Abstract
The objective of this study was to investigate the effect of storage temperature and time on nutrients, bioactive compounds, and antioxidant activities of walnut male inflorescences. The results showed that the moisture, saccharides, fat, protein, amino acids, ascorbic acid, phenolic and flavonoid compound contents, and antioxidant activities of walnut male inflorescences were markedly influenced by storage temperature, and different degrees of decrease in these parameters were observed during the entire storage period. Moreover, higher storage temperature had a more significant effect on the nutrients, bioactive compounds, and antioxidant activities of walnut male flowers, and the loss rate of these components at 25°C was higher than that determined at 4°C. However, the results also presented that the ash and mineral contents did not appear to be influenced significantly by the storage temperature, and slightly significant changes were observed in crude fiber throughout storage, which indicated that the influence of storage on the individual mineral and crude fiber content was minimal. Based on the findings in this study, in order to maximize nutrients concentration, walnut male inflorescences should be kept at 4°C for <6 days and be consumed as fresh as possible.
Keywords: antioxidant activity, physicochemical changes, storage temperature, walnut male inflorescences
1. Introduction
The quality of horticulture products is a combination of attributes, properties, and characteristics that give each commodity value in terms of human food. Consumers are concerned primarily about the color and flavor of dietary components, as well as aspects of nutritional quality that include energy, vitamins, minerals, dietary fiber, and the many bioactive compounds that enhance human health [1]. During postharvest storage, soluble sugars, organic acids, and the solid/acid ratio are important components of fruit quality that can be affected by both internal and external factors [2]. Senescence is the most important internal factor, whereas temperature plays a major role in causing horticulture commodity senescence [3–5]. Low temperature (LT) is the most effective way to delay postharvest ripening and deterioration of horticultural crops and to schedule ripening according to marketing needs [6]. During this period, the main sources of damage to products are physiological disorders, such as senescence and water loss by evaporation, and pathogenic diseases. LT is usually chosen to extend storage life and maintain fruit and vegetable quality during postharvest storage, when the quality of horticulture crops declines gradually [6,7]. Generally speaking, products can maintain greater flesh firmness and higher concentrations of organic matter and vitamin levels because most enzyme activities decrease and the expression of many genes is inhibited during cold storage [8].
Walnut male inflorescence, a traditional vegetable in the minority ethnic areas of Guizhou province and Yunnan Province, China, can be used as an ingredient in fried dishes, soup, and Chinese vegetable salads. It is also a good source of quality nutrients that include energy, vitamins, minerals, dietary fiber [9,10], and many bioactive compounds, such as phenols and flavonoids [11,12], which provide remarkable antihypoxic, anti-inflammatory, antioxidant, antidepressant, and antihemolytic activities in safe doses [12–14]. Moreover, the alcoholic extract of walnut male flowers can increase serum levels of insulin, and decrease blood glucose levels in diabetic rats [14]. Therefore, the male inflorescence of walnut was also known as longevity food in China. In addition, there are about 2000 male flowers in every adult walnut tree, which could translate to a harvest yield of about 1,000,000 tons every year in China. Furthermore, it blooms in the early spring, which results in less pollution because there was fewer diseases and pests in this time. As a result, it has been used and exploited by some companies as a natural edible flower resource, which can be used as seasonal fresh vegetable or dehydrated vegetable. In general, dried vegetables have several disadvantages such as bad mouth taste and loss of nutrients during the dried progress, and people prefer dishes made from fresh walnut male flowers. However, consumers usually place them in plastic bags and do not remove the field heat of flowers by using pre-chill treatment. As the same time, walnut male flower has a short postharvest life compared with other horticulture crops such as fruits and some vegetables. During postharvest storage, it easily decays and consequently loses much of its commodity value. However, little information is available on nutrition change about walnut male flowers in postharvest storage. Such knowledge is of great importance in developing storage strategies designed to maintain walnut flower quality in postharvest storage and thus extend its shelf life and increase its value. The main objective of the present research was to investigate the variation of sensory characteristics, the common nutritional components (proteins, fats, saccharides, minerals, vitamins, etc.), and the changes in total phenolic and flavonoid contents as well as antioxidant properties of male flowers of Juglans sigillata stored at different time intervals at room temperature and LT.
2. Material and methods
2.1. Plant materials and treatments
Walnut male inflorescences at flowering stage (J. sigillata cv. “Qianhe-7”) were harvested from Shuitang farm (27°13′ N, 104°71′ E) in Hezhang county, China. The evaluation criterion for the flowering stage was made based on the method described by Wang et al [10]. About 3 kg of fresh male flowers was handpicked, and care was taken to pick only those of good quality. The flowers were transported in an ice box to the Technology and Engineering Research Center of Fruit Crop, Guizhou University, southwest of China. The samples were divided into two sets and placed in clean enamel disk sealed with a plastic wrap. The first set was stored at ambient temperature (about 25 ± 2°C), and the other was stored at 4 ± 0.2°C in a refrigerator. The relative humidity was maintained at 85 ± 5% for all samples. Three biological replicates per storage temperature and duration were taken on sampling Days 0, 2, 4, 6, 8, 10, 12, and 14. The quality parameters measured in the three biological replicates were sensory properties, proximate indexes (ash, protein, fat, total soluble sugars, starch, dietary fiber), minerals (K, P, Ca, Mg, Fe, Mn, and B), amino acids, bioactive compounds, and antioxidant activity.
2.1.1. Reagents and standards
Test reagents, standard compounds, and solvents were obtained from sources as previously described [10].
2.2. Sensory properties
This evaluation session was based on the color, flavor (the inherent aroma of walnut male flowers), retained freshness, and degree to which the original shape was maintained (retain freshness, whether or not deterioration occurred).
2.3. Proximate analysis
The ash content was determined by incinerating the samples in a furnace at 525°C for 4 hours until a constant weight was obtained [15]. The protein content was obtained from the total nitrogen content by multiplying by 6.25, as estimated using the Kjeldahl method [15]. The fat was extracted using the soxhlet refluxing apparatus, with petroleum ether to extract the lipid [15]. The contents of total soluble sugars and starch were analyzed using the method of anthrone colorimetry. The content of total dietary fiber was determined using an acid–base scrubbing method by refluxing in sulfuric acid (1.25%) and potassium hydroxide (1.25%) [16]. All samples were undertaken in three replicates.
2.4. Mineral analysis
To determine the contents of nitrogen (N), potassium (K), and phosphorous (P), 0.50 g oven-dried powder of the sample was digested in flask using a diluted oxidant mixture (8 mL H2SO4 + 10 mL H2O2 + 1 mL H2O). The temperature was increased gradually, starting from 50°C and increasing up to 200–220°C. The digestion was completed in about 45–50 minutes as indicated by the appearance of a transparent liquid mixture. After complete digestion and cooling, the solution was diluted to 50 mL with ultrapure water. The contents of N, K, and P were analyzed using the Kjeldahl method, flame photometry method, and spectrophotometric method, respectively. The total N content was expressed as crude protein. The contents of boron (B), calcium (Ca), iron (Fe), zinc (Zn), manganese (Mn), copper (Cu), and magnesium (Mg) were analyzed according to the method described by the Association of the Official Analytical Chemists [15]. About 1.0 g of sample was incinerated in the muffle furnace (SX2-2.5-12; Boxun Industry & Commerce Co., Ltd., Shanghai, China) for 4 hours. The ash was dissolved by hydrochloric acid (6 mol/L) and diluted to 50 mL with ultrapure water. The content of B was measured using the spectrophotometric method. The contents of Ca, Fe, Zn, Mn, Cu, and Mg were evaluated by atomic absorption spectrophotometer (TAS-990; PERSEE, Beijing, China). All samples were analyzed in five replicates.
2.5. Amino acids analysis
To determine the content of amino acids, acid hydrolysis was performed on proteins. Briefly, accurately weighed samples (500 mg) were hydrolyzed in 10 mL of 6M HCl and heated at 110°C for 24 hours. The hydrolyzed product was vacuumed and filtered, and then diluted to 50 mL in a volumetric flask [17]. Next, 2 mL of the solution was concentrated in the oven at 60°C; the concentrated samples were dissolved in 2 mL borate buffer and agitated for 15 seconds, then filtered through a Millipore syringe filter (0.45 μm filter). Total amino acids (TAAs) were separated and quantified by injecting a 20 μL sample of this solution into the column of the automatic amino acid analyzer (A-300; MembraPure GmbH, Berlin, Germany).
The automatic amino acid analytic system was equipped with an eluent unit, an autosampler, a main unit with a double piston pump, a rack for two glass bottles containing reagent and reactor washing solution, two photometers, a damping unit, a reactor, a separating column, a precolumn and a two/three-way valve, an online solvent degasser, and a chromatography data handling system. The amino acid analytic conditions were as follows: separation of the amino acid derivatives was achieved by a flow of 200 μL/min at a column temperature of 40°C. The reactor temperature was 115°C. The presses of buffer and reagent were 60 and 7 bar, respectively. Amino acid samples were separated by ion exchange chromatography and determined by reaction with ninhydrin. Detection was conducted by a photometric detector using the wavelengths of emission at 570 nm (except proline at 440 nm). Seventeen kinds of amino acids were identified by comparison with retention times for amino acid stock solutions. For determination of retention times, the reference standards were injected individually. The concentration of each amino acid was obtained by direct interpolation of the peak area in the correspondent linear calibration curve (peak area vs. concentration). These calibration curves were obtained over a wide concentration range in concordance with the level of each amino acid found in the samples that were analyzed. Three replications were done for amino acid determination.
2.6. Bioactive compounds analysis
2.6.1. Determination of ascorbic acid
The ascorbic acid (AsA) content of walnut male flowers was determined using the high-performance liquid chromatography (HPLC) method with ultraviolet detection; the process may be summarized as follows. Three replicates of 2.0 g of fresh sample were diluted with 7 mL metaphosphoric acid (6%, w/v) in a mortar. The homogenate was centrifuged at 10,000 rpm at 4°C for 10 minutes; next, the supernatant was collected and diluted to 50 mL ultrapure water. The solution was filtered with a membrane filter (0.45 μm filter; Millipore, Boston, MA, USA), and then a 20 μL sample of it or the standard solutions was injected into the column of the HLPC system.
The HLPC system was a Shimadzu system model LC-15C (Shimadzu, Kyoto, Japan) and equipped with an autosampler (SIL-10AF), an online solvent degasser, a system controller for chromatography data analysis, a pump (2LC-15C), a column oven (CTO-15C), and a UV detector (SPD-15C). Chromatographic analysis was performed using an analytical scale (150 × 4.6 mm, i.d.) and Alltima C18 column with a particle size of 5 μm (Supelco, Bellefonte, PA, USA). The HPLC conditions were as follows: the mobile phase was 0.2% (w/v) metaphosphoric acid solution; the flow rate was maintained at 1 mL/min, and the column was maintained at 30°C. Spectral data from the UV detector were collected over the wavelength range 210–400 nm. AsA was monitored at 254 nm [18].
2.6.2. Determination of total phenolic content
The total phenolic content (TPC) was assayed using the Folin–Ciocalteu method as described by Conde-Hernández and Guerrero-Beltrán [19] with several modifications. Briefly, 1.5 g (d = 0.0001 g) of dried flower powder was extracted with 60 mL methanol (40%, v/v) at 50°C for 50 minutes and subjected to ultrasonic wave treatment. The reaction mixture—-which consisted of 0.5 mL extracted solution, 5.0 mL Folin–Ciocalteau reagent, and 15 mL Na2CO3 solution (20%, w/v)—was incubated at room temperature for 2 hours in the dark and then diluted to 50 mL with ultrapure water. The absorbance of the reaction mixture was measured at 765 nm against deionized water blank on a UV–Vis spectrophotometer (UV-2550; Shimadzu). Gallic acid (GA) was chosen as a standard. The TPC in walnut male flowers was determined, and results are expressed as mg GA equivalent per 100 g dry sample.
2.6.3. Determination of total flavonoid content
The extraction of total flavonoid content (TFC) was performed using the colorimetric method described by Feng et al [20] with minor modifications. Rutin was used as the standard for a calibration curve. In brief, dried flower powder (0.5 g, d = 0.0001 g) was added with 30 mL ethanol (30%, v/v) and incubated for 1 hour at 70°C in a water bath. The extracted production was filtered, and the filtrate was used for the TFC assay. The TFC was assayed by measuring the absorption at 510 nm using a spectrophotometer in 3 mL reaction mixture containing 0.8 mL extract, 1.2 mL ethanol (30%, v/v), 0.5 mL NaNO2 solution (5%, w/v), and 0.5 mL Al(NO3)3 solution (10%, w/v). The TFC concentration was calculated from a calibration curve using rutin as the standard, and results are expressed as mg rutin equivalents per 100 g dry sample.
2.7. Antioxidant activity analysis
2.7.1. 1,1-Diphenyl-2-picrylhydrazyl radical-scavenging activity
Dried flower powder (1.5 g, d = 0.0001 g) was extracted with 30 mL ethanol (50%, v/v) for 50 minutes at 50°C in ultrasonic cleaner and then filtered. The filter was used for assaying the antioxidant activity. The antioxidant activity of the extracts, on the basis of the scavenging activity of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, was determined according to the method described by Motamed and Naghibi [21] with a slight modification. In brief, 2 mL DPPH ethanol solution (0.2 mM) was prepared and mixed with 2 mL sample ethanol solution. The mixture was incubated for 30 minutes at room temperature, and the absorbance was measured at 517 nm. The activity of radical scavenging was given as % DPPH radical scavenging that was calculated using the following equation (the DPPH solution without sample solution was used as a control; AsA was used as standard):
2.7.2. Ferric reducing ability of plasma
The ferric reducing ability of plasma (FRAP) was determined according to the method described by Benzie and Strain [22] with minor modifications. FRAP assay is a simple, reproducible, rapid, and inexpensive method that measures the reductive ability of antiradical, and it is evaluated by the transformation of Fe3+-TPTZ to a blue color Fe2+-TPTZ, as a measure of total antioxidant capacity [23]. Briefly, 2.7 mL FRAP reagent {10:1:1 of 0.3 mmol/L sodium acetate buffer (pH 3.6), 10 mmol/L TPTZ [2,4,6-Tris(2-pyridyl)-s-triazine] solution, and 20 mmol/L FeCl3·6H2O solution}, prepared freshly and incubated in a water bath at 37°C 30 minutes, mixed with 0.3 mL sample solution, was used to measure the absorbance at 593 nm, with methanol as the reagent blank. The antioxidant potential of the extract was determined against the standard curve of FeSO4 (0, 25, 50, 100, 200, 400, and 800 μmol/L) in 0.1% (v/v) HCl. The FRAP was expressed as mmol FeSO4 per 100 g dry weight (DW).
2.8. Statistical analysis
Statistical analyses were conducted using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Analysis of variance in a completely randomized design and Duncan’s multiple range tests were used to compare any significant differences in storage temperature, time, and the interaction between time and temperature. Values were expressed as means ± standard deviations. All determinations were done at least in triplicate, and all values were averaged. The confidence limits used in this study were based on p < 0.05, p < 0.01, p < 0.001, or p < 0.0001.
3. Results and discussion
3.1. Proximate composition
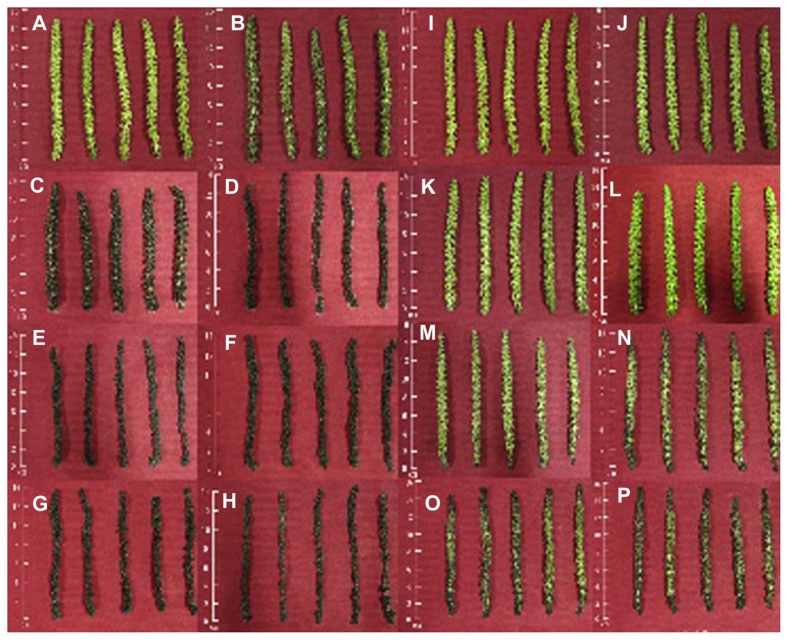
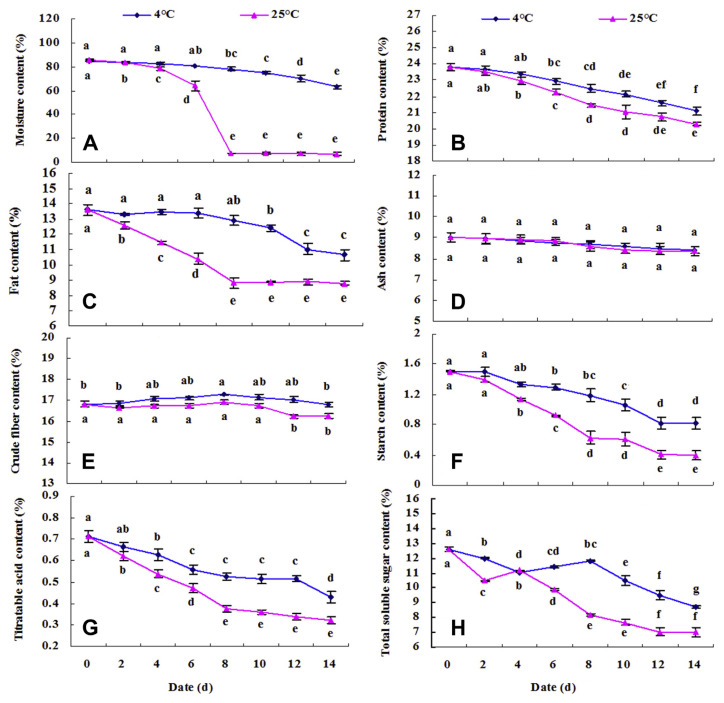
The appearance and proximate compositions of walnut male inflorescences at different storage temperature (4°C and 25°C) are shown in Figures 1 and 2, respectively. The water content of the male inflorescences reached up to 85.53% (Figure 2A). The data indicated that the initial moisture values of the samples were high. It is worthwhile to keep in mind that water content is the parameter that is, to some degree, variable for “fresh” male inflorescences. At room temperature, a gradual decrease in water content occurred from the 1st to 8th days (reduced about 91.09%) and then remained unchanged up to the last day (Figures 1 and 2A). The present finding indicated that significant differences were not observed between the 1st day and the 4th day, and then revealed a gradual decrease as the cold storage was extended. The water content reduced by about 8.37% in the first 4 days, and walnut male flowers began wilting and browning at 25°C (Figure 1). However, 8 days later, the water in walnut male inflorescences decreased by about 7.61%, and they only appeared wilting and browning under cold stage. In this study, significant effects of temperature (p < 0.0001), time (p < 0.0001), and their interaction (temperature × time, p < 0.0001) were observed based on Duncan’s multiple range tests. According to Zhao and Zhang [24], in many horticultural products a water loss of more than 5% would cause loss of freshness, wilting appearance, and even loss of commodity value (Figure 1). The highest protein content (23.808 g/100 g) of walnut male flowers was observed on the 1st day of storage (Figure 2B). A slight decrease in protein content was found throughout the storage period. However, the decreased amplitude of protein content during the whole storage at room temperature was 14.81%, which was higher than that observed under cold storage (11.35%). According to Duncan’s multiple range tests, storage temperature, time, and their interaction all affected protein content at a significant level (p < 0.0001). These might be due to the inhibition of the proteolytic activity at cold storage [7]. The crude fat content in walnut male flowers stored at room temperature decreased significantly (decreased by about 34.43%) between the 1st and 6th days, and then remarkable differences were not observed between the 8th and 14th days (Figure 2C). Although the fat content remained unchanged up to the 8th day, it then showed a slight decrease when cold storage was extended, and there were also obvious effects of temperature, time, and temperature × time on fat content according Duncan’s multiple analysis (p < 0.0001). The losses might be attributable to the fact that fats are rich in unsaturated fatty acid, which is susceptible to oxidation degradation [6,7]. And these results also indicated that cold storage could reduce the loss of fats in comparison with room temperature storage. No remarkable changes (p > 0.05) in ash values occurred during the whole storage period of this evaluation (Figure 2D). These results indicated that ash was insusceptible to environment conditions and less physiological activity. Different significant levels were found in crude fiber (Figure 2E); temperature (p < 0.0001) affects it more significantly than the time factor (p < 0.001) and the interaction between time and temperature (p < 0.05). Both groups of samples had a similar behavior in starch and titratable acid under different temperature conditions, with a gradual decrease during the storage period, whereas no significant differences were found in the first 2 days at 4°C (Figures 2F and 2G). During the whole storage period, the starch and titratable acid decreased by about 64.67% and 55.02% at 25°C, respectively, whereas their loss rates were 46% and 39.76% at 4°C, respectively. Both were significantly influenced by storage temperature (p < 0.0001), storage time (p < 0.0001), and their interaction (p < 0.01), which indicated that cold storage could reduce the rate of respiration and loss of energy substrate. The same decrease was observed in titratable acid in loquat fruits during storage [4]. The two kinds of treatment methods showed the same changing trend in sugars from the first day to the last day (Figure 2H). However, at room temperature, an increase in sugars was observed from the 2nd to the 4th days; under cold storage, the same increase from the 4th to the 8th days might be attributable to the accumulation of more sugars because of the hydrolysis of starch. According to Duncan’s multiple range tests, storage temperature, time, and their interaction all affect sugar content at a significant level (p < 0.0001), and a significant decline during the whole storage period was attributable to utilization of sugar respiration process [5,6]. Rodríguez et al [25] had reported that the saccharide content in vegetables changes because it is the most important energy substrate in the different metabolic processes that continue after the product has been harvested.
Figure 1.
Appearance of walnut male inflorescence at 4°C (A–H) and 25°C (I–P) for storage at 0 day (A, I), 2 days (B, J), 4 days (C, K), 6 days (D, L), 8 days (E, M), 10 days (F, N), 12 days (G, O), and 14 days (H, P).
Figure 2.
Contents dynamic change in (A) moisture, (B) protein, (C) fat, (D) ash, (E) crude fiber, (F) starch, (G) titratable acid, and (H) total soluble sugar of fresh walnut male flower stored for 14 days at 4°C and 25°C. Data are shown as mean ± standard deviation (n =3). * The lowercase letters near the bar indicate statistically different mean among storage days at p < 0.05.
3.2. Mineral composition
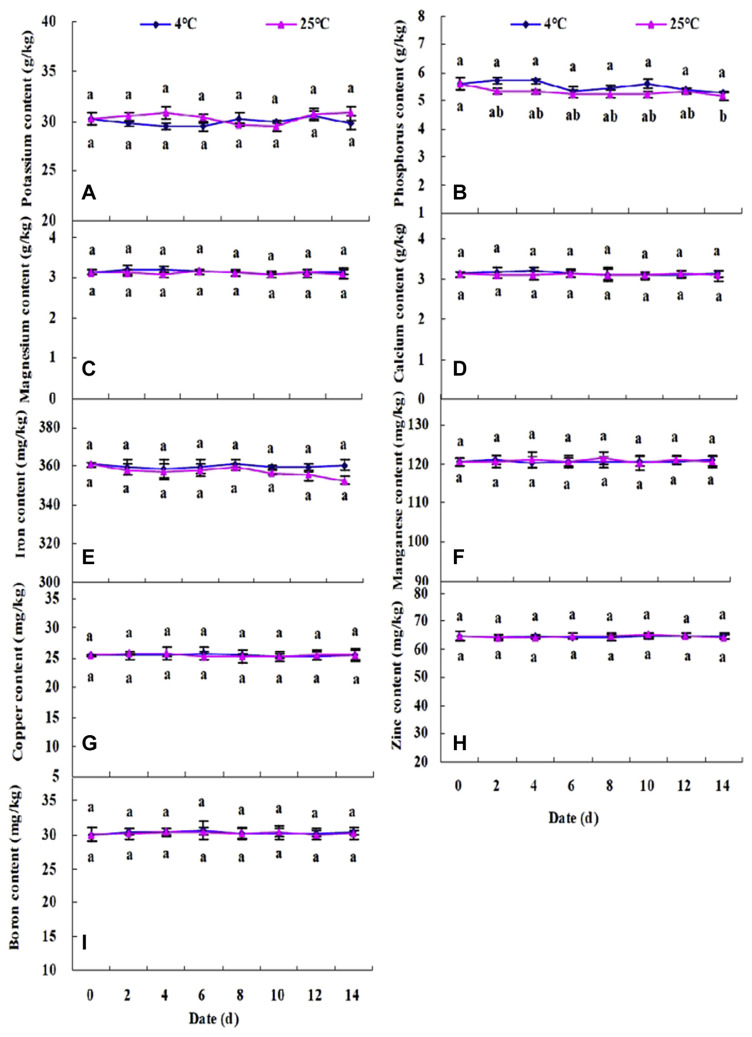
The mineral contents of walnut male flowers stored at 4°C and 25°C are shown in Figures 3A–3I. Among all the mineral elements, potassium content (30.08 g/kg DW) was the highest, followed by phosphorus (5.61 g/kg DW), calcium (3.14 g/kg DW), and magnesium (2.85 g/kg DW). Among all the micronutrients, the highest contents were found in iron elements (360.73 mg/kg DW), followed by manganese (120.54 mg/kg DW), zinc (64.59 mg/kg DW), and boron (30.04 mg/kg DW). The content of copper (25.34 mg/kg DW) was the lowest. These values were similar to those presented earlier [10], which further confirmed that walnut male inflorescences are a good source of minerals. Moreover, our results showed that no remarkable changes (p > 0.05) in mineral values occurred during the whole storage period at 4°C and at 25°C. A slightly significant change in phosphorus content was only observed on the last day in walnut flowers stored at room temperature. These indicated that the property of mineral elements was stable and was insusceptible to external environment. Rodríguez et al [25] reported that mineral losses were low after the vegetables were harvested, as well as during storage prior to consumption.
Figure 3.
Dynamic changes of (A) potassium, (B) phosphorus, (C) magnesium, (D) calcium, (E) iron, (F) manganese, (G) copper, (H) zinc, and (I) boron contents in fresh walnut male flower stored for 14 days at 4°C and 25°C. The data are shown as mean ± standard deviation (n =3). * The lowercase letters near the bar indicate statistically different mean among storage days at p < 0.05.
3.3. Amino acid
As presented in Table 1, the content of amino acid components in walnut male flowers changed with different trends during the whole storage period. Moreover, the content of amino acid components differed under different storage temperatures (4°C and 25°C). The contents of threonine, glutamic acid, glycine, valine, leucine, isoleucine, tyrosine, phenylalanine, and proline increased in the initial stage and then declined with the passing of storage time. The content of aspartic acid and serine showed a trend of decreasing first, then increasing and declining eventually. Furthermore, a gradual decrease in alanine, cysteine and methionine, histidine, lysine, and arginine contents occurred from the first to the last day. An increase in some amino acid components might be attributable to the hydrolysis of protein in the initial stage, and the remarkable degradation may be attributable to the result of the nonenzymatic browning of male flowers (Maillard reaction), which involves condensation between amino groups of amino acids with sugars. During the storage process, subsequent reactions were not reversible, and some amino acids were easily destroyed especially tyrosine, alanine, glutamine, and cysteine. This result agrees with observations of Liu et al (change in alanine and cysteine) [26]. However, in our results, histidine, lysine, and arginine were also easily decomposed during storage. Moreover, our results showed that no significant changes in essential amino acid (EAA) and total amino acid (TAA) values occurred in the first eight days, but they declined later for walnut flowers stored at 25°C; however, the EAAs/TAAs of walnut male inflorescence were all above 40%. Furthermore, at 4°C, the contents of amino acid components in walnut male flowers showed no remarkable difference in the first 2 days, then declined, and the reduction was less than that at 25°C, which indicated that LT can effectively reduce the degradation of amino acid components in walnut male flowers. These results suggested that some amino acids decreased at a slower pace under the lower storage temperature because of the relatively lower enzyme activity and respiration rate [2].
Table 1.
Changes in amino acids of walnut male inflorescence during cold storage.
| Amino acids Storage method | Storage days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | ||
| Asp | CS | 1.08 ± 0.08a | 1.07 ± 0.11a | 0.96 ± 0.04bc | 0.90 ± 0.02c | 1.06 ± 0.06ab | 0.93 ± 0.02c | 0.87 ± 0.01c | 0.86 ± 0.04c |
| RTS | 1.08 ± 0.08ab | 0.98 ± 0.02bc | 0.92 ± 0.01cd | 1.05 ± 0.07ab | 1.12 ± 0.10a | 0.92 ± 0.01cd | 0.83 ± 0.02de | 0.79 ± 0.02e | |
| Thrz | CS | 0.61 ± 0.04b | 0.61 ± 0.02b | 0.61 ± 0.02b | 0.69 ± 0.02a | 0.69 ± 0.02a | 0.59 ± 0.02b | 0.52 ± 0.02c | 0.42 ± 0.02d |
| RTS | 0.61 ± 0.04c | 0.69 ± 0.04b | 0.75 ± 0.01a | 0.58 ± 0.03c | 0.51 ± 0.01d | 0.44 ± 0.03e | 0.34 ± 0.02f | 0.29 ± 0.04g | |
| Ser | CS | 0.80 ± 0.02a | 0.81 ± 0.02a | 0.72 ± 0.02b | 0.62 ± 0.03c | 0.78 ± 0.02a | 0.70 ± 0.02b | 0.61 ± 0.02c | 0.56 ± 0.03d |
| RTS | 0.80 ± 0.02a | 0.69 ± 0.05bc | 0.64 ± 0.02cd | 0.72 ± 0.03b | 0.80 ± 0.03a | 0.71 ± 0.01b | 0.62 ± 0.03d | 0.52 ± 0.02e | |
| Glu | CS | 1.49 ± 0.04c | 1.50 ± 0.02bc | 1.50 ± 0.02bc | 1.57 ± 0.02a | 1.54 ± 0.01ab | 1.49 ± 0.02c | 1.43 ± 0.02d | 1.34 ± 0.02e |
| RTS | 1.49 ± 0.04b | 1.53 ± 0.01b | 1.59 ± 0.02a | 1.47 ± 0.06b | 1.39 ± 0.03c | 1.30 ± 0.04d | 1.23 ± 0.03e | 1.13 ± 0.03f | |
| Gly | CS | 1.16 ± 0.06bc | 1.15 ± 0.01bc | 1.16 ± 0.03bc | 1.18 ± 0.01ab | 1.23 ± 0.01a | 1.19 ± 0.02ab | 1.17 ± 0.01ab | 1.11 ± 0.03c |
| RTS | 1.16 ± 0.06bc | 1.20 ± 0.02ab | 1.21 ± 0.02a | 1.24 ± 0.01a | 1.20 ± 0.01ab | 1.16 ± 0.01bc | 1.12 ± 0.01c | 1.04 ± 0.01d | |
| Ala | CS | 1.20 ± 0.04a | 1.20 ± 0.02a | 1.20 ± 0.02a | 1.08 ± 0.02b | 1.01 ± 0.01c | 0.92 ± 0.02d | 0.83 ± 0.03e | 0.62 ± 0.01f |
| RTS | 1.20 ± 0.04a | 1.03 ± 0.05b | 0.93 ± 0.05c | 0.80 ± 0.01d | 0.59 ± 0.03e | 0.46 ± 0.05f | 0.35 ± 0.05g | 0.31 ± 0.02g | |
| Cys | CS | 0.06 ± 0.01a | 0.06 ± 0.01a | 0.06 ± 0.01a | 0.05 ± 0.00a | 0.04 ± 0.00b | 0.03 ± 0.00bc | 0.03 ± 0.01cd | 0.02 ± 0.00d |
| RTS | 0.06 ± 0.01a | 0.06 ± 0.01a | 0.04 ± 0.01b | 0.04 ± 0.01b | 0.03 ± 0.01c | 0.02 ± 0.01c | 0.02 ± 0.00c | 0.02 ± 0.00c | |
| Valz | CS | 0.90 ± 0.02b | 0.91 ± 0.02b | 0.90 ± 0.02b | 0.97 ± 0.03a | 0.96 ± 0.02a | 0.89 ± 0.01b | 0.81 ± 0.01c | 0.76 ± 0.05d |
| RTS | 0.90 ± 0.02c | 0.95 ± 0.01c | 1.03 ± 0.04b | 1.14 ± 0.09a | 0.97 ± 0.02bc | 0.71 ± 0.02d | 0.58 ± 0.02e | 0.36 ± 0.03f | |
| Metz | CS | 0.13 ± 0.03a | 0.13 ± 0.01a | 0.13 ± 0.01a | 0.11 ± 0.01ab | 0.10 ± 0.01bc | 0.09 ± 0.01cd | 0.08 ± 0.01de | 0.06 ± 0.00e |
| RTS | 0.13 ± 0.03a | 0.13 ± 0.02a | 0.11 ± 0.01b | 0.08 ± 0.01c | 0.06 ± 0.00cd | 0.06 ± 0.00cd | 0.05 ± 0.00de | 0.04 ± 0.00e | |
| Ilez | CS | 0.55 ± 0.04bc | 0.56 ± 0.04bc | 0.56 ± 0.03bc | 0.61 ± 0.01ab | 0.66 ± 0.02a | 0.60 ± 0.03b | 0.54 ± 0.02c | 0.48 ± 0.03d |
| RTS | 0.55 ± 0.04c | 0.58 ± 0.03c | 0.64 ± 0.02b | 0.68 ± 0.02a | 0.59 ± 0.02c | 0.50 ± 0.01d | 0.41 ± 0.02e | 0.32 ± 0.01f | |
| Leuz | CS | 1.12 ± 0.09bc | 1.10 ± 0.06bc | 1.14 ± 0.06bc | 1.27 ± 0.01a | 1.26 ± 0.02a | 1.20 ± 0.01ab | 1.06 ± 0.07c | 0.95 ± 0.06d |
| RTS | 1.12 ± 0.09c | 1.20 ± 0.02bc | 1.24 ± 0.03ab | 1.31 ± 0.01a | 1.20 ± 0.01bc | 1.03 ± 0.06d | 0.89 ± 0.08e | 0.74 ± 0.03f | |
| Tyr | CS | 0.30 ± 0.02cd | 0.31 ± 0.02cd | 0.30 ± 0.01cd | 0.35 ± 0.01b | 0.40 ± 0.02a | 0.33 ± 0.02bc | 0.30 ± 0.01cd | 0.29 ± 0.01d |
| RTS | 0.30 ± 0.02de | 0.34 ± 0.01c | 0.41 ± 0.02b | 0.46 ± 0.02a | 0.41 ± 0.01b | 0.33 ± 0.03cd | 0.29 ± 0.02ef | 0.26 ± 0.01f | |
| Phez | CS | 0.43 ± 0.11b | 0.44 ± 0.01b | 0.43 ± 0.02b | 0.52 ± 0.02a | 0.42 ± 0.01b | 0.39 ± 0.01bc | 0.34 ± 0.02c | 0.33 ± 0.02c |
| RTS | 0.43 ± 0.11cd | 0.44 ± 0.03cd | 0.53 ± 0.02ab | 0.60 ± 0.02a | 0.52 ± 0.01b | 0.50 ± 0.02bc | 0.40 ± 0.01d | 0.32 ± 0.01e | |
| Hisz | CS | 0.30 ± 0.03a | 0.30 ± 0.01a | 0.28 ± 0.01ab | 0.26 ± 0.02b | 0.23 ± 0.01c | 0.22 ± 0.01cd | 0.20 ± 0.01d | 0.20 ± 0.01d |
| RTS | 0.30 ± 0.03a | 0.26 ± 0.02b | 0.24 ± 0.01b | 0.20 ± 0.01c | 0.18 ± 0.01cd | 0.18 ± 0.01cd | 0.17 ± 0.01d | 0.14 ± 0.01e | |
| Lysz | CS | 0.58 ± 0.03a | 0.58 ± 0.02ab | 0.55 ± 0.02bc | 0.53 ± 0.01cd | 0.50 ± 0.01d | 0.46 ± 0.01e | 0.46 ± 0.01e | 0.42 ± 0.01f |
| RTS | 0.58 ± 0.03a | 0.53 ± 0.02b | 0.52 ± 0.02b | 0.48 ± 0.02c | 0.42 ± 0.02d | 0.42 ± 0.02d | 0.35 ± 0.01e | 0.35 ± 0.02e | |
| Argz | CS | 0.83 ± 0.05a | 0.84 ± 0.04a | 0.84 ± 0.02a | 0.79 ± 0.01a | 0.71 ± 0.01b | 0.60 ± 0.01c | 0.53 ± 0.04d | 0.46 ± 0.02e |
| RTS | 0.83 ± 0.05a | 0.77 ± 0.01b | 0.71 ± 0.03c | 0.53 ± 0.04d | 0.44 ± 0.06e | 0.40 ± 0.01ef | 0.36 ± 0.02f | 0.30 ± 0.02g | |
| Pro | CS | 1.22 ± 0.22b | 1.24 ± 0.04b | 1.24 ± 0.04b | 1.31 ± 0.02ab | 1.40 ± 0.01a | 1.27 ± 0.04ab | 1.21 ± 0.03b | 1.00 ± 0.02c |
| RTS | 1.22 ± 0.22b | 1.23 ± 0.10b | 1.31 ± 0.01ab | 1.45 ± 0.02a | 1.40 ± 0.01a | 1.16 ± 0.06bc | 1.03 ± 0.03c | 0.82 ± 0.06d | |
| EAA | CS | 5.47 ± 0.41a | 5.47 ± 0.12a | 5.44 ± 0.03a | 5.76 ± 0.08a | 5.53 ± 0.02a | 5.05 ± 0.07b | 4.53 ± 0.17c | 4.06 ± 0.12d |
| RTS | 5.47 ± 0.41a | 5.54 ± 0.08a | 5.75 ± 0.07a | 5.60 ± 0.04a | 4.89 ± 0.10b | 4.25 ± 0.08c | 3.57 ± 0.07d | 2.86 ± 0.04e | |
| TAA | CS | 12.78 ± 0.37a | 12.80 ± 0.29a | 12.58 ± 0.09a | 12.82 ± 0.07a | 12.98 ± 0.12a | 11.90 ± 0.15b | 10.98 ± 0.26c | 9.86 ± 0.25d |
| RTS | 12.78 ± 0.37a | 12.62 ± 0.08a | 12.80 ± 0.08a | 12.83 ± 0.19a | 11.84 ± 0.05b | 10.31 ± 0.19c | 9.06 ± 0.17d | 7.75 ± 0.15e | |
| EAA/TAA (%) | CS | 42.83 | 42.69 | 43.27 | 44.89 | 42.63 | 42.40 | 41.27 | 41.15 |
| RTS | 42.83 | 43.92 | 44.94 | 43.63 | 41.33 | 41.16 | 39.41 | 36.88 | |
Data are presented as mean ± standard deviation (n = 3).
Multiple comparison adopted the Duncan test; different lowercase letters (a–g) in each row indicate the significant difference among storage time at p < 0.05.
CS = cold storage; EAA = essential amino acid; RTS = ambient temperature storage; TAA = total amino acid.
Indicates the essential amino acids.
3.4. Bioactive compounds
The AsA content behavior in walnut male flowers during storage is shown in Figure 4. The AsA content of samples at the beginning of storage was about 102.32 mg/100 g. A relatively sharp decrease in AsA was noted during the whole storage period in walnut male inflorescences stored at 25°C, and the content became almost undetectable on the 8th day. It could be associated to the noticeable drying of male flowers, as a result of higher water loss at 25°C. We observed a gradual decrease in AsA at 4°C. Storage temperature, time, and temperature × time all significantly affected (p < 0.01) the AsA content during storage period according to Duncan’s multiple test. AsA was, by far, the least stable nutrient during storage, and it was highly sensitive to oxidation [27]. Loss of AsA was generally more rapid at higher storage temperatures [28]. Furthermore, Krüger et al [29] also confirmed that lower temperature after harvest was considered to be important for maintaining AsA content in horticultural products.
Figure 4.
Dynamic changes of (A) ascorbic acid, (B) total phenolic content, and (C) total flavonoid content of fresh walnut male flowers stored for 14 days at 4°C and 25°C. Data are shown as mean ± standard deviation (n =3). * The lowercase letters near the bar indicate statistically different mean among storage days at p < 0.05.
The total phenolic and total flavonoid contents of walnut male flowers are shown in Figures 4B and 4C. It is obvious that both temperature and storage time had a significant effect (p < 0.05) on total phenolics and total flavonoids of walnut male flowers and showed a trend of decline. The content of total phenolics and total flavonoids in walnut male inflorescences stored at room temperature decreased significantly (decreased by about 42.04% and 41.86%, respectively), and remarkable differences were not observed between the 8th and the 14th days, which might be attributable to the low water content in walnut male flowers in the next few days that could have inhibited their metabolism rate. However, there was a slow rate of decline (decreased by about 28.01%) in total phenolics for the first 12 days in walnut flowers stored at 4°C. A gradual decrease in total flavonoids occurred from the first to the last day (p < 0.05). Storage temperature, time, and their interaction had significant effects (p < 0.0001) on both phenolic and flavonoid contents during the storage period based on Duncan’s multiple test. These results also indicated that cold storage could be effective in maintaining the content of bioactive compounds. There are different results about the effects of storage temperature on total phenolics and total flavonoids of fruits and fruit juices. The TPC of apple juice, pear juice, and grape juice increased significantly after storage at 25°C for 9 months [30], but that of tomato juice stored at both 25°C and 4°C was stable within the guarantee period [31,32]. The TPC of pomegranate juice decreased by 20% after storage for 160 days at room temperature [33], and the decline in the rate of total phenolics and total flavonoids in plum fruit was slower at 2°C than at 20°C [34]. Phenolics and flavonoids had an unstable structure and high degradation rate at high temperatures because of the high oxidation and enzymatic activity [35,36], which coincides with our results in this study.
3.5. Antioxidant activity
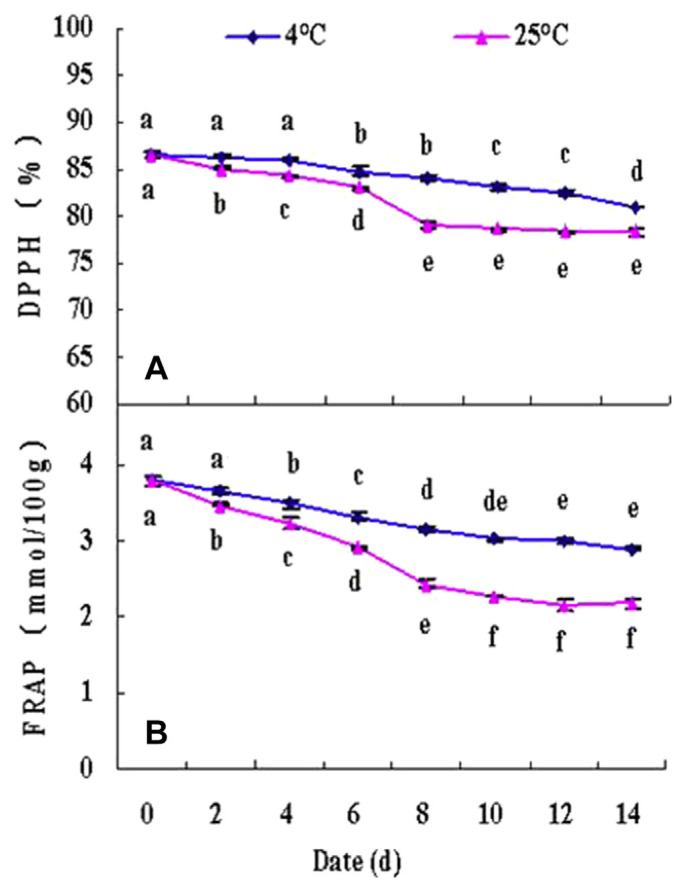
In Figures 5A and 5B, the DPPH radical scavenging activity (ARP) and FRAP activity results of male flower extracts under two storage temperatures (4°C and 25°C) are shown. ARP and FRAP values of extracts reaching up to 86.63% and 3.79 mmol FeSO4/100 g DW, respectively, on the 1st day may be attributed to the higher total phenolic and flavonoid contents at this time. The ARP and FRAP values showed a trend of significant decline with the passing of storage time (decreased by about 8.71% and 40.12%, respectively), and samples showed approximately the same ARP and FRAP values after 8 and 10 days, respectively, at room temperature. This might be ascribed to the different functional factors that played a leading role in the different antioxidant activity assay methods, and the different tolerance for external environment. Under cold storage, significant differences in ARP values were not observed between the 1st and the 4th days of storage, and then a gradual decrease in ARP values occurred when the storage period was prolonged; the value was 86.63% on the 14th day. A gradual decrease in FRAP values was observed after 2 days, and the value was 3.00 mmol FeSO4/100 g DW on the 12th day, after which no remarkable changes were found. The FRAP value of walnut male flowers was higher than those determined in the last year (2.06 mmol FeSO4/100 g DW). The effects of temperature, time, and temperature × time interaction on antioxidant activities were the same as in bioactive compounds and reached a significant level (p < 0.0001), according to Duncan’s multiple test. It could be possible that the variations in the TPC of walnut flowers were influenced by the environmental conditions of growing fields in different years. Most studies indicated that there is a significant relationship between total phenolics and flavonoid content and antioxidant capability, but during storage, the changes in total phenols and antioxidant capability were different depending on the experimental materials used, storage temperature, and so on [30–38]. The present study suggests that storage would decrease the antioxidant potential of walnut male flowers because of the oxidation of phenolic and flavonoid compounds, which agrees with previous research results, such as those reported in pomegranate juice [33], plum [34], and California almond [37]. However, to some extent, cold storage could reduce the oxidation that maintained the antioxidant capacity effectively. A similar behavior was highlighted by Bolling et al [37], who studied the influence of storage on polyphenol content and antioxidant capacity of California almond skins, and by Guo et al [34], who reported that lower storage temperature alleviated the declining trends of total antioxidant capacity, DPPH radicals, and hydroxyl radicals.
Figure 5.
Dynamic changes of (A) DPPH radical scavenging activity and (B) FRAP of fresh walnut male flower stored for 14 days at 4°C and 25°C. Data are shown as mean ± standard deviation (n =3). * The lowercase letters near the bar indicate statistically different mean among storage days at p < 0.05. DPPH =1,1-diphenyl-2-picrylhydrazyl; FRAP =ferric reducing ability of plasma.
4. Conclusion
In this study, the contents of saccharides, protein, amino acids, mineral elements, AsA, phenolic and flavonoid compounds, and the antioxidant activities of walnut male flowers under different storage temperatures (4°C and 25°C) with the passing of storage time were investigated. The results showed that storage temperature and time were important factors when studying the effects of storage in walnut male inflorescences especially on the following parameters: moisture, saccharides, protein, amino acids, fat, AsA, and antioxidant activities. During the entire storage period, we observed a different degree of decrease in moisture, saccharides, fat, protein, AsA, phenolic and flavonoid compound contents, and antioxidant activities by prolonging the storage period. Moreover, the loss rate of these components in walnut male flowers at 4°C was less than that observed at 25°C. It could be seen that the use of LT was more effective for maintaining a high level of nutritive constituents compared with that observed at room temperature. The effectiveness of LT in delaying quality loss in walnut male flowers was confirmed in our study. Furthermore, the data also showed that no remarkable changes in ash and mineral values occurred, and slightly significant changes were observed in crude fiber during the whole storage period of this evaluation, indicating that the influence of storage on the individual mineral and crude fiber content was minimal. In order to minimize the impact of storage conditions, it has been suggested that walnut male flowers be stored at 4°C for 4 days. In addition, to some degree, this study also confirmed that walnut male inflorescences were a good source of protein, fiber, mineral, and phenolic and flavonoid compounds, which could be utilized as food for human consumption.
In this study, new detailed information is presented on the effect of storage temperature on the nutrimental composition of walnut male flowers, which could generate useful information for consumers and may encourage them to consume walnut male flowers at appropriate storage conditions. However, more complete and effective storage methods to prevent nutrient losses are necessary because of the high water content in walnut male flowers, which leads the products to deteriorate and spoil easily by microorganism and chemical reactions.
Acknowledgments
This study was supported by the National Key Technology R&D Program (2014BAD23B03) and the Guizhou High Level Innovative Talents Project ([2016]4038).
Funding Statement
This study was supported by the National Key Technology R&D Program (2014BAD23B03) and the Guizhou High Level Innovative Talents Project ([2016]4038).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
REFERENCES
- 1. Yun ZE, Jin S, Ding YD, Wang Z, Gao HJ, Pan ZY, Xu J, Cheng YJ, Deng XX. Comparative transcriptomics and proteomics analysis of citrus fruit, to improve understanding of the effect of low temperature on maintaining fruit quality during lengthy post-harvest storage. J Exp Bot. 2012;63:2873–93. doi: 10.1093/jxb/err390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pareek S, Benkeblia N, Janick J, Cao SF, Yahiae EM. Postharvest physiology and technology of loquat (Eriobotrya japonica Lindl.) fruit. J Sci Food Agric. 2014;94:1495–504. doi: 10.1002/jsfa.6560. [DOI] [PubMed] [Google Scholar]
- 3. Song LL, Chen HJ, Gao HY, Fang XJ, Mu HL, Yuan Y, Yang Q, Jiang YM. Combined modified atmosphere packaging and low temperature storage delay lignification and improve the defense response of minimally processed water bamboo shoot. Chem Cent J. 2013;7:147–56. doi: 10.1186/1752-153X-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding CK, Chachin K, Ueda Y, Mochioka R. Effect of cold storage and harvest ripeness on the quality and chemical composition of loquat fruits. Food Sci Technol Int Tokyo. 1997;3:200–4. [Google Scholar]
- 5. Ding ZS, Tian SP, Wang YS, Li B, Chan ZL, Han J, Xu Y. Physiological response of loquat fruit to different storage conditions and its storability. Postharv Biol Technol. 2006;41:143–50. [Google Scholar]
- 6. Liu H, Song LL, You YL, Li YB, Duan XW, Jiang YM, Joyce DC, Ashraf M, Lu WJ. Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharv Biol Technol. 2011;60:24–30. [Google Scholar]
- 7. Lee JH, Cho KM. Changes occurring in compositional components of black soybeans maintained at room temperature for different storage periods. Food Chem. 2012;131:161–9. [Google Scholar]
- 8. Jin WW, Xu CJ, Li X, Zhang B, Wang P, Allan AC, Chen KS. Expression of ROP/RAC GTPase genes in postharvest loquat fruit in association with senescence and cold regulated lignification. Postharv Biol Technol. 2009;54:9–14. [Google Scholar]
- 9. Chen CY, Zhao SL, Cao JX, Zhang RQ, Guo JM. Analysis and study of edible value of walnut flower. Food Sci. 1998;19:35–7. [In Chinese, English abstract] [Google Scholar]
- 10. Wang CL, Zhang WE, Pan XJ. Nutritional quality of the walnut male inflorescences at four flowering stages. J Food Nutr Res. 2014;2:457–64. [Google Scholar]
- 11. Wang HB, Xiao JQ, Liu XK. Study on antioc/idant activity of Juglans regia L. flowers. Food Sci. 2008;29:140–2. [In Chinese, English abstract] [Google Scholar]
- 12. Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antihemolytic activity and mineral contents of Juglans regia L. flowers. Eur Rev Med Pharmacol Sci. 2013;17:1881–3. [PubMed] [Google Scholar]
- 13. Hosseini SE, Karimzadeh K, Vessal M. Effects of a hydroalcoholic extract of walnut male flowers on diabetic rats. Zah J Res Med Sci. 2013;15:29–32. [Google Scholar]
- 14. Nabavi SF, Ebrahimzadehma MA, Nabavi SM, Mahmoudi M, Keyvani RS. Biological activities of Juglans regia flowers. Rev Bras Farmacogn. 2011;21:465–70. [Google Scholar]
- 15.Association of Official Analytical Chemistry. Official methods of analysis. 18th ed. Washington, DC: AOAC; 2005. [Google Scholar]
- 16.China State Bureau of Quality and Technical Supervision. National Standard of the People’s Republic of China 2003 (GB/T 5009) Beijing: China; 2003. [Google Scholar]
- 17. Sánchez-Machado DI, Núñez-Gastélum JA, Reyes-Moreno C, Ramírez-Wong B, López-Cervantes J. Nutritional quality of edible parts of Moringa oleifera. Food Anal Method. 2010;3:175–80. [Google Scholar]
- 18. Park S, Arasu MV, Lee MK, Chun JH, Seo JM, Lee SW, Al-Dhabi NA, Kim SJ. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.) Food Chem. 2014;145:77–85. doi: 10.1016/j.foodchem.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 19. Conde-Hernández LA, Guerrero-Beltrán JÁ. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 2014;142:455–60. doi: 10.1016/j.foodchem.2013.07.078. [DOI] [PubMed] [Google Scholar]
- 20. Feng SM, Luo ZS, Zhang YB, Zhong Z, Lu BY. Phytochemical contents and antioxidant capacities of different parts of two sugarcane (Saccharum officinarum L.) cultivars. Food Chem. 2014;151:452–8. doi: 10.1016/j.foodchem.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 21. Motamed SM, Naghibi F. Antioxidant activity of some edible plants of the Turkmen Sahra region in northern Iran. Food Chem. 2010;119:1637–42. [Google Scholar]
- 22. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 23. Hatamnia AA, Abbaspour N, Darvishzadeh R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014;145:306–11. doi: 10.1016/j.foodchem.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 24.Zhao LQ, Zhang ZD. Storage and processing of horticultural product. Beijing: China Light Industry Press; 2011. [Google Scholar]
- 25. Rodríguez MJ, Villanueva MJ, Tenorio MD. Changes in chemical composition during storage of peaches (Prunus persica) Eur Food Res Technol. 1999;209:135–9. [Google Scholar]
- 26. Liu Y, Huang F, Yang H, Ibrahim SA, Wang YF, Huang W. Effects of preservation methods on amino acids and 5′-nucleotides of Agaricus bisporus mushrooms. Food Chem. 2014;149:221–5. doi: 10.1016/j.foodchem.2013.10.142. [DOI] [PubMed] [Google Scholar]
- 27. Patras A, Tiwari BK, Brunton NP. Influence of blanching and low temperature preservation strategies on antioxidant activity and phytochemical content of carrots, green beans and broccoli. LWT Food Sci Technol. 2011;44:299–306. [Google Scholar]
- 28. Paull RE. Effect of temperature and relative humidity on fresh commodity quality. Postharv Biol Technol. 1999;15:263–77. [Google Scholar]
- 29. Krüger E, Dietrich H, Schopplein E, Rasim S, Kürbel P. Cultivar, storage conditions and ripening effects on physical and chemical qualities of red raspberry fruit. Postharv Biol Technol. 2011;60:31–7. [Google Scholar]
- 30. Spanos GA, Wrolstab BE, Heatherbell DA. Influence of processing and storage on the phenolic composition of apple juice. J Agric Food Chem. 1990;38:1572–9. [Google Scholar]
- 31. Garcifa-Alonso FJ, Bravo S, Casas J, Pérez-Conesa D, Jacob K, Periago MJ. Changes in antioxidant compounds during the shelf life of commercial tomato juices in different packaging materials. J Agric Food Chem. 2009;57:6815–22. doi: 10.1021/jf900877c. [DOI] [PubMed] [Google Scholar]
- 32. Odriozola-Serrano I, Soliva-Fortuny R, Martín-Belloso O. Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innovative Food Sci Emerging Technol. 2008;9:272–9. [Google Scholar]
- 33. Pérez-Vicente A, Serrano P, García-Viguera C. Influence of packaging material on pomegranate juice colour and bioactive compounds, during storage. J Agric Food Chem. 2004;84:639–44. [Google Scholar]
- 34. Guo XM, An L, Wang YS, Wang GX, Wang ZY, Li LP. Changes of quality and antioxidant capacity of different parts of plum fruit at two storage temperature. Food Sci. 2010;31:425–9. [In Chinese, English abstract] [Google Scholar]
- 35. Sun JX, Zhang Y, Hu XS. Structural stability and degradation mechanisms of anthocyanins. Sci Agric Sin. 2009;42:996–1008. [In Chinese, English abstract] [Google Scholar]
- 36. Javadi N, Abas F, Mediani A, Hamid AA, Khatib A, Simoh S, Shaari K. Effect of storage time on metabolite profile and alpha-glucosidase inhibitory activity of Cosmos caudatus leaves – GCMS based metabolomics approach. J Food Drug Anal. 2015;23:433–41. doi: 10.1016/j.jfda.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bolling BW, Blumberg JB, Chen CYO. The influence of roasting, pasteurization, and storage on polyphenols content and antioxidant capacity of California almond skins. Food Chem. 2010;123:1040–7. doi: 10.1016/j.foodchem.2010.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma K, Ko EY, Assefa AD, Ha S, Nile SH, Lee ET, Park SW. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J Food Drug Anal. 2015;23:243–52. doi: 10.1016/j.jfda.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]