Abstract
Smog is created through the interactions between pollutants in the air, fog, and sunlight. Air pollutants, such as carbon monoxide, heavy metals, nitrogen oxides, ozone, sulfur dioxide, volatile organic vapors, and particulate matters, can induce oxidative stress in human directly or indirectly through the formation of reactive oxygen species. The outermost boundary of human skin and mucous layers are covered by a complex network of human-associated microbes. The relation between these microbial communities and their human host are mostly mutualistic. These microbes not only provide nutrients, vitamins, and protection against other pathogens, they also influence human’s physical, immunological, nutritional, and mental developments. Elements in smog can induce oxidative stress to these microbes, leading to community collapse. Disruption of these mutualistic microbiota may introduce unexpected health risks, especially among the newborns and young children. Besides reducing the burning of fossil fuels as the ultimate solution of smog formation, advanced methods by using various physical, chemical, and biological means to reduce sulfur and nitrogen contains in fossil fuels could lower smog formation. Additionally, information on microbiota disruption, based on functional genomics, culturomics, and general ecological principles, should be included in the risk assessment of prolonged smog exposure to the health of human populations.
Keywords: culturomics, human microbiota, nanoparticles, oxidative stress, PM2.5, smog
1. How smog is formed
Smog is an irritating mixture of gasses and particulates in the air formed by smoke and fog under the sun. Formation of smog is directly related to the weather and the topography of the land [1]. Air temperature decreases at about 6.4°C per kilometer in climbing altitude. Under normal conditions, warmer air on the ground rises to the upper atmosphere continuously. This constant air movement spreads pollutants around and thus prevent smog to accumulate on the ground. Sometimes, the temperature gradient is reversed; and the air on top is warmer than the air below it, a phenomenon called temperature inversion [2,3]. Temperature inversion can happen in many ways. When a warm and less dense air mass moves over a cold and dense air mass, or when warm air moves across a cold ground, the air temperatures near the ground, the troposphere, can be cooler than the air temperature above it. When an atmospheric inversion layer is closer to the ground, it works like a lid to prevent air upflow that spreads and dilute the pollutants. Temperature inversion explains why wintertime weather often favors smog formation on the ground [4]. The landscape of the land also plays a role in creating temperature inversion. When cold air from mountain peak flows down into valleys, the cold air pushes the warmer air in the valleys upward, creating an inversion. Because of their geographical location (they are built on low basins or valleys), cities such as Los Angeles [5], London [6], Taipei [7], Beijing [8], Tehran [9], and Mexico City [10] are prone to smog accumulation.
Smog can form naturally. Plants emit a plethora of volatile organic compounds (VOCs) as a defensive mechanism against herbivore attack [11]. Upon reaction with the sunlight, VOCs become a major source of ozone (O3) in the atmosphere [12–14]. Volcano eruption produces airborne ash and gasses with high concentrations of SO2 and H2S [15,16], which can contribute to smog formation. Likewise, forest fire also produces a significant amount of smog [17,18]. The 2015 massive fires in Sumatra and Kalimantan in Indonesian Borneo, for example, has produced a persistently hazardous air pollution across downwind Indonesia, Malaysia, and Singapore. It was estimated that the toxic haze from this fire has caused more than 100,000 premature adult deaths in areas closest to the blazes [19]. These pollutants have been related to childhood leukemia [20].
Smog is also anthropogenic. The first and second industrial revolutions have replaced manpower with machines in many production processes. The increase in production of goods and expansion in human population has changed the ways we communicate. The transportation evolution allows massive and rapid transfer of humans and goods. The energy generated from burning coal and other fossil fuels have replaced the traditional source of energy provided by animals. However, coal burning causes the pollution of the air. In 1948, for example, severe industrial air pollution created a deadly smog in Donora, Pennsylvania, which made thousands of people sick [21]. Similarly, in 1952, smog from factories and home fireplaces killed at least 4000 people in London over the course of several days [22–24]. Nowadays, cars, trucks, trains, airplanes, and ships are the major means of transportation on land, air, rivers, canals, and seas. They have become major sources of air pollution [25–31]. Human population expansion also complicates the air quality. Treatment of human wastes, such as incinerators, landfills, and sewage treatment plants, produces various toxic gasses that are air polluting [32–35]. More than 100 toxic gasses are released from landfills [36,37]. Some of them, such as methane and hydrogen sulfide, are most abundant. Others, such as those polyaromatic hydrocarbons and polychlorinated biphenyl released in landfill fires and incinerators, are carcinogenic [38]. Toxins leachates from landfills are a potential pollutant to ground waters [39].
2. Sources of pollutants
It is important to identify where the pollutants are released to the air. Pollutants generated from stationary sources, such as power plants, industrial and commercial boilers, paper and wood mills, smelters, refinery processing plants, chemical processing plants, and petroleum storage tanks, are considered point-source air pollution. The nonpoint source of air pollution includes some stationary and mobile sources that are individually small, but collectively, they generate a large volume of pollutants. Wood stoves, motor vehicles, ships and boats, and controlled burning of farm wastes by farmers are considered nonpoint sources of air pollution. Since 1972, The U.S. Environmental Protection Agency (EPA) has categorized information of different industrials and compiled them in its so-called AP-42 report [40,41] to regulate the emissions factors of the air of the United States.
The Clean Air Act passed by the U.S. Congress in 1970 and amended in 1990 sets limits on how much of a pollutant is allowed in the air in the United States [42]. The EPA has set national air quality standards for six major air pollutants: carbon monoxide (CO), lead (Pb), nitrogen oxides (NOx), ozone (O3), sulfur dioxide (SO2), and particulate matters (PMs). Whereas CO, Pb, NOx, and SO2 are the results of direct emissions from a variety of sources, the formation of PMs is the result of direct emissions and aggregates of emitted gasses in the atmosphere. Most of the fine particle pollutants are aggregated through a complex interaction of VOCs and sunlight. Sunlight also activates the reaction between NOx and VOCs, leading to the formation of O3. Because O3 is not produced directly from emission, O3 in the troposphere is often referred as secondary pollutant [43].
The EPA refers to chemicals that cause serious health and environmental impacts as air toxics. Many chemicals (total of 187), such as benzene, chloroform, acetaldehyde, dioxin, polycyclic organic matter, chromium, lead, nickel, and mercury compounds, are recognized among the many air toxics. Air toxics can cause cancer and other serious health, including human reproduction, and birth defects. Air toxics also cause adverse environmental and many ecological problems [44].
We briefly introduce the six main air pollutants related to human health.
VOCs are produced from trees, plants, cars, or industrial emissions. VOCs react with nitrogen oxides in the presence of sunlight to form ground-level ozone, a primary ingredient in smog. Many VOCs have been linked to birth defects, cancer, and other serious illnesses [45]. For example, polycyclic aromatic hydrocarbons, a common product of biomass combustion [46], is highly genotoxic [47]. Benzene in the air is strongly correlated to childhood leukemia [20]. The EPA estimates that the air toxics emitted from cars and trucks—which include benzene, acetaldehyde, and 1,3-butadiene—account for half of all cancers caused by air pollution. VOC emissions are tracked by the National Emissions Inventory [48,49].
Nitrogen oxides (NOx). Nitrogen can combine with oxygen in many forms, such as nitric oxide (NO), nitrogen dioxide (NO2), and nitrous oxide (N2O). NOx is a generic term for NO and NO2 [50]. While lighting, free-living bacteria and symbiotic nitrogen-fixing bacteria can produce NOx naturally [51]; combustion of nitrogenous containing compounds, such as the burning of coal, oil, or natural gas, and during processes such as arc welding, electroplating, engraving, and dynamite blasting, can produce NOx [52]. The toxicity of NOx has been well studied [53–55]. NOx not only causes lung irritation and weaken the body’s defenses against respiratory infections such as pneumonia and influenza [56], it is also found to be genotoxic [57].
Carbon monoxide (CO). This odorless, colorless, and poisonous gas is formed by the combustion of carbonaceous compounds such as coal, gasoline, and diesel fuels. CO binds to the hemoglobin of the red blood cell and prevents oxygen transport to various parts of the body. CO also binds to the terminal oxidase of the electron transport chain of the cells in the mitochondria and stops respiration. The major target of CO in humans is the cardiorespiratory system of the human body [58].
Sulfur dioxide (SO2) is created by coal-burning power plants and diesel fuels from motor vehicles. SO2 dissolves in the rain and become sulfuric acid (acid rain), which is toxic to plants [59]. Sulfur dioxide can react in the atmosphere to form fine particles and poses the largest health risk to young children and asthmatics. A comprehensive review on SO2 shows that exposure to relatively high levels of SO2 resulted in decreased fecundability in humans. Among females, SO2 interrupts fetal growth resulting in pregnancy loss. Among males, inhalation of SO2 interrupts male reproductive parameters such as the testicular histology and biochemistry of sperms. SO2 exposure also induces lipid peroxidation and interferes with the redox status in mouse organs [60].
PM, according to the EPA definition [61], is a complex mixture of extremely small particles and liquids that get into the air. Its sources include carbon dust, sulfates, and nitrates. Once inhaled, these particles can affect the heart and lungs, and cause serious health effects. PM10 is defined as particulates, such as dust, pollen, and mold, with a diameter smaller than 10 μm. PM2.5 refers to particulates with a diameter smaller than 2.5 μm. The PM2.5 particulates are the main cause of reduced visibility (haze) in parts of the United States [61]. Many trucks and ships use diesel engines. The burning of diesel engine is incomplete. Diesel engines running at its peak produce especially high concentrations of PM2.5 [62]. PM2.5 includes mainly combustion particles and organic compounds (27–40%) [63]. PM2.5 particulates also carries a large portion (22–54%) of secondary organic aerosols [63,64] that are formed by complicated photochemical reactions between numerous sulfates, nitrogen oxides, and other inorganic and organic chemicals [65]. Heavy metals in PM2.5 (~11–16%) often include Cd, Cr, Cu, Fe, Mn, Ni, As, and Pb [63,66–68]. The accelerated use of silver nanoparticles in many commercial products also raises concerns about the potential toxicity of Ag particles in the environment [69,70]. The sizes of some PM2.5 particulates can be very small, close to the range of nanoparticles (<0.1 μm). These nano-sized metals are not just nuisance dust. In fact, these fine particles pose the greatest health risk [69,71–73]. Many of them are bioactive. These particles can attach to the lungs and transport into the bloodstream [74]; some are even found in the human brain [75].
3. Smog in China
As early as the 1970s, China has recognized that the lung cancer rate at Xuanewi City northeastern of Yunnan Province was almost 5 times higher than that of the rest of the country [76]. The government attributed the mortality to exposure to indoor smoky coal emissions that contain very high levels of polycyclic aromatic hydrocarbons. Since then, the death rate from lung cancer had reached to 31 deaths per 100,000 population in 2004–2005 [77]. China’s economic growth requires that massive labor forces concentrate in a few large cities. Industrial growth also consumes a huge amount of fossil fuels. In 2014, Chinese people living in urban areas accounted for about 54% of the total population and is projected to be ~70% by 2030 [63,78]. Air pollution has imposed a serious threat on public health in China. The 2010 Global Burden of Disease Study reported that PM2.5 was one of the major public health risks to the Chinese people [77]. Air pollution also caused many social unrests in China [79]. In 2013, the Chinese government announced a plan to invest more than US$277 billion to reduce PM10 by 10% of the 2012 level in major Chinese cities. This plan also aimed to reduce PM2.5 in the Beijing–Tianjin–Hebei area, Yangtze River Delta, and Pearl River Delta by 25%, 20%, and 15%, respectively. To date, it seems that the 2012 “11th Five-Year Plan” for emission reduction has generally met its goals [80]. The Greenpeace East Asia’s 2015 annual city rankings show that PM2.5 in 189 cities in China, including Beijing, Guangzhou, and Shenzhen (average, ~77.1 μg/m3), have fallen by 10% compared to the 2014 levels (average ~92.6 μg/m3) and was approaching the 2017 target of 73 μg/m3. However, most of the 366 targeted cities still failed to meet the national standard of air quality [81]. Whether this reduction in air pollutants were related to the slowdown of the world’s economy, is still debatable [82].
4. Smog and human health
High death rates were observed in areas with elevated ambient pollution levels. It was estimated that more than 2 million premature baby deaths in China and in India are caused by smog [65]. The immediate effect was an increase in pneumonia deaths. The terms “acute respiratory distress syndrome” or “acute lung injury” are often used to describe many pneumonia deaths [74]. Numerous well-studied cases, including diseases of the heart [83], lung [84,85], skin and eye [86], reproduction [87], nervous system [88,89], inflammatory response [90], and cancer [91] are strongly related to smog exposure. Nanoparticles, because of their unique surface properties, may exhibit unexpected toxic biological effects [92,93]. Many heavy metal nanoparticles found in PM2.5 can induce reactive oxygen species (ROS) [73]. The mechanism of nanotoxins in inducing apoptosis and cancer via an oxidative route has been thoroughly reviewed by Fu and associates [94]. The human lipoprotein apolipoprotein E is produced by the liver and white blood cells, and by astrocytes in the brain. A recent study suggests that interaction of PM2.5 in the air and apolipoprotein E may cause brain aging and accelerates the development of Alzheimer’s disease [89]. Oxidative stress has been identified as a unifying feature underlying the toxic actions of smog [47,88,95–103]. This conclusion should not be surprising, because O3, VOCs, and the nanometal particles in smog can all induce free radicals [43,103–105]. Belyaeva and associates [106] proposed that the electron transport chain of the mitochondria is the prime target for heavy metal-induced neurotoxicity.
5. Smog induced oxidative stress
During respiration, foods are oxidized and the electrons in foodstuffs ultimately combine with molecular oxygen, producing water. Many reactive by-products of respiration, such as superoxide anion radicals, hydrogen peroxide (H2O2), and hydroxyl radicals (HO), are produced continuously in aerobically growing cells [107] through the Haber–Weiss reaction catalyzed by metals:
The reaction is followed by the Fenton reaction:
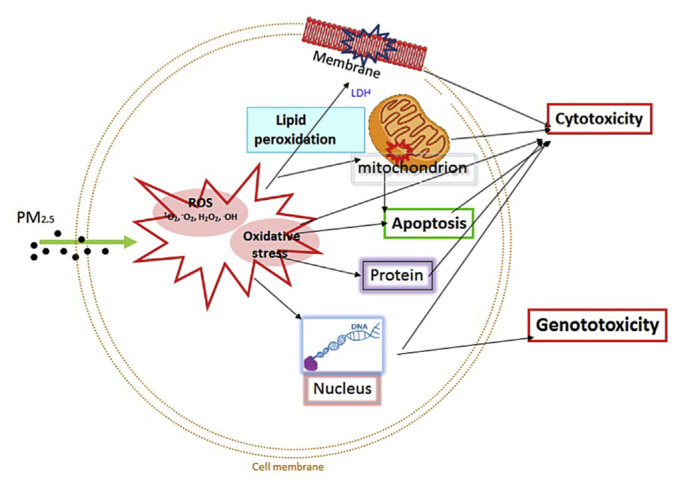
The free radicals generated from the above processes rapidly take part in other secondary reactions. Free radicals can cause oxidative damage to the cell via covalent bonds modification of DNA, proteins, lipids, and other biomolecules (Figure 1). Additionally, covalent binding to an unsaturated lipid can induce lipid peroxidation. Lipid peroxidation is a chain reaction (propagation). The end products of lipid peroxidation are more reactive aldehydes, lipid radical, and other reactive products. If lipid peroxidation is not terminated, the reaction not only damages a single cell—free radical propagation will affect the cells surrounding the propagation site. Arachidonic acid, a polyunsaturated fatty acid, is abundant in the brain, muscles, and liver [108]. This fatty acid is most sensitive to lipid peroxidation and has played an important role in inflammation [109]. The end products of lipid peroxidation, such as malondialdehyde, are mutagenic and carcinogenic [110]. The thiol groups of a protein are very sensitive to peroxidation. Protein damage by free radicals can induce cell death [111]. Peroxidation also causes DNA damage and formation of various DNA adducts [112,113].
Figure 1.
Cytotoxic and genotoxic mechanisms induced by PM2.5 via reactive oxidative species (ROS) in human cells. PM =particulate matter.
6. Human microbiota and health
Microbes are found everywhere. Humans and microbes have evolved together for millions of years. Humans harbor 10 times more microbes inside or on their bodies than the total number of their somatic and germ cells. It has been known for decades that the human microbiota contribute many vital compounds and vitamins to humans [114]. The Human Microbiome Project [115,116] is a summation of projects launched in multiple areas of the world, including the United States, European Union, and Asia [116]. Results from the Human Microbiome Project studies show that we have greatly underestimated the diversity, prevalence, and persistence of the bacteria in our body, partly because more than 99% of the bacteria, the so-call biological “dark matter,” cannot be cultured in the laboratory for detailed studies [117]. New methods that use the polymerase chain reaction approach to detect bacteria by their DNA or RNA sequences have allowed microbiologists to evaluate those otherwise unculturable bacteria in or on our body. Results from metagenomic studies show persistent and proliferate bacterial ecosystems in or on various parts of the human body. Gao et al [118] studied the microbiota of the forearms of some healthy individuals over a period of 10 months and described the bacteria as a “virtual zoo.” Bjorksten [119] equated the bacteria in the human gut to a “forest” with more than 40,000 different bacterial species. Bacteria in or on different parts of our body form their own complex and yet distinctive communities. The oral microflora, for example, is rich in Staphylococci, Propionibacteria, Streptococci, Enterococci, Pseudomonas, and Lactobacilli, whereas the gut microflora is rich in Lactococci, Lactobacilli, Bifidobacteria, Enterococci, Enterobacteriaceae, Clostridium, and Hellobacteira [116,120]. The traditional concept of microbes as pathogens or innocuous commensals is mostly disputed. More advanced studies found that microbes are not just living on or in our body, they are actively engaged, or sometimes directly influence many of our physiological events. Microbes in or on our body form a complex mutualistic relation with us. For examples, the microbiota of the lung enable the expansion of the virus-specific CD8 memory T lymphocyte [121]. During gestation, the mother’s microbes can influence her offspring’s immune system [122]. On the human skin, the potential pathogen, Staphylococcus epidermidis, can be beneficial by producing antimicrobial peptide (bacteriocins) and pheromone that inhibits other bacterial cell–cell communications, and activates the innate immune response of keratinocytes of the skin [123]. The gut microbiota can influence the outcomes of gallstones [124], obesity [125], colonic inflammation [126], and even the behavior of autism patients [127]. Compounds such as beta-methyl-d-galactoside and N-acetyl-d-mannosamine produced by some oral bacteria can be used as prebiotics to stimulate beneficial bacteria in the oral cavity and prevent tooth decay [128]. Microbes in the oral cavity of newborn babies induce the expression of the Growth-Arrest-Specific-Protein-6 (GAS6) in oral tissues; GAS6, in turn, regulates the antibacterial function of the oral cavity [129]. Some authors even suggested that the adaptive immunity may have been evolved in vertebrates because of the need to interact with the beneficial bacterial communities [130]. All these findings suggest that human and its human-associated microbiota are inseparable. Gill et al [120] consider the human body as a “superorganism” with distinctive contributions from both microbes and the human host. The diversity of microbiota in humans fluctuates considerably, especially during disease and in young children [131,132]. Knowledge on the part of bacterial physiology, therefore, is important to appreciate human as a superorganism.
7. Bioenergetic and ROS formation in bacteria
Bacteria must respond to their environment rapidly and effectively. The forms of nutrients provided to bacteria may differ from time to time. A bacterium must be able to adjust its trophic modes to satisfy its needs for carbon and energy [133–136]. However, the amount of carbons and energy in food sources are not necessarily the same [134,135,137]. Fermentation is a simple way for a bacterium to balance its redox by removing its surplus carbons (in forms such as lactic acid or alcohol) and to regenerate its oxidative capacity (NAD and FAD). When NADH is in surplus, some bacteria can regenerate its NAD via the formation of hydrogen gas [126]. Another way to regenerate the NAD is through the electron transport chain. The electron transport chains in bacteria are branched [133,138–141]. The electrons from various reductants (such as hydrogen gas [142], NADH, malate, and succinate [138,143]) are regulated by an energy-conserving electron transport pathway and by an energy-uncoupled pathway (Figure 2) [144]. Disruption of cellular metabolism, or uncoupling the electron transport chain, would likely interrupt the regulation of electron flow. Excessive electron flow through the uncoupled pathway could induce a futile cycle, leading to increases in ROS formation, and induce oxidative stress (Figure 3). All bactericidal antibiotics are known to induce cell death such as oxidative stress [145,146]. Lipid peroxidation [147,148], heavy metals (e.g., Cr, As, Hg) [106], and O3 from smog can uncouple the electron transport chain readily, leading to bacterial mutation and death [45,149–152].
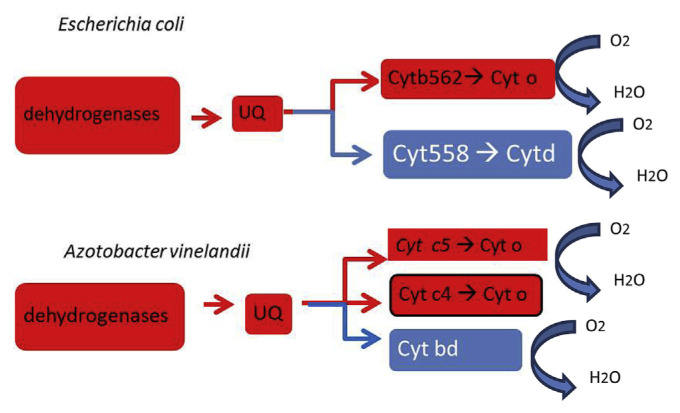
Figure 2.
Diagram of the electron transport chain of Escherichia coli and Azotobacter vinelandii with special reference to the energy-coupled (red) pathways and the energy-uncoupled (blue) pathways. Reductants such as NADH, succinate, and malate are oxidized by the dehydrogenases. Electrons from the dehydrogenase are transferred to the ubiquinone (UQ), which subsequently transfer the electrons to the cytochromes (cyt). There are two electron transport pathways, the energy-conserving pathway is coupled to a proton pump (red), whereas the energy-wasting pathway (blue) is not coupled to proton translocation. The diagram is compiled from published data [133,139,141,142]. Free radicals can generate via the Haber–Weiss reaction when electrons are passing the electron transport chain.
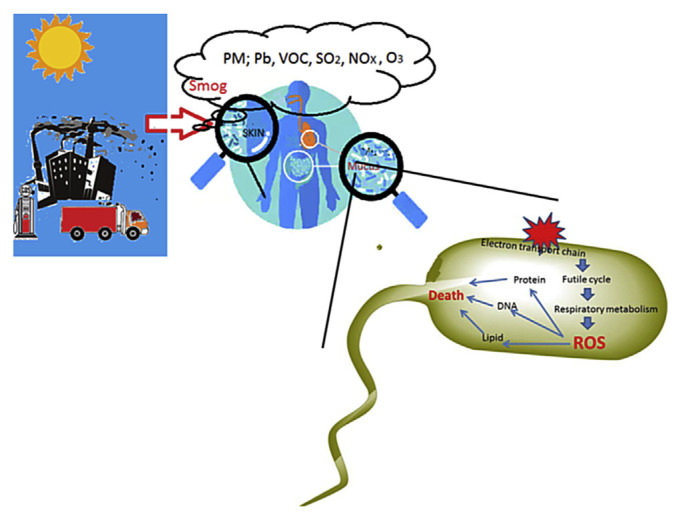
Figure 3.
Sunlight activates the pollutants in the air and fog, causing the formation of smog. Bacteria on the surface of our skin and mucus are the first to encounter the harmful elements in smog. These elements could uncouple the electron transport chain of the bacterium and induce ROS formation, which subsequently damage protein, DNA and lipid, leading to cell death. ROS =reactive oxygen species; PM =particulate matter.
8. Perspective
Humans evolved with microbes for millions of years. These human-associated microbes have developed many remarkable strategies to sustain their alliances with each other and with us. Their physical locations on the epithelium also make them the first to be exposed to the harmful elements in smog. Chronic exposure to smog would undoubtedly disrupt the equilibrium of the microbial communities in the human body. How changes in the microbiota would affect the human health, especially among newborns and children, is unclear. Our understanding of human-associated microbiota is still in its infancy. Most of the available data are structural— they describe the species diversity, richness, and evenness of the community based on sequence homologies. Metagenomics data do not provide meaningful information on metabolic capacities of these microbes, partly because only about 1% of the bacteria are culturable, and partly because a large number of genes have no known function [153]. How the inner working of the human and human-associated microbiota is less clear [154,155]. Functional genomics involves the use of various “omics” to predict the physiological dynamics of a biological system [156]. Research in functional genomics of human-associated microbiota should provide a better interpretation of diseases caused by smog. Culturomics [157–160], a new approach that uses high-throughput culture techniques and matrix-assisted laser desorption/ionization-time of flight mass spectrometry to grow and identify bacteria, should provide a more meaningful interpretation of microbiota successions and these biological dark matters. Although the consequence of smog-induced microbiota changes in human health is far from clear, general ecological principles should help to predict the consequences of population shifts in microbiota. Like a forest, ecological studies suggest that plants, animals, and the environment are interrelated. Species diversity is indicative of the healthiness of the forest; environmental changes can unbalance the equilibrium of the biocommunity, leading to the collapse of the ecosystem [153,161]. The successes in fecal transplant [162,163] show that it is possible to replace a collapsed microbial community. This implies that other forms of activity, such as exercise, diet, and a change in lifestyle, may alter the epithelium microbiota to prevent or mediate the harmful effects of pollution-induced changes in microbiota. Microbial succession can occur rapidly or gradually. The long-term effects of smog to the regime change of the human-associated microbes remain to be studied.
Obviously, reduction of fossil-fuel burning is the ultimate method to reduce smog formation. However, this goal is unlikely archived in countries with less economic wealth. Coal cleaning is a process by which impurities such as sulfur, ash, and rock are removed from coal prior to burning. Currently, both physical and chemical processes are available. Physical coal cleaning processes, the mechanical separation of coal from its contaminants using differences in density, are by far the major processes in use today [164,165]. Physical cleaning of coal is essentially based on the differences in either specific gravity or surface properties between the organic matter and the associated minerals, although a few separations that are conducted on the basis of their magnetic and electrostatic properties have been proposed. Chemical methods of cleaning coal [166], such as using molten caustic leaching, have been shown to remove more than 90% of the sulfur and ash from coal. The SNOX process is a very energy-efficient way to convert NOx in the flue gas into nitrogen and SOx into concentrated sulfuric acid of commercial quality without using any absorbents and without producing waste products or wastewater [167]. The SNOX technology is especially suitable for cleaning flue gasses from combustion of high-sulfur fuels in refineries. Biodesulfurization in fossil fuels [168] is an ideal alternative to remove sulfur in petroleum products. Many bacteria, both natural [169,170] and genetically altered [171], have shown promising results to remove SOx and NOx from fuels. However, the speed of bioconversion is still too slow to fulfill the industrial requirements. The remediation process could increase at the higher temperature. Some bacteria, called thermophiles (optimal temperature ~70°C) and extreme thermophiles (optimal temperature above 100°C), can grow at high temperatures. More research is needed to isolate suitable strains to release sulfur and nitrogen from fossil fuels.
REFERENCES
- 1. Chang L, Xu J, Tie X, Wu J. Impact of the 2015 El Nino event on winter air quality in China. Sci Rep. 2016;6:34275. doi: 10.1038/srep34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bytnerowicz A, Fenn ME. Nitrogen deposition in California forests: a review. Environ Pollut. 1996;92:127–46. doi: 10.1016/0269-7491(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 3. Snow JA, Dennison JB, Jaffe DA, Price HU, Vaughan JK, Lamb B. Aircraft and surface observations of air quality in Puget Sound and a comparison to a regional model. Atmos Environ. 2003;37:4019–32. [Google Scholar]
- 4. Ayres J, Fleming D, Williams M, McInnes G. Measurement of respiratory morbidity in general practice in the United Kingdom during the acid transport event of January 1985. Environ Health Perspect. 1989;79:83–8. doi: 10.1289/ehp.897983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Senn CL. General atmospheric pollution; Los Angeles smog. Am J Public Health Nations Health. 1948;38:962–5. doi: 10.2105/ajph.38.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whittaker A, BeruBe K, Jones T, Maynard R, Richards R. Killer smog of London, 50 years on: particle properties and oxidative capacity. Sci Total Environ. 2004;334–5:435–45. doi: 10.1016/j.scitotenv.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 7. Lai L-W. Effect of photochemical smog associated with synoptic weather patterns on cardiovascular and respiratory hospital admissions in metropolitan Taipei. Int J Environ Health Res. 2012;22:287–304. doi: 10.1080/09603123.2011.634390. [DOI] [PubMed] [Google Scholar]
- 8. Chen R, Zhao Z, Kan H. Heavy smog and hospital visits in Beijing, China. Am J Respir Crit Care Med. 2013;188:1170–1. doi: 10.1164/rccm.201304-0678LE. [DOI] [PubMed] [Google Scholar]
- 9. Mohammadi H, Cohen D, Babazadeh M, Rokni L. The effects of atmospheric processes on tehran smog forming. Iran J Public Health. 2012;41:1–12. [PMC free article] [PubMed] [Google Scholar]
- 10. Blake DR, Rowland FS. Urban leakage of liquefied petroleum gas and its impact on Mexico City air quality. Science. 1995;269:953–6. doi: 10.1126/science.269.5226.953. [DOI] [PubMed] [Google Scholar]
- 11. Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–4. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 12. Wood YA, Fenn M, Meixner T, Shouse PJ, Breiner J, Allen E, Wu LS. Smog nitrogen and the rapid acidification of forest soil, San Bernardino Mountains, southern California. Scientific World Journal. 2007;7:175–80. doi: 10.1100/tsw.2007.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heinrich A. An estimate of biogenic emissions of volatile organic compounds during summertime in China (7 pp) Environ Sci Pollut Res Int. 2007;14:69–75. doi: 10.1065/espr2007.02.376. [DOI] [PubMed] [Google Scholar]
- 14. Tsimpidi AP, Trail M, Hu Y, Nenes A, Russell AG. Modeling an air pollution episode in northwestern United States: identifying the effect of nitrogen oxide and volatile organic compound emission changes on air pollutants formation using direct sensitivity analysis. J Air Waste Manag Assoc. 2012;62:1150–65. doi: 10.1080/10962247.2012.697093. [DOI] [PubMed] [Google Scholar]
- 15. Tam E, Miike R, Labrenz S, Sutton AJ, Elias T, Davis J, Chen YL, Tantisira K, Dockery D, Avol E. Volcanic air pollution over the Island of Hawai’i: emissions, dispersal, and composition. Association with respiratory symptoms and lung function in Hawai’i Island school children. Environ Int. 2016;92–3:543–52. doi: 10.1016/j.envint.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hobbs PV, Radke LF, Eltgroth MW, Hegg DA. Airborne studies of the emissions from the volcanic eruptions of Mount St. Helens. Science. 1981;211:816–8. doi: 10.1126/science.211.4484.816. [DOI] [PubMed] [Google Scholar]
- 17. Lubick N. Northern fires feed southern smog. Environ Sci Technol. 2006;40:6528. [PubMed] [Google Scholar]
- 18. Thompson KA, Shimabuku KK, Kearns JP, Knappe DRU, Summers RS, Cook SM. Environmental comparison of biochar and activated carbon for tertiary wastewater rreatment. Environ Sci Technol. 2016;50:11253–62. doi: 10.1021/acs.est.6b03239. [DOI] [PubMed] [Google Scholar]
- 19. Koplitz SN, Mickley LJ, Marlier ME, Buonocore JJ, Kim PS, Liu T, Sulprizio MP, DeFries RS, Jacob DJ, Schwartz J, Pongsiri M, Myers SS. Public health impacts of the severe haze in Equatorial Asia in September–October 2015: demonstration of a new framework for informing fire management strategies to reduce downwind smoke exposure. Environ Res Lett. 2016;11:094023. [Google Scholar]
- 20. Filippini T, Heck JE, Malagoli C, Giovane CD, Vinceti M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J Environ Sci Healtht C. 2015;33:36–66. doi: 10.1080/10590501.2015.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helfand WH, Lazarus J, Theerman P. Donora, Pennsylvania: an environmental disaster of the 20th century. Am J Public Health. 2001;91:553. doi: 10.2105/ajph.91.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fry J, Dillane JB, Fry L. Smog: 1962 v. 1952. Lancet. 1962;2:1326. doi: 10.1016/s0140-6736(62)90871-1. [DOI] [PubMed] [Google Scholar]
- 23. Smith RN. Smog: 1962 v. 1952. Lancet. 1963;1:57. [PubMed] [Google Scholar]
- 24. Lyster WR. Altered sex ratio after the London smog of 1952 and the Brisbane flood of 1965. J Obstet Gynaecol Br Commonw. 1974;81:626–31. doi: 10.1111/j.1471-0528.1974.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 25.Taylor GR. The transportation revolution, 1815–1860. New York: Rinehart; 1951. p. xvii.p. 490. [Google Scholar]
- 26.Schwantes CA. Going places: transportation redefines the twentieth-century West. Bloomington, IN: Indiana University Press; 2003. p. xix.p. 419. [Google Scholar]
- 27.Brasseaux CA, Fontenot KP. Steamboats on Louisiana’s bayous: a history and directory. Baton Rouge, LA: Louisiana State University Press; 2004. p. xi.p. 277. [Google Scholar]
- 28.Ray K. New roads, canals, and railroads in early 19th-century America: the transportation revolution. 1st ed. New York: Rosen Pub. Group; 2004. p. 32. [Google Scholar]
- 29.Hu A, Shi B. Zhongguo jiao tong ge ming : kua yue shi fa zhan zhi lu [China transportation revolution: path to leap-forward development]. Di 1 ban. Beijing Shi: Ren Min Jiao Tong Chu Ban She; 2009. pp. 2–5.pp. 155 [Google Scholar]
- 30.Kapsch RJ. Over the Alleghenies: early canals and railroads of Pennsylvania. 1st ed. Morgantown, WV: West Virginia University Press; 2013. p. 449. [Google Scholar]
- 31.Farndon J, Hart-Davis A. Engineers. New York, NY: DK Publishing; 2015. p. 360. [Google Scholar]
- 32. Jeswani HK, Azapagic A. Assessing the environmental sustainability of energy recovery from municipal solid waste in the UK. Waste Manag. 2016;50:346–63. doi: 10.1016/j.wasman.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 33. Mu D, Horowitz N, Casey M, Jones K. Environmental and economic analysis of an in-vessel food waste composting system at Kean University in the U.S. Waste Manag. 2016;59:476–86. doi: 10.1016/j.wasman.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 34. Kirkeby JT, Birgisdottir H, Bhander GS, Hauschild M, Christensen TH. Modelling of environmental impacts of solid waste landfilling within the life-cycle analysis program EASEWASTE. Waste Manag. 2007;27:961–70. doi: 10.1016/j.wasman.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 35. Sharma R, Sharma M, Sharma R, Sharma V. The impact of incinerators on human health and environment. Rev Environ Health. 2013;28:67–72. doi: 10.1515/reveh-2012-0035. [DOI] [PubMed] [Google Scholar]
- 36. Brosseau J, Heitz M. Trace gas compound emissions from municipal landfill sanitary sites. Atmos Environ. 1994;28:285–93. [Google Scholar]
- 37.Young P, Parker A. Hazardous and Industrial Waste Management and Testing: Third Symposium. Philadelphia, PA: American Society for Testing and Materials; 1984. Vapors, odors, and toxic gases from landfills. [Google Scholar]
- 38. Ruokojärvi P, Ruuskanen J, Ettala M, Rahkonen P, Tarhanen J. Formation of polyaromatic hydrocarbons and polychlorinated organic compounds in municipal waste landfill fires. Chemosphere. 1995;31:3899–908. [Google Scholar]
- 39. Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Albrechtsen HJ, Heron G. Biogeochemistry of landfill leachate plumes. Appl Geochem. 2001;16:659–718. [Google Scholar]
- 40.Duprey RL.United States Environmental Protection Agency Office of Air Programs; Research Triangle Park NC; For sale by the Supt. of Docs, editor. Compilation of air pollutant emission factors Rev. Washington, DC: U.S. Govt. Print. Off; 1972. [Google Scholar]
- 41.United States Environmental Protection Agency Office of Air Quality Planning and Standards. Compilation of air pollutant emission factors. 5th ed. Research Triangle Park, NC: U.S. Environmental Protection Agency; 1995. pp. 18–86. [Google Scholar]
- 42. Tattersfield AE. Air pollution: brown skies research. Thorax. 1996;51:13–22. doi: 10.1136/thx.51.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gaffney JS, Marley NA. Atmospheric chemistry and air pollution. ScientificWorldJournal. 2003;3:199–234. doi: 10.1100/tsw.2003.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chakraborty J. The geographic distribution of potential risks posed by industrial toxic emissions in the U.S. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39:559–75. doi: 10.1081/ese-120027725. [DOI] [PubMed] [Google Scholar]
- 45. Claxton LD, Matthews PP, Warren SH. The genotoxicity of ambient outdoor air, a review: Salmonella mutagenicity. Mutat Res. 2004;567:347–99. doi: 10.1016/j.mrrev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 46. Straka P, Havelcova M. Polycyclic aromatic hydrocarbons and other organic compounds in ashes from biomass combustion. Acta Geodyn Geomater. 2012;9:481–90. [Google Scholar]
- 47. Fu PP, Von Tungeln LS, Chiu LH, Own ZY. Halogenated-polycyclic aromatic hydrocarbons: a class of genotoxic environmental pollutants. J Environ Sci Health C. 1999;17:71–109. [Google Scholar]
- 48. Pennington DW, Potting J, Finnveden G, Lindeijer E, Jolliet O, Rydberg T, Rebitzer G. Life cycle assessment: Part 2. Current impact assessment practice. Environ Int. 2004;30:721–39. doi: 10.1016/j.envint.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 49. Rebitzer G, Ekvall T, Frischknecht R, Hunkeler D, Norris G, Rydberg T, Schmidt WP, Suh S, Weidema BP, Pennington DW. Life cycle assessment: Part 1. Framework, goal and scope definition, inventory analysis, and applications. Environ Int. 2004;30:701–20. doi: 10.1016/j.envint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 50. Chock DP, Dunker AM, Kumar S, Sloane CS. Effect of nitrogen oxides (NOx) emission rates on smog formation in the California South Coast Air Basin. Environ Sci Technol. 1981;15:933–9. doi: 10.1021/es00090a009. [DOI] [PubMed] [Google Scholar]
- 51. Dhar NR, Singh PN. Role of light energy, organic matter and rock phosphate in fixation of atmospheric nitrogen in alkali soil. Proc Natl Acad Sci India A. 1968;38:377–9. [Google Scholar]
- 52. Renner MG. Car sick. World Watch. 1988;1:36–43. [PubMed] [Google Scholar]
- 53. Gray EL, Goldberg SB, Patton FM. Toxicity of the oxides of nitrogen: III. Effect of chronic exposure to low concentrations of vapors from red fuming nitric acid. AMA Arch Ind Health. 1954;10:423–5. [PubMed] [Google Scholar]
- 54. Gray EL, Patton FM, Goldberg SB, Kaplan E. Toxicity of the oxides of nitrogen: II. Acute inhalation toxicity of nitrogen dioxide, red fuming nitric acid, and white fuming nitric acid. AMA Arch Ind Health. 1954;10:418–22. [PubMed] [Google Scholar]
- 55. Gray EL, Goldberg SB, Patton FM. Toxicity of the oxides of nitrogen: I. Introduction and apparatus. AMA Arch Ind Health. 1954;10:409–17. [PubMed] [Google Scholar]
- 56. Gray EL. Oxides of nitrogen: their occurrence, toxicity, hazard; a brief review. AMA Arch Ind Health. 1959;19:479–86. [PubMed] [Google Scholar]
- 57. Victorin K. Review of the genotoxicity of nitrogen oxides. Mutat Res. 1994;317:43–55. doi: 10.1016/0165-1110(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 58. Turino GM. Effect of carbon-monoxide on the cardiorespiratory system — carbon-monoxide toxicity — physiology and biochemistry. Circulation. 1981;63:A253–9. [PubMed] [Google Scholar]
- 59. Hackney JD, Linn WS, Avol EL. Acid fog: effects on respiratory function and symptoms in healthy and asthmatic volunteers. Environ Health Perspect. 1989;79:159–62. doi: 10.1289/ehp.8979159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaufman FL, Kim AN, Campbell MA, Wu KL, Donald JM. Sulfur dioxide—evidence of reproductive and developmental toxicity. Birth Defects Res A. 2011;91:378. [Google Scholar]
- 61. EPA. Particulate Matter (PM) Pollution. 2017 [Google Scholar]
- 62. Omidvarborna H, Kumar A, Kim D-S. Recent studies on soot modeling for diesel combustion. Renew Sustainable Energy Rev. 2015;48:635–47. [Google Scholar]
- 63. Chan CK, Yao X. Air pollution in mega cities in China. Atmos Environ. 2008;42:1–42. [Google Scholar]
- 64. Jaoui M, Corse E, Kleindienst TE, Offenberg JH, Lewandowski M, Edney EO. Analysis of secondary organic aerosol compounds from the photooxidation of d-limonene in the presence of NOx and their detection in ambient PM2.5. Environ Sci Technol. 2006;40:3819–28. doi: 10.1021/es052566z. [DOI] [PubMed] [Google Scholar]
- 65. Jia H, Wang L. Peering into China’s thick haze of air pollution. Chem Eng News. 2017;95:19–22. [Google Scholar]
- 66. Pastuszka JS, Rogula-Kozlowska W, Zajusz-Zubek E. Characterization of PM10 and PM2.5 and associated heavy metals at the crossroads and urban background site in Zabrze, Upper Silesia, Poland, during the smog episodes. Environ Monit Assess. 2010;168:613–27. doi: 10.1007/s10661-009-1138-8. [DOI] [PubMed] [Google Scholar]
- 67. Fang W, Yang Y, Xu Z. PM10 and PM2.5 and health risk assessment for heavy metals in a typical factory for cathode ray tube television recycling. Environ Sci Technol. 2013;47:12469–76. doi: 10.1021/es4026613. [DOI] [PubMed] [Google Scholar]
- 68. Luo K, Zhang X, Chen C, Lu Y. Estimate of arsenic emission amount from the coal power stations in china. Chin Sci Bull. 2004;49:2183–9. [Google Scholar]
- 69. Swidwinska-Gajewska AM, Czerczak S. Nanosilver — occupational exposure Limits. Med Pracy. 2015;66:429–42. doi: 10.13075/mp.5893.00177. [DOI] [PubMed] [Google Scholar]
- 70. McShan D, Ray PC, Yu HT. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22:116–27. doi: 10.1016/j.jfda.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moitra S, Puri R, Paul D, Huang Y-CT. Global perspectives of emerging occupational and environmental lung diseases. Curr Opin Pulm Med. 2015;21:114–20. doi: 10.1097/MCP.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 72. Wang H, Chen C, Zhang Y, Peng L, Ma S, Yang T, Guo H, Zhang Z, Su DS, Zhang J. In situ oxidation of carbon-encapsulated cobalt nanocapsules creates highly active cobalt oxide catalysts for hydrocarbon combustion. Nat Commun. 2015;6:7181. doi: 10.1038/ncomms8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. He W, Liu Y, Wamer WG, Yin JJ. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J Food Drug Anal. 2014;22:49–63. doi: 10.1016/j.jfda.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Leiva GMA, Santibañez DA, Ibarra ES, Matus CP, Seguel R. A five-year study of particulate matter (PM2.5) and cerebrovascular diseases. Environ Pollut. 2013;181:1–6. doi: 10.1016/j.envpol.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 75. Maher BA, Ahmed IAM, Karloukovski V, MacLaren DA, Foulds PG, Allsop D, Mann DMA, Torres-Jardon R, Calderon-Garciduenas L. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci. 2016;113:10797–801. doi: 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xiao Y, Shao Y, Yu X, Zhou G. The epidemic status and risk factors of lung cancer in Xuanwei City, Yunnan Province, China. Front Med. 2012;6:388–94. doi: 10.1007/s11684-012-0233-3. [DOI] [PubMed] [Google Scholar]
- 77. Chen Z, Wang JN, Ma GX, Zhang YS. China tackles the health effects of air pollution. Lancet. 2013;382:1959–60. doi: 10.1016/S0140-6736(13)62064-4. [DOI] [PubMed] [Google Scholar]
- 78. Gaughan AE, Stevens FR, Huang Z, Nieves JJ, Sorichetta A, Lai S, Ye X, Linard C, Hornby GM, Hay SI, Yu H, Tatem AJ. Spatiotemporal patterns of population in mainland China, 1990 to 2010. Sci Data. 2016;3:160005. doi: 10.1038/sdata.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fu B. Blue skies for China. Science. 2008;321:611. doi: 10.1126/science.1162213. [DOI] [PubMed] [Google Scholar]
- 80. Liu Q, Wang Q. How China achieved its 11th Five-Year Plan emissions reduction target: a structural decomposition analysis of industrial SO2 and chemical oxygen demand. Sci Total Environ. 2017;574:1104–16. doi: 10.1016/j.scitotenv.2016.08.176. [DOI] [PubMed] [Google Scholar]
- 81.Baxter T. China saw average PM2.5 levels fall by 10% in 2015, but 80% of cities still fail to meet national air quality standards. Greenpease Greenpeace International Press Desk; 2016. [Google Scholar]
- 82. van der Ronald A, Mijling B, Ding J, Koukouli ME, Liu F, Li Q, Mao H, Theys N. Cleaning up the air: effectiveness of air quality policy for SO2 and NOx emissions in China. Atmos Chem Phys. 2017;17:1775–89. [Google Scholar]
- 83. Routledge HC, Ayres JG. Air pollution and the heart. Occup Med (Lond) 2005;55:439–47. doi: 10.1093/occmed/kqi136. [DOI] [PubMed] [Google Scholar]
- 84. Pain MC. Causes of chronic airway diseases. Chest. 1999;115:4–6. doi: 10.1378/chest.115.1.4. [DOI] [PubMed] [Google Scholar]
- 85. Zhang F, Li M-Y, Lan Y-T, Wang C-B. Imbalance of Th17/Tregs in rats with smoke inhalation-induced acute lung injury. Sci Rep. 2016;6:21348. doi: 10.1038/srep21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mucke W. The environment and the eye. Topics of ophthalmic toxicology. J Med Liban. 1996;44:146–50. [PubMed] [Google Scholar]
- 87. Fukuda M, Fukuda K, Shimizu T, Moller H. Decline in sex ratio at birth after Kobe earthquake. Hum Reprod. 1998;13:2321–2. doi: 10.1093/humrep/13.8.2321. [DOI] [PubMed] [Google Scholar]
- 88. Martinez-Lazcano JC, Gonzalez-Guevara E, del Carmen Rubio M, Franco-Perez J, Custodio V, Hernandez-Ceron M, Livera C, Paz C. The effects of ozone exposure and associated injury mechanisms on the central nervous system. Rev Neurosci. 2013;24:337–52. doi: 10.1515/revneuro-2012-0084. [DOI] [PubMed] [Google Scholar]
- 89. Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, Serre ML, Vizuete W, Sioutas C, Morgan TE, Gatz M, Chui HC, Shumaker SA, Resnick SM, Espeland MA, Finch CE, Chen JC. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry. 2017;7:e1022. doi: 10.1038/tp.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Doyle M, Sexton KG, Jeffries H, Jaspers I. Atmospheric photochemical transformations enhance 1,3-butadiene-induced inflammatory responses in human epithelial cells: the role of ozone and other photochemical degradation products. Chem Biol Interact. 2007;166:163–9. doi: 10.1016/j.cbi.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 91. Knox EG. Atmospheric pollutants and mortalities in English local authority areas. J Epidemiol Community Health. 2008;62:442–7. doi: 10.1136/jech.2007.065862. [DOI] [PubMed] [Google Scholar]
- 92. Lee J, Donahue NM. Secondary organic aerosol coating of synthetic metal-oxide nanoparticles. Environ Sci Technol. 2011;45:4689–95. doi: 10.1021/es104147z. [DOI] [PubMed] [Google Scholar]
- 93. Gwinn MR, Vallyathan V. Nanoparticles: Health effects—pros and cons. Environ Health Perspect. 2006;114:1818–25. doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yukihiro M, Hiramatsu T, Bouteau F, Kadono T, Kawano T. Peroxyacetyl nitrate-induced oxidative and calcium signaling events leading to cell death in ozone-sensitive tobacco cell-line. Plant Signal Behav. 2012;7:113–20. doi: 10.4161/psb.7.1.18376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Aibo DI, Birmingham NP, Lewandowski R, Maddox JF, Roth RA, Ganey PE, Wagner JG, Harkema JR. Acute exposure to ozone exacerbates acetaminophen-induced liver injury in mice. Toxicol Sci. 2010;115:267–85. doi: 10.1093/toxsci/kfq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Afaq F, Zaid MA, Pelle E, Khan N, Syed DN, Matsui MS, Maes D, Mukhtar H. Aryl hydrocarbon receptor is an ozone sensor in human skin. J Invest Dermatol. 2009;129:2396–403. doi: 10.1038/jid.2009.85. [DOI] [PubMed] [Google Scholar]
- 98. Sharma VP, Singh HP, Kohli RK, Batish DR. Mobile phone radiation inhibits Vigna radiata (mung bean) root growth by inducing oxidative stress. Sci Total Environ. 2009;407:5543–7. doi: 10.1016/j.scitotenv.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 99. Bessac BF, Jordt S-E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360–70. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sathishkumar K, Murthy SN, Uppu RM. Cytotoxic effects of oxysterols produced during ozonolysis of cholesterol in murine GT1-7 hypothalamic neurons. Free Radic Res. 2007;41:82–8. doi: 10.1080/10715760600950566. [DOI] [PubMed] [Google Scholar]
- 101. Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ Health Perspect. 2006;114:627–33. doi: 10.1289/ehp.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. He QC, Tavakkol A, Wietecha K, Begum-Gafur R, Ansari SA, Polefka T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int J Cosmet Sci. 2006;28:349–57. doi: 10.1111/j.1467-2494.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 103. Fu PP, Xia Q, Sun X, Yu H. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)-light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012;30:1–41. doi: 10.1080/10590501.2012.653887. [DOI] [PubMed] [Google Scholar]
- 104. Andersen VF, Wallington TJ, Nielsen OJ. Atmospheric chemistry of i-butanol. J Phys Chem A. 2010;114:12462–9. doi: 10.1021/jp107950d. [DOI] [PubMed] [Google Scholar]
- 105. Hurley MD, Ball JC, Wallington TJ. Sulbaek Andersen MP, Nielsen OJ, Ellis DA, Martin JW, Mabury SA. Atmospheric chemistry of n-C(x)F(2)(x)(+1)CHO (x = 1, 2, 3, 4): fate of n-C(x)F(2)(x)(+1)C(O) radicals. J Phys Chem A. 2006;110:12443–7. doi: 10.1021/jp064029m. [DOI] [PubMed] [Google Scholar]
- 106. Belyaeva EA, Sokolova TV, Emelyanova LV, Zakharova IO. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper. ScientificWorldJournal. 2012;2012:136063. doi: 10.1100/2012/136063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. González-Flecha B, Demple B. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J Biol Chem. 1995;270:13681–7. doi: 10.1074/jbc.270.23.13681. [DOI] [PubMed] [Google Scholar]
- 108. Brenna JT. Arachidonic acid needed in infant formula when docosahexaenoic acid is present. Nutr Rev. 2016;74:329–36. doi: 10.1093/nutrit/nuw007. [DOI] [PubMed] [Google Scholar]
- 109. Pryor WA, Das B, Church DF. The ozonation of unsaturated fatty acids: aldehydes and hydrogen peroxide as products and possible mediators of ozone toxicity. Chem Res Toxicol. 1991;4:341–8. doi: 10.1021/tx00021a014. [DOI] [PubMed] [Google Scholar]
- 110. Yin J-J, Lao F, Fu PP, Wamer WG, Zhao Y, Wang PC, Qiu Y, Sun B, Xing G, Dong J, Liang XJ, Chen C. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials. 2009;30:611–21. doi: 10.1016/j.biomaterials.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. He QC, Krone K, Scherl D, Kotler M, Tavakkol A. The use of ozone as an oxidizing agent to evaluate antioxidant activities of natural substrates. Skin Pharmacol Physiol. 2004;17:183–9. doi: 10.1159/000078821. [DOI] [PubMed] [Google Scholar]
- 112. Ni YC, Kadlubar FF, Fu PP. Formation of malondialdehyde-modified 2′-deoxyguanosinyl adduct from metabolism of chloral hydrate by mouse-liver microsomes. Biochem Bioph Res Commun. 1995;216:1110–7. doi: 10.1006/bbrc.1995.2735. [DOI] [PubMed] [Google Scholar]
- 113. Ni YC, Wong TY, Lloyd RV, Heinze TM, Shelton S, Casciano D, Kadlubar FF, Fu PP. Mouse liver microsomal metabolism of chloral hydrate, trichloroacetic acid, and trichloroethanol leading to induction of lipid peroxidation via a free radical mechanism. Drug Metab Dispos. 1996;24:81–90. [PubMed] [Google Scholar]
- 114. Martens JH, Barg H, Warren MJ, Jahn D. Microbial production of vitamin B12. Appl Microbiol Biotechnol. 2002;58:275–85. doi: 10.1007/s00253-001-0902-7. [DOI] [PubMed] [Google Scholar]
- 115. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Marcy Y, Ouverney C, Bik EM, Losekann T, Ivanova N, Martin HG, Szeto E, Platt D, Hugenholtz P, Relman DA, Quake SR. Dissecting biological “dark matter” with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc Natl Acad Sci U S A. 2007;104:11889–94. doi: 10.1073/pnas.0704662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A. 2007;104:2927–32. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bjorksten B. The gut microbiota: a complex ecosystem. Clin Exp Allergy. 2006;36:1215–7. doi: 10.1111/j.1365-2222.2006.02579.x. [DOI] [PubMed] [Google Scholar]
- 120. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tanaka K, Sawamura S, Satoh T, Kobayashi K, Noda S. Role of the indigenous microbiota in maintaining the virus-specific CD8 memory T cells in the lung of mice infected with murine cytomegalovirus. J Immunol. 2007;178:5209–16. doi: 10.4049/jimmunol.178.8.5209. [DOI] [PubMed] [Google Scholar]
- 122. Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 123. Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158:442–55. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fremont-Rahl JJ, Ge Z, Umana C, Whary MT, Taylor NS, Muthupalani S, Carey MC, Fox JG, Mauer KJ. An analysis of the role of the indigenous microbiota in cholesterol gallstone pathogenesis. PLoS One. 2013;8:e70657. doi: 10.1371/journal.pone.0070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chassaing B, Van de Wiele T, Gewirtz A. O-013 Dietary emulsifiers directly impact the human gut microbiota increasing its pro-inflammatory potential and ability to induce intestinal inflammation. Inflamm Bowel Dis. 2017;23:S5. [Google Scholar]
- 126. Portune KJ, Benítez-Paez A, Del Pulgar EMG, Cerrudo V, Sanz Y. Gut microbiota, diet, and obesity-related disorders—the good, the bad, and the future challenges. Mol Nutr Food Res. 2017;61:1600252–8. doi: 10.1002/mnfr.201600252. [DOI] [PubMed] [Google Scholar]
- 127. Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Slomka V, Hernandez-Sanabria E, Herrero ER, Zaidel L, Bernaerts K, Boon N, Quirynen M, Teughels W. Nutritional stimulation of commensal oral bacteria suppresses pathogens: the prebiotic concept. J Clin Periodontol. 2017 doi: 10.1111/jcpe.12700. [DOI] [PubMed] [Google Scholar]
- 129. Nassar M, Tabib Y, Capucha T, Mizraji G, Nir T, Pevsner-Fischer M, Zilberman-Schapira G, Heyman O, Nussbaum G, Bercovier H, Wilensky A, Elinav E, Burstyn-Cohen T, Hovav AH. GAS6 is a key homeostatic immunological regulator of host–commensal interactions in the oral mucosa. Proc Natl Acad Sci. 2017;114:E337–46. doi: 10.1073/pnas.1614926114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McFall-Ngai M. Adaptive immunity: care for the community. Nature. 2007;445:153. doi: 10.1038/445153a. [DOI] [PubMed] [Google Scholar]
- 131. Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, Segre JA. NISC Comparative Sequencing Program. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–50. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wong TY, Maier RJ. H2-dependent mixotrophic growth of N2-fixing Azotobacter vinelandii. J Bacteriol. 1985;163:528–33. doi: 10.1128/jb.163.2.528-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wong TY, Murdock CA, Concannon SP, Lockey TD. Simultaneous uptake of galactose and glucose by Azotobacter vinelandii. Biochem Cell Biol. 1991;69:711–4. doi: 10.1139/o91-106. [DOI] [PubMed] [Google Scholar]
- 135. Wong TY, Pei H, Bancroft K, Childers GW. Diauxic growth of Azotobacter vinelandii on galactose and glucose: regulation of glucose transport by another hexose. Appl Environ Microbiol. 1995;61:430–3. doi: 10.1128/aem.61.2.430-433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ferenci T. ‘Growth of bacterial cultures’ 50 years on: towards an uncertainty principle instead of constants in bacterial growth kinetics. Res Microbiol. 1999;150:431–8. doi: 10.1016/s0923-2508(99)00114-x. [DOI] [PubMed] [Google Scholar]
- 137. Wong TY, Yao XT. The DeLey–Doudoroff pathway of galactose metabolism in Azotobacter vinelandii. Appl Environ Microbiol. 1994;60:2065–8. doi: 10.1128/aem.60.6.2065-2068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jurtshuk P, Jr, Mueller TJ, Wong TY. Isolation and purification of the cytochrome oxidase of Azotobacter vinelandii. Biochim Biophys Acta. 1981;637:374–82. doi: 10.1016/0005-2728(81)90176-6. [DOI] [PubMed] [Google Scholar]
- 139. Haddock BA, Jones CW. Bacterial respiration. Bacteriol Rev. 1977;41:47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jones CW, Brice JM, Downs AJ, Drozd JW. Bacterial respiration-linked proton translocation and its relationship to respiratory-chain composition. Eur J Biochem. 1975;5:265–71. doi: 10.1111/j.1432-1033.1975.tb03994.x. [DOI] [PubMed] [Google Scholar]
- 141. Wong TY, Jurtshuk P., Jr Activation studies by phospholipids on the purified cytochrome c4:o oxidase of Azotobacter vinelandii. J Bioenerg Biomembr. 1984;16:477–89. doi: 10.1007/BF00743240. [DOI] [PubMed] [Google Scholar]
- 142. Wong TY, Maier RJ. Hydrogen-oxidizing electron transport components in nitrogen-fixing Azotobacter vinelandii. J Bacteriol. 1984;159:348–52. doi: 10.1128/jb.159.1.348-352.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wong TY, Maier RJ. Chlorpromazine inhibition of electron transport in Azotobacter vinelandii membranes. Biochim Biophys Acta. 1985;807:320–3. doi: 10.1016/0005-2728(85)90264-6. [DOI] [PubMed] [Google Scholar]
- 144. Liu J, Lee F, Lin C, Yao X, Davenport JW, Wong T. Alternative function of the electron transport system in Azotobacter vinelandii: removal of excess reductant by the cytochrome d pathway. Appl Environ Microbiol. 1995;61:3998–4003. doi: 10.1128/aem.61.11.3998-4003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dwyer DJ, Belenky PA, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CT, Lobritz MA, Braff D, Schwarz EG, Ye JD, Pati M, Vercruysse M, Ralifo PS, Allison KR, Khalil AS, Ting AY, Walker GC, Collins JJ. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A. 2014;111:E2100–9. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lobritz MA, Belenky P, Porter CB, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ. Antibiotic efficacy is linked to bacterial cellular respiration. Proc Natl Acad Sci U S A. 2015;112:8173–80. doi: 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–66. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 148. Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hannigan MP, Cass GR, Lafleur AL, Busby WF, Jr, Thilly WG. Seasonal and spatial variation of the bacterial mutagenicity of fine organic aerosol in southern california. Environ Health Perspect. 1996;104:428–36. doi: 10.1289/ehp.96104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Krueger AP, Smith RF, Go IG. The action of air ions on bacteria: I. Protective and lethal effects on suspensions of Staphylococci in droplets. J Gen Physiol. 1957;41:359–81. doi: 10.1085/jgp.41.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nover H, Botzenhart K. Bactericidal effects of photochemical smog constituents produced by a flow reactor: III. Communication: determination of mutagenic effects of photochemical smog on E. coli K 12 343/113. Zentralbl Bakteriol Mikrobiol Hyg B. 1985;181:71–80. [PubMed] [Google Scholar]
- 152. Gou H, Lu J, Li S, Tong Y, Xie C, Zheng X. Assessment of microbial communities in PM1 and PM10 of Urumqi during winter. Environ Pollut. 2016;214:202–10. doi: 10.1016/j.envpol.2016.03.073. [DOI] [PubMed] [Google Scholar]
- 153. Wong T-Y, Kuo J. A new drug design strategy: killing drug resistant bacteria by deactivating their hypothetical genes. J Environ Sci Health C. 2016;34:276–92. doi: 10.1080/10590501.2016.1236605. [DOI] [PubMed] [Google Scholar]
- 154. Morgan XC, Segata N, Huttenhower C. Biodiversity and functional genomics in the human microbiome. Trends Genet. 2013;29:51–8. doi: 10.1016/j.tig.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhou J, Miller JH. Microbial genomics—challenges and opportunities: the 9th International Conference on Microbial Genomes. J Bacteriol. 2002;184:4327–33. doi: 10.1128/JB.184.16.4327-4333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hieter P, Boguski M. Functional genomics: It’s all how you read it. Science. 1997;278:601. doi: 10.1126/science.278.5338.601. [DOI] [PubMed] [Google Scholar]
- 157. Lagier J-C, Hugon P, Khelaifia S, Fournier P-E, La Scola B, Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015;28:237–64. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dickson I. Gut microbiota: culturomics: illuminating microbial dark matter. Nat Rev Gastroenterol Hepatol. 2017;14:3. doi: 10.1038/nrgastro.2016.189. [DOI] [PubMed] [Google Scholar]
- 159. Lagier JC, Armougom F, Million M, Hugon P, Pagnier I, Robert C, Bittar F, Fournous G, Gimenez G, Maraninchi M, Trape JF, Koonin EV, La Scola B, Raoult D. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol. 2012;18:1185–93. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 160. Greub G. Culturomics: a new approach to study the human microbiome. Clin Microbiol Infect. 2012;18:1157–9. doi: 10.1111/1469-0691.12032. [DOI] [PubMed] [Google Scholar]
- 161.Begon MH, Townsend RWCR. Essentials of ecology. 4th ed. New York: Wiley; 2014. pp. 230–89. [Google Scholar]
- 162. Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29:79–84. doi: 10.1097/MOG.0b013e32835a4b3e. [DOI] [PubMed] [Google Scholar]
- 163. Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, Huerta-Cepas J, Nieuwdorp M, Salojarvi J, Voigt AY, Zeller G, Sunagawa S, de Vos WM, Bork P. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352:586–9. doi: 10.1126/science.aad8852. [DOI] [PubMed] [Google Scholar]
- 164. Mohanta S, Meikap BC. Pre-combustion removal possibility of hazardous trace elements from Indian high-ash coal by coal preparation technologies. Energ Source Part A. 2016;38:1693–8. [Google Scholar]
- 165. Çelik MS, Yildirim I. A new physical process for desulfurization of low-rank coals. Fuel. 2000;79:1665–9. [Google Scholar]
- 166. Chriswell CD, Shah ND, Markuszewski R. Countercurrent washing of Pittsburgh No-8 Coal after leaching with molten mixtures of sodium and potassium hydroxides. Sep Sci Technol. 1991;26:961–75. [Google Scholar]
- 167. Zhou Q, Huang GH, Chan CW. Development of an intelligent decision support system for air pollution control at coal-fired power plants. Expert Syst Appl. 2004;26:335–56. [Google Scholar]
- 168. Soleimani M, Bassi A, Margaritis A. Biodesulfurization of refractory organic sulfur compounds in fossil fuels. Biotechnol Adv. 2007;25:570–96. doi: 10.1016/j.biotechadv.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 169. Lin WC, Chang-Chien GP, Kao CM, Newman L, Wong TY, Liu JK. Biodegradation of polychlorinated dibenzo-p-dioxins by Pseudomonas mendocina strain NSYSU. J Environ Qual. 2014;43:349–57. doi: 10.2134/jeq2013.06.0215. [DOI] [PubMed] [Google Scholar]
- 170. Papizadeh M, Roayaei Ardakani M, Motamedi H. Growth-phase dependent biodesulfurization of dibenzothiophene by Enterobacter sp. strain NISOC-03. Pollution. 2017;3:101–11. [Google Scholar]
- 171. Wang J, Butler RR, III, Wu F, Pombert J-F, Kilbane JJ, II, Stark BC. Enhancement of microbial biodesulfurization via genetic engineering and adaptive evolution. PLoS One. 2017;12:e0168833. doi: 10.1371/journal.pone.0168833. [DOI] [PMC free article] [PubMed] [Google Scholar]