also to enhance the antioxidant activity in green tea powder [20]. Homogenizers have been used conventionally for reducing the size of fat globules to augment the stability of emulsions [21]. A liquid product is subjected to very high stress causing the formation of extremely fine emulsion droplets in high-pressure homogenization. High-pressure homogenizers are proficient in producing finer milk emulsions than conventional homogenizers [22]. Micofluidization is a form of homogenization in which auxiliary chambers are used for size reduction and emulsion formation, thereby it enhances texture and mouth feel [18]. It has effectively been used in the production of salad dressing, creams, yoghurts, syrups, chocolate and malted drinks, flavor oil emulsions, fillings, and icings [23]. More recently, reported during the time period 2011–2016, ultrasound emulsification is used to prepare oil and water nanoemulsions using high-intensity ultrasound waves that can change the characteristics of treated matter through intensive shear forces, pressure, and temperature due to cavitation [24–26].
4. Nanostructured materials in food
Some food products contain the ingredients that are nano-sized and different from synthetically manufactured nanomaterials. Many food proteins are of globular structures between 10 nm and 100 nm in size, and others include the majority of polysaccharides and lipids, which are linear polymers of <1 nm in thickness (one-dimensional nanostructures). Milk and milk products, such as milk proteins and casein, are also natural nanostructures.
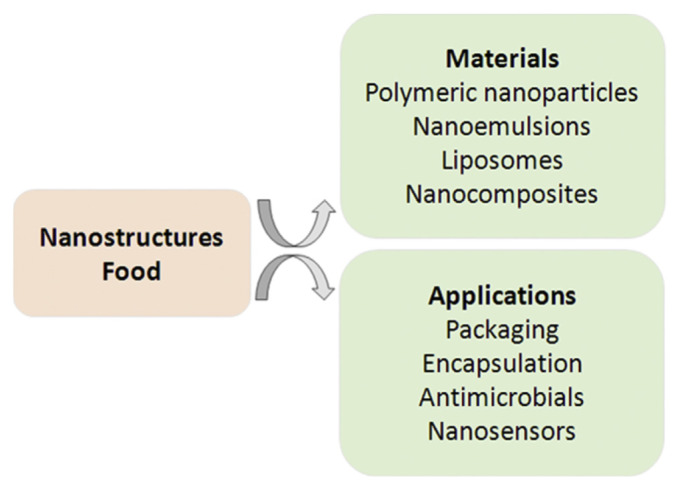
The most significant synthetic nanostructured systems in food are polymeric NPs, liposomes, nanoemulsions, and microemulsions. These materials enhance solubility, improve bioavailability, facilitate controlled release, and protect bioactive components during manufacture and storage [27]. An overview of nanostructures in food and their applications in food science are illustrated in Figure 1.
Figure 1.
Overview of nanostructures in food and their applications in food science.
4.1. Biopolymeric nanostructured particles (proteins)
Micelles (spherical structures of 5–100 nm in diameter) are able to encapsulate nonpolar molecules such as lipids, vitamins, and antioxidants. Elements that are not water soluble can be solubilized using micelles and are referred to as microemulsions. Microemulsions have also been used to produce glycerides for applications in food products. An important application of microemulsion is to provide improved antioxidation effectiveness because of the possibility of a synergistic effect between hydrophilic and lipophilic antioxidants.
Food-grade biopolymers such as proteins or polysaccharides can be used to produce nanometer-sized particles [28–30]. One of the most common components of many biodegradable biopolymeric NPs is polylactic acid. It is commonly available from a number of manufacturers. Polylactic acid is regularly used to encapsulate and deliver drugs, vaccines, and proteins, but it has certain limitations: it is quickly removed from the bloodstream and remains isolated in the liver and kidneys. As its purpose as an NP is to deliver active components to other areas of the body, polylactic acid needs an associative compound such as polyethylene glycol to be successful in this regard [31].
4.2. Liposomes
Liposomes are spherical bilayered vesicles of phospholipids [32]. Liposomes have been used in the food industry to encapsulate functional ingredients, and more recently, they have been explored for their ability to integrate food antimicrobials that could aid in the protection of food products against growth of spoilage and pathogenic microorganisms [33]. Lipid-based nanoencapsulation can potentially improve the solubility, stability, and bioavailability of foods, thus preventing unwanted interactions with other food components. Nanoliposomes are some of the most promising lipid-based carriers for antioxidants. Nanoliposomes also help in controlled and specific delivery of nutraceuticals, nutrients, enzymes, vitamins, antimicrobials, and additives [32]. Liposomes have a smaller size and a larger interfacial surface area for contact with biological tissues, and thereby provide greater bioavailability of encapsulated compounds [27,34,35].
4.3. Nanoemulsions
Nanoemulsions are colloidal dispersions with droplet sizes ranging from 50 nm to 1000 nm. They are used to produce food products for flavored oils, salad dressing, personalized beverages, sweeteners, and other processed foods [36]. Nanoemulsions present many advantages such as decontamination of equipment and high clarity without compromising product appearance and flavor. Nanosized functional compounds that are encapsulated by the self-assembled nanoemulsions are used for targeted delivery of lutein; β-carotene; lycopene; vitamins A, D, and E3; co-enzymeQ10; and omega-3-fatty acids [37]. Stable double-layered capsaicin-loaded nanoemulsions were stabilized with natural polymers such as alginate and chitosan for use as a functional ingredient delivery system [38]. Functional food components can be integrated within the droplets [39] regularly to allow a slowdown of chemical degradation processes by engineering the properties of the interfacial layer surrounding them [40]. Another application of nanoemulsion includes bottled drinking water and milk fortified with vitamins, minerals, and antioxidants [41].
4.4. Nanocomposites
Using the polymers in nanocomposite food packaging is one of the good alternatives for conventional packaging materials (glass, paper, and metals) due to their functionality and low cost [42]. Nanocomposites are polymer matrices reinforced in the nanofillers (nanoclays, nanooxides, carbon nanotubes, and cellulose microfibrils), where one of the phases has at least one, two, or three dimensions less than 100 nm in size [43]. Several synthetic (polyamide, polystyrene, nylon, and polyolefins) and natural (chitosan, cellulose, and carrageenan) polymers have been used in food packaging [43,44]. However, due to environmental concerns, there is a growing demand for biodegradable packaging with the use of biopolymers that are either natural or synthetic (polyvinyl alcohol, polylactide, and polyglycolic acid) [45].
5. Applications of nanostructures in the food industry
Potential applications in food science and technology are mainly categorized into packaging, process technology, antimicrobials, and food ingredients. Its usage in foodstuff can be categorized as “direct” or “indirect.” Direct use refers to the incorporation of nanostructured substances and materials in foodstuff and must also be declared as such. Some of the direct applications include fragrances, coloring agents, antioxidants, preservatives, and biologically active components (vitamins, omega-3 fatty acids, polyphenols, etc.). Indirect use comprises the use of nanostructured materials in packaging technology [13] and sensors, or the use of proficiently nanostructured catalyzers for the hydration of fats [46,47]. Therefore, greater parts of applications of nanostructured materials are included in this category. However, it should be noted that indirect use of nanostructures come into contact with the foodstuff [1,48], as they are directly incorporated into matrix to benefit food production such as catalyzed hydration of fats with low trans-fatty acid contents.
5.1. Nanosensors
Nanodevices or nanosensors in conjunction with polymers are used to monitor food pathogens and chemicals during storage and transit processes in smart packaging [49,50]. Additionally, smart packaging ensures integrity of the food package and authenticity of the food product. Furthermore, these devices may also track the history of time, temperature, and expiration date. Several recent reports indicated that nanosensors are able to detect toxins and food pathogens in the packaging [43,51,52]. Nanosensors for food analysis, flavors, drinking water, and clinical diagnostics have also been developed [53], and NPs can also be incorporated as nanostructured transducers of biosensor devices [54].
A low-cost nanobioluminescent spray [55] was developed, which reacts with microbes making the food to produce a visual glow, thereby indicating microbial contamination. Nanocantilevers are used for pathogen detection, and recently a rapid biosensor (i.e., micromechanical oscillators) was developed for the detection of bacterial growth of Escherichia coli [56]. The basis of the detection scheme is the change in resonance frequency as a function of the increasing mass on a cantilever array, which can detect E. coli within 1 hour, which is considerably faster than any conventional plating method that needs at least 24 hours. Cantilevers can also be used to detect other changes such as temperature, superficial tension, and mass. Using a series of cantilevers with different molecular recognition elements together in an array mounted on a single chip can be used to detect multiple toxins and microorganisms [57,58]. Molecular imprinted polymers and nanomaterials, being developed for food quality control, are able to recognize a large number of small molecules, large proteins, and macromolecules. Recently, an imprinted core–shell NPs with a silica core has been developed for the detection of tert-butylhydroquinone in foodstuff [59]. Additional nanosensors developed based on the molecular imprinted polymer technology include those used for the detection of trypsin, glucose, catechol, and ascorbic acid [60–64].
5.2. Food packaging
Nanotechnology offers food safety in terms of packaging for ensuring a longer shelf life by avoiding spoilage or loss of food nutrients. Active packaging has a desirable role in food preservation other than providing an inert barrier to the external conditions. It mainly refers to the packaging systems that respond to changes in the environment. They act by releasing desirable molecules such as antimicrobial or antioxidant agents, or act as gas scavengers. These interactions result in improved food stability, and some of these packaging systems include the antimicrobials, oxygen scavengers, and enzyme immobilization systems. Another application in active packaging is the controlled-release packaging, where nanocomposites can also be used as delivery systems, thereby helping the migration of functional additives, such as minerals, probiotics, and vitamins, into food [65]. Silver NPs are used in packaging materials to preserve food for longer periods by killing microorganisms in 6 minutes [18,66]. Nylon nanocomposites providing barriers to oxygen and carbon dioxide flow have also been used in food packaging to maintain freshness and block out smells in food. A typical example is multilayer polyethylene terephthalate (PET) bottles for beer and alcoholic beverages [66,67].
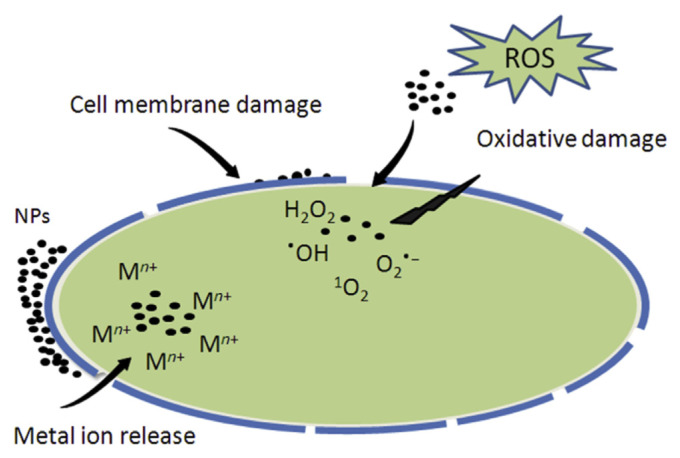
Active packaging includes usage of metal and metal oxide NPs as antimicrobial agents in the form of nanocomposites for food packaging. Titanium dioxide, zinc oxide, copper, copper oxide, and silver-based nanofillers are used due to their antimicrobial properties [11]. TiO2- and SiO2-based nanofillers are used for applications in self-cleaning surfaces [11]. Among them silver NPs are the most common NPs, which are effective against a wide variety of microorganisms [13,68]. Adhesion to cell surface, disruption of cell membrane, DNA damage, and release of silver ions are the mechanisms of silver antimicrobial agent [11,69,70]. The antibacterial activity of metal nanostructures is mostly dependent on various factors such as large surface area, size, shape, particle internalization, and chemical functionalization [71]. Our earlier studies and reports from others demonstrated that these nanostructures can penetrate into outer and inner bacterial membranes [70,72–74]. Three major mechanisms of bacterial toxicity of metal-containing nanomaterials (Figure 2) that are widely accepted in literature include metal ions uptake causing depletion of intracellular ATP [75], generation of ROS causing oxidative damage to cells [76,77], and bacterial membrane damage [78]. ROS include free radicals such as superoxide anion (O2•−), hydroxyl radical (•OH), as well as nonradical molecules such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) [77].
Figure 2.
Schematic representation of possible mechanisms of toxicity involved in metal-containing nanomaterials: (1) metal ion uptake into cells, (2) generation of reactive oxygen species, and (3) bacterial cell membrane damage. NP =nanoparticle; ROS =reactive oxygen species.
Titanium dioxide has a broad range of applications, for example, as a UV blocker, pigment, photocatalyst, and antimicrobial agent [79]. Moreover, TiO2 NPs are effective against food spoilage-related bacteria [80,81], and they are also used in food packaging [11,79]. Besides TiO2, it was reported that a plastic wrap containing ZnO NPs can be used to keep packaging surfaces hygienic under indoor lighting conditions [5]. Oxygen scavenger films were developed by adding TiO2 NPs into different polymers [82] under UV illumination, but one major drawback of TiO2 NPs is due to its photocatalytic mechanisms, i.e., becoming active mainly under UV light because of the large bandgap [83]. The major toxicity mechanism of TiO2 nanomaterials is the production of ROS under visible and UV light (photocatalytic activity) causing lipid peroxidation, which leads to oxidative stress and cell death [74,84–86].
Graphene nanoplate-based nanocomposites are reported for their heat resistance and barrier properties, which have an improved food-packaging application [87]. Carbon nanotubes and nanofibers are used due to their electrical and mechanical properties, but their applications in food packaging are limited because of the cost factor and difficulty in processing dispersions [88]. Nanoclays with montmorillonite NPs in different starch-based materials (biodegradable polymers) were developed to improve barrier and mechanical properties [89]. Most commonly used nanoclay material montmorillonite (also known as bentonite) is used to achieve the gas barrier properties, and it can restrict permeation of gases when incorporated into a polymer. It is also widely available and is relatively inexpensive. Some of the biodegradable nanocomposites in starch clay have been reported for several applications including food packaging [90–94].
5.3. Encapsulated food components and edible supplements
The potential applications of nanotechnology in functional food, and design of nutritional supplements and nutraceuticals containing nanosized ingredients and additives such as vitamins, antimicrobials, antioxidants, and preservatives are currently available for enhanced taste, absorption, and bioavailability [95]. To avoid accumulation of cholesterol, some of the nutraceuticals incorporated in the carriers consist of lycopene, beta-carotenes, and phytosterols [96]. A green tea product containing nanoselenium has many health benefits resulting from better uptake of selenium. Nanoencapsulation is the process of packing materials at nanoscale with the help of nanocapsules, and it provides final product functionality that includes controlled release of the core. Thus, encapsulated forms of ingredients have several advantages, which include a longer shelf life, enhanced stability, consecutive delivery of multiple active ingredients, and pH-triggered controlled release. Functional ingredients such as vitamins, antioxidants, probiotics, carotenoids, preservatives, omega fatty acids, proteins, peptides, and lipids as well as carbohydrates are incorporated into a nanodelivery system [97]. This increases the functionality and stability of these foods, as they are not used in their pure form. Lipid based nanoencapsulation can potentially improve the solubility, stability, and bioavailability of foods, thus preventing unwanted interactions with other food components. Nanoliposomes and nanocochleates are some of the most promising lipid-based carriers for antioxidants. Nanoliposomes also help in controlled and specific delivery of nutraceuticals, nutrients, enzymes, vitamins, antimicrobials, and additives [32]. Nanocochleates have the ability to stabilize micronutrients and can enhance the nutritional value of processed foods. Nanoencapsulated probiotics can be selectively delivered to certain parts of the gastrointestinal tract, where they have the capability to modulate immune responses [98]. In the current market, Tip-Top Up bread in Western Australia is fortified with omega-3 fatty acids, which makes it one of the best examples of the above-mentioned application. HydraCel, a natural mineral product (5 nm in size) can be used to improve the absorption of water and other nutrients in the body by decreasing the surface tension of drinking water [99]. Other nanoencapsulated products include α-lactalbumin (a hydrolyzed milk protein) as a carrier of nutrients [100], casein micelles for delivery of sensitive food products [101], dextrins for bioactive products, and hydrophobically modified starch to encapsulate curcumin [102].
6. Toxicological aspects
In contrast to the beneficial applications as antimicrobials and antioxidants, overproduction of ROS by nanostructures is expected to cause a cell-damaging effect. Overall, literature reports on the toxicity of nanostructures used in food science are very limited. The common properties shared by nanostructures and NPs, such as nanoscale, large surface area, and high reactivity, may pose a health threat to humans and other organisms. Nanostructures in the food sector may not create a direct effect on human health; however, their nanoscale property may cause some unavoidable side effects. Mechanisms of toxicity induced by nanomaterials or NPs have been studied intensively [77,103,104]. Nanotoxicity is principally mediated through the overproduction of ROS, resulting in the induction of oxidative stress and thereby resulting in cells failing to maintain normal physiological redox-regulated functions. Thus, nanotoxicity can lead to DNA damage, unregulated cell signaling, change in cell motility, cytotoxicity, apoptosis, and cancer initiation [77]. As described previously [77], the critical determinants of nanotoxicity include size, shape, particle surface, surface positive charges, surface-containing groups, particle dissolution, metal ion release from nanometals and nanometal oxides, UV light activation, aggregation, mode of interaction with cells, inflammation, and pH of the medium. As such, it is apparent that the severity of toxicity of nanomaterials should be in the following order: NPs > nanotubes (nanowires) > nanostructures. Thus, the information concerning the nanotoxicity caused by NPs can provide some useful reference for the nanostructure-induced toxicity. Besides, these nanostructured materials or NPs used in packaging materials may migrate into food. For example, silver and zinc oxide NPs may migrate into food when they are used as packaging materials, and silver ions of a detectable level were leeched into the meat exudates (although not into the meat itself) [105]; it was also reported that the metal ions of silver and zinc leached into food substance (orange juice), which may accelerate the degradation of ascorbic acid [106]. Hence, further research is needed before these antimicrobial nanocomposite packaging materials are commercialized. Other studies showed that migration of NPs into actual food occurs [107–111]. However, a recent report by ToxConsult Pty Ltd [112] indicated that based on the case studies of food packing with nanomaterials migration of intact NPs into food simulants were found to be negligible. Nevertheless, validity of the testing results may be compromised, as the complex matrix of most foods could interfere with precise measurements of migrated NPs or ions within the food [107,113]. Therefore, more studies need to be conducted to evaluate the potential toxicity of nanomaterials or nanostructures to be used in food science and related industries.
7. Future perspectives
While scientific advances in the application of nanotechnology to the food industry have been progressing rapidly, development of nanotechnology associated with nanostructures is relatively much slower. As the research in food nanotechnology increases, it also raises public concerns about safety of the products of nanotechnology for human consumption and usage [114]. Therefore, a comprehensive assessment of potential risks to human health is essential before the nano food products are commercially available. However, there has been a lack of a universal guideline that is specifically developed for the safety assessment of nanomaterials in food. Recently, EU regulations established that any food ingredient resulting from nanotechnological applications must undergo safety assessment before being approved for its use [115]. Some guidelines were also released recently from the United States Food and Drug Administration, which are related to the use of nanotechnology in food [116]. Without a doubt, in the near future more studies and regulations concerning the impacts of these nanomaterials on human and environmental health need to be conducted and established to assure our food safety.
Acknowledgments
This study was supported by NSF-CREST program [the National Science Foundation-Centers of Research Excellence in Science and Technology (NSF-CREST)] with grant #HRD-157754 to Jackson State University.
Funding Statement
This study was supported by NSF-CREST program [the National Science Foundation-Centers of Research Excellence in Science and Technology (NSF-CREST)] with grant #HRD-157754 to Jackson State University.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. European Food Safety Authority (EFSA) The potential risks arising from nanoscience and nanotechnologies on food and feed safety. EFSA J. 2009;958:1–39. [Google Scholar]
- 2. Chen H, Weiss J, Shahidi F. Nanotechnology in nutraceuticals and functional foods. Food Technol. 2006;60:30–6. [Google Scholar]
- 3. Sekhon BS. Nanotechnology in agri-food production: an overview. Nanotechnol Sci Appl. 2014;7:31–53. doi: 10.2147/NSA.S39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dasgupta N, Ranjan S, Mundekkad D, Ramalingam C, Shanker R, Kumar A. Nanotechnology in agro-food: from field to plate. Food Res Int. 2015;69:381–400. [Google Scholar]
- 5. Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, Aitken R, Watkins R. Applications and implications of nanotechnologies for the food sector. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:241–58. doi: 10.1080/02652030701744538. [DOI] [PubMed] [Google Scholar]
- 6. Kour H, Malik AA, Ahmad N, Wani TA, Kaul RK, Bhat A. Nanotechnology—new lifeline for food industry. Crit Rev Food Sci Nutr. 2015 [Epub ahead of print] [Google Scholar]
- 7. Narayanan A, Sharma P, Moudgil BM. Applications of engineered particulate systems in agriculture and food industry. Kona Powder Part J. 2012;30:221–35. [Google Scholar]
- 8. Pradhan N, Singh S, Ojha N, Shrivastava A, Barla A, Rai V, Bose S. Facets of nanotechnology as seen in food processing, packaging, and preservation industry. Biomed Res Int. 2015 doi: 10.1155/2015/365672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sekhon BS. Food nanotechnology—an overview. Nanotechnol Sci Appl. 2010;3:1–15. [PMC free article] [PubMed] [Google Scholar]
- 10. He X, Hwang HM. Nanotechnology in food science: functionality, applicability, and safety assessment. J Food Drug Anal. 2016;24:671–81. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee KT. Quality and safety aspects of meat products as affected by various physical manipulations of packaging materials. Meat Sci. 2010;86:138–50. doi: 10.1016/j.meatsci.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 12. de Azeredo HMC. Nanocomposites for food packaging applications. Food Res Int. 2009;42:1240–53. [Google Scholar]
- 13. McClements DJ, Xiao H. Potential biological fate of ingested nanoemulsions: influence of particle characteristics. Food Funct. 2012;3:202–20. doi: 10.1039/c1fo10193e. [DOI] [PubMed] [Google Scholar]
- 14.Labrune JC, Palmino F. Nanowires. In: Dupas C, Houdy P, Lahmani M, editors. Nanoscience, nanotechnologies and nanophysics. Berlin: Springer; 2004. pp. 325–79. [Google Scholar]
- 15. Hsieh YHP, Ofori JA. Innovation in food technology for health. Asia Pac J Clin Nutr. 2007;16:65–73. [PubMed] [Google Scholar]
- 16. Moraru CI, Panchapakesan CP, Huang Q, Takhistov P, Liu S, Kokini JL. Nanotechnology: a new frontier in food science. Food Technol. 2003;57:24–9. [Google Scholar]
- 17. Sanguansri P, Augustin MA. Nanoscale materials development: a food industry perspective. Trends Food Sci Technol. 2006;17:547–56. [Google Scholar]
- 18. Degant O, Schwechten D. Wheat flour with increased water binding capacity and process and equipment for its manufacture. DE10107885A1. German Patent. 2002
- 19. Shibata T. Method for producing green tea in microfine powder. US6416803B1. United States Patent. 2002
- 20. Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today. 2011;16:354–60. doi: 10.1016/j.drudis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 21. Thiebaud M, Dumay E, Picart L, Guiraud JP, Cheftel JC. High-pressure homogenisation of raw bovine milk—effects on fat globule size distribution and microbial inactivation. Int Dairy J. 2003;13:427–39. [Google Scholar]
- 22. Swientek RJ. Microfluidizing technology enhances emulsion stability. Food Process. 1990:152–3. [Google Scholar]
- 23. Kentish SE, Wooster TJ, Ashokkumar M, Balachandran S, Mawson R, Simons L. The use of ultrasonics for nanoemulsion preparation. Innov Food Sci Emerg. 2008;9:170–5. [Google Scholar]
- 24. Rao J, McClements DJ. Formation of flavor oil microemulsions, nanoemulsions and emulsions: influence of composition and preparation method. J Agric Food Chem. 2011;59:5026–35. doi: 10.1021/jf200094m. [DOI] [PubMed] [Google Scholar]
- 25. Li PH, Chiang BH. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason Sonochem. 2012;19:192–7. doi: 10.1016/j.ultsonch.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 26. Singh H. Nanotechnology applications in functional foods; Opportunities and challenges. Prev Nutr Food Sci. 2016;21:1–8. doi: 10.3746/pnf.2016.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang YC, Chen DGH. Adsorption kinetics and thermodynamics of acid dyes on a carboxymethylated chitosan-conjugated magnetic nano-adsorbent. Macromol Biosci. 2005;5:254–61. doi: 10.1002/mabi.200400153. [DOI] [PubMed] [Google Scholar]
- 28. Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 29. Ritzoulis C, Scoutaris N, Papademetriou K, Stavroulias S, Panayiotou C. Milk protein-based emulsion gels for bone tissue engineering. Food Hydrocol. 2005;19:575–81. [Google Scholar]
- 30. Riley T, Govender T, Stolnik S, Xiong CD, Garnett MC, Illum L, Davis SS. Colloidal stability and drug incorporation elements of micellar-like PLA-PEG nanoparticles. Colloids Surf B. 1999;16:147–59. [Google Scholar]
- 31. Taylor TM, Davidson PM, Bruce BD, Weiss J. Liposomal nanocapsules in food science and agriculture. Crit Rev Food Sci Nutr. 2005;45:587–605. doi: 10.1080/10408390591001135. [DOI] [PubMed] [Google Scholar]
- 32. Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C. Nanoliposomes and their applications in food nanotechnology. J Liposome Res. 2008;18:309–27. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- 33.Singh H, Thompson A, Liu W, Corredig M. Liposomes as food ingredients and nutraceutical delivery systems. In: Garti N, McClements DJ, editors. Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Cambridge, UK: Woodhead Publishing Ltd; 2012. pp. 287–318. [Google Scholar]
- 34.Liu W, Ye A, Singh H. Progress in applications of liposomes in food systems. In: Sagis LMC, editor. Microencapsulation and microspheres for food applications. New York, NY: Academic Press; 2015. pp. 151–70. [Google Scholar]
- 35. Kumar MNVR. A review of chitin and chitosan applications. React Funct Polym. 2000;46:1–27. [Google Scholar]
- 36.Garti N. Delivery and controlled release of bioactives in foods and nutraceuticals. Cambridge, England: Elsevier; Woodland Publishing Co; 2008. [Google Scholar]
- 37. Choi AJ, Kim CJ, Cho YJ, Hwang JK, Kim CT. Characterization of capsaicin-Loaded nanoemulsions stabilized with alginate and chitosan by self-assembly. Food Bioprocess Tech. 2011;4:1119–26. [Google Scholar]
- 38. Jasinska M, Dmytrow I, Mituniewicz-Małek A, Wąsik K. Cow feeding system versus milk utility for yoghurt manufacture. Acta Sci Pol Technol Aliment. 2010;9:189–99. [Google Scholar]
- 39. McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci. 2000;65:1270–82. [Google Scholar]
- 40. Gu YS, Decker AE, McClements DJ. Production and characterization of oil-in-water emulsions containing droplets stabilized by multilayer membranes consisting of beta-lactoglobulin, iota-carrageenan and gelatin. Langmuir. 2005;21:5752–60. doi: 10.1021/la046888c. [DOI] [PubMed] [Google Scholar]
- 41. Huang JY, Li X, Zhou W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci Technol. 2015;45:187–99. [Google Scholar]
- 42. Silvestre C, Duraccio D, Cimmino S. Food packaging based on polymer nanomaterials. Prog Polym Sci. 2011;36:1766–82. [Google Scholar]
- 43. Bastarrachea L, Dhawan S, Sablani SS. Engineering properties of polymeric-based antimicrobial films for food packaging: a review. Food Eng Rev. 2011;3:79–93. [Google Scholar]
- 44. Rhim JW, Park HM, Ha CS. Bio-nanocomposites for food packaging applications. Prog Polym Sci. 2013;38:1629–52. [Google Scholar]
- 45. Shiju NR, Guliants VV. Recent developments in catalysis using nanostructured materials. Appl Catal A. 2009;356:1–17. [Google Scholar]
- 46. Stankovic M, Gabrovska M, Krstic J, Tzetkov, Shopska M, Tsacheva T, Bankovic P, Edreva-Kardjieva R, Jovanović D. Effect of silver modification on structure and catalytic performance of Ni–Mg/diatomite catalysts for edible oil hydrogenation. J Mol Catal A Chem. 2009;297:54–62. [Google Scholar]
- 47. Moraru CI, Huang Q, Takhistov P, Dogan H, Kokini JL. Food nanotechnology: current developments and future prospects. Global Issues Food Sci Technol. 2009;21:369–99. [Google Scholar]
- 48. Yam KL, Takhistov PT, Miltz J. Intelligent packaging: concepts and applications. J Food Sci. 2005;70:R1–10. [Google Scholar]
- 49. Baeumner A. Nanosensors identify pathogens in food. Food Technol. 2004;58:51–5. [Google Scholar]
- 50. Lerner MB, Goldsmith BR, McMillon R, Dailey J, Pillai S, Singh SR, Johnson ATC. A carbon nanotube immunosensor for Salmonella. AIP Adv. 2011;1:042127. [Google Scholar]
- 51. Yang JY, Li Y, Chen SM, Lin KC. Fabrication of a cholesterol biosensor based on cholesterol oxidase and multiwall carbon nanotube hybrid composites. Int J Electrochem Sci. 2011;6:2223–34. [Google Scholar]
- 52. Li Z, Sheng C. Nanosensors for food safety. J Nanosci Nanotechnol. 2014;14:905–12. doi: 10.1166/jnn.2014.8743. [DOI] [PubMed] [Google Scholar]
- 53. Vo-Dinh T, Cullum BM, Stokes DL. Nanosensors and biochips: frontiers in biomolecular diagnostics. Sens Actuators B: Chem. 2001;74:2–11. [Google Scholar]
- 54.Plexus Institute. New nanotechnology food research-if it glows don’t eat it. 2006. [Accessed 20 Apr 2009]. Available from: http://www.plexusinstitute.org/news-events/show_news.cfm?id=164.
- 55. Gfeller KY, Nugaeva N, Hegner M. Micromechanical oscillators as rapid biosensor for the detection of active growth of Escherichia coli. Biosens Bioelectron. 2005;21:528–33. doi: 10.1016/j.bios.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 56. Lange D, Hagleitner C, Hierlemann A, Brand O, Baltes H. Complementary metal oxide semiconductor cantilever arrays on a single chip: mass-sensitive detection of volatile organic compounds. Anal Chem. 2002;74:3084–95. doi: 10.1021/ac011269j. [DOI] [PubMed] [Google Scholar]
- 57. Mabeck JT, Malliaras GG. Chemical and biological sensors based on organic thin-film transistors. Anal Bioanal Chem. 2006;384:343–53. doi: 10.1007/s00216-005-3390-2. [DOI] [PubMed] [Google Scholar]
- 58. Zhao P, Hao J. Tert-butylhydroquinone recognition of molecular imprinting electrochemical sensor based on core–shell nanoparticles. Food Chem. 2013;139:1001–7. doi: 10.1016/j.foodchem.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 59. Hayden O, Haderspo C, Krassnig S, Chen X, Dickert FL. Surface imprinting strategies for the detection of trypsin. Analyst. 2006;131:1044–50. doi: 10.1039/b608354b. [DOI] [PubMed] [Google Scholar]
- 60. Male KB, Hrapovic S, Liu YL, Wang DS, Luong JHT. Electrochemical detection of carbohydrates using copper nanoparticles and carbon nanotubes. Anal Chim Acta. 2004;516:35–41. [Google Scholar]
- 61. Wang SG, Zhang Q, Wang RL, Yoon SF. A novel multi-walled carbon nanotube-based biosensor for glucose detection. Biochem Biophys Res Commun. 2003;311:572–6. doi: 10.1016/j.bbrc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 62. Wolfrum B, Zevenbergen M, Lemay S. Nanofluidic redox cycling amplification for the selective detection of catechol. Anal Chem. 2008;80:972–7. doi: 10.1021/ac7016647. [DOI] [PubMed] [Google Scholar]
- 63. Ambrosi A, Morrin A, Smyth MR, Killard AJ. The application of conducting polymer nanoparticle electrodes to the sensing of ascorbic acid. Anal Chim Acta. 2008;609:37–43. doi: 10.1016/j.aca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 64. Rodriguez F, Sepulveda HM, Bruna J, Guarda A, Galotto MJ. Development of cellulose eco-nanocomposites with antimicrobial properties oriented for food packaging. Packag Technol Sci. 2013;26:149–60. [Google Scholar]
- 65. Graveland-Bikkera JF, de Kruifa CG. Unique milk protein based nanotubes: food and nanotechnology meet. Trends Food Sci Tech. 2006;17:196–203. [Google Scholar]
- 66.Sherman LM.Chasing nanocomposites. 2005. [Accessed 5 Sept 2012]. Available from: http://www.ptonline.com/articles/200411fa2.html.
- 67. Duncan TV. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci. 2011;363:1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Becaro AA, Puti FC, Correa DS, Paris EC, Marconcini JM, Ferreira MD. Polyethylene films containing silver nanoparticles for applications in food packaging: characterization of physico-chemical and anti-microbial properties. J Nanosci Nanotechnol. 2015;15:2148–56. doi: 10.1166/jnn.2015.9721. [DOI] [PubMed] [Google Scholar]
- 69. Dallas P, Sharma VK, Zboril R. Silver polymeric nanocomposites as advanced antimicrobial agents: classification, synthetic paths, applications, and perspectives. Adv Colloid Interface Sci. 2011;166:119–35. doi: 10.1016/j.cis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 70. Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 71.Diaz-Visurraga J, Garcia A, Cardenas G. Morphological changes induced in bacteria as evaluated by electron microscopy. In: Mendez-Vilas A, Díaz J, editors. Microscopy: science, technology, applications and education. Badajoz, Spain: Formatex; 2010. pp. 307–15. [Google Scholar]
- 72.Díaz-Visurraga J, Gutiérrez C, von Plessing C, García A. Metal nanostructures as antibacterial agents. In: Méndez-Vilas A, editor. Science against microbial pathogens: communicating current research and technological advances. Badajoz, Spain: Formatex; 2011. pp. 210–8. [Google Scholar]
- 73. Dasari TP, Pathakoti K, Hwang HM. Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria. J Environ Sci. 2013;25:882–8. doi: 10.1016/s1001-0742(12)60152-1. [DOI] [PubMed] [Google Scholar]
- 74. Pathakoti K, Morrow S, Han C, Pelaez M, He X, Dionysiou DD, Hwang HM. Photoinactivation of Escherichia coli by sulfur-doped and nitrogen-fluorine-codoped TiO2 nanoparticles under solar simulated light and visible light irradiation. Environ Sci Technol. 2013;47:9988–96. doi: 10.1021/es401010g. [DOI] [PubMed] [Google Scholar]
- 75. Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–24. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 76. Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 77. Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Amro NA, Kotra LP, Wadu-Mesthrige K, Bulychev A, Mobashery S, Liu GY. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: structural basis for permeability. Langmuir. 2000;16:2789–96. [Google Scholar]
- 79. Yemmireddy VK, Hung YC. Effect of binder on the physical stability and bactericidal property of titanium dioxide (TiO2) nanocoatings on food contact surfaces. Food Control. 2015;57:82–8. doi: 10.1111/1750-3841.12962. [DOI] [PubMed] [Google Scholar]
- 80. Chawengkijwanich C, Hayata Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int J Food Microbiol. 2008;123:288–92. doi: 10.1016/j.ijfoodmicro.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 81. Robertson JMC, Robertson PKJ, Lawton LA. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms. J Photochem Photobiol A. 2005;175:51–6. [Google Scholar]
- 82. Xiao EL, Green ANM, Haque SA, Mills A, Durrant JR. Light-driven oxygen scavenging by titania/polymer nanocomposite films. J Photochem Photobiol A. 2004;162:253–9. [Google Scholar]
- 83. Mills A, Doyle G, Peiro AM, Durrant JR. Demonstration of a novel, flexible, photocatalytic oxygen-scavenging polymer film. J Photochem Photobiol A. 2006;177:328–31. [Google Scholar]
- 84. Pathakoti K, Huang MJ, Watts JD, He X, Hwang HM. Using experimental data of Escherichia coli to develop a QSAR model for predicting the photo-induced cytotoxicity of metal oxide nanoparticles. J Photochem Photobiol B. 2014;130:234–40. doi: 10.1016/j.jphotobiol.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 85. Mateejka V, Tokarsky J. Photocatalytical nanocomposites: a review. J Nanosci Nanotechnol. 2014;14:1597–616. doi: 10.1166/jnn.2014.9081. [DOI] [PubMed] [Google Scholar]
- 86. Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65:4094–8. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ramanathan T, Abdala AA, Stankovich S, Dikin DA, Herrera-Alonso M, Piner RD, Adamson DH, Schniepp HC, Chen X, Ruoff RS, Nguyen ST, Aksay IA, Prud’Homme RK, Brinson LC. Functionalized graphene sheets for polymer nanocomposites. Nat Nanotechnol. 2008;3:327–31. doi: 10.1038/nnano.2008.96. [DOI] [PubMed] [Google Scholar]
- 88. Arora A, Padua GW. Review: nanocomposites in food packaging. J Food Sci. 2010;75:43–9. doi: 10.1111/j.1750-3841.2009.01456.x. [DOI] [PubMed] [Google Scholar]
- 89. Avella M, De Vlieger JJ, Errico ME, Fischer S, Vacca P, Volpe MG. Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem. 2005;93:467–74. [Google Scholar]
- 90. Huang J, He C, Liu X, Xu J, Tay CSS, Chow SY. Organic–inorganic nanocomposites from cubic silsesquioxane epoxides: direct characterization of interphase, and thermomechanical properties. Polymer. 2005;46:7018–27. [Google Scholar]
- 91. Cyras VP, Manfredi LB, Ton-That MT, Vazquez A. Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr Polym. 2008;73:55–63. [Google Scholar]
- 92. Chen B, Julian RGE. Thermoplastic starch–clay nanocomposites and their characteristics. Carbohydr Polym. 2005;61:455–63. [Google Scholar]
- 93. Huang J, Xiao Y, Mya KY, Liu X, He C, Dai J, Siow YP. Thermomechanical properties of polyimide-epoxy nanocomposites from cubic silsesquioxane epoxides. J Mater Chem. 2004;14:2858–63. [Google Scholar]
- 94. Park HM, Li X, Jin CZ, Park CY, Cho WJ, Ha CS. Preparation and properties of biodegradable thermoplastic starch/clay hybrids. Macromol Mater Eng. 2002;287:553–8. [Google Scholar]
- 95. Momin JK, Jayakumar C, Prajapati JB. Potential of nanotechnology in functional foods. Emir J Food Agric. 2013;25:10–9. [Google Scholar]
- 96. Mozafari M, Flanagan J, Matia-Merino L, Awati A, Omri A, Suntres ZE, Singh H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J Sci Food Agric. 2006;86:2038–45. [Google Scholar]
- 97. Elliott R, Ong TJ. Nutritional genomics. BMJ. 2002;324:1438–42. doi: 10.1136/bmj.324.7351.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vidhyalakshmi R, Bhakyaraj R, Subhasree RS. Encapsulation “the future of probiotics”—a review. Adv Biol Res. 2009;3:96–103. [Google Scholar]
- 99.Mazzocchi S.5 Things you need to know about nanofoods. 2011. [Accessed 9 February 2017]. Available from: http://www.pbs.org/wnet/need-to-know/five-things/nanofoods/6682/
- 100. Bugusu B, Mejia C, Magnuson B, Tafazoli S. Global regulatory policies on food nanotechnology. Food Technol. 2009;63:24–8. [Google Scholar]
- 101. Semo E, Kesselman E, Danino D, Livney YD. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocol. 2007;21:936–42. [Google Scholar]
- 102. Yu H, Huang Q. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem. 2010;119:669–74. [Google Scholar]
- 103. He W, Liu Y, Wamer WG, Yin JJ. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J Food Drug Anal. 2014;22:49–63. doi: 10.1016/j.jfda.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22:116–27. doi: 10.1016/j.jfda.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fernández A, Picouet P, Lloret E. Reduction of the spoilage-related microflora in absorbent pads by silver nanotechnology during modified atmosphere packaging of beef meat. J Food Prot. 2010;73:2263–9. doi: 10.4315/0362-028x-73.12.2263. [DOI] [PubMed] [Google Scholar]
- 106. Emamifar A, Kadivar M, Shahedi M, Solaimanianzad S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control. 2011;22:408–13. [Google Scholar]
- 107. Cushen M, Kerry J, Morris M, Cruz-Romero M, Cummins E. Silver migration from nanosilver and a commercially available zeolite filler polyethylene composites to food simulants. Food Addit Contam Part A. 2014;31:1132–40. doi: 10.1080/19440049.2014.905874. [DOI] [PubMed] [Google Scholar]
- 108. Artiaga G, Ramos K, Ramos L, Cámara C, Gómez-Gómez M. Migration and characterisation of nanosilver from food containers by AF4-ICP-MS. Food Chem. 2015;166:76–85. doi: 10.1016/j.foodchem.2014.05.139. [DOI] [PubMed] [Google Scholar]
- 109. Alfirevic M, KriZanec B, Voncina E, Brodnjak-Voncina D. Presence of nonylphenols in plastic films and their migration into food simulants. Acta Chim Slov. 2011;58:127–33. [PubMed] [Google Scholar]
- 110. von Goetz N, Fabricius L, Glaus R, Weitbrecht V, Günther D, Hungerbühler K. Migration of silver from commercial plastic food containers and implications for consumer exposure assessment. Food Addit Contam Part A. 2013;30:612–20. doi: 10.1080/19440049.2012.762693. [DOI] [PubMed] [Google Scholar]
- 111. Metak A, Ajaal T. Investigation on polymer based nanosilver as food packaging materials. Int J Biol Vet Agric Food Eng. 2013;7:772–8. [Google Scholar]
- 112.Drew R, Hagen T. Prepared for Food Standards Australia New Zealand. Science Media Centre New Zealand; New Zealand: 2016. Nanotechnologies in food packaging: an exploratory appraisal of safety and regulation. [Google Scholar]
- 113. Kuorwel K, Cran MJ, Orbell JD, Buddhadasa S, Bigger SW. Review of mechanical properties, migration, and potential applications in active food packaging systems containing nanoclays and nanosilver. Compr Rev Food Sci Food Saf. 2015;14:411–30. [Google Scholar]
- 114. Rastogi ID. Nanotechnology: safety paradigms. J Toxicol Environ Health Sci. 2012;4:1–12. [Google Scholar]
- 115. Cubadda F, Aureli FD, Amato M, Raggi A, Mantovani A. Nanomaterials in the food sector: new approaches for safety assessment. Rapporti ISTISAN. 2013;13:48. [Google Scholar]
- 116.Division of Dockets Management, HFA-305, U.S. Food and Drug Administration. FDA issues guidance on the use of nanomaterials in food for animals. 2015. [Accessed 9 February 2017]. Available from: http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm457112.htm.