Abstract
Essential oils from the seed, pulp, and leaf of sea buckthorn were obtained with hydrodistillation, and their phytochemical composition was analyzed through gas chromatography–mass spectrometry. Furthermore, the antibacterial activity of the oils was tested on five food-borne bacteria by spectrometry and evaluated in terms of minimum inhibitory concentration. The results indicate that the composition of all essential oils is dominated by free fatty acids, esters, and alkanes. Minimum inhibitory concentration values on each bacterium were obtained for oils from different parts. The oils from different parts exhibited nearly equal inhibitory effect on Staphylococcus aureus. The pulp oil was found to be the most effective for the rest of bacteria tested except Escherichia coli, on which seed oil shows twice the inhibitory effect to that of leaf or pulp oil. Three natural inhibitory examples were found comparable with or even better than the positive control: pulp oil on Bacillus subtilis, and pulp oil and leaf oil on Bacillus coagulans.
Keywords: antibacterial activity, essential oil, gas chromatography-mass spectrometry, Hippophae rhamnoides L., phytochemical composition
1. Introduction
Sea buckthorn (Hippophae rhamnoides L.), a thorny bush with yellow-orange, pearl-shaped fruits, has a very wide natural distribution throughout Asia and Europe [1]. Sea buckthorn is resistant to drought, cold, salt, and alkali. With its fast-developing, strong root system, and nitrogen fixing nodules [2], sea buckthorn is widely used in water and soil conservation and reforestation in China.
The pulp of sea buckthorn is rich in carotenoids, tocopherols, sterols, lipids, ascorbic acid, flavonoids, triterpenes, etc. [3]. These compounds have biological and therapeutic activities such as antioxidant, antitumoral, and immunomodulatory properties [4]. In addition to medicinal use, the pulp is processed into various products such as juice and marmalade [5]. The valuable components in pulp and their biological activity make the plantation of sea buckthorn not only a measure of ecological management but also an economical improvement.
Studies based on gas chromatography (GC), liquid chromatography, and mass spectrometry (MS) have been reported on the compositions of the pulp of sea buckthorn [5,6], whereas there are few reports regarding the compositions of the leaves [7,8], despite the fact that the leaves contain a number of nutrients and bioactive substances, which are all favorable ingredients for animal foods [9]. As a byproduct of the sea buckthorn processing industry, the chemical composition of seed is also yet to be exploited [1,10].
To our knowledge, no information is available on comparing the compositions and antibacterial activities of essential oils from different parts of sea buckthorn, although the antimicrobial and antioxidant activities of ethanolic extracts from leaf, stem, root, and seed of sea buckthorn have been investigated [11]. GC/MS is known to be very helpful in the discrimination of sample information based on its massive identification. Vítová et al [12] and Socaci et al [13], coincidently, combined GC/MS with certain sampling techniques (solid-phase microextraction and in-tube extraction, respectively) to successfully discriminate sea buckthorn samples from different sources. The objective of the present study is to ascertain and compare the compositions of the essential oils from different parts of sea buckthorn with GC/ MS, as well as to investigate their antibacterial activities on five food-borne bacteria by spectrometry with the aid of 96-well plate for their potential utility.
2. Methods
2.1. Plant material
Sea buckthorn was collected from Yijun, Shaanxi Province, China. The plant was authenticated in Key Laboratory of the Ministry of Education for Medicinal Resources and Natural Pharmaceutical Chemistry, Xi'an, China. It was identified as Hippophae rhamnoides subsp. sinensis.
The sample was dried in a shady ventile place, and the voucher specimen had been deposited in the School of Life sciences, Shaanxi Normal University, Shaanxi, China (specimen number SNNU-h015). The dried sample was ground into certain size particles with a blender.
2.2. Reagents
The solvents and chemicals were obtained from the following sources: N2, 99.99% purity, was from Xi'an Yangguang Gas Factory, China. HPLC grade methanol was purchased from Fisher Sci.Co., USA. Tetracycline hydrochloride was purchased from Wolsen Co., Ltd, China; Tween 80 was purchased from Sigma-Aldrich Co. LLC., USA; Mueller-Hinton broth (MHB) medium was purchased from Beijing Aoboxing Biotechnology Co., Ltd, China. All the water used was from the purification system (Millipore, USA); all chemicals used were of analytical reagent grade or higher unless otherwise specified.
2.3. Instruments
The essential oil analysis was performed on a GC/MS instrument (Shimadzu GC/MS-QP2010; Shimadzu, Japan). The volatile compounds were separated on RTX-5MS fused-silica capillary column (30 m in length, 0.25 mm in diameter, 0.25-m film thickness) coated with 5% diphenyl and 95% dimethylpolysiloxane.
Other instruments include BS-1EA Oscillating Incubator (Guohua Electrical Appliance Co., Ltd., China); FZ102 Mini plant grinder (Zhongxing Co., Ltd., Huangye, China); Q/ BKYY31-2000 Electrothermal oven (Shanghai Yuejin Medical Instrument Factory, China); super clean bench (Suzhou Purification Equipment Co., Ltd., China); Zenyth 3100 enzyme-labeling instrument (Anthos, Austria).
2.4. Preparation of bacteria suspension
Bacillus subtilis, Bacillus cereus, Escherichia coli, Staphylococcus aureus, and Bacillus coagulans were obtained from Microbiology Institute of Shaanxi Province, china. The bacteria were activated on MHB medium and then diluted with 0.9% NaCl.
2.5. Preparation of the essential oil
The seed, pulp, and leaf of sea buckthorn (100.0 g) were cut into small pieces and ground to particles of certain size, and 4.0 g of particles then underwent hydrodistillation at 95°C for 6 hours using a Clevenger-type apparatus as recommended by British Pharmacopoeia [14]. The distillate evaporated along with water vapor were collected in a condenser. The essential oil was dried over anhydrous sodium sulfate and stored at 4°C in the dark pending analysis by GC/MS and antibacterial test.
2.6. GC/MS analysis
GC/MS (electron ionization; EI) conditions: Helium was used as the carrier gas (1.26 mL/min). Oil samples (1 L) were injected into the column with a split ratio set at 20:1. The GC program was initiated by a column temperature set at 80°C, and then increased to 160°C and 250°C at a rate of 20°C /min and 8°C /min, respectively. Temperatures of the ion source (positive) and interface were 200°C and 250°C, respectively. The mass spectrometer was operated with EI ion source at 70 eV, and the mass range was from m/z 10 to m/z 600.
GC/MS (chemical ionization, CI) conditions: The pressure CI and negative CI mass spectra were recorded on the same apparatus equipped with the same column and specific ionization chemical source. Ionizing gas: methane, other experimental conditions were the same as those in the EI analysis.
All essential oils were freshly diluted 50 times with diethyl ether before GC-MS analysis.
2.7. Antibacterial test
Antibacterial activity of the essential oils from different parts of sea buckthorn was tested on five bacteria, including B. subtilis, B. cereus, E. coli, S. aureus, and B. coagulans.
The essential oils from sea buckthorn were tested on B. subtilis, B. cereus, E. coli, S. aureus, and B. coagulans in super clean bench. The minimum inhibitory concentration (MIC) of every oil sample was determined using the microdilution method [15,16]. A 100-μL aliquot of MHB medium (2% V/V Tween80) was added into each well of 96-well plate, and then 100 μL essential oil (200 mg/mL) was added into the first well. Through serial two-fold dilution, the following 10 wells contained decreasing concentrations of essential oil. The mixture in the 11th well was discarded. Next, 5 μL of bacterial suspension (5 × 105 colony-forming units/mL) was added into each well, where the concentrations of essential oils were from 97.56 mg/mL (in Well 1) to 0.10 mg/mL (in Well 11). The sample without bacteria (100 μL MHB medium, 5 μL essential oil) and the sample without essential oil (100 μL MHB medium, 5 μL bacterial suspension) were used as a negative control. Tetracycline hydrochloride (the concentrations were the same as aforementioned corresponding essential oils) was used as the positive control.
The plate was kept in the refrigerator (4°C) for 1 hour, and was then incubated at 37°C for 24 hours. After the incubation, MIC of every essential oil acting on each bacterium was ascertained by determining the absorbance of samples at the wavelength of 630 nm. MICs value were an average of six replicate determinations.
3. Results and discussion
3.1. Yields of oil
The qualities of yellowish oils from three different parts (seed, pulp, and leaf) and their corresponding yields are listed in Table 1.
Table 1.
Yields of oil from different parts of sea buckthorn.
| Parts | Weight of oil (g) | Weight of material (g) | Yields of oil (%) |
|---|---|---|---|
| Seed | 0.0013 | 4.000 | 0.033 |
| Pulp | 0.0505 | 4.000 | 1.260 |
| Leaf | 0.0146 | 4.000 | 0.365 |
3.2. Qualitative and quantitative analysis
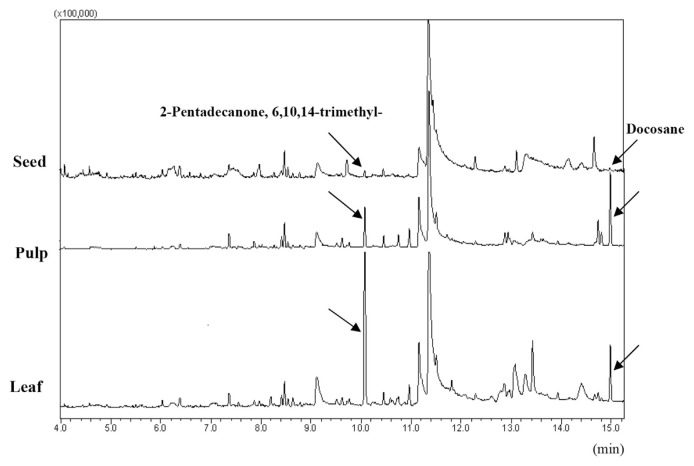
Total ion current (TIC) was obtained on GC/MS under full scan mode; typical TIC of essential oil samples from different parts of sea buckthorn is shown in Figure 1. The unambiguous identification of the most compounds was done by comparing their fragmentation pattern in EI mass spectra with those of a mass spectral database (NIST11); as for those without adequate assurance, the identification was based on the joint information from EI and chemical ionization mass spectra. Some of them were further confirmed by comparing their fragmentation pattern in EI mass spectra with those of authentic compounds available in our laboratory or from literature data [17–19].
Figure 1.
Total ion current of essential oils from different parts of sea buckthorn.
Quantification was expressed in peak area percent. Relative content (%) of individual component was calculated based on GC peak areas without response factors correction. In terms of TIC area, 84.61%, 89.14%, and 86.03% of components were identified for essential oil from seed, pulp, and leaf, respectively. The results are listed in Table 2, where the compounds are arranged in the order of elution on the RTX-5MS silica capillary column.
Table 2.
Composition of the essential oils from different parts of sea buckthorn.
| No. | Retention time (min) | Name | Formula | Molecular weight | Normalized contents (%) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Seed | Pulp | Leaf | |||||
| 1 | 4.57 | Decanal | C10H20O | 156 | 0.74 | ||
| 2 | 6.38 | Tetradecane | C14H30 | 198 | 1.45 | 0.41 | 0.43 |
| 3 | 6.79 | Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | C11H30O3 | 210 | 0.75 | ||
| 4 | 7.85 | Hexadecane | C16H34 | 226 | 2.27 | 1.12 | 0.58 |
| 5 | 7.91 | 2,6,10-trimethyl-pentadecane | C18H38 | 254 | 0.87 | 0.43 | |
| 6 | 7.97 | NI | 1.91 | 0.38 | |||
| 7 | 8.20 | 2-methyl-1-decanol | C11H24O | 172 | 0.41 | ||
| 8 | 8.41 | Heneicosane | C21H44 | 296 | 0.99 | 1.00 | 0.54 |
| 9 | 8.47 | NI | 2.20 | 2.17 | 1.10 | ||
| 10 | 8.53 | 1,1′-Biphenyl, 2,2′,5,5′-tetramethyl- | C16H18 | 210 | 0.92 | 0.62 | |
| 11 | 8.78 | 10-Octadecenal | C18H34O | 266 | 0.37 | ||
| 12 | 9.13 | Tetradecanoic acid | C14H28O2 | 228 | 4.07 | 4.20 | 3.90 |
| 13 | 9.52 | 2,6,11-trimethyl-dodecane | C15H32 | 212 | 0.93 | 0.43 | |
| 14 | 9.62 | 2,6,10,14-tetramethyl-hexadecane | C20H42 | 282 | 1.09 | 0.57 | |
| 15 | 9.75 | NI | 2.26 | 0.49 | 0.42 | ||
| 16 | 10.07 | 6,10,14-trimethyl-2-pentadecanone | C18H36O | 268 | 0.77 | 3.74 | 12.60 |
| 17 | 10.45 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 278 | 0.80 | 1.03 | 0.69 |
| 18 | 10.75 | (Z)-9-hexadecenoic acid, methyl ester | C17H32O2 | 268 | 1.35 | 0.77 | |
| 19 | 10.96 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 1.57 | 0.98 | |
| 20 | 11.16 | Oleic acid | C18H34O2 | 282 | 7.70 | 9.06 | 6.73 |
| 21 | 11.36 | n-Hexadecanoic acid | C16H32O2 | 256 | 36.64 | 32.88 | 26.07 |
| 22 | 11.51 | Dibutyl phthalate | C16H22O4 | 278 | 14.72 | 9.76 | 7.37 |
| 23 | 11.75 | Stearic acid, ethyl ester | C20H40O2 | 312 | 2.58 | 2.51 | |
| 24 | 11.82 | NI | 2.03 | 1.30 | 1.05 | ||
| 25 | 12.08 | 13-Tetradecen-1-ol acetate | C16H30O2 | 254 | 1.66 | ||
| 26 | 12.29 | 8,11-Octadecadienoic acid, methyl ester | C19H34O2 | 294 | 1.12 | 2.67 | 3.49 |
| 27 | 12.88 | (Z)-9-octadecenoic acid, methyl ester | C19H36O2 | 296 | 1.30 | 0.68 | |
| 28 | 13.07 | 5-dodecyldihydro-2(3H)-furanone | C16H30O2 | 254 | 5.89 | ||
| 29 | 13.12 | NI | 2.81 | ||||
| 30 | 13.29 | Eicosane | C20H42 | 282 | 4.39 | ||
| 31 | 13.31 | Nonacosane | C29H60 | 408 | 7.23 | ||
| 32 | 13.43 | NI | 6.90 | 5.61 | |||
| 34 | 13.60 | Ethyl 9-decenoate | C12H22O2 | 198 | 0.91 | ||
| 35 | 13.93 | Heptacosane | C27H56 | 380 | 0.95 | 1.07 | |
| 36 | 14.17 | 2,6,10,15-tetramethyl-heptadecane | C21H44 | 296 | 4.43 | ||
| 37 | 14.41 | NI | 4.89 | ||||
| 38 | 14.67 | NI | 4.17 | 0.52 | |||
| 39 | 14.73 | (Z)-9-tricosene | C23H46 | 322 | 3.13 | 0.87 | |
| 40 | 14.80 | 1-Nonadecene | C19H38 | 266 | 1.43 | ||
| 41 | 15.01 | Docosane | C22H46 | 310 | 6.54 | 2.60 | |
NI = not identified.
3.3. Analysis and comparison of the compositions of the essential oils from different parts of sea buckthorn
As shown in Table 3, the composition of every essential oil is dominated by free fatty acids, esters, and alkanes, which amount to a total of 82.18%, 80.22%, 65.89%, respectively, for seed, pulp, and leaf. The three major free fatty acids are n-hexadecanoic acid (26–37%), oleic acid (6–9%), and tetradecanoic acid (~4%). Two major esters are dibutyl phthalate (7–14%) and 8,11-octadecadienoic acid, methyl ester (1–3%). Dibutyl phthalate is a common plasticizer and may be released from the plastic material when in contact with solvents [20].
Table 3.
Main components of the essential oils from different parts of sea buckthorn.
| Acids (%) | Esters (%) | Alkane (%) | Ketone (%) | Others (%) | |||
|---|---|---|---|---|---|---|---|
| Seed | 48.41 | A (36.64) | 17.39 | D (14.72) | 16.38 | 0.92 | 1.51 |
| B (7.70) | E (1.12) | ||||||
| C (4.07) | |||||||
| Pulp | 46.14 | A (32.88) | 21.17 | D (9.76) | 12.91 | 3.74 | 5.18 |
| B (9.06) | E (2.67) | ||||||
| C (4.20) | F (2.58) | ||||||
| Leaf | 36.70 | A (26.07) | 18.15 | D (7.37) | 11.04 | 18.49 | 1.65 |
| B (6.73) | E (3.49) | ||||||
| C (3.90) | F (2.51) | ||||||
A = n-hexadecanoic acid; B = oleic acid; C = tetradecanoic acid; D = dibutyl phthalate; E = 8,11-octadecadienoic acid, methyl ester; F = stearic acid, ethyl ester.
The contents of certain components identified apparently vary according to from which part the essential oil was exacted. The contents of tetradecane and hexadecane in the seed oil were about three times and, two to four times, respectively, that of the other two parts, while some alkanes and esters that are not detected in the seed oil are discovered in the pulp and leaf oils. They are 2,6,10-trimethyl-pentadecane, 2,6,11-trimethyl-dodecane, 2,6,10,14-tetramethyl-hexadecane, heptacosane, docosane, (Z)-9-hexadecenoic methyl ester, hexadecanoic methyl ester, stearic ethyl ester, and (Z)-9-octadecenoic methyl ester.
The normalized contents of putative component of 6,10,14-trimethyl-2-pentadecanone in sea buckthorn decrease drastically in the order of leaf (12.60%), pulp (3.74%), and seed (0.77%), while the contents of another putative component of docosane decrease in the order of pulp (6.54%), leaf (2.60%), and seed (0.00%). These observations (as indicated in Figure 1) are consistent with the report from Liu [21].
3.4. Antibacterial activities of the essential oils from sea buckthorn
MIC was reported as the lowest concentration of the compound capable of inhibiting the complete growth of the bacterium being tested. Practically, throughout this experiment, the MICs were defined as the concentrations at which the absorbance of the analyte tested reaches 0.015, while the absorbance of the negative control without any bacterium was 0.008 ± 0.007.
To varying extents, essential oils inhibited the growth of five food-borne bacteria. The results of the inhibition effect on each bacterium obtained for the essential oils from different parts are presented in Table 4. Oils from different parts exhibited a nearly equal inhibition effect on S. aureus. The pulp oil was found to be the most effective, followed by seed and leaf for the rest of bacteria except E. coli, on which seed oil shows twice inhibitory effect that of leaf or pulp oil. By comparison, there were three natural inhibitory examples comparable with or even better than that with the positive control in terms of inhibitory effect: pulp oil on B. subtilis, and pulp oil and leaf oil on B. coagulans.
Table 4.
Minimum inhibitory concentration of essential oils and positive control on different bacteria (mean, mg/mL).
| Bacillus subtilis | Bacillus cereus | Bacillus coagulans | Staphylococcus aureus | Escherichia coli | |
|---|---|---|---|---|---|
| Seed | 1.52 | 24.39 | 6.10 | 12.20 | 6.10 |
| Pulp | 0.19 | 3.05 | 0.10 | 12.20 | 12.20 |
| Leaf | 3.05 | 48.78 | 1.52 | 12.20 | 12.20 |
| Tetracycline hydrochloride | 0.76 | 0.76 | 3.05 | 1.52 | 0.76 |
To the best of our knowledge this is the first time that the essential oils from different parts of sea buckthorn were investigated and compared. The antibacterial experiment has demonstrated that essential oils from different parts exhibit, more or less, antibacterial activities, which may be part of the reason that sea buckthorn is widely used in pharmaceutical, cosmetic, and food industries. Being a natural antibacterial source, the utility of sea buckthorn is yet to be explored in more fields. This research provides reference for further promotion and deep processing of sea buckthorn, as well as for transgenic tissue culture and planting regulation.
Acknowledgments
The research was supported by the National Natural Science Foundation of China (No. 21305084), the National Natural Science Foundation of Shaanxi Province, China (No. 2014JQ2-8048), and the Fundamental Research Funds for the Central Universities (GK 201503003).
Funding Statement
The research was supported by the National Natural Science Foundation of China (No. 21305084), the National Natural Science Foundation of Shaanxi Province, China (No. 2014JQ2-8048), and the Fundamental Research Funds for the Central Universities (GK 201503003).
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
REFERENCES
- 1. Lalit DK, Sushant CP, Anuradha SP, Rekha SS, Vandana BP. Separation of bioactives from seabuckthorn seeds by supercritical carbon dioxide extraction methodology through solubility parameter approach. Sep Purif Technol. 2011;80:533–40. [Google Scholar]
- 2. Yang BR, Heikki K. Composition and physiological effects of sea buckthorn lipids. Trends Food Sci Tech. 2002;13:160–7. [Google Scholar]
- 3. Alam Z, Sana U. Sea buckthorn seed oil protects against the oxidative stress produced by thermally oxidized lipids. Food Chem. 2015;186:6–12. doi: 10.1016/j.foodchem.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 4. Paweł G, Elga S, Aleksander S, Dalija S. Sea buckthorn (Hippophae rhamnoides L.) leaves as valuable source of lipophilic antioxidants: the effect of harvest time, sex, drying and extraction methods. Ind Crop Prod. 2014;60:1–7. [Google Scholar]
- 5. Yang BR, Markku A, Petri M, Heikki K. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res Int. 2011;44:2009–17. [Google Scholar]
- 6. Sindhu M, Carl G, Kimmo R, Patrick A. Analysis of carbonyl compounds in sea buckthorn for the evaluation of triglyceride oxidation, by enzymatic hydrolysis and derivatisation methodology. Food Chem. 2011;126:1399–405. [Google Scholar]
- 7. Tian CJ, Nan P, Chen JK, Zhong Y. Volatile composition of Chinese Hippophae rhamnoides and its chemotaxonomic implications. Biochem Syst Ecol. 2004;32:431–41. [Google Scholar]
- 8. Nitin K, Upadhyay MS, Yogendra K, Asheesh G. Antioxidant, cytoprotective and antibacterial effects of sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol. 2010;48:3443–8. doi: 10.1016/j.fct.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 9. Yang B, Karlsson RM, Oksman PH, Kallio HP. Phytosterols in sea buckthorn (Hippophae rhamnoides L.) berries: identification and effects of different origins and harvesting times. J. Agr Food Chem. 2001;49:5620–9. doi: 10.1021/jf010813m. [DOI] [PubMed] [Google Scholar]
- 10. Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS. Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005;92:119–24. [Google Scholar]
- 11. Thomas M, Emilie D, Gaetan LF, Marie EL, Claire E. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophae rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012;131:754–60. [Google Scholar]
- 12. Vítová E, Sůkaloá K, Mahdalová M, Butorová L, Melikantová M. Comparison of selected aroma compounds in cultivars of sea buckthorn (Hippophae rhamnoides L.) Chem Pap. 2015;69:881–8. [Google Scholar]
- 13. Socaci SA, Socaciu C, Tofanǎ M, Raţi IV, Pintea A. In-tube extraction and GC-MS analysis of volatile components from wild and cultivated sea buckthorn (Hippophae rhamnoides L. ssp. Carpatica) berry varieties and juice. Phytochem Anal. 2013;24:319–28. doi: 10.1002/pca.2413. [DOI] [PubMed] [Google Scholar]
- 14.British Pharmacopoeia Commission. British pharmacopeia, Appendix XI F. London: HMSO; 1988. [Google Scholar]
- 15. Ebrahimabadia AH, Ebrahimabadia EH, Djafari-Bidgolia Z, Kashia FJ, Mazoochia A, Batoolib H. Composition and antioxidant and antimicrobial activity of the essential oil and extracts of Stachys inflata Benth from Iran. Food Chem. 2010;119:452–8. [Google Scholar]
- 16. Mirjana ZM, Katarina GM, Olgica DS, Sava MV, Ljiljana RC. Extracts of Agrimonia eupatoria L. as sources of biologically active compounds and evaluation of their antioxidant, antimicrobial and antibiofilm activities. J Food Drug Anal. 2016;24:539–47. doi: 10.1016/j.jfda.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masada Y. Analysis of essential oils by gas chromatography and mass spectrometry. New York: John Wiley; 1976. [Google Scholar]
- 18.Adams RP. Identification of essential oils components by gas chromatography/ quadrupole mass spectroscopy. Carol Stream, IL: Allured Publishing; 1995. [Google Scholar]
- 19. Tao NP, Wu R, Zhou PG, Gu SQ, Wu W. Characterization of odor-active compounds in cooked meat of farmed obscure puffer (Takifugu obscurus) using gas chromatography-massspectrometry-olfactometry. J Food Drug Anal. 2014;22:431–8. doi: 10.1016/j.jfda.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bianco A, Venditti A, Foddai S, Toniolo C, Nicoletti M. A new problem. Contamination of botanicals by phthalates. Rapid detection tests. Nat Prod Res. 2014;28:134–7. doi: 10.1080/14786419.2013.842997. [DOI] [PubMed] [Google Scholar]
- 21. Liu Z. The chemical composition of Seabuckthorn. Chin J Ethnomed. 2004;68:147–8. [Google Scholar]