Abstract
Phytonutrients may play important roles in human health and yet only recently a few studies have described phytonutrient consumption patterns, using data obtained from daily consumption methods. We aimed to estimate the phytonutrient content in Taiwanese diets and analyzed main food sources of 10 major phytonutrients. In this study, food items and dietary data gathered with the 24-hour dietary recall from 2908 participants in the 2005–2008 Nutrition and Health Survey in Taiwan were used to create a food phytonutrient database with 933 plant-based foods through integrating database, literature search, and chemical analysis and to appraise phytonutrient consumption status of participants. SUDAAN (Survey Data Analysis) was used for generating weighted phytonutrient intake estimates and for statistical testing. In Taiwanese adults, ~20% met the recommended number of servings for fruits and 30% met that for vegetables from the Taiwan Food-Guide recommendations. However, only 7.4% consumed the recommended numbers for both fruits and vegetables. Those meeting the recommendations tended to be older and with more females compared with those who did not. Phytonutrient intake levels were higher in meeters than nonmeeters. More than 60% of α-carotene, lycopene, hesperetin, epigallocatechin 3-gallate, and isoflavones came from a single phytonutrient-specific food source. In addition, sweet potato leaf, spinach, and water spinach were among the top three sources of multiple phytonutrients. Cross-comparison between this study and two previous studies with similar methodology showed higher mean levels of lycopene and quercetin in the United States, anthocyanidins in Korea, and lutein and zeaxanthin in Taiwan. The Taiwanese phytonutrient pattern is different from that of the Korean and American. It would be interesting to relate phytonutrient patterns to health profiles in the future.
Keywords: 24-hour recall, database, pattern, phytonutrient, Taiwan
1. Introduction
Encouraging increase in vegetable and fruit consumption is among the top ranking health promotion policies. Vegetables and fruits contain a large number of phytonutrients in addition to their rich contents of vitamins, minerals, and dietary fiber. A large body of research has demonstrated that increased intake of fruits and vegetables lowers the risk of noncommunicable diseases such as cardiovascular disease, stroke, and cancer [1–3].
Among a large numbers of phytonutrients; carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, lycopene), flavonoids (anthocyanidins, epigallocatechin 3-gallate, hesperetin, quercetin), and isoflavones are more extensively studied and profiled for plant foods. Laboratory studies showed that almost all phytonutrients have anti-oxidative and anti-inflammatory effects. Their functions also include immune function modulation [4], hormonal regulation [5], antibacterial and antiviral effects [6,7], signal transduction [8], and nerve conduction [9]. Epidemiologic evidences point to their potential roles in reducing risk of various chronic diseases. For example, low blood concentrations of carotenoids have been associated with cardiovascular disease and cancer [10,11]; lutein and zeaxanthin with bone health [12], polyarthritis [13] and age related muscular degenerative diseases [14–16]; lycopene with prostate cancer [17,18]. Anthocyanidins and epigallocatechin 3-gallate (EGCG) has been associated with cognitive impairment [19,20], Alzheimer’s disease [21], and lung cancer [22]. Greater intake of hesperetin has been associated with decreased incidence of cerebrovascular disease and asthma [23]. Providing quercetin has helped reduce low density lipoprotein (LDL)-cholesterol and blood pressure levels [24] and prevent bone loss [5]. Isoflavones have estrogen-like and antioxidant effects, which have been associated with reduced risk of lung cancer [25,26] and breast cancer.
Vegetables, fruits, and some other plant foods are major food sources of phytonutrients. These phytonutrients contributing to the different colors of various vegetables and fruits are often grouped into five-colored groups such as: green, red, white, purple/blue, and yellow/orange. According to the Taiwan Food-Guide recommendations, people are advised to consume three to five servings of vegetables and two to four servings of fruit per day depending on their total caloric intake levels from 1200 Kcal to 2700 Kcal. Although general understanding is that intake of vegetables and fruits often fall short of the Food-Guide recommendations, data is lacking on phytonutrient intakes in Taiwan as well as in many parts of the world at the present time. Therefore, we used data from the 2005–2008 Nutrition and Health Survey in Taiwan to estimate the proportion of people with lower than Food-Guide recommendations for vegetables and fruits and intake levels of the 10 above-mentioned phytonutrients and to rank the main food sources of each phytonutrient. Finally, a comparison of phytonutrient intake patterns was made between estimates of Taiwanese and those previously reported for Korea [27] and for the United States (US) [28].
2. Methods
2.1. Nutrition and health survey in Taiwan 2005–2008
Dietary data for this study was taken from the Nutrition and Health Survey in Taiwan (NAHSIT) 2005–2008 for which the first author is the principle investigator. The survey adopted a multi-staged sampling scheme. The 358 counties and city districts in Taiwan were divided into five strata based on geographical location. In each stratum, probability–proportional-to-size method was used to select townships or city districts. Within each township or district, cluster sampling in two locations was carried out to generate sample lists. Information on socio-demographics, lifestyle, 24-hour dietary recall, and health-related questionnaires were gathered within households along with health examination in the temporarily established clinics. The survey details have been published elsewhere [29]. The Institutional Review Board from Academia Sinica, Taipei, Taiwan approved the study protocol, informed consent forms, and the questionnaires and every participant provided signed informed consent. We specifically analyzed the 24-hour dietary recall data from 2908 participants aged 19 years and older who had completed the dietary assessment.
2.2. Dietary data collected by 24-hour recall
Interviewers collected information through face-to-face interviews regarding foods consumed by participants in the past 24 hours. Interviewers used specifically designed food-piece models (for dishes containing chopped, sliced, or shredded foods), multiple hollow hemisphere models (for round-shaped foods) for Taiwanese, and other standard cooking measures to assist participants in providing information regarding the quantity of foods consumed [30]. Mixed dishes, e.g., the stir-fries were composed by interviewees with food piece models which were then disaggregated and weighed separately. The real weights of foods were estimated from the food model data using established polynomial equations between model measures and real food weights as described elsewhere [30]. The Nutrient Composition Database for Foods in Taiwan was used to estimate participants’ calorie and nutrient intakes.
2.3. Establishing the phytonutrient content table
A total of 2412 food items were consumed by 2908 participants in the 24-hour recall. After removing items with very low phytonutrients such as meat, sea foods, etc., there were 933 food items left in the categories of grains, tubers, vegetables, fruits, nuts and seeds, beans, soy-products, and tea. Phytonutrient supplements were not considered in this study. A phytonutrient content table was created for these 933 plant-based food items commonly consumed by Taiwanese people (Taiwan Phytonutrient Database, Taipei, Taiwan, https://goo.gl/sqNWdQ). For these food items, we combined phytonutrient data from databases of the Food Industry Research Development Institutes, Taiwan [31]; United States Department of Agriculture [32–34]; and Korea [27]; as well as those provided in the relevant literature [35–50]. In addition, we sent 172 Taiwanese commonly consumed plant foods to AVRDC – The World Vegetable Center, Taiwan to examine their phytonutrient contents.
Fresh samples were sent within one day of purchase to the laboratory. All food samples were freeze-dried, ground into fine powder, and stored at −80°C subjected to analysis. Eighteen compounds were selected and classified to four groups based on high performance liquid chromatography (HPLC) methods. The phytonutrient groups included carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, lycopene), isoflavones (daidzein, glycitein, and genistein), anthocyanidins (delphinidin, cyanidin, petunidin, pelargonidin, peonidin, and malvidin), and other flavonoids (epigallocatechin 3-gallate-EGCG, hesperetin, and quercetin).
Carotenoids were extracted with 80% acetone in water, and the compounds were profiled with C30 column (YMC™, 3.0 μm, 4.6 × 150 mm, Waters, Milford, MA, USA) equipped with HPLC system (Waters Alliance 2695 and PDA 996, Milford, MA, USA). Isoflavones were extracted with 70% ethanol from defatted fine powder samples and compounds were separated with RP-18 column (LiChroCART, 4.0 × 250 mm, Munchen, Bavaria, Germany) with the same HPLC system mentioned above. Anthocyanidins were extracted with 3.7% acetic acid and 50% methanol in water, hydrolyzed in 1.2 M HCl in 50% methanol at 90°C water bath for two hours, and the aglycones were separated with RP-18 column (Agilent ZORBAX ODS, C18, 4.6 × 150 mm, 3.5 μm, Santa Clara, California, USA). Other flavonoids were extracted with 1% formic acid and 80% methanol in water from powder samples, hydrolyzed under the same condition mentioned above, and the aglycones were separated with RP-18 column (Agilent Zorbax ODS, SB-C18, 4.6 × 150 mm, 3.5 μm). All compounds were identified by comparing with commercially available and HPLC-grade standards. Concentration was measured through the calibration of each standard, and adjusted based on recovery tests. Detail methods for carotenoids and flavonoids were described in previous reports [51,52].
2.4. Phytonutrient intake estimation and major food sources
Phytonutrient intakes were estimated by multiplying the amount and phytonutrient density of each food. Then intakes from different food sources were summed to obtain total phytonutrient intake. Major food sources were derived by ranking the mean weight of phytonutrients provided by each food item in an averaged Taiwanese diet. We also estimated the phytonutrient density by dividing individual phytonutrient intake level with his or her caloric level and multiplying with 2000 Kcal.
2.5. Phytonutrient intakes by fruit and vegetable consumption status
The Taiwan Food-Guide provides recommendations on number of servings for vegetables and fruits per day, based on caloric levels [53]. The Food-Guide recommends an intake of minimum three servings of vegetables and at least two servings of fruits for caloric levels from 1200 to 2700. The recommended number of servings of vegetables and fruits increases with calories. When daily intake is ~2700 kilocalories, five servings of vegetables and four servings of fruit are recommended. In this study, the caloric intake category that most closely corresponded to a participant’s caloric intake was used to estimate whether one has taken more than the recommended number of servings or not. Participants were then grouped into those meeting and those not meeting the recommendations.
2.6. Statistical analysis
SUDAAN (version 11.01, 2012, Research Triangle Institute) was used for generating standard errors and for the statistical testing, since NAHSIT adopted a complex sampling scheme. The Chi-square test was used to compare the proportions meeting recommended numbers of vegetables and fruits and also to compare differences in sex distribution between meeters and nonmeeters. The t test was used to compare age and body mass index (BMI) between groups. Due to severe skewness in all phytonutrient distributions, Wilcoxon rank sum test conducted by SAS (version 9.4, 2013, SAS Institute Inc.) was used to compare phytonutrient intake density level between meeters and nonmeeters.
3. Results
Table 1 shows by sex and age groups the percentage of participants consuming the recommended number of servings of vegetables and fruits. In adults, ~19.5% met the recommended servings for fruits and 30.8% met that for vegetables. However, only 7.4% consumed the recommended number of servings for both fruits and vegetables. Proportion of women who followed the recommendations was significantly higher than that of men. The percentage of men consuming the recommended servings of fruits and vegetables increased with age. In men who were aged 65 years and over, 10.9% of them were meeters of both fruit and vegetable recommendations, which represented the highest percentage in all three age groups. However, in women, the highest percentage was observed in the 45–64 year group. If looking at whether the total combined number of servings for fruit and vegetables was met, not considering their relative proportion; ~20% of men and 29% of women reached the recommendation.
Table 1.
Age and sex-specific percentages of participants with intakes of fruits and vegetables at or above recommended levels, NAHSIT, 2005–2008, N = 2908.
| Sex | Age group (y) | Weighted proportion of those meeting recommended no. of servings (%) | |||
|---|---|---|---|---|---|
|
| |||||
| For fruitsa | For vegetablesb | For fruits & for vegetablesc | For fruits & vegetables combinedd | ||
| Men | 19–44 | 13.5 | 23.5 | 5.0 | 14.7 |
| 45–64 | 21.8 | 32.1 | 6.5 | 25.1 | |
| 65+ | 23.5 | 36.4 | 10.9 | 33.5 | |
| All ages | 17.4 | 27.8 | 6.2 | 20.4 | |
| Women | 19–44 | 17.8 | 26.9 | 4.2 | 23.1 |
| 45–64 | 30.0 | 43.5 | 15.6 | 40.3 | |
| 65+ | 17.1 | 39.2 | 9.9 | 28.8 | |
| All ages | 21.6 | 33.8 | 8.6 | 29.3 | |
| All | 19–44 | 15.6 | 25.2 | 4.6 | 18.9 |
| 45–64 | 25.9 | 37.8 | 11.1 | 32.8 | |
| 65+ | 20.2 | 37.8 | 10.4 | 31.1 | |
| All ages | 19.5 | 30.8 | 7.4 | 24.8 | |
NAHSIT = Nutrition and Health Survey in Taiwan.
Proportion of people whose fruit consumption meeting the recommendation of his/her energy intake level.
Proportion of people whose vegetable consumption meeting the recommendation of his/her energy intake level.
Proportion of people whose vegetable consumption meeting the recommendation and fruit consumption also meeting the recommendation of his/her energy intake level.
Proportion of people whose consumption exchange number for vegetable and that for fruit combined is equal or beyond the sum of vegetable and fruit recommended numbers at his/her energy intake level.
Comparison of characteristics between those meeting and those not meeting the recommendations (Table 2) showed that there were significantly more women in meeters. In addition, either men or women who met the recommendations were significantly older than those who did not. No statistically significant difference in BMI was observed between the meeters and nonmeeters.
Table 2.
Comparison of sex, age, and BMI between those meeting and those not meeting recommended numbers of servings for fruit and vegetables.
| Meeters (n = 262) | Nonmeeters (n = 2646) | p | |
|---|---|---|---|
| Sex (%) | |||
| Male | 42.2 | 50.9 | 0.004b |
| Female | 57.8 | 49.1 | |
| Age (y) | |||
| Men | 49.8 ± 2.0 | 43.1 ± 0.4 | <0.001c |
| Women | 51.2 ± 1.2 | 43.3 ± 0.4 | <0.001 |
| Total | 50.6 ± 1.2 | 43.2 ± 0.2 | <0.001 |
| BMIa | |||
| Men | 24.8 ± 0.7 | 24.2 ± 0.2 | NSc,d |
| Women | 23.5 ± 0.5 | 23.3 ± 0.2 | NS |
| Total | 24.1 ± 0.5 | 23.7 ± 0.1 | NS |
Data are presented as % or mean ± standard error.
BMI = body mass index.
Sample size was 175 meeters and 1477 nonmeeters for BMI, because some participants did not attend physical examination.
Chi-square test.
t test.
NS (Not statistically significantly different) for both BMI and log-transformed BMI.
Comparisons of phytonutrient intakes between meeters and nonmeeters were made separately in men and women. Phytonutrient intakes were calibrated to a 2000 kilocalorie dietary intake to avoid the influence of caloric intakes on comparisons. The Wilcoxon rank sum test showed that densities of almost all phytonutrients were significantly higher in those meeting compared with those not meeting recommendations except for EGCG (Table 3). Mean EGCG was lower in meeters than in nonmeeters. But median in meeters was higher in women (not in men) than the counterpart.
Table 3.
Mean phytonutrient densitya levels per 2000 kcal in those meeting and those not meeting fruit and vegetable recommendations.
| Nutrients (Mean ± SE) | Male | Female | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Meeters (n = 109) | Nonmeeters (n = 1337) | p a | Meeters (n = 153) | Nonmeeters (n = 1309) | p b | |
| ➝ | ➝ | |||||
| Energy (kcal) | 2508 ± 233 | 2262 ± 40 | 1758 ± 61 | 1670 ± 42 | ||
| Adjustedb | Adjustedb | |||||
| Carotenoids (μg) | ||||||
| α-Carotene | 1238 ± 329 (381) | 450 ± 69 (48) | <0.001 | 932 ± 158 (372) | 520 ± 41 (88) | <0.001 |
| β-Carotene | 9678 ± 1027 (7079) | 3459 ± 172 (1548) | <0.001 | 10829 ± 1148 (7658) | 4870 ± 247 (2565) | <0.001 |
| β-Cryptoxanthin | 351 ± 70 (107) | 108 ± 14 (5) | <0.001 | 403 ± 89 (188) | 154 ± 17 (15) | <0.001 |
| Lutein/zeaxanthin | 18517 ± 2461 (8914) | 6309 ± 386 (1175) | <0.001 | 22416 ± 2645 (12621) | 9546 ± 682 (1720) | <0.001 |
| Lycopene | 2299 ± 823 (1356c) | 1466 ± 181 (0c) | <0.001 | 2957 ± 709 (3365c) | 1777 ± 262 (0c) | <0.001 |
| Flavonoids (mg) | ||||||
| Anthocyanidins | 13 ± 2 (4) | 5 ± 1 (0) | <0.001 | 19 ± 4 (9) | 6 ± 1 (0) | <0.001 |
| Hesperetin | 18 ± 4 (12c) | 5 ± 1 (0c) | <0.001 | 20 ± 7 (14c) | 7 ± 2 (0c) | <0.001 |
| Quercetin | 18 ± 2 (13) | 15 ± 3 (7) | <0.001 | 17 ± 2 (10) | 11 ± 1 (5) | <0.001 |
| EGCG | 198 ± 57 (0.1) | 294 ± 86 (0.5) | <0.001 | 68 ± 15 (0.1) | 127 ± 13 (0) | <0.001 |
| Isoflavones (mg) | 16 ± 3 (1) | 14 ± 1 (1) | <0.001 | 19 ± 3 (3) | 15 ± 1 (0.2) | <0.001 |
Data are presented as mean ± standard error or mean ± standard error (median).
EGCG = epigallocatechin 3-gallate.
Phytonutrient/caloric intake × 2000 kilocalories.
Wilcoxon rank-sum test was used to compare phytonutrient intakes between those meeting and not meeting recommended fruit and vegetable intakes.
75 percentile value is shown because the median for both meeters and nonmeeters is zero.
The top five food sources for each phytonutrient are shown in Table 4. More than 60% intake of α-carotene, lycopene, hesperetin, EGCG, and isoflavones were provided by a single phytonutrient-specific food item. For seven phytonutrients: α-carotene, β-cryptoxanthin, lycopene, anthocyanidins, hesperetin, EGCG, and isoflavones, the top five food sources listed provided >80% of the phytonutrients. This shows that the above seven phytonutrients are found in a limited number of fruits, vegetables, or other plant foods. By contrast, a lower percentage intake of β-carotene (58.7%), lutein/zeaxanthin (74.1%), and quercetin (77.4%) are provided by the top five foods. Table 4 also pinpoints some foods ranked among the top five sources for several different phytonutrients. For example, sweet potato leaf was an important source of five phytonutrients: lutein/zeaxanthin and, β-carotene, quercetin, β-cryptoxanthin, and α-carotene in that order, providing 4.1–36.1% of the intake of these phytonutrients. The most commonly appeared food sources of the listed phytonutrients were the sweet potato leaf (for five phytonutrients), spinach (for three phytonutrients), and water spinach (for three phytonutrients).
Table 4.
Percentage contribution of top five food sources for each phytonutrient.
| α-carotene | β-carotene | β-cryptoxanthin | Lutein/zeaxanthin | Lycopene | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||
| Food | Contribution (%) | Food | Contribution (%) | Food | Contribution (%) | Food | Contribution (%) | Food | Contribution (%) |
| Carrot and related products | 62.9 | Sweet potato leaf | 19.8 | Mandarin oranges & related products | 39.1 | Sweet potato leaf | 36.1 | Tomato & related products | 63.1 |
| Pumpkin | 11.9 | Carrot & related products | 14.3 | Papaya & related products | 18.7 | Spinach | 16.9 | Watermelon & related products | 31.0 |
| Seaweed | 7.5 | Spinach | 11.0 | Oranges & related products | 12.0 | Water spinach | 13.7 | Papaya & related products | 4.8 |
| Sweet potato leaf | 4.1 | Tomato & related products | 9.3 | Sweet potato leaf | 8.0 | Seaweed | 4.8 | Grapefruit & related products | 1.0 |
| Leek | 2.0 | Water spinach | 4.2 | Watermelon & related products | 6.4 | Kale | 2.6 | Plums | 0.05 |
|
| |||||||||
| Cumulative contribution | 88.4 | Cumulative contribution | 58.7 | Cumulative contribution | 84.1 | Cumulative contribution | 74.1 | Cumulative contribution | 100.0 |
|
| |||||||||
| Anthocyanidins | Hesperetin | Quercetin | EGCG | Isoflavones | |||||
|
| |||||||||
| Food | Contribution (%) | Food | Contribution (%) | Food | Contribution (%) | Food | Contribution (%) | Food | Contribution (%) |
|
| |||||||||
| Radishes | 40.7 | Oranges & related products | 73.7 | Tea | 54.5 | Tea | 99.97 | Soybean & related products | 92.4 |
| Bananas | 17.9 | Mandarin oranges & related products | 16.8 | Sweet potato leaf | 9.7 | Peaches | 0.009 | Vegetarian meat products | 6.9 |
| Grapes and related products | 12.2 | Lemons & related products | 8.9 | Spinach | 5.5 | Apples | 0.008 | Bean sprouts | 0.6 |
| Strawberries | 10.8 | Mixed juice | 0.5 | Water spinach | 3.9 | Onion | 0.003 | Brown rice | 0.01 |
| Eggplant | 7.9 | Grapefruit & related products | 0.1 | Nashi pears | 3.8 | Strawberries | 0.002 | Alfalfa sprouts | 0.01 |
|
| |||||||||
| Cumulative contribution | 89.5 | Cumulative contribution | 99.9 | Cumulative contribution | 77.4 | Cumulative contribution | 100.0 | Cumulative contribution | 99.9 |
EGCG = epigallocatechin 3-gallate.
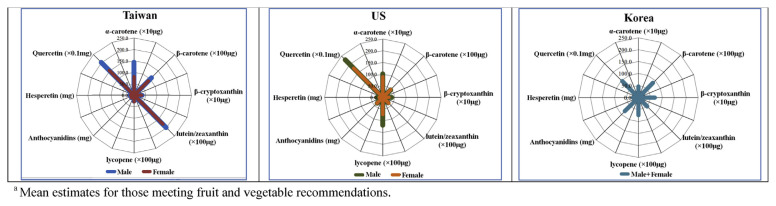
Phytonutrient intake patterns were compared between data of the vegetable and fruit meeters from this study (NAHSIT), and those reported in the literature from the US (NHANES) [28] and Korea (KNHANES) [27] (not statistically tested). Figure 1 provides radar maps established using estimated population means of each phytonutrient, which shows that mean intake of α-carotene, β-carotene, and lutein/zeaxanthin was higher in Taiwan than in the US and Korea. Mean lycopene was in the descending order from the US, Korea, to Taiwan. Quercetin was descending from US, Taiwan, to Korea. In addition, mean intake of anthocyanidins was descending from Korea, US, to Taiwan. Overall, three countries have unique phytonutrient patterns.
Figure 1.
Comparison of phytonutrient intake a patterns between Taiwan, the United States (US), and Korea.
4. Discussion
This is one of the three studies (from US, Korea, and Taiwan) examining the phytonutrient intake status using daily consumption data derived from 24-hour recall. In this study, we not only profiled the phytonutrient intake status in Taiwanese adult men and women during 2005–2008, but established a phytonutrient database through integrating existing databases, searching literature, and carrying out chemical analysis for Asian/Taiwanese plant foods. There were 172 newly analyzed plant foods in this database.
We found that women and elders tend to approach the Taiwan Food-Guide recommendations for vegetables and fruits more so than their male and younger counterparts. These findings are consistent with those of the US [28] and Korea [27] using the same dietary method. More subtle but a similar gender difference was seen in the World Health Survey carried out in 13 regions of the World [54], which employed semiquantitative food frequency data to estimate numbers of vegetables and fruits consumed per day, used food balance sheet data to estimate phytonutrient concentrations in vegetables and in fruits, and weighted into mean phytonutrient estimates.
As expected, in our study, phytonutrient intakes were significantly higher in those meeting recommended intakes of fruit and vegetables compared with their counterpart. But for EGCG, a prominent phytonutrient in tea, the mean was lower in meeters than nonmeeters and the direction of the median is opposite for women, potentially due to its very extreme skewness in distribution and poor association between tea drinking and vegetable/fruit consumption.
Comparison between Taiwan, the US, and Korea showed that around 20% of Taiwanese adults met recommended number of servings for fruits, which is slightly lower than that observed in the US (21%) and Korea (23.5%) [27,55]. In addition, 31% of people in Taiwan met recommended number of servings for vegetables, which is higher than that in the US (22%) and Korea (21.4%). Overall speaking, Taiwanese vegetable and fruit consumption status (7.4% meeting recommendation) is slightly better than that of the US (6%) and Korea (5.3%).
The radar charts of phytonutrient consumption patterns in Taiwan, the US, and Korea show distinct phytonutrient consumption patterns, with relatively higher mean values of lycopene and quercetin in US population, anthrocyanidin in Korean, and lutein and zeaxanthin in Taiwanese. Although all of these phytonutrients are known to be involved in anti-oxidative and anti-inflammatory reactions in human body, their specific or unique roles are not fully elucidated. Future research should investigate whether differences in disease rates between these countries are related to these variations in phytonutrient intake patterns.
The mean intake of lutein/zeaxanthin was higher in Taiwan compared with the other two countries [27,28]. Examination of phytonutrient source foods shows that sweet potato leaf, water spinach and spinach, key contributors of lutein/zeaxanthin, are commonly consumed vegetable sources of several phytonutrients in Taiwan (Table S1). These vegetables do not appear as phytonutrient sources in the US or Korean reports. By contrast, phytonutrients from red or yellow/orange colored foods, such as: hesperetin, lycopene, were lower in Taiwan. This could be due to less consumption of tomatoes and carrots in Taiwan where stir-frying green leafy vegetables is a common practice. In addition, mean intakes of EGCG (data from this study) and isoflavones [56] are high in Taiwan compared with the US probably due to a cultural preference of tea and soy products. Although we do not know whether it is absolutely necessary to consume all five color phytonutrients in certain proportions; as it is generally accepted to consume a wide variety of foods, Taiwanese people may consider increasing their consumption of red and yellow/orange colored fruits and vegetables.
Similar to the findings of the US and Korea, major plant food contributors of phytonutrients are limited in numbers, indicating relatively low diversity in plant food consumption in each geographic region. However; because carrot, sweet potato leaf, mandarin orange, tomato, radish, orange, tea, soy-products are key providers, respectively, of α-carotene, β-carotene/lutein and zeaxanthin, β-cryptoxanthin, lycopene, anthrocyanidin, hesperetin, EGCG/quercetin, and isoflavones. Low intake of some of these foods may be used as markers of low level of the corresponding phytonutrients for the Taiwanese.
This study has several limitations. Similar to the US and Korean studies, we used 24-hour dietary recall method to collect dietary data, which has considerable day-to-day variations. Conclusions made on proportions should be interpreted with cautions. Future research may consider measuring intake for multiple days or to adjust for daily variations. In addition, we only examined the intake of 10 phytonutrients, because data are lacking for other less consumed phytonutrients. Future research shall compile a list of major phytonutrients for vegetables and fruits of different colors as well as expand on the number of phytonutrients examined. Consensus shall be made in the future on the correspondence between phytonutrients and five colored groups. Future research in this area is urgently needed to better understand the relationship between phytonutrient profiles, plant food color grouping, and long-term health in order to improve quality of life of humans.
In this study, we found that the majority of Taiwanese people did not consume vegetables and fruits to the recommended levels of Taiwan Food-Guide. However, the proportion of people fulfilling the recommendation is close to the estimates of US and Korea. With the exception of β-carotene and lutein/zeaxanthin, most phytonutrients are provided from a few major sources including some local plant foods such as sweet potato leaf and water spinach. Taiwan, Korea, or the US have their own unique phytonutrient profile, probably influenced by weather, plantations, and food cultures.
Acknowledgments
This study was supported by Ministry of Health and Welfare through a grant (DOH94-FS-6-4) supporting Nutrition and Health Survey in Taiwan and a grant from the Nutrilite Health Institute (103A30203) for phytonutrient analysis.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2016.12.015.
Funding Statement
This study was supported by Ministry of Health and Welfare through a grant (DOH94-FS-6-4) supporting Nutrition and Health Survey in Taiwan and a grant from the Nutrilite Health Institute (103A30203) for phytonutrient analysis.
Footnotes
Conflicts of interest
No potential conflict of interest was reported by the authors.
REFERENCES
- 1. Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr. 2003;78(Suppl):S544–51. doi: 10.1093/ajcn/78.3.544S. [DOI] [PubMed] [Google Scholar]
- 2. Boffetta P, Couto E, Wichmann J, Ferrari P, Trichopoulos D, Bueno-de-Mesquita HB, van Duijnhoven FJ, Buchner FL, Key T, Boeing H, Nothlings U, Linseisen J, Gonzalez CA, Overvad K, Nielsen MR, Tjonneland A, Olsen A, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Lagiou P, Naska A, Benetou V, Kaaks R, Rohrmann S, Panico S, Sieri S, Vineis P, Palli D, van Gils CH, Peeters PH, Lund E, Brustad M, Engeset D, Huerta JM, Rodriguez L, Sanchez MJ, Dorronsoro M, Barricarte A, Hallmans G, Johansson I, Manjer J, Sonestedt E, Allen NE, Bingham S, Khaw KT, Slimani N, Jenab M, Mouw T, Norat T, Riboli E, Trichopoulou A. Fruit and vegetable intake and overall cancer risk in the European prospective investigation into cancer and nutrition (EPIC) J Natl Cancer Inst. 2010;102:529–37. doi: 10.1093/jnci/djq072. [DOI] [PubMed] [Google Scholar]
- 3. He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367:320–6. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 4. Santos MS, Gaziano JM, Leka LS, Beharka AA, Hennekens CH, Meydani SN. Beta-carotene-induced enhancement of natural killer cell activity in elderly men: an investigation of the role of cytokines. Am J Clin Nutr. 1998;68:164–70. doi: 10.1093/ajcn/68.1.164. [DOI] [PubMed] [Google Scholar]
- 5. Son YO, Kook SH, Choi KC, Jang YS, Choi YS, Jeon YM, Kim JG, Hwang HS, Lee JC. Quercetin accelerates TNF-alpha-induced apoptosis of MC3T3-E1 osteoblastic cells through caspase-dependent and JNK-mediated pathways. Eur J Pharmacol. 2008;579:26–33. doi: 10.1016/j.ejphar.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 6. Bhat RS, Al-Daihan S. Phytochemical constituents and antibacterial activity of some green leafy vegetables. Asian Pac J Trop Biomed. 2014;4:189–93. doi: 10.1016/S2221-1691(14)60230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ozcelik B, Kartal M, Orhan I. Cytotoxicity, antiviral, and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm Biol. 2011;49:396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- 8. Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarkozi K, Papp A, Mate Z, Horvath E, Paulik E, Szabo A. Rutin, a flavonoid phytochemical, ameliorates certain behavioral and electrophysiological alterations and general toxicity of oral arsenic in rats. Acta Biol Hung. 2015;66:14–26. doi: 10.1556/ABiol.66.2015.1.2. [DOI] [PubMed] [Google Scholar]
- 10. Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr. 2008;138:344–50. doi: 10.1093/jn/138.2.344. [DOI] [PubMed] [Google Scholar]
- 11. Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev. 2006;7:533–46. [PubMed] [Google Scholar]
- 12. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24:1086–94. doi: 10.1359/JBMR.090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pattison DJ, Symmons DP, Lunt M, Welch A, Bingham SA, Day NE, Silman AJ. Dietary beta-cryptoxanthin and inflammatory polyarthritis: results from a population-based prospective study. Am J Clin Nutr. 2005;82:451–5. doi: 10.1093/ajcn.82.2.451. [DOI] [PubMed] [Google Scholar]
- 14. Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 15. Moore LE, Brennan P, Karami S, et al. Glutathione S-transferase polymorphisms, cruciferous vegetable intake and cancer risk in the Central and Eastern European Kidney Cancer Study. Carcinogenesis. 2007;28:1960–4. doi: 10.1093/carcin/bgm151. [DOI] [PubMed] [Google Scholar]
- 16. Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272(18):1413–20. [PubMed] [Google Scholar]
- 17. Giovannucci E, Ascherio A, Rimm EB, et al. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–76. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 18. Schabath MB, Hernandez LM, Wu X, et al. Dietary phytoestrogens and lung cancer risk. JAMA. 2005;294:1493–504. doi: 10.1001/jama.294.12.1493. [DOI] [PubMed] [Google Scholar]
- 19. Joseph JA, Shukitt-Hale B, Willis LM. Grape juice, berries, and walnuts affect brain aging and behavior. J Nutr. 2009;139(Suppl):S1813–7. doi: 10.3945/jn.109.108266. [DOI] [PubMed] [Google Scholar]
- 20. Lau FC, Shukitt-Hale B, Joseph JA. Nutritional intervention in brain aging: reducing the effects of inflammation and oxidative stress. Subcell Biochem. 2007;42:299–318. [PubMed] [Google Scholar]
- 21. Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–80. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim CY, Lee C, Park GH, Jang JH. Neuroprotective effect of epigallocatechin-3-gallate against beta-amyloid-induced oxidative and nitrosative cell death via augmentation of antioxidant defense capacity. Arch Pharm Res. 2009;32:869–81. doi: 10.1007/s12272-009-1609-z. [DOI] [PubMed] [Google Scholar]
- 23. Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–8. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 24. Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G, Wolffram S, Muller MJ. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102:1065–74. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 25. Dwiecki K, Neunert G, Polewski P, Polewski K. Antioxidant activity of daidzein, a natural antioxidant, and its spectroscopic properties in organic solvents and phosphatidylcholine liposomes. J Photochem Photobiol B. 2009;96:242–8. doi: 10.1016/j.jphotobiol.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 26. Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S, Group CS. Carotenoids and antioxidants in age-related maculopathy Italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology. 2008;115:324e2–33e2. doi: 10.1016/j.ophtha.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 27. Lee HS, Cho YH, Park J, Shin HR, Sung MK. Dietary intake of phytonutrients in relation to fruit and vegetable consumption in Korea. J Acad Nutr Diet. 2013;113:1194–9. doi: 10.1016/j.jand.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 28. Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet. 2012;112:222–9. doi: 10.1016/j.jada.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 29. Tu SH, Chen C, Hsieh YT, Chang HY, Yeh CJ, Lin YC, Pan WH. Design and sample characteristics of the 2005–2008 Nutrition and Health Survey in Taiwan. Asia Pac J Clin Nutr. 2011;20:225–37. [PubMed] [Google Scholar]
- 30.Huang SY. Mater thesis. Taiwan: Chinese Cultural University; 1996. Validation study on household 24-hour recall method and food models. [Google Scholar]
- 31.Food Industry Research and Development Institute. [Accessed 20 December 2015]. Available at http://www.firdi.org.tw.
- 32.US Department of Agriculture. USDA Food and Nutrient Database for Dietary Studies, 3.0. Beltsville, MD: Agricultural Research Service, Food Surveys Research Group; 2008. [Accessed 22 August 2016]. Available at http://bit.ly/2lUQpLB. [Google Scholar]
- 33.US Department of Agriculture. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1. Beltsville, MD: US Department of Agriculture, Agricultural Research Service; 2013. [Accessed 22 August 2016]. Available at http://bit.ly/2mrudLW. [Google Scholar]
- 34.US Department of Agriculture. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0. Beltsville, MD: U.S. Department of Agriculture; Agricultural Research Service; 2008. [Accessed 22 August 2016]. Available at http://bit.ly/2mrqONs. [Google Scholar]
- 35. Barros L, Falcão S, Baptista P, Freire C, Vilas-Boas M, Ferreira ICFR. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008;111:61–6. [Google Scholar]
- 36. Burns J, Fraser PD, Bramley PM. Identification and quantification of carotenoids, tocopherols and chlorophylls in commonly consumed fruits and vegetables. Phytochemistry. 2003;62:939–47. doi: 10.1016/s0031-9422(02)00710-0. [DOI] [PubMed] [Google Scholar]
- 37. Chukwumah Y, Walker L, Vogler B, Verghese M. Profiling of bioactive compounds in cultivars of Runner and Valencia peanut market-types using liquid chromatography/APCI mass spectrometry. Food Chem. 2012;132:525–31. doi: 10.1016/j.foodchem.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 38. Franke AA, Custer LJ, Arakaki C, Murphy SP. Vitamin C and flavonoid levels of fruits and vegetables consumed in Hawaii. J Food Compost Anal. 2004;17:1–35. [Google Scholar]
- 39. Isabelle M, Lee BL, Lim MT, Koh WP, Huang D, Ong CN. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010;123:77–84. [Google Scholar]
- 40. Justesen U, Knuthsen P, Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J Chromatogr A. 1998;799:101–10. doi: 10.1016/s0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- 41. Kornsteiner M, Wagner KH, Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98:381–7. [Google Scholar]
- 42. Lako J, Trenerry VC, Wahlqvist M, Wattanapenpaiboon N, Sotheeswaran S, Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–41. [Google Scholar]
- 43. Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–12. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 44. Moo-Huchin VM, Estrada-Mota I, Estrada-Leon R, Cuevas-Glory L, Ortiz-Vazquez E, Vargas y Vargas Mde L, Betancur-Ancona D, Sauri-Duch E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014;152:508–15. doi: 10.1016/j.foodchem.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 45. Patil BS, Pike LM, Yoo KS. Variation in the quercetin content in different colored onions (Allium cepa L.) J Am Soc Hortic Sci. 1995;120:909–13. [Google Scholar]
- 46. Pichaiyongvongdee S, Haruenkit R. Investigation of Limonoids, Flavanones, Total polyphenol content and antioxidant activity in seven Thai pummelo cultivars. Kasetsart J (Nat Sci) 2009;43:458–66. [Google Scholar]
- 47. Pugliese A, Loizzo MR, Tundis R, O’Callaghan Y, Galvin K, Menichini F, O’Brien N. The effect of domestic processing on the content and bioaccessibility of carotenoids from chili peppers (Capsicum species) Food Chem. 2013;141:2606–13. doi: 10.1016/j.foodchem.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 48. Vuong QV, Hirun S, Phillips PA, Chuen TL, Bowyer MC, Goldsmith CD, Scarlett CJ. Fruit-derived phenolic compounds and pancreatic cancer: perspectives from Australian native fruits. J Ethnopharmacol. 2014;152:227–42. doi: 10.1016/j.jep.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 49. Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54:4069–75. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 50. Zhang B, Hu Z, Zhang Y, Li Y, Zhou S, Chen G. A putative functional MYB transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale (Brassica oleracea var. acephala f. tricolor) Plant Cell Rep. 2012;31:281–9. doi: 10.1007/s00299-011-1162-3. [DOI] [PubMed] [Google Scholar]
- 51. Hanson P, Yang R-y, Chang L-c, Ledesma L, Ledesma D. Carotenoids, ascorbic acid, minerals, and total glucosinolates in choysum (Brassica rapa cvg. parachinensis) and kailaan (B. oleraceae Alboglabra group) as affected by variety and wet and dry season production. J Food Compost Anal. 2011;24:950–62. [Google Scholar]
- 52. Yang RY, Lin S, Kuo G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac J Clin Nutr. 2008;17(Suppl 1):275–9. [PubMed] [Google Scholar]
- 53.Dietary guidelines for Taiwanese. Health promotion Administration, Ministry of Health and Walfare; Taipei, Taiwan: 2012. [Accessed 22 August 2016]. Available at, http://obesity.hpa.gov.tw/upload/e_docs/Manual.pdf. [Google Scholar]
- 54. Murphy MM, Barraj LM, Spungen JH, Herman DR, Randolph RK. Global assessment of select phytonutrient intakes by level of fruit and vegetable consumption. Br J Nutr. 2014;112:1004–18. doi: 10.1017/S0007114514001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nutrilite Phytonutrient Spectrum. Nutrilite’s America’s Phytonutrient Report: Quantifying the Gap. 2009. [Accessed 22 August 2016]. Available at http://www.pwrnewmedia.com/2009/nutrilite90921nmr/downloads/AmerciasPhytonutrientReport.pdf.
- 56. Sebastian RS, Wilkinson Enns C, Goldman JD, Martin CL, Steinfeldt LC, Murayi T, Moshfegh AJ. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among US Adults. J Nutr. 2015;145:1239–48. doi: 10.3945/jn.115.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]