Abstract
Human triple-negative breast cancer (TNBC) is the most aggressive and poorly understood subclass of breast cancer. Glucose transporters (GLUTs) are required for glucose uptake in malignant cancer cells and are ideal targets for cancer therapy. To determine whether the inhibition of GLUTs could be used in TNBC cell therapy, the apple polyphenol phloretin (Ph) was used as a specific antagonist of GLUT2 protein function in human TNBC cells. Interestingly, we found that Ph (10–150 μM, for 24 h) inhibited cell growth and arrested the cell cycle in MDA-MB-231 cells in a p53 mutant-dependent manner, which was confirmed by pre-treatment of the cells with a p53-specific dominant-negative expression vector. We also found that Ph treatment (10–150 μM, for 24 h) significantly decreased the migratory activity of the MDA-MB-231 cells through the inhibition of paxillin/FAK, Src, and alpha smooth muscle actin (α-sMA) and through the activation of E-cadherin. Furthermore, the anti-tumorigenic effect of Ph (10, 50 mg/kg or DMSO twice a week for six weeks) was demonstrated in vivo using BALB/c nude mice bearing MDA-MB-231 tumor xenografts. A decrease in N-cadherin, vimentin and an increase in p53, p21 and E-cadherin were detected in the tumor tissues. In conclusion, inhibition of GLUT2 by the apple polyphenol Ph could potentially suppress TNBC tumor cell growth and metastasis.
Keywords: Apple polyphenol, Breast cancer, Glucose transporter 2, Phloretin, Triple-negative breast cancer
1. Introduction
Glucose is a major source of energy for cancer cells. In proliferating cancer cells, the transport of glucose across the cell membrane by glucose transporters is the rate-limiting step during metabolism [1]. There are several different subtypes of the mammalian glucose transporter (GLUT1–12, 14) family, which can be identified in various human organs [2]. In this study, we focused on the type 2 glucose transporter (GLUT2), which is detected primarily in the pancreas, intestine, liver and kidney [3]. GLUT2 expression can be regulated by the extracellular glucose concentrations and insulin [4]. Metastasis is the major cause of death in many different types of cancer. Previous studies have demonstrated that the apple polyphenol phloretin (Ph) [5] and an extracted modified Fuji apple polysaccharide [6] could have significant anti-tumorigenic effects on colon cancer cells. Many previous studies have also demonstrated that apple extracts have significant anti-tumorigenic effects in breast cancer cells [7–10]. However, the mechanisms that relate the apple-derived components with potential tumor preventative or therapeutic effects remain unclear.
Ph is detected in apples or apple-derived products and is conjugated to glucosidic to form phloridzin (phloretin 2′-O-glucose) [11]. An in vitro study demonstrated that Ph can be produced in Erwinia herbicola Y46, which degrades phloridzin to yield Ph [12]. Furthermore, Ph glycosides have been detected at a high level in apple purees and in commercial juices as a consequence of the processing conditions [13]. Apples also contain other phytochemicals or polysaccharides with anti-tumorigenic effects in breast cancer cells [7]. In addition, apple components have chemopreventive activity in breast cancer [14,15]. Increased consumption of apples and their derivatives has been associated with the prevention of breast and colon cancer [14,16]. Most previous reports have focused on apple polysaccharides, which affect breast cancer cell growth or induce apoptosis [17,18]. However, many studies have reported that the phytochemicals produced by apples may function as antioxidants and have anti-proliferative effects in breast cancer cells [19,20]. In our previous studies, it was shown that Ph is a specific inhibitor of GLUT2 and that the significant anti-tumorigenic effects are due to the suppression of trans-membrane glucose transport [5,21,22]. In vivo studies also demonstrated that Ph suppresses the growth of xenograft tumors including bladder and liver cancer [21–23]. These findings suggest that Ph has potential anti-tumorigenic activity. However, the mechanism of the effects of Ph in human breast cancer cells is not well known.
In this study, we demonstrate that Ph can significantly inhibit TNBC cancer cell growth in an in vivo xenograft mouse model. Our results showed that apple polyphenols inhibit GLUT2 and can be effectively used for breast cancer chemoprevention.
2. Materials and methods
2.1. Cell lines
The human mammary gland epithelial adenocarcinoma cell line MDA-MB-231 (ATCC HTB-26) [24] and the normal human breast epithelial cell line MCF-10A (ATCC CRL-10317) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). MCF-10A cells were maintained in a MCF-10A culture media consisting of DMEM/F12 (Thermo Fisher Scientific, Passau, Germany) supplemented with 20 ng/mL epidermal growth factor, 10 g/mL insulin, 0.5 g/mL hydrocortisone, and 1× non-essential amino acids (Thermo Fisher Scientific). MDA-MB-231 cells were maintained in DMEM (Thermo Fisher Scientific). The cells were cultured according to standard protocols [25].
2.2. Cell proliferation and viability assays
Cell growth and proliferation were determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) and trypan blue assays [26]. This assay was repeated four times with two technical replicates each time.
2.3. Protein extraction, Western blotting analysis and antibodies
Cells treated with DMSO and Ph were harvested for immunoblotting analysis [25]. Primary antibodies were purchased from multiple sources. Antibodies against WAF1/Cip (p21, #2947), Rb (#9309), cyclin D1 (#2922), cyclin E1 (#4129), phospho-FAK (Tyr397, #3283), FAK (#3285), phospho-Src (Tyr416, #2101) and E-cadherin (#14472) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-Kip1 (p27, # 610241) was purchased from BD Bioscience Pharmingen (San Diego, CA, USA). Anti-GAPDH (ab9485), anti-paxillin (ab32084), anti-alpha smooth muscle actin (α–SMA, ab21027), anti-N-cadherin (ab18203) and anti-Src (ab47405) antibodies were purchased from Abcam (Cambridge, UK). Antibodies against GLUT2 (H-67), p53 (DO-1), and PARP (F2) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
2.4. Flow cytometry analysis
The populations of cells treated with Ph or DMSO were sorted and analyzed based on cell cycle phase using flow cytometry [27]. The population of cells in each of the different phases of the cell cycle was evaluated using the FACSCAN laser flow cytometric analysis software (Becton Dickenson, CA, USA).
2.5. Treatment of MDA-MB-231 cells-derived xenografts in vivo
MDA-MB-231 cells (5 × 106) were transplanted into BALB/c nude mice (6–7 weeks of age) [27,28]. Tumor size was measured after transplantation using calipers and estimated according to the following formula: tumor volume (mm3) = L × W2/2, where L is the length and W is the width [28]. The mice were treated with DMSO or Ph at a dose of 25 mg/kg three times per day for 6 weeks until the tumors reached a mean volume of more than 200 mm.3 The study was approved by the Association for Assessment and Accreditation of Laboratory Animal Care, and all processes were performed based on the Taipei Medical University animal care and use rules (License No. LAC-2015-0098).
2.6. Wound-healing cell migration and in vitro invasion assay
MDA-MB-231 cells were seeded into six-well plates and treated with Ph (25–100 μM for 24 h) for an in vitro wound-healing migration assay according to a previously reported protocol [29].
2.7. Statistical methods
All results are expressed as the mean value of at least three experiments with 95% confidence intervals (CIs), unless otherwise stated. A paired t-test was used to analyze the MTT assay, the flow cytometry analysis, and the wound-healing migration assay. The statistical software SigmaPlot graphing (San Jose, CA, USA) and the Statistical Package for the Social Sciences, v. 16.0 (SPSS, Chicago, IL, USA) were used to compare the control and the study groups. A p-value of 0.05 or less was considered significant.
3. Results
3.1. GLUT2 inhibition-induced cell cycle arrest in breast cancer cells
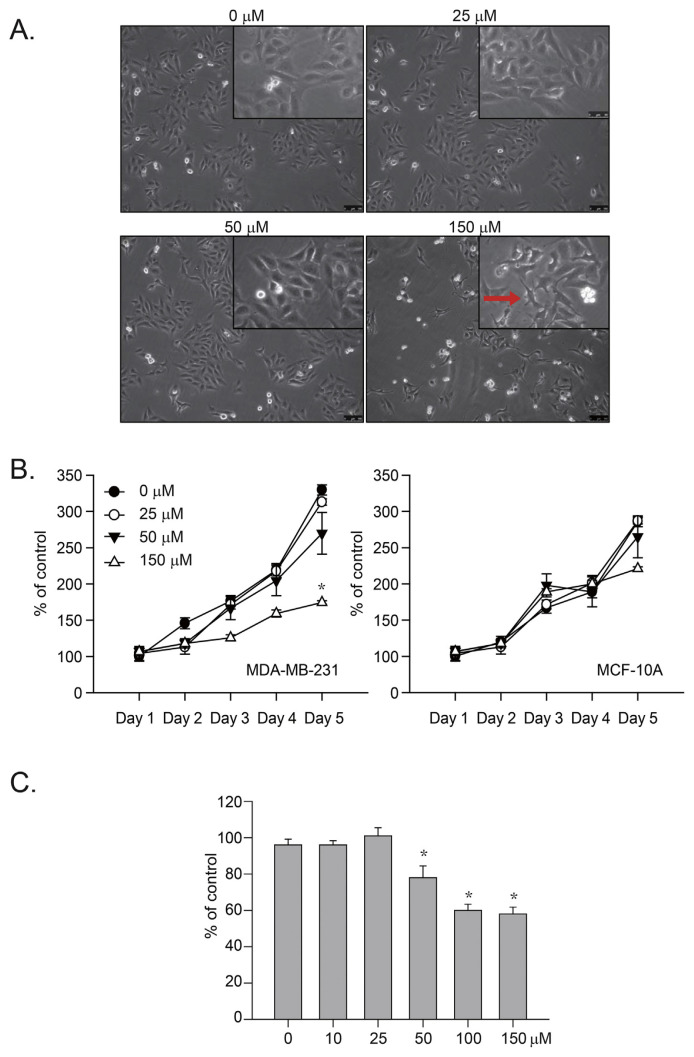
Our recent studies demonstrated that GLUT-2 inhibition by Ph significantly inhibited liver and colon cancer cell proliferation [5,21,22]. To determine whether GLUT2 inhibition could cause significant cell growth arrest in human cancer cells, we treated human breast cancer (MDA-MB-231) cells with Ph (25–150 μM) for 24 h and observed the gross morphology of the cells (Fig. 1A). The results indicated that a high dose of Ph (>150 μM) caused significant changes in the cell morphology (Fig. 1A, indicated by a red arrow). We also treated human breast cancer (MDA-MB-231) and normal (MCF-10A) cells with Ph (25–150 μM) for 24 h and measured cell proliferation with an MTT assay (Fig. 1B). Similarly, the results indicated that a high dose of Ph (>150 μM) significantly inhibited the growth of cancer cells but not of normal cells (Fig. 1B, *p < 0.05). A trypan blue exclusion assay was performed, and the results indicated that Ph at a dose of more than 50 μM caused significant cell death (Fig. 1C, *p < 0.05). Together, these results indicated that Ph preferentially inhibits the proliferation of breast cancer cells compared with normal cells.
Fig. 1.
Ph-induced human TNBC cell growth. (A) Human breast TNBC cancer (MDA-MB-231) cells were treated with 25–150 μM Ph for 24 h. Some cells were also treated with 0.05% DMSO as a control. The gross morphology of the cells was determined, and the scale bar represents 200 μm. (B) The human breast TNBC cancer (MDA-MB-231) and normal (MCF-10A) cells were treated with 25–150 μM Ph for 24 h. Cells were also treated with 0.05% DMSO as a control. MTT assays were performed, and the results were observed for 1–5 days; *p < 0.05. (C) The Ph-induced cytotoxic effects were determined by trypan blue exclusion assay. The results are presented as percentage of the control; *p < 0.05. All results presented above were repeated at least three times.
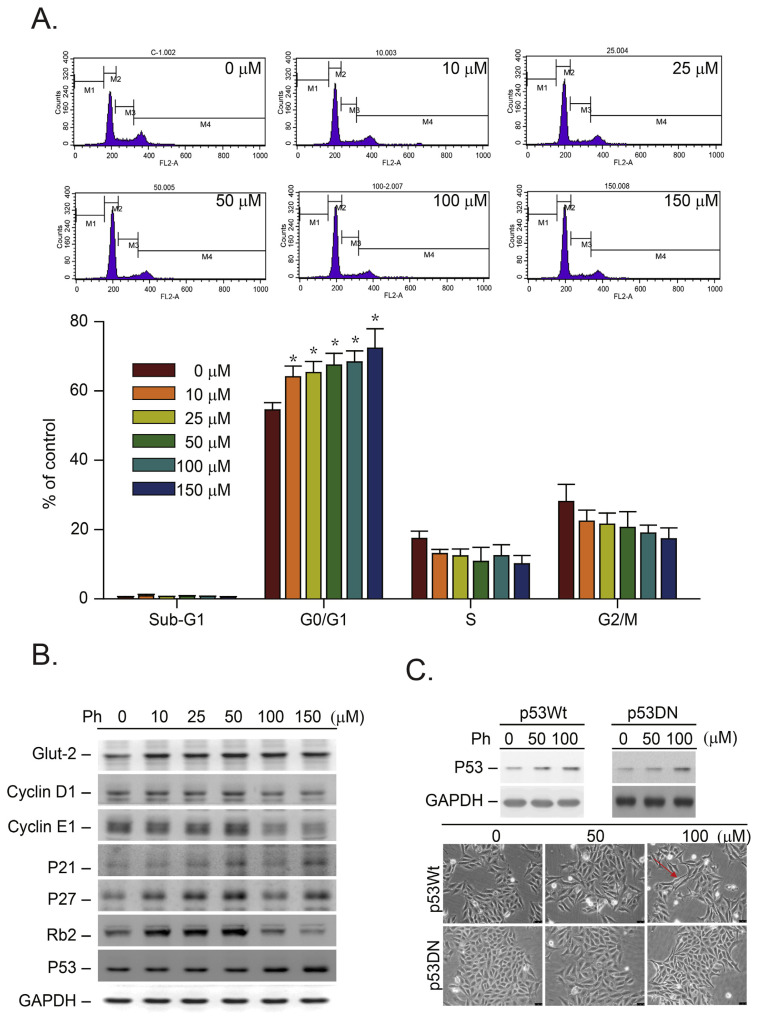
To determine whether the inhibition of cell growth that was induced by Ph due to a blockage of the cell cycle, a flow cytometry analysis was performed (Fig. 2A). To demonstrate the effects of Ph on a specific phase of the cell cycle, we synchronized the breast cancer (MDA-MB-231) cells by switching them to media with 0.04% FCS for 24 h to render them quiescent [27]. The synchronized MDA-MB-231 cells were then returned to culture media containing 10% FCS (control) and 10–150 μM Ph, which was the start of a new cell cycle. The cells were harvested after 16 h, which represents the S phase peak for the flow cytometry analysis [27]. Fig. 2A shows a representative FACS analysis result of the DMSO- (0 μM) and Ph- (10–150 μM) treated cells 16 h after the cells were released from quiescence. The results demonstrated that Ph-treatment (>10 μM) induced a significant accumulation of MDA-MB-231 cells in the G0/G1 phase compared with the control cells, suggesting that the observed growth inhibition was due to the inhibition of GLUT2 by Ph (Fig. 2A, *p < 0.05).
Fig. 2.
Effects of Ph-induced G0/G1 phase cell cycle arrest in TNBC cancer cells. (A) Flow cytometry (FACS) analysis of DNA was conducted after MDA-MB-231 cells were synchronized by 0.04% FCS for 24 h and then switched to culture media supplemented with 10% FBS containing 0.05% DMSO (control) or Ph (10–150 μM in 0.05% DMSO) for an additional 16 h. The lower panel shows the flow cytometry chart of all these cells. The data were analyzed by nonparametric two-sided tests (Kruskal–Wallis and Mann–Whitney tests, *p = 0.05 for all comparisons). (B) The MDA-MB-231 cells were treated with or without Ph (10–150 μM) for 16 h. The protein levels of GLUT2, p21/Cip1, p27/Kip1, cyclin E1, cyclin D1, and p53 were determined by immunoblotting analysis. In each case, GADPH expression served as a control. (C) The MDA-MB-231 cells were pre-treated with a p53-specific dominant-negative expression vector (indicated as p53DN cells) for 24 h. Both the MDA-MB-231 and p53DN cells were treated with Ph (50–100 μM) for 16 h, and the protein was harvested for detection of p53 protein by immunoblotting analysis. Membranes were also probed with anti-GADPH antibodies to correct for differences in protein loading. All results presented above were repeated at least three times, and representative results are shown.
3.2. The effect of Ph on the expression of proteins involved in regulating the G0/G1 phase of the cell cycle
Based on the FACS analysis results shown in our previous paper [27], the MDA-MB-231 cells were released from quiescence at 0, 16, 18 and 24 h, representing the G0/G1, S, G2/M and 2nd G0/G1 phases of the cell cycle, respectively. Accordingly, the 16-h point (representing the lowest G0/G1 or highest S phase) was selected for immunoblotting analysis. As shown in Fig. 2B, the protein level of GLUT2 was increased in both cell lines after Ph treatment (10–150 μM). We hypothesized that this finding was due to a Ph-induced inhibition of glucose uptake that led to a compensatory upregulation of GLUT2 protein expression in both cancer cell lines.
As described above, Fig. 2B shows that the Ph-induced G0/G1 phase cell cycle arrest was observed in MDA-MB-231 cancer cells containing mutant p53 (p53 mt) [30]. According to that study, the results indicated that the p53 mt in MDA-MB-231 breast cancer cells is stabilized by elevated phospholipase D, which contributes to the carcinogenic effects of the breast cancer cell proliferation. The authors proposed that the p53 mutation is required for the survival of MDA-MB-231 breast cancer cells subjected to the stress of serum withdrawal [30]. Ph treatment caused intracellular glucose deprivation through the inhibition of GLUT2 function in breast cancer cells. In our study (Fig. 2B), we found that the expression of p53 mt was induced by a high dose of Ph (>100 μM) treatment. These results indicated that p53 mt is involved in glucose deprivation-induced G0/G1 cell cycle arrest in human breast cancer cells. According to the previous paper, the p53 mt protein in MDA-MB-231 cells is relatively stable compared with the wild-type p53 in MCF7 cells (p53 wild type) [30]. p53 mt is believed to be a transcription factor involved in the regulation of cell cycle arrest and apoptosis [31]. We selected p27/Kip1 and p21/Cip1 as two representative protein markers to evaluate the Ph-induced cell growth arrest and apoptosis. After Ph (10–50 μM) treatment, p27/Kip1 and p21/Cip1 were prominently induced in MDA-MB-231 cells (Fig. 2B). These results suggest that p53 mt-mediated signals were important for Ph-induced cell growth inhibition in these cells. We also demonstrated that the cyclins E1 and D1 were inhibited in the cells treated with a high dose of Ph (>100 μM).
3.3. The mutant p53-mediated signals were important for Ph-induced inhibition of cell growth
To test whether the mutant p53-mediated signals were important for Ph-induced inhibition of cell growth, we pre-treated the MDA-MB-231 cells with a p53-specific dominant-negative expression vector (named p53DN cells) [32,33]. The results indicated that an upregulation of the Ph-induced p53 mt was also detected in the p53DN cells. However, the Ph-induced cytotoxic effects were attenuated (Fig. 2C, red arrow indicated). These results implied that even in cancer cells with p53 mt, which make up a majority of cancer cells, the Ph-induced cytotoxic effects were also prominent. These results are valuable for clinical applications. Similar results were also indicated in our previous papers indicated that in the colon cancer (HT 29, p53 His273 mutated) cells the drugs which induced G0/G1 arrest were also detected [27,34–36].
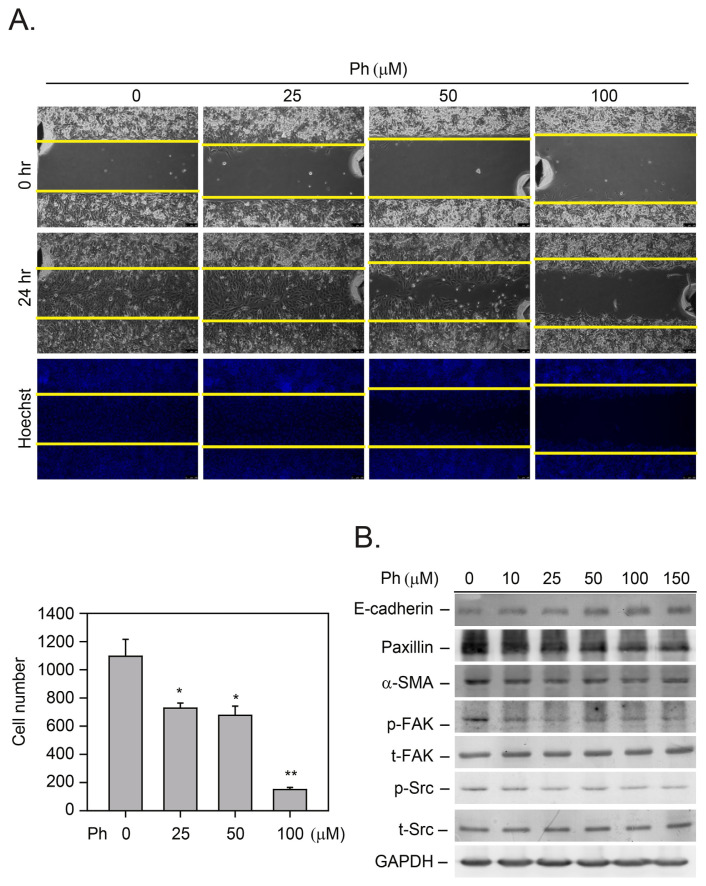
We then evaluated whether the Ph treatment could effectively inhibit tumor cell migration by performing a wound-healing migration assay (Fig. 3A). The results indicated that a low concentration of Ph (>25 μM) effectively inhibited cancer cell migration (Fig. 3A, *p < 0.05). We then explored the mechanism underlying the Ph-induced anti-migratory effects. The MDA-MB-231 cells were treated with Ph (10–150 μM) for 24 h, and the expression of relevant proteins in the migratory cells was detected by immunoblotting analysis (Fig. 3B). Previous studies have demonstrated that the FAK/Src complex mediates the phosphorylation of paxillin, an adaptor protein that is a component of the FAK/Src adaptor complex [37]. In this study, the results demonstrated that Ph inhibited the phosphorylation of FAK and Src and decreased the expression of paxillin (Fig. 3B). In addition, α-SMA is a protein in humans that is encoded by the ACTA2 gene located on 10q22-q24 [38]. α-SMA is commonly used as a marker of myofibroblast formation; however, it is also known to be related to cell migration. For example, Docetaxel-conjugate nanoparticles known as Cellax have been used as a therapy in MDA-MB-231 orthotopic breast tumor models. Cellax therapy significantly reduced α-SMA content by 70% [39]. Similar results were also shown in this study, as we found that a low dose of Ph (>10 μM, 24 h) significantly inhibited α-SMA protein levels (Fig. 3B). E-cadherin has been known to play a role in tumor metastasis, especially of breast cancer. Lower expression of E-cadherin was detected in breast cancer tissues and was associated with lymph node metastasis and poor prognosis [40]. The results shown in Fig. 3B indicated that increased expression of E-cadherin was detected in the Ph-treated breast cancer cells (>50 μM). All these results indicate that Ph has the potential to prevent breast cancer cell migration through the inhibition of these migratory-related signaling proteins.
Fig. 3.
The mechanisms of Ph-induced TNBC cell migration. (A) The MDA-MB-231 tumor cells were treated with Ph (25–100 μM) for 24 h, and tumor cell growth and migration was calculated by the wound-healing method. The data were analyzed by paired t-tests. The mean value determined from the DMSO-treated cells was significantly different from that of Ph-treated cells (*p = 0.05 for all comparisons). (B) Total protein from the Ph-treated cells was harvested for detection of proteins related to cell migration by immunoblotting analysis. Membranes were also probed with anti-GADPH antibodies to correct for differences in protein loading. All results presented above were repeated at least three times, and representative results are shown.
3.4. The in vivo anti-tumor effects of Ph in MDA-MB-231 xenograft mice
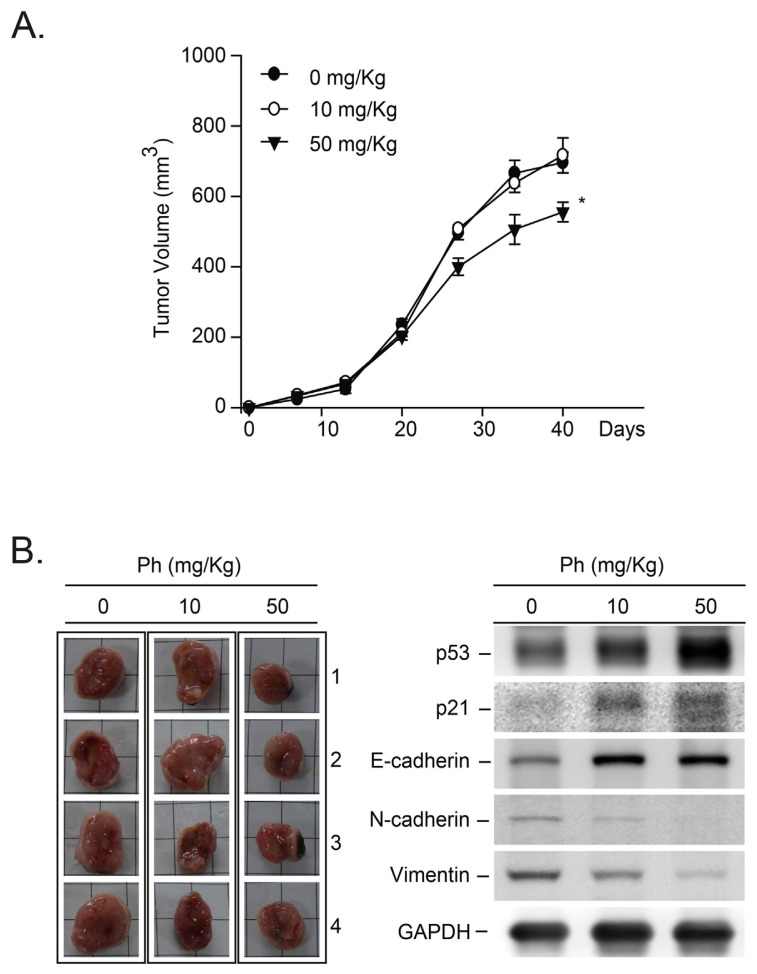
We further examined the anti-tumorigenic effects of Ph in vivo by assessing BALB/c nude mice bearing MDA-MB-231 tumor xenografts. After establishing palpable tumors (mean tumor volume, 200 mm3), the animals received Ph at a dose of 10–50 mg/kg or a DMSO control three times a week. The tumor size was measured from the first to the sixth week, and the final tumor weight was significantly smaller in the Ph-treated group than that of the control group (Fig. 4A, *p < 0.05). In mice receiving these treatment regimens, no gross signs of toxicity were observed after a determination of the body weight, a visible inspection of the general appearance, and a microscopic examination of the individual organs (data not shown). To confirm the involvement of p53-mediated signaling pathways in the Ph-induced suppression of tumor growth, we performed a Western blot analysis (Fig. 4B, right panel). As shown in Fig. 4B, an upregulation of p53-mediated proteins, such as p21/Cip1, was detected in the Ph-treated MDA-MB-231 tumor-bearing mice compared with the control mice. These findings suggest that the p53-mediated anti-tumor effects in tumor tissues are involved in the Ph-mediated inhibition of xenograft growth in mice.
Fig. 4.
The Ph-induced in vivo anti-tumor effects in MDA-MB-231-xenografted tumors. (A) The in vivo anti-tumor effect of Ph was evaluated by treating mice bearing MDA-MB-231 tumor xenografts. After transplantation, tumor size was measured using calipers, and tumor volume was estimated according to the following formula: tumor volume (mm3) = L × W2/2, where L is the length and W is the width. Once the tumors reached a mean volume of 200 mm3, the animals received intraperitoneal injections of DMSO or Ph at 10 mg/kg, or 50 mg/kg three times per week for 6 weeks. All animal studies were performed according to the local guidelines for animal care and protection. (B) After the mice were sacrificed, protein lysates were isolated from the MDA-MB-231 xenograft tumors, and proteins related to cell growth, arrest or migration were detected by immunoblotting analysis. Membranes were also probed with anti-GADPH antibodies to correct for differences in protein loading. All results presented above were repeated at least three times, and representative results are shown.
We further demonstrated that Ph induced E-cadherin expression in MDA-MB-231 tumor-bearing mice. These results are consistent with the results observed in the in vitro study (Fig. 3B). As described above, the loss of E-cadherin-mediated adhesion has been shown to play an important role in the transition of epithelial tumors from a benign to an invasive state [39]. However, recent evidence indicates that another member of the cadherin family, N-cadherin, is expressed in highly invasive breast tumor cell lines that lack E-cadherin expression [41]. We found that Ph treatment significantly inhibited N-cadherin expression (Fig. 4B). Furthermore, increased expression of Vimentin has been shown to be a novel predictive biomarker for lymph node metastasis and poor prognosis in colorectal and gallbladder carcinoma [42,43]. We also demonstrated that Ph inhibited Vimentin expression in the MDA-MB-231 tumor-bearing mice (Fig. 4B). Together, these results demonstrated that Ph has potential effects on the anti-tumor growth and metastasis mechanisms by inhibiting cell growth and the expression of migratory-related proteins.
4. Discussion
Our previous studies have demonstrated that increased GLUT2 expression is a unique phenotype of liver cancer cells, which have a high glucose requirement and increased glucose uptake [5,21,22]. Based on these mechanisms, GLUT2 plays a critical role in the uptake of glucose across the membrane of cancer cells. Our previous studies demonstrated a higher expression level of GLUT2 mRNA in breast, colon, and liver tumor tissues [5,21,22]. Based on these observations, inhibition of the GLUT2 by its specific functional inhibitors should be useful for cancer therapy. Our study demonstrated that in vivo inhibition of the GLUT2 by Ph significantly decreased the uptake of glucose as assayed by positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-d-glucose integrated with computed tomography (18F-FDG PET/CT), which has emerged as a powerful imaging tool for the detection of various cancers [21]. Our results demonstrated that Ph has potential antitumor effects when used as a therapy in liver cancer. Recently, we examined the mRNA levels of GLUT2 in 27 colon tumor vs. normal paired tissue samples [5]. The results indicated that greater quantities of GLUT2 mRNA were detected in the tumor than in normal cells (>2.4-fold, *p = 0.027). We also demonstrated that GLUT2 was required for the advanced-stage colorectal cancer cell growth. Similar results have been published indicating that an increase in glucose uptake is significantly associated with poor prognosis in metastatic colon cancer cells [44]. Such results suggest that the activation of the GLUT2 in cancer cells has an important role in metastatic tumor growth.
To assess this hypothesis, we used an apple polyphenol, Ph, which has been shown to be an inhibitor of GLUT2 [21,22], and determined that targeting GLUT2 significantly inhibited MDAMB-231 cancer cell proliferation and migration in vitro and in vivo. Inhibition of GLUT2 by Ph caused the cancer cells to undergo G0/G1 cell cycle arrest. The results showed that this inhibition the cytotoxic effects on cancer cells via GLUT2 inhibition was attenuated by pre-treatment with a dominant-negative p53 expression plasmid. Many previous reports have demonstrated that cancer cells have more stringent glucose requirements than normal cells. In this study, the protein level of the GLUT2 was increased in the Ph-treated MDA-MB-231 cells. We also demonstrated that the GLUT2 mRNA expression was increased in response to high glucose medium treatment. The results indicated that high GLUT2 protein expression is required for the uptake of the glucose that is present in the microenvironment. Ph treatment inhibited the glucose uptake process, and as a result, increased GLUT2 protein expression was required for cancer cell survival.
GLUT2 is a key rate-limiting factor in the transport of glucose into cancer cells. Enhanced GLUT2 expression and accelerated glycolysis promote aggressive growth in a wide range of tumor types. A previous study provides functional evidence that increased GLUT1 expression in melanoma cells enhanced their metastatic behavior [45]. These findings specify GLUT1 as an attractive therapeutic target and prognostic marker for this highly aggressive tumor. Another study indicated that Ph could modify the amount of glucose entering into the cells by either modulating GLUT (GLUT1 and GLUT4) protein expression or altering glucose binding [46]. Previous studies have also demonstrated that Ph could induce cancer cell apoptosis in B16 melanoma and HL60 leukemia cells via the p53-dependent pathway [47]. Our study is the first to demonstrate that the p53 mutant was also involved in Ph-induced antitumor effects. A previous study using MCF-7 cells demonstrated that prominent upregulation of p53 and Bax and cleavage of poly (ADP)-ribose polymerase were detected in the Ph-treated cells [48]. Such results implied that Ph-induced glucose deprivation-induced ATP depletion [49] initiated the mitochondrial death pathway cascade [50] and increased oxidative stress, which triggered p53-associated apoptosis [51].
The therapeutic importance of the Warburg effect is increasingly recognized, and blocking glucose transporters has become a common anticancer strategy. We previously identified Ph as a novel small compound that inhibits the glucose transport in hepatoma and colon cancer cells and reduces cancer cell growth through a mechanism involving glucose deprivation [5,21,22]. We hypothesized that compounds targeting GLUT2 should be efficacious in vivo as anti-cancer metastasis agents (Fig. 5). Here, we report that Ph inhibited not only cell growth in a breast cancer cell line but also cancer growth in a nude mouse model. We also demonstrated that Ph could inhibit cell migration through different molecules (Fig. 5). Similar observations have also been reported in many previous papers. For example, abnormal vascular smooth muscle cell proliferation and migration are key factors in many cardiovascular diseases. Thus, Ph may be a potential treatment against atherosclerosis and restenosis after vascular injury [52]. A previous study found that treatment with streptozotocin, which can induce type-1 diabetes in animals, significantly upregulated GLUT2 expression in liver cells [53]. These observations suggested that for increased glucose uptake into cells, upregulation of the GLUTs was required. Two recent papers demonstrated that Ph has significant antitumor effects on the lung cancer cells through the activation of apoptosis-related signals [54,55].
Fig. 5.
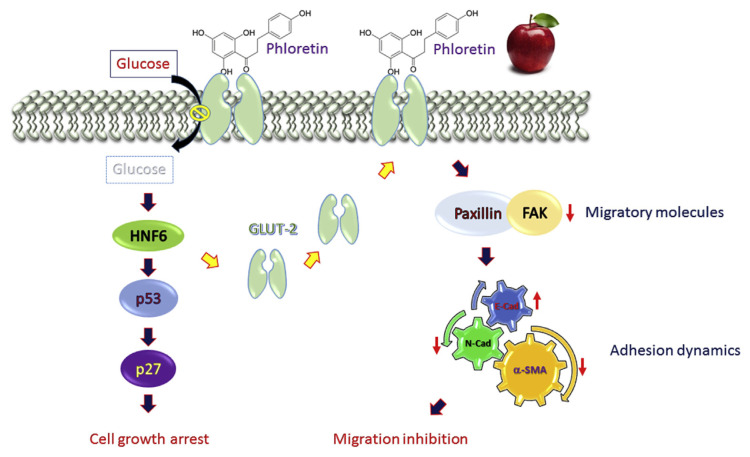
The molecular mechanisms underlying the Ph-induced anti-tumor effects in human MDA-MB-231 cancer cells. In response to Ph treatment, cell glucose uptake was suppressed through GLUT2. Subsequently, low concentrations of intracellular glucose transcriptionally activated HNF6 protein expression as described in our previous paper [5]. The overexpressed HNF6 was reported to be a transcription factor [56] that induced GLUT2 compensatory upregulation in response to glucose deprivation (shown in Fig. 2B). HNF6 also acts as a transcription factor [57] to induce p53-mediated signals and trigger G0/G1 cell cycle arrest, which then caused the in vivo anti-tumor effects in the MDA-MB-231 xenograft mice (right panel). In this study, we also found that Ph could inhibit Paxillin/FAK, N-cadherin, and α-SMA-mediated migratory signals in the tumor-bearing mice treated with Ph. Our results indicated that apple polyphenol Ph could be an important chemopreventive or therapeutic agent for TNBC breast cancer patients.
Acknowledgments
This study was supported by the Health and Welfare Surcharge on tobacco products (MOHW105-TDU-B-212-134001) and by the Ministry of Science and Technology (MOST 104-2320-B-038-066-MY2).
Funding Statement
This study was supported by the Health and Welfare Surcharge on tobacco products (MOHW105-TDU-B-212-134001) and by the Ministry of Science and Technology (MOST 104-2320-B-038-066-MY2).
Footnotes
Disclosure of potential conflicts of interest
The authors declare that no financial competing interests or financial relationships exist with other people or organizations involved in this study.
REFERENCES
- 1. Roy A, Hashmi S, Li Z, Dement AD, Cho KH, Kim JH. The glucose metabolite methylglyoxal inhibits expression of the glucose transporter genes by inactivating the cell surface glucose sensors Rgt2 and Snf3 in yeast. Mol Biol Cell. 2016;27:862–71. doi: 10.1091/mbc.E15-11-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deng D, Sun P, Yan C, Ke M, Jiang X, Xiong L, et al. Molecular basis of ligand recognition and transport by glucose transporters. Nature. 2015;526:391–6. doi: 10.1038/nature14655. [DOI] [PubMed] [Google Scholar]
- 3. Cha JY, Kim H, Kim KS, Hur MW, Ahn Y. Identification of transacting factors responsible for the tissue-specific expression of human glucose transporter type 2 isoform gene. Cooperative role of hepatocyte nuclear factors 1alpha and 3beta. J Biol Chem. 2000;275:18358–65. doi: 10.1074/jbc.M909536199. [DOI] [PubMed] [Google Scholar]
- 4. Peng BJ, Zhu Q, Zhong YL, Xu SH, Wang Z. Chlorogenic acid maintains glucose homeostasis through modulating the expression of SGLT-1, GLUT-2, and PLG in different intestinal segments of Sprague-Dawley rats fed a high-fat diet. Biomed Environ Sci. 2015;28:894–903. doi: 10.3967/bes2015.123. [DOI] [PubMed] [Google Scholar]
- 5. Lin ST, Tu SH, Yang PS, Hsu SP, Lee WH, Ho CT, et al. Apple polyphenol phloretin inhibits colorectal cancer cell growth via inhibition of the type 2 glucose transporter and activation of p53-mediated signaling. J Agric Food Chem. 2016;64:6826–37. doi: 10.1021/acs.jafc.6b02861. [DOI] [PubMed] [Google Scholar]
- 6. Zhang D, Li YH, Mi M, Jiang FL, Yue ZG, Sun Y, et al. Modified apple polysaccharides suppress the migration and invasion of colorectal cancer cells induced by lipopolysaccharide. Nutr Res. 2013;33:839–48. doi: 10.1016/j.nutres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 7. Delphi L, Sepehri H. Apple pectin: a natural source for cancer suppression in 4T1 breast cancer cells in vitro and express p53 in mouse bearing 4T1 cancer tumors, in vivo. Biomed Pharmacother. 2016;84:637–44. doi: 10.1016/j.biopha.2016.09.080. [DOI] [PubMed] [Google Scholar]
- 8. Schiavano GF, De Santi M, Brandi G, Fanelli M, Bucchini A, Giamperi L, et al. Inhibition of breast cancer cell proliferation and in vitro tumorigenesis by a new red apple cultivar. PLoS One. 2015;10:e0135840. doi: 10.1371/journal.pone.0135840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He X, Wang Y, Hu H, Zhang Z. In vitro and in vivo antimammary tumor activities and mechanisms of the apple total triterpenoids. J Agric Food Chem. 2012;60:9430–6. doi: 10.1021/jf3026925. [DOI] [PubMed] [Google Scholar]
- 10. Reagan-Shaw S, Eggert D, Mukhtar H, Ahmad N. Antiproliferative effects of apple peel extract against cancer cells. Nutr Cancer. 2010;62:517–24. doi: 10.1080/01635580903441253. [DOI] [PubMed] [Google Scholar]
- 11. Crespy V, Aprikian O, Morand C, Besson C, Manach C, Demigne C, et al. Bioavailability of phloretin and phloridzin in rats. J Nutr. 2001;131:3227–30. doi: 10.1093/jn/131.12.3227. [DOI] [PubMed] [Google Scholar]
- 12. Chatterjee AK, Gibbins LN. Metabolism of phloridzin by Erwinia herbicola: nature of the degradation products, and the purification and properties of phloretin hydrolase. J Bacteriol. 1969;100:594–600. doi: 10.1128/jb.100.2.594-600.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parpinello GP, Versari A, Galassi S. Phloretin glycosides: bioactive compounds in apple fruit, purees, and juices. J Med Food. 2000;3:149–51. doi: 10.1089/jmf.2000.3.149. [DOI] [PubMed] [Google Scholar]
- 14. Slattery ML. Does an apple a day keep breast cancer away? JAMA. 2001;285:799–801. doi: 10.1001/jama.285.6.799. [DOI] [PubMed] [Google Scholar]
- 15. An apple a day may help prevent breast cancer. Health News. 2005;11:6. [PubMed] [Google Scholar]
- 16. Jaganathan SK, Vellayappan MV, Narasimhan G, Supriyanto E, Octorina Dewi DE, Narayanan AL, et al. Chemopreventive effect of apple and berry fruits against colon cancer. World J Gastroenterol. 2014;20:17029–36. doi: 10.3748/wjg.v20.i45.17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson MD, Stushnoff C, McGinley JN, Thompson HJ. In vitro measures used to predict anticancer activity of apple cultivars and their comparison to outcomes from a rat model of experimentally induced breast cancer. Nutr Cancer. 2009;61:510–7. doi: 10.1080/01635580902825563. [DOI] [PubMed] [Google Scholar]
- 18. Yang J, Liu RH. Synergistic effect of apple extracts and quercetin 3-beta-d-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J Agric Food Chem. 2009;57:8581–6. doi: 10.1021/jf8039796. [DOI] [PubMed] [Google Scholar]
- 19. Sun J, Liu RH. Apple phytochemical extracts inhibit proliferation of estrogen-dependent and estrogen-independent human breast cancer cells through cell cycle modulation. J Agric Food Chem. 2008;56:11661–7. doi: 10.1021/jf8021223. [DOI] [PubMed] [Google Scholar]
- 20. Yoon H, Liu RH. Effect of selected phytochemicals and apple extracts on NF-kappaB activation in human breast cancer MCF-7 cells. J Agric Food Chem. 2007;55:3167–73. doi: 10.1021/jf0632379. [DOI] [PubMed] [Google Scholar]
- 21. Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, et al. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer. 2009;124:2210–9. doi: 10.1002/ijc.24189. [DOI] [PubMed] [Google Scholar]
- 22. Yang KC, Tsai CY, Wang YJ, Wei PL, Lee CH, Chen JH, et al. Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol Carcinog. 2009;48:420–31. doi: 10.1002/mc.20480. [DOI] [PubMed] [Google Scholar]
- 23. Nelson JA, Falk RE. The efficacy of phloridzin and phloretin on tumor cell growth. Anticancer Res. 1993;13:2287–92. [PubMed] [Google Scholar]
- 24. Cailleau R, Young R, Olive M, Reeves WJ., Jr Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974;53:661–74. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ, Chang YJ, et al. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J Natl Cancer Inst. 2010;102:1322–35. doi: 10.1093/jnci/djq300. [DOI] [PubMed] [Google Scholar]
- 26. Chou YH, Ho YS, Wu CC, Chai CY, Chen SC, Lee CH, et al. Tubulozole-induced G2/M cell cycle arrest in human colon cancer cells through formation of microtubule polymerization mediated by ERK1/2 and Chk1 kinase activation. Food Chem Toxicol. 2007;45:1356–67. doi: 10.1016/j.fct.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 27. Lee WS, Chen RJ, Wang YJ, Tseng H, Jeng JH, Lin SY, et al. In vitro and in vivo studies of the anticancer action of terbinafine in human cancer cell lines: G0/G1 p53-associated cell cycle arrest. Int J Cancer. 2003;106:125–37. doi: 10.1002/ijc.11194. [DOI] [PubMed] [Google Scholar]
- 28. Ho YS, Duh JS, Jeng JH, Wang YJ, Liang YC, Lin CH, et al. Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int J Cancer. 2001;91:393–401. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1070>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29. Lee CH, Chang YC, Chen CS, Tu SH, Wang YJ, Chen LC, et al. Crosstalk between nicotine and estrogen-induced estrogen receptor activation induces alpha9-nicotinic acetylcholine receptor expression in human breast cancer cells. Breast Cancer Res Treat. 2011;129:331–45. doi: 10.1007/s10549-010-1209-0. [DOI] [PubMed] [Google Scholar]
- 30. Hui L, Zheng Y, Yan Y, Bargonetti J, Foster DA. Mutant p53 in MDA-MB-231 breast cancer cells is stabilized by elevated phospholipase D activity and contributes to survival signals generated by phospholipase D. Oncogene. 2006;25:7305–10. doi: 10.1038/sj.onc.1209735. [DOI] [PubMed] [Google Scholar]
- 31. Strano S, Dell’Orso S, Di Agostino S, Fontemaggi G, Sacchi A, Blandino G. Mutant p53: an oncogenic transcription factor. Oncogene. 2007;26:2212–9. doi: 10.1038/sj.onc.1210296. [DOI] [PubMed] [Google Scholar]
- 32. Hsu SP, Lee WS. Progesterone receptor activation of extranuclear signaling pathways in regulating p53 expression in vascular endothelial cells. Mol Endocrinol. 2011;25:421–32. doi: 10.1210/me.2010-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ho PY, Hsu SP, Liang YC, Kuo ML, Ho YS, Lee WS. Inhibition of the ERK phosphorylation plays a role in terbinafine-induced p21 up-regulation and DNA synthesis inhibition in human vascular endothelial cells. Toxicol Appl Pharmacol. 2008;229:86–93. doi: 10.1016/j.taap.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 34. Lin JC, Ho YS, Lee JJ, Liu CL, Yang TL, Wu CH. Induction of apoptosis and cell-cycle arrest in human colon cancer cells by meclizine. Food Chem Toxicol. 2007;45:935–44. doi: 10.1016/j.fct.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 35. Chen RJ, Lee WS, Liang YC, Lin JK, Wang YJ, Lin CH, et al. Ketoconazole induces G0/G1 arrest in human colorectal and hepatocellular carcinoma cell lines. Toxicol Appl Pharmacol. 2000;169:132–41. doi: 10.1006/taap.2000.9062. [DOI] [PubMed] [Google Scholar]
- 36. Ho YS, Wang YJ, Lin JK. Induction of p53 and p21/WAF1/CIP1 expression by nitric oxide and their association with apoptosis in human cancer cells. Mol Carcinog. 1996;16:20–31. doi: 10.1002/(SICI)1098-2744(199605)16:1<20::AID-MC4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37. Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–70. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ueyama H, Bruns G, Kanda N. Assignment of the vascular smooth muscle actin gene ACTSA to human chromosome 10. Jinrui Idengaku Zasshi. 1990;35:145–50. doi: 10.1007/BF01876459. [DOI] [PubMed] [Google Scholar]
- 39. Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li SD. Docetaxel conjugate nanoparticles that target alpha-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Cancer Res. 2013;73:4862–71. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- 40. Yu Z, Sun M, Jin F, Xiao Q, He M, Wu H, et al. Combined expression of ezrin and E-cadherin is associated with lymph node metastasis and poor prognosis in breast cancer. Oncol Rep. 2015;34:165–74. doi: 10.3892/or.2015.3967. [DOI] [PubMed] [Google Scholar]
- 41. Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–90. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toiyama Y, Yasuda H, Saigusa S, Tanaka K, Inoue Y, Goel A, et al. Increased expression of Slug and Vimentin as novel predictive biomarkers for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis. 2013;34:2548–57. doi: 10.1093/carcin/bgt282. [DOI] [PubMed] [Google Scholar]
- 43. Dong P, He XW, Gu J, Wu WG, Li ML, Yang JH, et al. Vimentin significantly promoted gallbladder carcinoma metastasis. Chin Med J Engl. 2011;124:4236–44. [PubMed] [Google Scholar]
- 44. Ha TK, Chi SG. CAV1/caveolin 1 enhances aerobic glycolysis in colon cancer cells via activation of SLC2A3/GLUT3 transcription. Autophagy. 2012;8:1684–5. doi: 10.4161/auto.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koch A, Lang SA, Wild PJ, Gantner S, Mahli A, Spanier G, et al. Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget. 2015;6:32748–60. doi: 10.18632/oncotarget.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gonzalez-Menendez P, Hevia D, Rodriguez-Garcia A, Mayo JC, Sainz RM. Regulation of GLUT transporters by flavonoids in androgen-sensitive and -insensitive prostate cancer cells. Endocrinology. 2014;155:3238–50. doi: 10.1210/en.2014-1260. [DOI] [PubMed] [Google Scholar]
- 47. Kobori M, Iwashita K, Shinmoto H, Tsushida T. Phloretin-induced apoptosis in B16 melanoma 4A5 cells and HL60 human leukemia cells. Biosci Biotechnol Biochem. 1999;63:719–25. doi: 10.1271/bbb.63.719. [DOI] [PubMed] [Google Scholar]
- 48. Kim MS, Kwon JY, Kang NJ, Lee KW, Lee HJ. Phloretin induces apoptosis in H-Ras MCF10A human breast tumor cells through the activation of p53 via JNK and p38 mitogen-activated protein kinase signaling. Ann NY Acad Sci. 2009;1171:479–83. doi: 10.1111/j.1749-6632.2009.04692.x. [DOI] [PubMed] [Google Scholar]
- 49. Ye P, Liu Y, Chen C, Tang F, Wu Q, Wang X, et al. An mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose- and amino acid-deprivation. Mol Cell. 2015;57:708–20. doi: 10.1016/j.molcel.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agarwal NR, Maurya N, Pawar JS, Ghosh I. A combined approach against tumorigenesis using glucose deprivation and mitochondrial complex 1 inhibition by rotenone. Cell Biol Int. 2016;40:821–31. doi: 10.1002/cbin.10619. [DOI] [PubMed] [Google Scholar]
- 51. Moley KH, Mueckler MM. Glucose transport and apoptosis. Apoptosis. 2000;5:99–105. doi: 10.1023/a:1009697908332. [DOI] [PubMed] [Google Scholar]
- 52. Wang D, Wang Q, Yan G, Qiao Y, Tang C. Phloretin inhibits platelet-derived growth factor-BB-induced rat aortic smooth muscle cell proliferation, migration, and neointimal formation after carotid injury. J Cardiovasc Pharmacol. 2015;65:444–55. doi: 10.1097/FJC.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 53. Sun Y, Jia Z, Yang G, Kakizoe Y, Liu M, Yang KT, et al. mPGES-2 deletion remarkably enhances liver injury in streptozotocin-treated mice via induction of GLUT2. J Hepatol. 2014;61:1328–36. doi: 10.1016/j.jhep.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma L, Wang R, Nan Y, Li W, Wang Q, Jin F. Phloretin exhibits an anticancer effect and enhances the anticancer ability of cisplatin on non-small cell lung cancer cell lines by regulating expression of apoptotic pathways and matrix metalloproteinases. Int J Oncol. 2016;48:843–53. doi: 10.3892/ijo.2015.3304. [DOI] [PubMed] [Google Scholar]
- 55. Min J, Huang K, Tang H, Ding X, Qi C, Qin X, et al. Phloretin induces apoptosis of non-small cell lung carcinoma A549 cells via JNK1/2 and p38 MAPK pathways. Oncol Rep. 2015;34:2871–9. doi: 10.3892/or.2015.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gannon M, Ray MK, Van Zee K, Rausa F, Costa RH, Wright CV. Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of beta cell function. Development. 2000;127:2883–95. doi: 10.1242/dev.127.13.2883. [DOI] [PubMed] [Google Scholar]
- 57. Yuan XW, Wang DM, Hu Y, Tang YN, Shi WW, Guo XJ, et al. Hepatocyte nuclear factor 6 suppresses the migration and invasive growth of lung cancer cells through p53 and the inhibition of epithelial-mesenchymal transition. J Biol Chem. 2013;288:31206–16. doi: 10.1074/jbc.M113.480285. [DOI] [PMC free article] [PubMed] [Google Scholar]