Abstract
A total of 825 samples of retail raw meats (chicken, turkey, pork, and beef) were examined for the presence of Escherichia coli and Salmonella serovars, and 719 of these samples were also tested for Campylobacter spp. The samples were randomly obtained from 59 stores of four supermarket chains during 107 sampling visits in the Greater Washington, D.C., area from June 1999 to July 2000. The majority (70.7%) of chicken samples (n = 184) were contaminated with Campylobacter, and a large percentage of the stores visited (91%) had Campylobacter-contaminated chickens. Approximately 14% of the 172 turkey samples yielded Campylobacter, whereas fewer pork (1.7%) and beef (0.5%) samples were positive for this pathogen. A total of 722 Campylobacter isolates were obtained from 159 meat samples; 53.6% of these isolates were Campylobacter jejuni, 41.3% were Campylobacter coli, and 5.1% were other species. Of the 212 chicken samples, 82 (38.7%) yielded E. coli, while 19.0% of the beef samples, 16.3% of the pork samples, and 11.9% of the turkey samples were positive for E. coli. However, only 25 (3.0%) of the retail meat samples tested were positive for Salmonella. Significant differences in the bacterial contamination rates were observed for the four supermarket chains. This study revealed that retail raw meats are often contaminated with food-borne pathogens; however, there are marked differences in the prevalence of such pathogens in different meats. Raw retail meats are potential vehicles for transmitting food-borne diseases, and our findings stress the need for increased implementation of hazard analysis of critical control point (HACCP) and consumer food safety education efforts.
Microbial food safety is an increasing public health concern worldwide. It is estimated that each year in the United States there are approximately 76 million food-borne illnesses (23). While most of these illnesses are undiagnosed and thus unreported, approximately 325,000 cases result in hospitalization, and 5,000 cases are fatal. Nearly 2.4 million cases are caused by Campylobacter spp., 1.4 million cases are caused by nontyphoidal Salmonella serovars, and 270,000 cases are caused by pathogenic Escherichia coli, including E. coli O157:H7 (23). Although these pathogens usually cause mild to moderate self-limiting gastroenteritis, invasive diseases and complications may occur, resulting in more severe cases. For example, Campylobacter has been identified as the predominant cause of Guillain-Barré syndrome and reactive arthritis (3). Systemic salmonellosis infections can be life threatening, and Shiga toxin-producing E. coli (STEC), particularly E. coli O157:H7, can cause bloody diarrhea and hemolytic uremic syndrome (12).
Campylobacter, Salmonella, and pathogenic E. coli all colonize the gastrointestinal tracts of a wide range of wild and domestic animals, especially animals raised for human consumption (24). Food contamination with these pathogens can occur at multiple steps along the food chain, including production, processing, distribution, retail marketing, and handling or preparation. Numerous epidemiological reports have implicated foods of animal origin as the major vehicles associated with illnesses caused by food-borne pathogens (30, 34). Contaminated raw or undercooked poultry and red meats are particularly important in transmitting these food-borne pathogens. Other sources of human infections with Campylobacter, Salmonella, and STEC include contaminated produce and contact with farm animals and pets. Person-to-person transmission has also been described (33).
Studies worldwide have shown that Campylobacter, Salmonella, and E. coli are often present in fresh meat and poultry (34). However, there is a paucity of data concerning the prevalence of contamination with multiple food-borne pathogens in retail meats in the United States. The objectives of this study were to determine the prevalence of Campylobacter, Salmonella, and E. coli in retail raw meats obtained in the Greater Washington, D.C., area and to investigate the association of microbial contamination with product type, season, and supermarket chain.
MATERIALS AND METHODS
Sample collection and preparation.
Meat samples (n = 825), including chicken carcasses, turkey breasts, beef steaks, and pork chops, were randomly collected from retail stores of four supermarket chains in the Greater Washington, D.C., area, including suburban Maryland. Stores of the four supermarket chains in the area were identified by using phone books, store web sites, and store maps. Each store was assigned an identification number in order to form a store database. Sampling visits were made on every other Monday for 14 months (June 1999 to July 2000). On each sampling day, four stores were randomly chosen from the store database by using a statistical program (SAS Institute Inc., Cary, N.C.). Eight prepackaged raw meat products (two of each meat type) were randomly selected and transported on ice to the laboratory. Each sample was aseptically removed and placed in a plastic bag that contained 200 to 500 ml of buffered peptone (Difco Laboratories, Detroit, Mich.), depending on the sample size. The bag was shaken manually for 3 min and left on ice for 20 min. The rinse solution was used for isolation of Campylobacter, E. coli, and Salmonella.
Bacterial isolation.
Modifications of methods described in the Food and Drug Administration Bacteriological Analytical Manual were used to isolate Campylobacter, E. coli, and Salmonella from the retail raw meat samples (11). Isolation and culturing of Campylobacter were always conducted with the AnaeroPak system (Mitsubish Gas Chemical Co., Inc., Osaka, Japan) under microaerophilic conditions created by using a 10% CO2–10% H2–80% N2 gas mixture and Campy pack (Becton Dickinson, Cockeysville, Md.). A 20-ml portion of a meat sample rinse solution was mixed with the same volume of double-concentrated Bolton broth (Oxoid Inc., Ogdensburg, N.Y.) and incubated at 42°C overnight with shaking. The overnight enrichment broth was used to inoculate Campylobacter onto blood-free selective agar (Oxoid) plates using a cotton swab. After 48 h of incubation at 42°C, the plates were examined for typical Campylobacter colonies, which were small, gray, and droplike or small and shiny or slimy. Presumptive Campylobacter colonies were subcultured on blood agar plates and incubated for 48 h at 42°C. Single colonies (3–5) on a blood agar plate were selected for Gram staining and oxidase and catalase tests.
For isolation of E. coli, 200 μl of a meat rinse solution was streaked onto MacConkey agar (Difco) plates and incubated at 35°C for 24 h. Following incubation, lactose-positive colonies (3–5) were streaked onto eosin-methylene blue (Difco) agar plates. Typical E. coli colonies on eosin-methylene blue agar (green and shiny or with dark or purple centers) were subcultured in 10 ml of Trypticase soy broth (Difco) and incubated for 24 h at 37°C. The broth cultures were tested for indole production, and indole-positive cultures were confirmed to be E. coli by using API 20E (Biomerieux Vitek, Inc., Hazelwood, Mo.).
To isolate Salmonella, 20 ml of a meat rinse solution was mixed with the same volume of double-concentrated lactose broth (Difco). After incubation at 35°C for 24 h, 1.0 ml of the enrichment broth was transferred into 9.0 ml of tetrathionate broth and incubated at 42°C for 24 h. Following 24 h of incubation, the broth culture was streaked onto XLT4 (Difco) agar plates and incubated for 24 h at 37°C. Presumptive Salmonella colonies (3–5) on an XLT4 plate were selected and used to inoculate triple sugar iron (Difco) slants, which were then incubated for 24 h at 37°C. The identities of Salmonella isolates were confirmed by using API 20E.
PCR assays.
Presumptive Campylobacter isolates that were gram-negative, curved organisms as determined by microscopic examination and were oxidase and catalase positive were to be confirmed members of the genus Campylobacter by performing a PCR assay. Primers BO4263 and BO4264 amplified a 256-bp unique fragment of Campylobacter genomes (17). A multiplex PCR method, based on two PCR assays described by Linton et al., were developed to identify Campylobacter species with primers HIP 400F and HIP 1134R for Campylobacter jejuni and primers CC18F and CC519R for Campylobacter coli (20). Multiplex PCR assays were also performed for E. coli to identify genes encoding Shiga toxins 1 and 2 and heat-labile and heat-stable enterotoxins (17, 25, 36). The targets, primer sequences, and sizes of amplicons for the PCR assays are shown in Table 1.
TABLE 1.
Targets and oligonucleotide primers used in PCR assays for identification of Campylobacter and virulence genes of E. coli isolated from retail meats
| Target | PCR product size (bp) | Primer | Primer sequence | Reference |
|---|---|---|---|---|
| ORF common to C. jejuni, C. coli, and C. upsaliensisa | 256 | BO4263 | 5′-AGAACACGCGGACCTATATA-3′ | 17 |
| BO4264 | 5′-CGATGCATCCAGGAATGTAT-3′ | |||
| Hippurase | 735 | HIP 400F | 5′-GAAGAGGGTTTGGGTGGTG-3′ | 20 |
| HIP 1134R | 5′-AGCTAGCTTCGCATAATAACTTG-3′ | |||
| ORF specific for C. coli | 500 | CC18F | 5′-GGTATGATTTCTACAAAGCGAG-3′; | 20 |
| CC519R | 5′-ATAAAAGACTATCGTCGCGTG-3′ | |||
| Shiga toxin 1 | 210 | VT1-f | 5′-TGTAACTGGAAAGGTGGAGTATACA-3′ | 25 |
| VT1-r | 5′-GCTATTCTGAGTCAACGAAAAATAAC-3′ | |||
| Shiga toxin 2 | 484 | VT2-f | 5′-GTTTTTCTTCGGTATCCTATTCC-3′ | 25 |
| VT2-r2 | 5′-GATGCATCTCTGGTCATTGTATTAC-3′ | |||
| Heat-labile enterotoxin | 110 | LT 51 | 5′-CCGGTATTACAGAAATCTGA-3′ | 36 |
| LT 31 | 5′-GTGCATGATGAATCCAGGGT-3′ | |||
| Heat-stable enterotoxin | 368 | STII-FP | 5′-GCAATAAGGTTGAGGTGAT-3′ | 21 |
| STII-RP | 5′-GCCTGCAGTGAGAAATGGAC-3′ |
ORF, open reading frame.
The PCR procedures used have been described previously (20, 26). Briefly, bacterial templates were prepared by heating broth cultures at 98°C for 10 min. PCR reagents were obtained from PE Applied Biosystems, Foster City, Calif. Each PCR mixture consisted of 1× reaction buffer, 1.5 mM MgCl2, 200 μM (each) dATP, dCTP, dGTP, and dTTP, 10 pmol of each primer, 1 U of AmpliTaq polymerase, and 10 μl of bacterial template. Deionized water was added to bring the final volume to 50 μl. The PCR was performed with a thermal cycler (GeneAmp PCR System 9600; Perkin-Elmer, Norwalk, Conn.) by using 30 cycles of denaturation at 94°C for 1 min, primer annealing at 60°C for 1 min, and primer extension at 72°C for 1 min. PCR products were stained with ethidium bromide and visualized under UV light after gel electrophoresis on 2% agarose.
Data analysis.
Prevalence data for the microorganisms sorted by meat type, season, and store chain were analyzed by using the analysis of variance of SAS for Windows (version 6.12; SAS Institute Inc.).
RESULTS
Fifty-nine stores, including 29 chain A stores, 17 chain B stores, 9 chain C stores, and 4 chain D stores, were visited a total of 107 times from June 1999 to July 2000. Thirty of these stores were visited once, 15 stores were visited twice, 9 stores were visited three times, and 5 stores were visited four times. A total of 825 samples of retail raw meats were collected and examined for the presence of E. coli and Salmonella; 719 of these samples were also tested for the presence of Campylobacter. (Table 2).
TABLE 2.
Prevalence of Campylobacter, E. coli, and Salmonella in retail raw meats
| Meat | No. of samplesa | No. (%) of samples positive for:
|
||
|---|---|---|---|---|
| Campylobacter | E. coli | Salmonella | ||
| Chicken | 212 (184) | 130 (70.7) | 82 (38.7) | 9 (4.2) |
| Turkey | 194 (172) | 25 (14.5) | 23 (11.9) | 5 (2.6) |
| Pork | 209 (181) | 3 (1.7) | 34 (16.3) | 7 (3.3) |
| Beef | 210 (182) | 1 (0.5) | 40 (19.0) | 4 (1.9) |
| Total | 825 (719) | 159 (22.1) | 179 (21.7) | 25 (3.0) |
The numbers in parentheses are the numbers of samples analyzed for Campylobacter.
Prevalence of Campylobacter, E. coli, and Salmonella.
Table 2 shows the prevalence of Campylobacter, E. coli, and Salmonella in retail chicken, turkey, pork, and beef obtained from the 59 stores. Of the four raw meat products, chicken was most frequently contaminated with Campylobacter (70.7%), followed by turkey (14.5%). Compared to poultry, red meats had much lower rates of contamination with Campylobacter. Less than 1% of beef samples and less than 2% of pork samples were positive for this pathogen. Chicken also had the highest rate of E. coli contamination (38.7%). Interestingly, beef (19.0%) and pork (16.3%) were more likely contaminated with E. coli than turkey was (11.9%). In contrast, Salmonella was isolated from only 3.0% of the 825 meat samples, and chicken had the highest rate of Salmonella contamination (4.2%).
A number of meat samples were contaminated either with Campylobacter and E. coli or with Campylobacter and Salmonella. Of 184 chicken samples tested, 54 (29.3%) were contaminated with both Campylobacter and E. coli, and 2 were positive for all three bacteria. Only five pork samples and four turkey samples had more than one type of organism present. The five pork samples contained E. coli and Salmonella, whereas only one turkey sample contained E. coli and Salmonella. Two turkey samples were contaminated with Campylobacter and E. coli, and one turkey sample was contaminated with Campylobacter and Salmonella. In contrast, none of the beef samples contained detectable numbers of more than one of the three enteric bacteria.
Isolation of Campylobacter, E. coli, and Salmonella sorted by store and supermarket chain.
Most (91%) of the stores during 92 sampling visits had Campylobacter-contaminated chicken. Only 22 (24%) of the store visits yielded Campylobacter-positive turkey samples. E. coli was recovered from chicken after nearly 60% of 106 store visits, whereas E. coli was recovered from pork, beef, and turkey after 24, 23, and 19% of the store visits, respectively. However, very few stores had Campylobacter-contaminated beef (1%) or pork (3%). Due to the low prevalence of Salmonella, no significant difference was observed among the stores that were positive for the presence of Salmonella regardless of the type of meat tested.
During the 14-month sample collection period, five stores of three supermarket chains were visited four times. Regardless of the store visited, Campylobacter was repeatedly found in one or two of the two chicken samples analyzed except for the initial visit to one store. Chicken samples were also frequently (60% of the visits) contaminated with E. coli. Salmonella, however, was isolated only from one turkey sample and one beef sample from one store after the fourth visit.
The microbial contamination rates for the four supermarket chains ranged from 20.6 to 32.6% for Campylobacter, from 18.1 to 28.3% for E. coli, and from 0 to 3.4% for Salmonella (Table 3). Similar to the findings obtained when the retail meats were compared, there were not significant differences in the levels of Salmonella contamination among the four chains. However, the Campylobacter and E. coli contamination rates for the four supermarket chains were significantly different (P < 0.05). Chain D had higher microbial contamination rates for both Campylobacter and E. coli than chains A and B and a higher E. coli contamination rate than chain C.
TABLE 3.
Prevalence of Campylobacter, E. coli, and Salmonella in meat products from four supermarket chains
| Supermarket chain | No. of samplesa | No. (%) of samples positive forb:
|
||
|---|---|---|---|---|
| Campylobacter | E. coli | Salmonella | ||
| A | 473 (413) | 85 (20.6) A | 99 (20.9) A | 16 (3.4) A |
| B | 190 (152) | 33 (21.7) A | 43 (22.6) A | 3 (1.6) A |
| C | 116 (108) | 31 (28.7) B | 21 (18.1) A | 3 (2.6) A |
| D | 46 (46) | 15 (32.6) B | 13 (28.3) B | 0 (0) A |
| Total | 825 (719) | 164 (22.8) | 179 (21.7) | 25 (3.0) |
The numbers in parentheses are the numbers of samples analyzed for Campylobacter.
Values in the same column followed by different letters are significantly different (P < 0.05).
PCR results for Campylobacter identification and E. coli toxins.
A total of 722 isolates (three to five isolates per sample) from 159 meat samples that were presumptively Campylobacter positive (Table 4) were identified based on Gram staining and oxidase and catalase tests. A PCR assay specific for C. jejuni, C. coli, and Campylobacter upsaliensis confirmed that almost all of the isolates were Campylobacter isolates; the only exceptions were three isolates from chicken and one isolate from turkey. Approximately one-half (53.6%) of the isolates were identified as C. jejuni, 41.3% were identified as C. coli, and 5.1% were identified as other species. Both C. jejuni and C. coli were isolated more frequently from retail chicken than from turkey, pork, or beef (Table 4). Interestingly, C. coli was recovered more often from retail turkey samples than C. jejuni was. Twenty retail meat samples (18 chicken samples, one turkey sample, and one pork sample) contained more than one Campylobacter species. Two chicken samples yielded three species of Campylobacter. Most of these retail meat samples were collected from different stores or at different times.
TABLE 4.
Campylobacter species identified in retail meats
| Species | No. of isolates (no. of meat samples)
|
||||
|---|---|---|---|---|---|
| Chicken | Turkey | Pork | Beef | Total | |
| C. jejuni | 365 (83) | 16 (4) | 2 (1) | 4 (1) | 387 (89) |
| C. coli | 203 (54) | 86 (19) | 9 (3) | 0 | 298 (76) |
| Other Campylobactera | 27 (13) | 10 (3) | 0 | 0 | 37 (16) |
| Totalb | 595 (150) | 112 (26) | 11 (4) | 4 (1) | 722 (181) |
Organisms not identified by the multiplex PCR specific for C. jejuni and C. coli.
More than one species was isolated from 20 meat samples.
Based on the PCR assays specific for genes encoding Shiga toxins and enterotoxins of E. coli, none of the 179 E. coli isolates tested possessed Shiga toxin genes, whereas one pork isolate was positive for the heat-labile enterotoxin and two isolates (one pork isolate and one beef isolate) were positive for the heat-stable enterotoxins (data not shown).
Seasonality component.
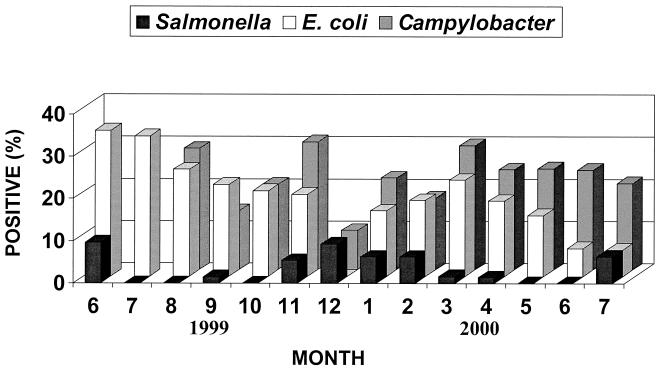
The prevalence of Campylobacter, Salmonella, and E. coli in the four meats varied during the 14-month sampling period (Fig 1). However, no seasonality component was observed, and these enteric pathogens were found in retail meats in both warm and cold months.
FIG. 1.
Prevalence of Campylobacter, E. coli, and Salmonella in raw chicken, turkey, pork, and beef samples from four retail supermarket chains in the Greater Washington area from June 1999 to July 2000. Examination of samples for Campylobacter contamination started in August 1999.
DISCUSSION
The present study demonstrated that three major enteric bacterial taxa were present in retail raw meat products obtained from supermarkets in the Greater Washington, D.C., area, including suburban Maryland, over a 14-month period. Chicken carcasses, turkey breasts, beef steaks, and pork chops were used because they are widely available in grocery stores and are representative of meat products that are handled and prepared in the raw state in domestic kitchens. Additionally, these retail meats are often associated with direct hand-to-mouth exposure to enteric pathogens and cross-contamination of the kitchen environment and ready-to-eat foods.
Several studies have indicated that Campylobacter is present in retail raw meats. Raw poultry meats are commonly contaminated with Campylobacter; this is particularly true of chicken products, and the rates of contamination that have been reported are as high as 100% (1, 2). The reported rates of contamination of pork products vary from 1.3% in the United States (10) to 2% in Belgium (18) and 16.9% in Canada (14). The prevalence of Campylobacter in beef is generally low (22, 28). Other studies demonstrated that this pathogen was isolated from only 2 to 10% of the beef samples tested (18, 29). The lower levels of Campylobacter in pork and beef may be due to a lower incidence of these organisms in swine and cattle populations than in poultry, as well as the sensitivity of Campylobacter to atmospheric oxygen and other environmental stresses during transport, processing, and storage of the products tested. Our study also indicated that multiple Campylobacter species are present in raw meats, which has also been observed in other studies (16, 19, 27). More than one species of Campylobacter was identified in 20 meat samples (primarily chicken samples). It is likely that different serotypes or genotypes of the same species (multiple clones) can also be present in one sample, which presents a challenge to molecular subtyping methods used for epidemiological or outbreak investigations. Recent studies have also suggested that coinfection with multiple strains of Campylobacter occurs in 5 to 10% of human cases of acute enteritis (19). Therefore, it is important that more than one bacterial colony per sample be selected for identification and subtyping of Campylobacter. Multiple isolates may be obtained from different isolation steps, such as direct selective plating and selective enrichment, and/or may be identified on the basis of variations in colonial morphology. The Campylobacter isolates recovered in this study are now being analyzed by ribotyping and pulsed-field gel electrophoresis to gain a better understanding of the population genetics of these organisms.
The rates of microbial contamination of retail meats with E. coli in this study ranged from 39% for chicken samples to 12% for turkey samples. The rates of E. coli contamination in the different retail meats were not as dissimilar as the rates observed for Campylobacter contamination. This may have been due to the frequent presence of E. coli in the animal production and food processing environments. In fact, all but three E. coli isolates identified in this study were negative for virulence-associated Shiga toxin or enterotoxin genes. This most likely indicates that the E. coli isolates identified were part of the normal enteric flora that is present in animals and often identified in food production, processing, and distribution environments. The absence of Shiga toxin-producing E. coli strains in the retail meats analyzed in this study is interesting. Several studies have shown that E. coli O157:H7 and other STEC are present in retail meat products, mostly beef products (5, 6, 9, 15, 31). It is likely that STEC could have been recovered from the meat samples tested if an enrichment procedure had been used in this study. However, the overall aim of our research was to investigate general E. coli contamination of retail meats. Also, our study was not designed to determine the levels of microbial contamination in retail meats; hence, our results might not reflect contamination levels.
The reported prevalence of Salmonella in retail meats varies widely in different countries. Salmonella is found less frequently in retail meats in developed countries, although as much as 36% of poultry meat samples were contaminated in a recent study in Belgium (35) and 43% of poultry meat samples were contaminated in a previous study in the United States (4). The rates of Salmonella contamination in pork and beef appear to be much lower, ranging from 0.8 to 10.4% in the United States (10, 32). The difference could be due in part to the types of samples analyzed (whole birds versus steaks; fresh versus frozen). The results of this study indicate that the rates of Salmonella contamination in retail meat samples were low, ranging from 1.9% for beef samples to 4.2% for chicken samples.
The Centers for Disease Control Foodborne Diseases Active Surveillance Network (FoodNet) data indicate that outbreaks and clusters of food-borne infections peak during the warmest months of the year (7). The reasons for this seasonal pattern are not known, but they may include (i) increased prevalence of the pathogens in cattle or other livestock or vehicles of transmission during the summer; (ii) greater human exposure to contaminated foods during the cook-out months; and/or (ii) more improper handling (e.g., temperature abuse) or incomplete cooking of products, such as ground beef, during warm months. Some studies also have shown that the rate of microbial contamination of food products follows the same trend (8, 13, 37). Our results did not provide a clear picture of a seasonality component of microbial contamination of retail meats. It does appear that more meat samples were positive for Campylobacter and E. coli contamination in some of the traditionally warmer months. However, no significant difference in microbial meat contamination was observed when data for warm and cold months were compared. In fact, the rates of Salmonella contamination were higher in cold months than in warm months. This may be explained by the fact that the Salmonella contamination rates in our study were too low to draw any statistically significant conclusions. The findings of this research suggest that future food safety studies focusing on seasonality components may require larger sample sizes and longer analysis periods. An interesting finding of the present study was that the rates of enteric organism contamination of retail meats, particularly chicken carcasses, were significantly different for the four supermarket chains, although all 59 stores of the four chains sold the same product brands. The possible explanations for this finding include differences in store handling practices, sampling times, and product batches. Most studies of retail meats have involved isolation and identification of multiple organisms in different products. We believe that our study was the first study in which the same retail meat samples were examined for Campylobacter, Salmonella, and E. coli contamination in the United States. In a recent study of microbial contamination of pork retail products, the researchers collected samples from six cities in the United States; however, no information concerning differences in store contamination rates in the six cities was given (10). In conclusion, we found that retail raw meats were often contaminated with Campylobacter and E. coli and less often contaminated with Salmonella. The contamination was dependent on the type of meat. Some retail meats were also contaminated with more than one food-borne pathogen. The presence of Campylobacter and Salmonella in retail meats remains a significant public health concern. Our data confirm that raw retail meats may be vehicles for transmitting food-borne diseases. To diminish Campylobacter, E. coli, and Salmonella contamination rates in retail meats, it is critical that risk reduction strategies are used throughout the food chain. These strategies include on-farm practices that reduce pathogen carriage, increased hygiene at both slaughter and meat processing, continued implementation of HACCP systems, and increased consumer education efforts. Additionally, consumption of undercooked meat products and cross-contamination during food handling and preparation must be avoided to ensure food safety at home and in the food service industry. Further research focusing on effective prevention of food-borne illness is essential for developing intervention and mitigation strategies to reduce the presence of food-borne bacterial pathogens at the retail level.
ACKNOWLEDGMENTS
We are indebted to Robert D. Walker from the Division of Animal and Food Microbiology, Center for Veterinary Medicine, U. S. Food and Drug Administration, for his assistance and comments during preparation of the manuscript.
This study was supported in part by a grant from the Maryland Agricultural Experimental Station.
REFERENCES
- 1.Atanassova V, Ring C. Prevalence of Campylobacter spp. in poultry and poultry meat in Germany. Int J Food Microbiol. 1999;51:187–190. doi: 10.1016/s0168-1605(99)00120-8. [DOI] [PubMed] [Google Scholar]
- 2.Baker R C, Paredes M D, Qureshi R A. Prevalence of Campylobacter jejuni in eggs and poultry meat in New York State. Poult Sci. 1987;66:1766–1770. doi: 10.3382/ps.0661766. [DOI] [PubMed] [Google Scholar]
- 3.Blaser M J. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis. 1997;176(Suppl. 2):S103–S105. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 4.Bokanyi R P, Jr, Stephens J F, Foster D N. Isolation and characterization of Salmonella from broiler carcasses or parts. Poult Sci. 1990;69:592–598. doi: 10.3382/ps.0690592. [DOI] [PubMed] [Google Scholar]
- 5.Bolton F J, Crozier L, Williamson J K. Isolation of Escherichia coli O157 from raw meat products. Lett Appl Microbiol. 1996;23:317–321. doi: 10.1111/j.1472-765x.1996.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 6.Brooks H J, Mollison B D, Bettelheim K A, Matejka K, Paterson K A, Ward V K. Occurrence and virulence factors of non-O157 Shiga toxin-producing Escherichia coli in retail meat in Dunedin, New Zealand. Lett Appl Microbiol. 2001;32:118–122. doi: 10.1046/j.1472-765x.2001.00868.x. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Preliminary FoodNet data on the incidence of foodborne illnesses–selected sites, United States, 2000. Morb Mortal Wkly Rep. 2001;50:241–246. [PubMed] [Google Scholar]
- 8.Chapman P A, Cerdan Malo A T, Ellin M, Ashton R, Harkin Escherichia coli O157 in cattle and sheep at slaughter, on beef and lamb carcasses and in raw beef and lamb products in South Yorkshire, UK. Int J Food Microbiol. 2001;64:139–150. doi: 10.1016/s0168-1605(00)00453-0. [DOI] [PubMed] [Google Scholar]
- 9.Doyle M P, Schoeni J L. Isolation of Escherichia coli O157:H7 from retail fresh meats and poultry. Appl Environ Microbiol. 1987;53:2394–2396. doi: 10.1128/aem.53.10.2394-2396.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffy E A, Belk K E, Sofos J N, Bellinger G R, Pape A, Smith G C. Extent of microbial contamination in United States pork retail products. J Food Prot. 2001;64:172–178. doi: 10.4315/0362-028x-64.2.172. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Bacteriological analytical manual. 8th ed. 1998. (rev. A). AOAC International, Gaithersburg, Md. [Google Scholar]
- 12.Griffin P M. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 739–761. [Google Scholar]
- 13.Hanninen M L, Perko-Makela P, Pitkala A, Rautelin H. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J Clin Microbiol. 2000;38:1998–2000. doi: 10.1128/jcm.38.5.1998-2000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hariharan H, Wright T, Long J R. Isolation and antimicrobial susceptibility of Campylobacter coli and Campylobacter jejuni from slaughter hogs. Microbiologica. 1990;13:1–6. [PubMed] [Google Scholar]
- 15.Heuvelink A E, Wernars K, De Boer E. Occurrence of Escherichia coli O157 and other verocytotoxin-producing E. coli in retail raw meats in the Netherlands. J Food Prot. 1996;59:1267–1272. doi: 10.4315/0362-028X-59.12.1267. [DOI] [PubMed] [Google Scholar]
- 16.Hudson J A, Nicol C, Wright J, Whyte R, Hasell S K. Seasonal variation of Campylobacter types from human cases, veterinary cases, raw chicken, milk and water. J Appl Microbiol. 1999;87:115–124. doi: 10.1046/j.1365-2672.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 17.Jackson C J, Fox A J, Jones D M. A novel polymerase chain reaction assay for the detection and speciation of thermophilic Campylobacter spp. J Appl Bacteriol. 1996;81:467–473. doi: 10.1111/j.1365-2672.1996.tb03534.x. [DOI] [PubMed] [Google Scholar]
- 18.Korsak N, Daube G, Ghafir Y, Chahed A, Jolly S, Vindevogel H. An efficient sampling technique used to detect four foodborne pathogens on pork and beef carcasses in nine Belgian abattoirs. J Food Prot. 1998;61:535–541. doi: 10.4315/0362-028x-61.5.535. [DOI] [PubMed] [Google Scholar]
- 19.Kramer J M, Frost J A, Bolton F J, Wareing D R. Campylobacter contamination of raw meat and poultry at retail sale: identification of multiple types and comparison with isolates from human infection. J Food Prot. 2000;63:1654–1659. doi: 10.4315/0362-028x-63.12.1654. [DOI] [PubMed] [Google Scholar]
- 20.Linton D, Lawson A J, Owen R J, Stanley J. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J Clin Microbiol. 1997;35:2568–2572. doi: 10.1128/jcm.35.10.2568-2572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lortie L A, Dubreuil J D, Harel J. Characterization of Escherichia coli strains producing heat-stable enterotoxin b (STb) isolated from humans with diarrhea. J Clin Microbiol. 1991;29:656–659. doi: 10.1128/jcm.29.3.656-659.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madden R H, Moran L, Scates P. Frequency of occurrence of Campylobacter spp. in red meats and poultry in Northern Ireland and their subsequent subtyping using polymerase chain reaction-restriction fragment length polymorphism and the random amplified polymorphic DNA method. J Appl Microbiol. 1998;84:703–708. doi: 10.1046/j.1365-2672.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 23.Mead P S, Slutsker L, Dietz V, McCaig L F, Bresee J S, Shapiro C, Griffin P M, Tauxe R V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng J, Doyle M P. Emerging and evolving microbial foodborne pathogens. Bull Inst Pasteur. 1998;96:151–164. [Google Scholar]
- 25.Meng J, Zhao S, Doyle M, Mitchell S, Kresovich S. A multiplex PCR for identifying Shiga-like toxin-producing Escherichia coli O157:H7. Lett Appl Microbiol. 1997;24:172–176. doi: 10.1046/j.1472-765x.1997.00375.x. [DOI] [PubMed] [Google Scholar]
- 26.Meng J, Zhao S, Doyle M P. Virulence genes of Shiga toxin-producing Escherichia coli isolated from food, animals and humans. Int J Food Microbiol. 1998;45:229–235. doi: 10.1016/s0168-1605(98)00163-9. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen E M, Nielsen N L. Serotypes and typability of Campylobacter jejuni and Campylobacter coli isolated from poultry products. Int J Food Microbiol. 1999;46:199–205. doi: 10.1016/s0168-1605(98)00194-9. [DOI] [PubMed] [Google Scholar]
- 28.Ono K, Yamamoto K. Contamination of meat with Campylobacter jejuni in Saitama, Japan. Int J Food Microbiol. 1999;47:211–219. doi: 10.1016/s0168-1605(99)00015-x. [DOI] [PubMed] [Google Scholar]
- 29.Osano O, Arimi S M. Retail poultry and beef as sources of Campylobacter jejuni. East Afr Med J. 1999;76:141–143. [PubMed] [Google Scholar]
- 30.Petersen K E, James W O. Agents, vehicles, and causal inference in bacterial foodborne disease outbreaks: 82 reports (1986–1995) J Am Vet Med Assoc. 1998;212:1874–1881. [PubMed] [Google Scholar]
- 31.Samadpour M, Ongerth J E, Liston J, Tran N, Nguyen D, Whittam T S, Wilson R A, Tarr P I. Occurrence of Shiga-like toxin-producing Escherichia coli in retail fresh seafood, beef, lamb, pork, and poultry from grocery stores in Seattle, Washington. Appl Environ Microbiol. 1994;60:1038–1040. doi: 10.1128/aem.60.3.1038-1040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sofos J N, Kochevar S L, Reagan J O, Smith G C. Incidence of Salmonella on beef carcasses relating to the U.S. meat and poultry inspection regulations. J Food Prot. 1999;62:467–473. doi: 10.4315/0362-028x-62.5.467. [DOI] [PubMed] [Google Scholar]
- 33.Tauxe R V. Emerging foodborne diseases: an evolving public health challenge. Emerg Infect Dis. 1997;3:425–434. doi: 10.3201/eid0304.970403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todd E C. Epidemiology of foodborne diseases: a worldwide review. World Health Stat Q. 1997;50:30–50. [PubMed] [Google Scholar]
- 35.Uyttendaele M, De Troy P, Debevere J. Incidence of Salmonella, Campylobacter jejuni, Campylobacter coli, and Listeria monocytogenes in poultry carcasses and different types of poultry products for sale on the Belgian retail market. J Food Prot. 1999;62:735–740. doi: 10.4315/0362-028x-62.7.735. [DOI] [PubMed] [Google Scholar]
- 36.Victor T, du Toit R, van Zyl J, Bester A J, van Helden P D. Improved method for the routine identification of toxigenic Escherichia coli by DNA amplification of a conserved region of the heat-labile toxin A subunit. J Clin Microbiol. 1991;29:158–161. doi: 10.1128/jcm.29.1.158-161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willis W L, Murray C. Campylobacter jejuni seasonal recovery observations of retail market broilers. Poult Sci. 1997;76:314–317. doi: 10.1093/ps/76.2.314. [DOI] [PubMed] [Google Scholar]