Abstract
Black garlic produced from fresh garlic under controlled high temperature and humidity has strong antioxidant properties. To determine these compounds, five fractions (from F1 to F5) were separated and purified by elution with chloroform:methanol at different ratios (8:1, 6:1, 4:1, 2:1, and 0:1; v/v). The antioxidant activity of each fraction was analyzed. The results showed that F3 and F4 had higher phenolic contents and stronger 2,2-diphenyl-2-picrylhydrazyl radical scavenging activity than the others. Seven purified individual components were further separated using semipreparation high-performance liquid chromatography from these two intensely antioxidant fractions (F3 and F4), their structures were elucidated by high-performance liquid chromatography coupled to diode array detection, electrospray ionization, mass spectrometry, 1H nuclear magnetic resonance, and 13C nuclear magnetic resonance spectrometry. Three compounds including adenosine, uridine, and 2-acetylpyrrole were first identified in black garlic, except for 5-hydroxymethylfurfural, (1S, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, and (1R, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid. The cellular antioxidant activities of uridine, adenosine, carboline alkaloids, 5-hydroxymethylfurfural, and ethyl acetate extracts were consistent with the results of in vitro experimental anti-oxidant properties. The results provide useful information for understanding the health benefits of black garlic products.
Keywords: antioxidant activity, black garlic, composition, identification
1. Introduction
Garlic (Allium sativum L.) is one of the most important foods and spices for centuries. Extensive studies have shown that garlic can provide favorable biological and pharmacological effects in vitro and in animal models in vivo, such as antimicrobial and anticancer activities [1,2], and also has hypoglycemic and antioxidant effects [3]. Garlic extracts have also been shown to have a strong radical scavenging activity [1,1-diphenyl-2-picrylhydrazyl (DPPH)] [4] and superoxide dismutase activity in vitro [5]. A number of studies have demonstrated garlic’s medicinal effects such as exhibiting antifatigue effects, regulating blood glucose [6] and blood pressure [3], helping in digestion, and improving appetite [7]. Experiments with small white rats reported that black garlic had antibiosis and antitumor functions, which could induce the human body to produce intense immune response of TH1 [8]. Black garlic could restrain the development of atherosclerosis by cleaning cholesterol to improve hyperlipidemia [9], and reduce weight and blood lipids [10,11]. Furthermore, researchers found that black garlic had a strong antioxidant activity both in vivo and in vitro [12].
The antioxidant activity of garlic could be affected by processing methods and conditions [4]. Black garlic, endowed with antioxidant activity, is produced from fresh garlic under controlled high temperature and humidity, eliminating its unpleasant odor. After processing, the black garlic products have a high content of polysaccharides, reducing sugar, protein, phenolic compounds, organic sulfur compounds, and melanoidins [4]. As shown in our previous study, the freeze-drying process can significantly maintain a high content of functional bioactive components in black garlic [13]. The antioxidant properties of black garlic products are related to polyphenols [14]. Polyphenols are sensitive to some heat treatments such as frying, baking, and boiling of some vegetables [15]. Researchers found the antioxidant capacity of whole garlic was very similar to that of peeled garlic cloves during the black garlic processing. The amount of polyphenols increased threefold in whole black garlic bulbs and about sixfold in peeled black garlic cloves [16]. In addition, total polyphenol and total flavonoid contents of black garlic significantly increased during the aging period [17]. Some researchers also mentioned that some components, such as (1S, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid and (1R, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, were present in high concentration in fresh and black garlic, and had a strong H2O2 scavenging capacity [18,19]. The objective of the present study was to determine the characteristic of bioactive components and health benefits of black garlic.
2. Materials and methods
2.1. Materials and reagents
Fresh garlic was purchased from the local market. Reagents such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). All other solvents/chemicals obtained from Tianjin Yongda Chemical Reagent Co., Ltd. (Tianjin, China) were of analytical grade or high-performance liquid chromatography (HPLC) grade.
2.2. Preparation of black garlic extract
Black garlic was mixed with an appropriate amount of water and homogenized, then extracted by ethyl acetate. The ethyl acetate extract was placed on the top of a glass column (100 × 5 cm2 i.d.) filled with silica gel (200–300 meshes) in chloroform. Elution was performed with chloroform and methanol at different ratios of 8:1, 6:1, 4:1, 2:1, and 0:1 (v/v) at a flow rate of 2 mL/min. The effluents were monitored at 254 nm; five fractions were collected and designated as F1 (8:1), F2 (6:1), F3 (4:1), F4 (2:1), and F5 (0:1), and eventually desiccated at vacuum for antioxidant activity test and HPLC analysis. The polarity of the five fractions was consistent with that of the eluent, i.e., F1 < F2 < F3 < F4 < F5; thus, the crude extraction was separated based on the polarity of the component in this step.
Their antioxidant properties were determined by the DPPH radical scavenging activity and total phenolic content. In addition, intense antioxidant fractions obtained from the elution of chloroform and methanol were analyzed by HPLC and semipreparation HPLC (semiprep-HPLC), and then their structures elucidated by chromatographic (HPLC) and spectrometric [electrospray ionization–mass spectrometry (ESI–MS), 1H nuclear magnetic resonance, (1H NMR), and 13C nuclear magnetic resonance (13C NMR)] techniques.
2.3. Determination of total phenolic content
Total phenolic content was determined according to Zhu et al [20] and Sharma et al [21], with minor modifications. A sample of the black garlic extracts (200 μL), Folin–Ciocalten reagent (1 mL, diluted 10 times), and sodium carbonate (1 mL, 7.5%) were mixed and diluted with distilled water to 5 mL. Subsequently, the mixtures were incubated in the dark for 30 minutes. After incubation, the absorbance was recorded at 760 nm. Gallic acid was used as a standard for the calibration curve. The phenolic content was reported as gallic acid equivalents (μg) using the following linear equation based on the calibration curve: A = 0.0929C + 0.0131, R2 = 0.9993, where A is the absorbance at 760 nm and C is the concentration of gallic acid equivalents (μg/mL).
2.4. DPPH radical scavenging activity
DPPH radical scavenging activity was determined according to Xu et al [22] and Yokozawa et al [23], with slight modification. Briefly, 1 mL of 0.2mM DPPH radical solution prepared in ethanol was mixed with 1 mL of the test sample dissolved in 100mM Tris-HCl buffer (pH 7.4); the mixtures were then shaken up vigorously for 30 minutes at room temperature in the dark. The absorbance was measured at 517 nm. DPPH radical scavenging activity was given as percent DPPH radical scavenging and calculated as follows:
| (1) |
where Ai is the absorbance of the mixture of 1 mL sample and 1 mL 0.2mM DPPH, Aj is the absorbance of the mixture of 1 mL sample and 1 mL ethanol, and Ac is the absorbance of the mixture of 1 mL 0.2mM DPPH and 1 mL Tris-HCl buffer.
2.5. HPLC analysis and purification by semiprep-HPLC
The HPLC analysis was performed with an Agilent model 1100. Five fractions were separated by reverse-phase HPLC on an Agilent Zorbax SB-C18 column (4.6 mm × 250 mm) equilibrated in pure water solvent A and methanol solvent B. Elution was performed with a linear gradient by increasing the concentration of solvent B, as followed for the ethyl acetate extracts from black garlic: 0–30 minutes, 5–100%; 30–45 minutes, 100%; 45–55 minutes, 100–5%. F3 was detected by HPLC with the solution of methanol:water (1:9, v/v) for 45 minutes; F4 was detected by HPLC with the solution of methanol:water (2:8, v/v) for 55 minutes. The temperature of the column was maintained at 25°C. The flow rate was 0.8 mL/min, and the wavelength was set at 254 nm for UV detection [24].
The semiprep-HPLC was performed with analysis/circular semipreparative system of SHIMADZU LC-6AD system on an Agilent Zorbax SB-C18 column (9.4 mm × 250 mm); F3 was detected by semiprep-HPLC with the solution of methanol:-water (1:9, v/v) for 45 minutes. F4 was detected by semiprep-HPLC with the solution of methanol:water (2:8, v/v) for 55 minutes at a flow rate of 2 mL/min, and the wavelength was set at 254 nm for UV detection. The compounds we purified from F3 and F4 were detected by HPLC with the solution of methanol:water (2:8, v/v) for 55 minutes at a flow rate of 0.8 mL/min.
2.6. HPLC–DAD–ESI–MS analysis and NMR spectrometry
Liquid chromatography was performed on an Agilent 1200 series HPLC (Agilent, Palo Alto, CA, USA) equipped with an autoinjector and a quaternary HPLC pump. Chromatography was performed with a 4.6 mm × 250 mm i.d., 5 μm Agilent plus C18 column. The injection volume was 20 μL. Mobile phase A was water and B was methanol. The procedure was as follows: 0–30 minutes, B was 5–100%; 30–40 minutes, B was 100%; 40–50 minutes, B was 5% at a flow rate of 0.8 mL/min.
Mass spectrometry was performed with an Agilent 1100LC-MSD (Agilent). The optimized conditions were as follows: compounds were analyzed in the positive ion mode. Capillary and fragment voltages were 3500 V and 175 V, respectively. The skimmer was set at 65.0 V. The flow rate of the drying gas was 10.0 L/min, and the nebulizer was operated at 40 psi. Nitrogen was used as the collision gas. Mass spectra were acquired in a full scan analysis within an m/z range of 100–1000 using an extended dynamic range and a scan rate of 1.4 spectra/s, and by varying the collision energy with mass. The data station operating software was the Mass Hunter Workstation (version B.04.00). A reference mass solution containing reference ions 121.0508 and 922.0097 was used to maintain mass accuracy during run time.
For melanoidin compounds, 1H NMR and 13C NMR spectra were recorded on a Varian INOVO-600 (Varian, Palo Alto, USA) spectrometer working at 400 MHz for 1H NMR and at 100 MHz for 13C NMR. Purified samples (8–15 mg) dissolved in 0.4 mL of dimethyl sulfoxide was used as internal standards.
2.7. Cellular antioxidant activity of compounds from black garlic
The cellular antioxidant activity (CAA) assay protocol was described previously [25]. HepG2 cells were seeded at a density of 6 × 104/well on a 96-well microplate in 100 μL of growth medium/well. After 24 hours of seeding, the growth medium was removed, and the wells were washed with phosphate-buffered saline (PBS). Wells were treated in triplicate for 1 hour with 100 μL of treatment medium containing tested compounds from black garlic plus 25μM dichlorofluorescin diacetate. When a PBS wash was utilized, wells were washed with 100 μL of PBS. Then 600μM 2,2′-azobis (2-amidinopropane) dihydrochloride was applied to the cells in 100 μL of Hanks’ balanced salt solution, and the 96-well microplates were placed into a Fluoroskan Ascent FL plate-reader at 37°C. Emission at 538 nm was measured with excitation at 485 nm every 5 minutes for 1 hour.
2.8. Quantification of CAA
After blank subtraction and subtraction of initial fluorescence values, the area under the curve for fluorescence versus time was integrated to calculate the CAA value at each compound from black garlic as follows:
| (2) |
where ∫SA is the integrated area under the sample fluorescence versus time curve and ∫CA is the integrated area from the control curve.
3. Results and discussion
3.1. Total phenolic content in black garlic extracts
The total phenolic content and different fractions of black garlic extracts are shown in Table 1. It is well known that phenolic compounds belong to the bioactive components of plant products and have good health-promoting activities [26,27]. It was observed that total phenolic contents of fractions F3 and F4 were much higher than those of F1, F2, and F5 (p < 0.05). The total phenolic content in black garlic was increased by about four- to 10-fold compared with that in fresh garlic, and it was reported that hydroxycinnamic acid derivatives were found to be the major phenolic acids of garlic at different processing steps [28].
Table 1.
Total phenolic content in black garlic ethyl acetate extracts and individual black garlic fractions (F1–F5) (mean ± SD, n = 3).
| Samples | Gallic acid equivalents (mg/g) |
|---|---|
| Black garlic ethyl acetate extracts | 13.5 ± 0.12a |
| F1 | 1.0 ± 0.03c |
| F2 | 1.1 ± 0.04c |
| F3 | 4.5 ± 0.09b |
| F4 | 4.3 ± 0.08b |
| F5 | 1.2 ± 0.04c |
Means that do not share a letter at each line of measurement within each gallic acid equivalent are significantly different (p < 0.05).
SD = standard deviation.
3.2. DPPH radical scavenging activity
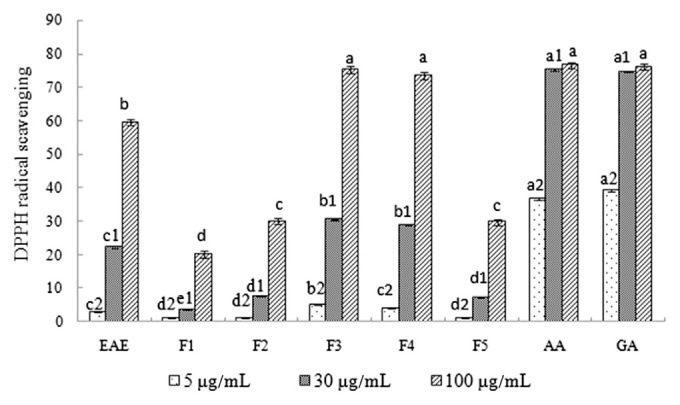
Owing to its odd electron, DPPH showed strong absorption at 517 nm (purple color). DPPH radical scavenging rates of black garlic ethyl acetate extraction, as well as those of all five fractions, were dose dependent (Figure 1). The highest DPPH radical scavenging rate was shown by F3 and F4, followed by F5. Fractions F3 and F4 have a DPPH radical scavenging rate of about 30% at a concentration of 30 μg/mL, close to 5 μg/mL of ascorbic or gallic acid. At a concentration of 100 μg/mL, F3 and F4 account for 70% of the DPPH radical scavenging rate. The lowest DPPH radical scavenging rate was shown by F1 at a concentration of 100 μg/mL, which is only 20.2%. Interestingly, fractions F3 and F4 also had higher phenolic contents; the phenolic contents of F2 were in the middle, but those of F1 and F5 were lowest (Table 1). There is a close relationship between the total phenolic content and the DPPH radical scavenging rate. According to the total phenolic content and antioxidant activity of different fractions, F3 and F4 fractions, which have the highest total phenolic content and antioxidant activity, were analyzed by HPLC and prepared by semiprep-HPLC. The single composition was isolated from F3 and F4, which would have antioxidant activities. Experiments showed that multi-composition can play a dominant role in antioxidant activity.
Figure 1.
DPPH radical scavenging activity of black garlic extracts and individual black garlic fractions (F1–F5). Ascorbic acid and gallic acid were used as positive controls. Means that do not share a letter at the same concentration measurement within each of different materials (EAE, F1, F2, F3, F4, F5, AA, and GA) are significantly different (p < 0.05). AA = ascorbic acid; DPPH = 2,2-diphenyl-2-picrylhydrazyl; EAE = ethyl acetate extracts; GA = gallic acid.
3.3. HPLC analysis and purification by semiprep-HPLC
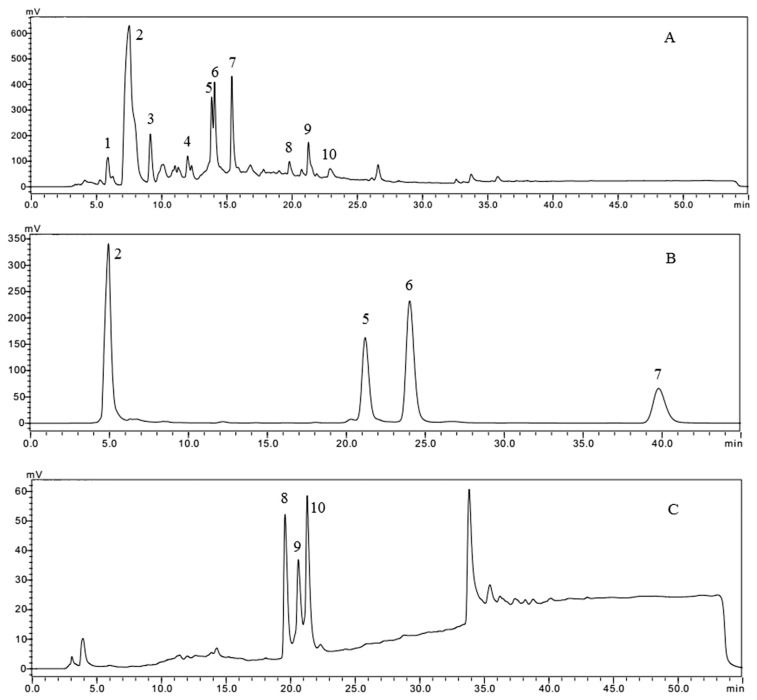
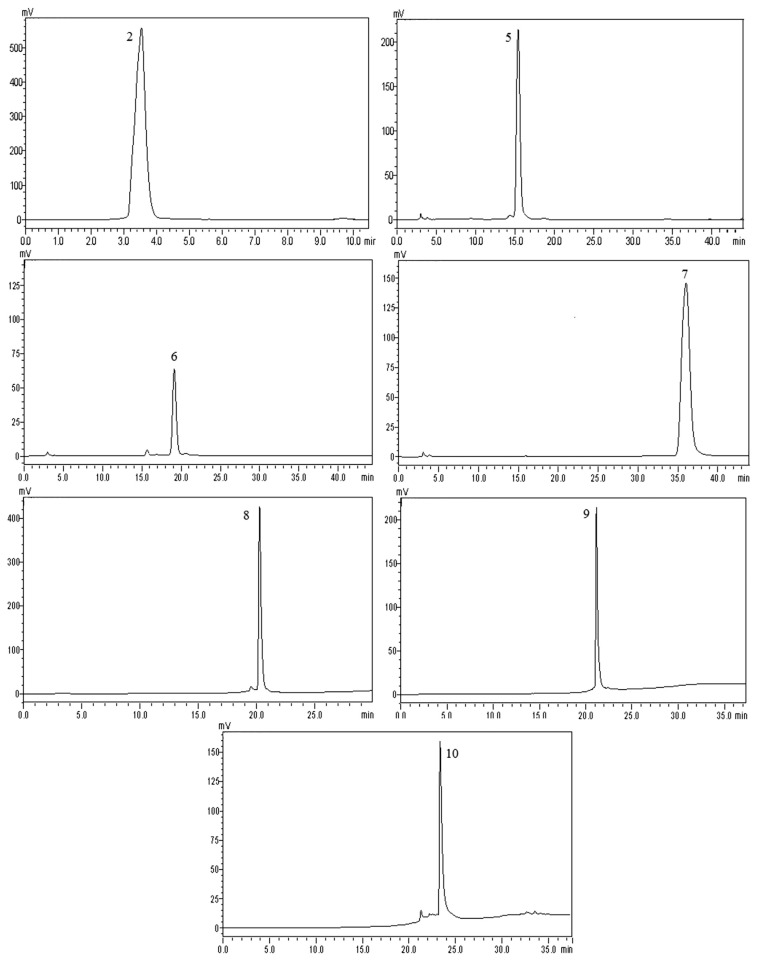
As indicated in the HPLC chromatogram using reversed-phase C-18 column, 10 separate peaks were detected for ethyl acetate extracts of black garlic (Figure 2A). Further four peaks were detected for F3 (Figure 2B) under the same conditions, corresponding to 2, 5, 6, and 7 in Figure 2A. Three peaks were further detected for F4 (Figure 2C) corresponding to 8, 9, and 10 in Figure 2A. Totally, seven individual components (Components 2, 5, 6, 7, 8, 9, and 10) were separated from black garlic after being purified through the silica gel column and semiprep-HPLC. The obtained seven individual substances were further analyzed by HPLC, as demonstrated in Figure 3. Seven individual substances were obtained by semiprep-HPLC, and then the structures of the seven single components were analyzed and identified by liquid chromatography/mass spectrometry, and 1H NMR and 13C NMR spectrometry. The aforementioned work would provide a theoretical basis for the function and mechanism of black garlic. Therefore, it is scientifically vital to look into the characteristics and mechanism of garlic, and control the browning process efficiently, which not only can shorten processing time and reduce energy consumption, but also can promote the generation of the black garlic functional components and guarantee garlic products.
Figure 2.
HPLC chromatogram of (A) the ethyl acetate extract, (B) F3, and (C) F4 detected at 254 nm. HPLC = high-performance liquid chromatography.
Figure 3.
HPLC chromatogram of seven individual compounds purified from F3 and F4 by semiprep-HPLC from black garlic detected at 254 nm. HPLC = high-performance liquid chromatography.
3.4. Structure elucidation of compounds by HPLC coupled with diode array detection analysis and NMR spectrometry
HPLC coupled with diode array detection (HPLC–DAD) analysis of the isolated compounds demonstrated their high purity, and they were identified by comparison of their UV spectra and HPLC retention times (TR) with those of reference standards, and where possible, by mass spectrometry (Table 2), chemical methods, as well as NMR spectrometry (Tables 3 and 4); their chemical structures are shown in Figure 4. Seven functional substances were obtained from crude extracts of black garlic by separation of silica gel column and semiprep-HPLC.
Table 2.
Analysis of compounds by HPLC–DAD–ESI–MS and NMR spectra.
| Compound | TR (min) | UV max (nm) | MW | ESI–MS + (m/z) | ESI–MS – (m/z) | Identification |
|---|---|---|---|---|---|---|
| 2 | 7.504 | 200 | 90.08 | 89.0247 (M – H) | DL-lactic acid a | |
| 5 | 13.824 | 280 | 126.11 | 127.0379 (M + H) | 5-HMF a | |
| 6 | 14.046 | 210,268 | 244.20 | 267.0 (M + NA) | Uridine b | |
| 7 | 15.367 | 200,260 | 267.24 | 268.1 (M + H) | Adenosine b | |
| 8 | 19.785 | 200,260 | 230 | 231.1 (M + H) | 229.0989 (M – H) | (1S, 3S)-MTCC b |
| 9 | 21.247 | 200,260 | 230 | 231.1 (M + H) | 229.0989 (M – H) | (1R, 3S)-MTCC b |
| 10 | 22.902 | 200,260 | 109.13 | 110.0576 (M + H) | 2-Acetylpyrrole a |
DAD = diode array detection; ESI = electrospray ionization; H = hydrogen; HPLC = high-performance liquid chromatography; MS = mass spectrometry; MTCC = 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid; MW = molecular weight; NA = natrium; NMR = nuclear magnetic resonance.
Identified by comparison with reference standards.
Identified by MS and NMR spectra.
Table 3.
1H NMR and 13C NMR spectra data of Compounds 6 and 7 in DMSO.
| 1H chemical shift values (ppm) | 13C chemical shift values (ppm, J = Hz) | |||
|---|---|---|---|---|
|
|
|
|||
| Compound 6 | Compound 7 | Compound 6 | Compound 7 | |
| 2 | 8.133 (1H, S) | 152.813 | 151.180 | |
| 4 | 149.486 | 163.630 | ||
| 5 | 119.780 | 102.165 | ||
| 6 | 7.892 (1H, J = 7.8 HZ) | 156.596 | 141.179 | |
| 7 | 5.900 (1H, d, J = 5.4 Hz) 5.915 | 88.113 | ||
| 8 | 8.351 (1H, S) | 140.340 | 70.292 | |
| 9 | ||||
| 10 | 5.869 (1H, d) | 88.334 | 73.985 | |
| 11 | 4.601 (1H, J = 6 Hz) | 73.893 | 85.252 | |
| 12 | 4.356 | 71.078 | 61.245 | |
| 13 | 4.144 (1H, J = 3 Hz) | 4.241 | 86.328 | |
| 14 | 3.925 | 62.107 | ||
| NH2 | 7.350 (2H, s) | |||
DMSO = dimethyl sulfoxide; NMR = nuclear magnetic resonance.
Table 4.
1H NMR and 13C NMR spectra data of Compounds 8 and 9 in DMSO.
| 1H chemical shift values (ppm) | 13C chemical shift values (ppm, J = Hz) | |||
|---|---|---|---|---|
|
|
|
|||
| Compound 8 | Compound 9 | Compound 8 | Compound 9 | |
| 10 | 1.498 | 1.377 | 19.164 | 21.285 |
| 4 | 2.605, 2.996 | 2.834,2.638 | 25.111 | 25.542 |
| 1 | 4.298 | 4.290 | 49.382 | 46.369 |
| 3 | 3.364 | 3.374 | 58.773 | 53.799 |
| 4a | 107.830 | 107.467 | ||
| 8 | 7.300 | 7.246 | 111.469 | 111.182 |
| 5 | 7.384 | 7.338 | 118.125 | 117.881 |
| 6 | 6.952 | 6.913 | 118.865 | 118.445 |
| 7 | 7.029 | 6.983 | 121.077 | 120.604 |
| 4b | 127.085 | 127.416 | ||
| 9a | 135.874 | 136.312 | ||
| 8a | 136.601 | 137.761 | ||
| 11 | 173.152 | 175.846 | ||
| 9 | 10.944 | 10.725 | ||
DMSO = dimethyl sulfoxide; NMR = nuclear magnetic resonance.
Figure 4.
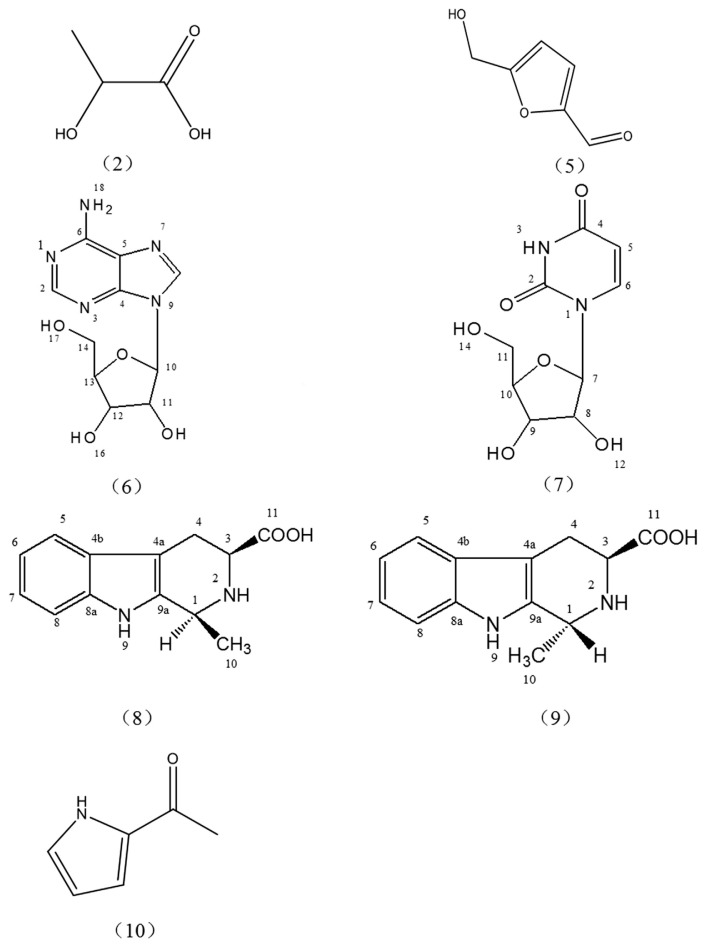
Chemical structures of Compounds 2, 5, 6, 7, 8, 9, and 10 from black garlic: DL-lactic acid (2), 5-hydroxymethyl-2-furfural (5), adenosine (6), uridine (7), (1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (8), (1R, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (9), and 2-acetylpyrrole (10).
Compound 2 was a light yellow clear liquid, which was directly identified as DL-lactic acid by comparison of TR and UV spectra with those of standards, and was also confirmed by ESI–MS spectra. Lactic acid should be formed by the fermentation of black garlic in hot and humid conditions, and the unique lactic sourness improved the taste of black garlic. In our previous study, it was found that black garlic contained many organic acids occurring in nature. Lactic acid is the major organic acid in black garlic, as found by liquid analysis, therefore, lactic acid may be responsible for the unique flavor of black garlic. Furthermore, lactic acid is also a strong antioxidant, which could have contributed to the strong antioxidant capacity of black garlic [29].
Compound 5 was colorless or white powder, which was directly identified as 5-hydroxymethylfurfural (5-HMF) by comparison of TR and UV spectra with those of standards and confirmed by ESI–MS spectra. As an intermediate product of Maillard reaction, presence of 5-HMF in black garlic also confirmed that Maillard reaction indeed took place during the formation of black garlic, and that the reaction occurred in an acidic environment, when the pH ≤ 7. Amadori rearrangement products mainly formed furfural (when sugar is a pentose) or HMF (when sugar is a hexose) by 1,2 enolization. Extensive research reported that 5-HMF has antioxidant activity, anti-ischemic function, and other beneficial effects on the human body. It has been proved to have the anti-inflammatory potential in tumor necrosis factor-α-stimulated human umbilical vein endothelial cells (HUVECs) [30]. In addition, 5-HMF is beneficial for the efficacy of traditional Chinese medicine. However, 5-HMF is probably an active ingredient that has not been understood in Chinese medicine. It was also confirmed that the fermentation process of black garlic was similar to the brewing process of Chinese medicine, which was also a part of the pharmacological effects of black garlic.
Compound 6 was white powder, the structure (Figure 4) of which was assigned as uridine based on its 1H NMR and 13C NMR data (Table 3), which was also confirmed by comparing with the literature data [31,32]. Uridine is a drug constituting components of nucleic acid in animal cells; it can also improve the body’s antibody levels. The combination of uridine and inosine can promote myocardial metabolism; accelerate biosynthesis of protein and nucleic acid, and production of energy; and promote and improve metabolism of brain cell. Isolation of this product from black garlic would lay the foundation for further elaboration of other functions of black garlic.
Compound 7 was white powder, the structure of which (Figure 4) was assigned as adenosine, on the basis of its 1H NMR and 13C NMR data (Table 3) and comparison with the literature data [33,34]. Adenosine was also separated from other herbs, which have various physiological effects on the cardiovascular system and many other systems of the body. The successful separation would have potential for further elaboration of other effects of black garlic.
Compound 8 was white powder, the structure of which (Figure 4) was assigned as (1S, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, on the basis of its 1H NMR and 13C NMR data (Table 4) and comparison with the literature data [35–37].
Compound 9 was white powder, the structure of which (Figure 4) was assigned as (1R, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, based on its 1H NMR and 13C NMR data (Table 4) and comparison with the literature data [35–37].
Compounds 8 and 9 are isomers of carboline alkaloids, and their reaction pathway has also been deduced clearly. These two substances have been isolated from black garlic in a previous study [19]; such alkaloids were found to have strong antioxidant capacity, so they would make a strong contribution to the antioxidant activity of black garlic.
Compound 10 was colorless or white powder, which was directly identified as 2-acetylpyrrole by comparison of TR and UV spectra with those of standards, and confirmed by ESI–MS spectra. The compound 2-acetylpyrrole is an important flavor substance of Maillard reaction and the main ingredient of the black garlic flavor because of its pleasant fragrance.
To investigate the compositions of black garlic, many efforts have been made by researchers worldwide. Wang et al [38] studied the changes of nutrients in garlic during Maillard reaction: the reducing sugar content was 214.9 mg/g, total acid content 2.14%, total phenol content 5.4 mg/g, and 5-HMF content 2729.12 μg/g. Liang et al [39] have reported an NMR-based comprehensive analysis of raw garlic and black garlic to determine the compositional changes resulting from thermal processing. They found that 38 components were altered by the thermal processing of raw garlic. Zhang et al [40] used simultaneous distill ion extraction for extracting the volatile substances in dormant garlic and black garlic; 50 kinds of chemical compounds in black garlic were detected by gas chromatography (GC) and MS, 28 of which are present in relatively high amounts. The main compounds extracted are 3-vinyl-1,2-dithiacyclohex-5-ene, diallyl disulfide, thiophene-2-ethyltetrahydro, and 2-vinyl-1,2-N,N′-dimethyl. The compounds 5-HMF, (1S, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, and (1R, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid have been reported in previous studies [35–39]. However, monomers of other three substances with important different functions, including adenosine, uridine, and 2-acetylpyrrole, were first isolated and identified in black garlic.
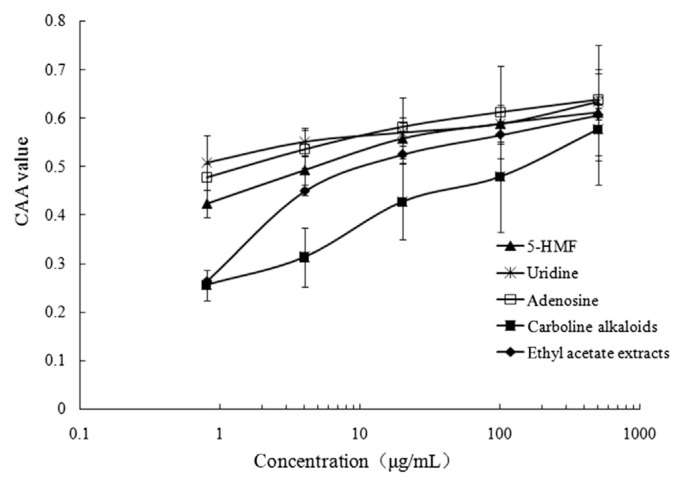
3.5. CAA activity of compounds from black garlic
We selected uridine, adenosine, carboline alkaloids, 5-HMF, and ethyl acetate extracts to verify the cellular antioxidant quality. The results showed that all of the compounds had high antioxidant properties, and the higher the concentration, the stronger the antioxidant capacity (Figure 5). We found that all compounds at a concentration of 500 μg/mL showed the highest inhibition of the proliferation of HepG2 cells, by more than 60%. The results of cellular antioxidant activity were consistent with those of antioxidant property in in vitro experiments.
Figure 5.
The CAA activity of compounds from black garlic. CAA = cellular antioxidant activity; 5-HMF = 5-hydroxymethylfurfural.
4. Conclusion
Black garlic extracts have been demonstrated to show DPPH radical scavenging activities. Among the five black garlic fractions extracted by chloroform and methanol mixed at different ratios, F3 and F4 showed the strongest antioxidant activities in a DPPH system. Seven substances were purified and separated by semiprep-HPLC, HPLC–DAD–ESI–MS, 1H NMR, and 13C NMR spectrometry from F3 and F4, especially, adenosine, uridine, and 2-acetylpyprrole were first identified in black garlic, except for 5-HMF, (1S, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, and (1R, 3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid. Generally, the whole function of black garlic would be a synergistic effect of all the components.
Acknowledgments
This project was supported by the Public Benefit Research Foundation, Agricultural Department of China (201303079); the National Natural Science Foundation of China (31371816); and the Public Benefit Research Foundation, Agricultural Department of China (201503142).
Funding Statement
This project was supported by the Public Benefit Research Foundation, Agricultural Department of China (201303079); the National Natural Science Foundation of China (31371816); and the Public Benefit Research Foundation, Agricultural Department of China (201503142).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Kodera Y, Suzuki A, Imada O, Kasuga S, Sumioka I, Kanezawa A, Ono K. Physical, chemical, and biological properties of S-allylcysteine, an amino acid derived from garlic. J Agric Food Chem. 2002;50:622–32. doi: 10.1021/jf0106648. [DOI] [PubMed] [Google Scholar]
- 2. Gonzalez RE, Burba JL, Camargo AB. A physiological indicator to estimate allicin content in garlic during storage. J Food Biochem. 2013;37:449–55. [Google Scholar]
- 3. Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- 4. Queiroz YS, Ishimoto EY, Bastos DHM, Sampaio GR, Torres EAFS. Garlic (Allium sativum L.) and ready-to-eat garlic products: in vitro antioxidant activity. Food Chem. 2009;115:371–4. [Google Scholar]
- 5. Jang EK, Seo JH, Lee SP. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J Food Sci Technol. 2008;40:443–8. [Google Scholar]
- 6. Al-Qattan K, Thomson M, Ali M. Garlic (Allium sativum) and ginger (Zingiber officinale) attenuate structural nephropathy progression in streptozotocin-induced diabetic rats. e-SPEN. 2008;3:e62–71. [Google Scholar]
- 7. Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–9. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 8. Wang D, Feng YH, Liu J, Yan JZ, Wang MR, Sasaki JI, Lu CL. Black garlic extracts enhance the immune system. Med Arom Plant Sci Biotech. 2010;4:37–40. [Google Scholar]
- 9. Seo YJ, Gweon OC, Im J, Lee YM, Kang MJ, Kim JI. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. J Food Sci Nutr. 2009;14:1–7. doi: 10.4162/nrp.2009.3.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim I, Kim JY, Hwang YJ, Hwang KA, Om AS, Kim JH, Cho KJ. The beneficial effects of aged black garlic extract on obesity and hyperlipidemia in rats fed a high-fat diet. J Med Plants Res. 2011;5:3159–68. [Google Scholar]
- 11. Dillon SA, Burmi RS, Lowe GM, Billington D, Rahman K. Antioxidant properties of aged garlic extract: an in vitro study incorporating human low density lipoprotein. Life Sci. 2003;72:1583–94. doi: 10.1016/s0024-3205(02)02475-x. [DOI] [PubMed] [Google Scholar]
- 12. Lee YM, Gweon OC, Seo YJ, Im J, Kang MJ, Kim MJ, Kim JI. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract. 2009;3:156–61. doi: 10.4162/nrp.2009.3.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li N, Lu X, Pei H, Qiao X. Effect of freezing pretreatment on the processing time and quality of black garlic. J Food Process Eng. 2014;38:329–35. [Google Scholar]
- 14. Sato E, Kohno M, Hamano H, Niwano Y. Increased anti-oxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum Nutr. 2006;61:157–60. doi: 10.1007/s11130-006-0017-5. [DOI] [PubMed] [Google Scholar]
- 15. Kao FJ, Chiu YS, Chiang WD. Effect of water cooking on antioxidant capacity of carotenoid-rich vegetables in Taiwan. J Food Drug Anal. 2014;22:202–9. [Google Scholar]
- 16. Medina MAT, Pérez-Aparicio J, Moreno-Rojas R, Merina-Amo T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016;199:135–9. doi: 10.1016/j.foodchem.2015.11.128. [DOI] [PubMed] [Google Scholar]
- 17. Choi IS, Cha HS, Lee YS. Physicochemical and antioxidant properties of black garlic. Molecules. 2014;19:16811–23. doi: 10.3390/molecules191016811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sato E, Kohno M, Niwano Y. Increased level of tetrahydro-β-carboline derivatives in short-term fermented garlic. Plant Foods Hum Nutr. 2006;61:175–8. doi: 10.1007/s11130-006-0028-2. [DOI] [PubMed] [Google Scholar]
- 19. Ichikawa M, Yoshida J, Ide N, Sasaoka T, Yamaguchi H, Ono K. Tetrahydro-β-carboline derivatives in aged garlic extract show antioxidant properties. J Nutr. 2006;136:726S–31S. doi: 10.1093/jn/136.3.726S. [DOI] [PubMed] [Google Scholar]
- 20. Zhu QY, Hackman RM, Ensunsa JL, Holt RR, Keen CL. Antioxidative activities of oolong tea. J Agric Food Chem. 2002;50:6929–34. doi: 10.1021/jf0206163. [DOI] [PubMed] [Google Scholar]
- 21. Sharma K, Ko EY, Assefa AD, Ha S, Nile SH, Lee ET, Park SW. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J Food Drug Anal. 2015;23:243–52. doi: 10.1016/j.jfda.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu QP, Tao WY, Ao ZH. Antioxidant activity of vinegar melanoidins. Food Chem. 2007;102:841–9. [Google Scholar]
- 23. Yokozawa T, Dong E, Natagawa T, Kashiwagi H, Nakagawa H, Takeuchi S, Chung HY. In vitro and in vivo studies on the radical-scavenging activity of tea. J Agric Food Chem. 1998;46:2143–50. [Google Scholar]
- 24. Bae SE, Cho SY, Won YD, Lee SH, Park HJ. A comparative study of the different analytical methods for analysis of s-allyl cysteine in black garlic by HPLC. LWT Food Sci Technol. 2012;46:532–5. [Google Scholar]
- 25. Wang HY, Qian H, Yao WR. Melanoidins produced by the Maillard reaction: structure and biological activity. Food Chem. 2011;128:573–84. [Google Scholar]
- 26. Liao DY, Chai YC, Wang SH, Chen CW, Tsai MS. Antioxidant activities and contents of flavonoids and phenolic acids of Talinum triangulare extracts and their immunomodulatory effects. J Food Drug Anal. 2015;23:294–302. doi: 10.1016/j.jfda.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Denardin CC, Hirsch GE, da Rocha RF. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J Food Drug Anal. 2015;23:387–98. doi: 10.1016/j.jfda.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JS, Kang OJ, Gweon OC. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J Funct Foods. 2013;51:80–6. [Google Scholar]
- 29. Groussard C, Morel I, Chevanne M, Monnier M, Cillard J, Delamarche A. Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. J Appl Physiol. 2000;89:169–75. doi: 10.1152/jappl.2000.89.1.169. [DOI] [PubMed] [Google Scholar]
- 30. Kim HK, Choi YW, Lee EN, Park JK, Kim SG. 5- Hydroxymethylfurfural from black garlic extract prevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phytother Res. 2011;25:965–74. doi: 10.1002/ptr.3351. [DOI] [PubMed] [Google Scholar]
- 31. Hu XY, Dou DQ, Pei YP, Fu WW. Chemical constituents of roots of Ranunculus ternatus Thunb. J Chin Pharm Sci. 2006;15:127. [Google Scholar]
- 32. Song Y, Chen GT, Sun BH, Huang J, Li X, Wu LJ. Chemical constituents of water-soluble part of Mentha spicata L. J Shenyang Pharm Univ. 2008;9:008. [Google Scholar]
- 33. He XJ, Qiu F, Yao XS. The active constituents research of gualou xiebai tang (IV): nitrogen-containing compounds and others. Nat Prod Res Dev. 2003;15:9–11. [Google Scholar]
- 34. Okuyama T, Fujita K, Shibata S, Hoson M, Kawada T, Masaki M, Yamate N. Effects of Chinese drugs “xiebai” and “dasuan” on human platelet aggregation (Allium bakeri, A. sativum) Planta Med. 1989;55:242–4. doi: 10.1055/s-2006-961993. [DOI] [PubMed] [Google Scholar]
- 35. Ichikawa M, Ryu K, Yoshida J, Ide N, Yoshida S, Yoshida T, Sumi SI. Antioxidant effects of tetrahydro-β-carboline derivatives identified in aged garlic extract. Biofactors. 2008;16:57–72. doi: 10.1002/biof.5520160302. [DOI] [PubMed] [Google Scholar]
- 36. Ide N, Lau BHS. Aged garlic extract attenuates intracellular oxidative stress. Phytomedicine. 1999;6:125–31. doi: 10.1016/S0944-7113(99)80047-6. [DOI] [PubMed] [Google Scholar]
- 37. Bosin TR, Krogh S, Mais D. Identification and quantitation of 1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid and 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid in beer. J Agric Food Chem. 1986;34:843–7. [Google Scholar]
- 38. Wang WD, Wang Y, Wang C, Sun YE, Gao MX. Effect of Maillard reaction on nutrients and antioxidant activities of garlic. Food Sci Technol. 2013;4:011. [Google Scholar]
- 39. Liang T, Wei F, Lu Y, Kodani Y, Nakada M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J Agric Food Chem. 2015;63:683–91. doi: 10.1021/jf504836d. [DOI] [PubMed] [Google Scholar]
- 40. Zhang ZY, Yang XJ, Zhang JS, Zhang WY. Identification of volatile compounds in fermented black garlic by GC–MS. China Condiment. 2012;7:74–6. [Google Scholar]