Abstract
Tea, a popular beverage made from leaves of the plant Camellia sinensis, has been studied extensively in recent decades for its beneficial health effects in the prevention of obesity, metabolic syndrome, diabetes, cancer, and other diseases. Whereas these beneficial effects have been convincingly demonstrated in most laboratory studies, results from human studies have not been consistent. Some studies demonstrated that weight reduction, alleviation of metabolic syndrome and risk reduction in diabetes were only observed in individuals who consume 3–4 cups of tea (600–900 mg tea catechins) or more daily. This chapter reviews some of these studies, the possible mechanisms of actions of tea constituents, and the challenges in extrapolating laboratory studies to human situations.
Keywords: Cancer, Diabetes, Metabolic syndrome, Obesity, Polyphenols
1. Introduction
The popular beverage, tea is made of the leaves of the plant Camellia sinensis. Historically, tea was used for medicinal purposes and in recent decades, it has been studied for its potential beneficial health effects. These include the prevention of excessive body weight gain, alleviation of metabolic syndrome (MetS), and prevention of diabetes, cardiovascular diseases (CVDs), cancer and other diseases (reviewed in Refs. [1–3]). Most of these beneficial effects are believed to be due to the polyphenols in green tea, although caffeine also contributes to some of the effects.
Overweight, obesity and diabetes are emerging major health issues, and the closely related MetS also predispose individuals to CVDs. CVDs and cancer are the two most common diseases and the top two leading causes of death in many countries. If tea could prevent or delay the development of these diseases, the public health implications would be tremendous. Because of this, there is immense scientific and public interest on this topic. A literature search on PubMed in May 2017 using the key words “Tea and Health” yielded 5168 publications, “Tea and weight control” – 2315 publications, “Tea and diabetes” – 894 publications, and “Tea and cancer” – 4355 publications. Some publications are based on strong evidence, while others appear to be superficial. Many of the beneficial effects found in the laboratory may have been carelessly extrapolated to human health and propagated in the news media or popular magazines.
This chapter reviews many of the key studies on these topics, especially those that are relevant to human health. Because of the large number of publications in this area of research, information from recently published meta-analyses and systematic reviews are used to help assess the relative strengths of the existing data. Selected examples are used to illustrate the beneficial health effects and possible mechanisms involved. Suggestions for future research are made.
2. Tea constituents, chemical properties, bioavailability and biotransformation
2.1. Major composition of tea
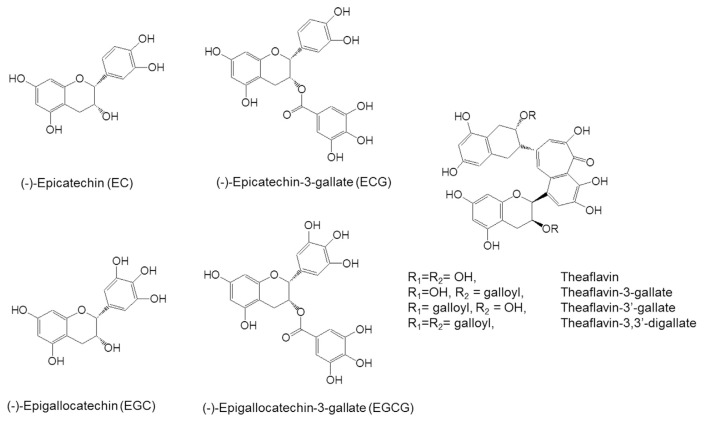
The most commonly consumed teas are green tea, black tea and oolong tea. In the manufacture of green tea, the tea leaves are heated or steamed, rolled and dried. The heating process inactivates the enzymes and the drying of the tea leaves help to stabilize the tea constituents during storage. The characteristic polyphenolic compounds in green tea are known as catechins, which include: (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epicatechin (EC). The structures of these catechins, together with theaflavins from black tea, are shown in Fig. 1. Tea leaves also contain lower quantities of other polyphenols such as quercetin, kaempferol, and myricetin as well as alkaloids, such as caffeine and theobromine. A typical brewed green tea beverage (e.g., 2.5 g tea leaves in 250 ml of hot water) contains 240–320 mg of catechins, of which 60–65% is EGCG, and 20–50 mg of caffeine [4,5].
Fig. 1.
Structures of catechins and theaflavins.
In the manufacture of black tea, the tea leaves are withered, crushed and allowed to undergo enzyme-mediated oxidation in a process commonly referred to as “fermentation.” During this process, most of the catechins are oxidized, dimerized and polymerized to form theaflavins and thearubigins [4,5]. Theaflavins exist in four forms (theaflavin, theaflavin-3 gallate, theaflavin-3′-gallate, and theaflavin-3,3′-digallate) and contribute to the orange color and characteristic taste of black tea. Thearubigins are heterogeneous polymers of tea catechins with a characteristic red-brown color, but the structures are poorly understood. In brewed black tea, catechins, theaflavins, and thearubigins each account for 3–10%, 2–6%, and greater than 20% of the dry weight, respectively. Oolong tea is manufactured by crushing only the rims of the leaves and limiting fermentation to a short period of time to produce specific flavor and taste of the tea. Oolong tea contains catechins, theaflavins, and thearubigins as well as some characteristic components: epigallocatechin esters, dimeric catechins (such as theasinensins), and dimeric proanthocyanidins [5].
2.2. Chemical properties of tea polyphenols
Tea catechins are strong antioxidants, which scavenge free radicals, and prevent the formation of reactive oxygen species (ROS) by chelating metal ions [5]. Among tea catechins, EGCG has the strongest antioxidant activity and has been studied extensively. EGCG is also known to undergo superoxide-catalyzed auto-oxidation in vitro to produce ROS that can induce cell death [6]. Nevertheless, such auto-oxidation of EGCG is not expected to occur extensively in vivo under normal physiological conditions, because of the lower oxygen partial pressure (than in solution in vitro) and the presence of anti-oxidant enzymes in animal tissues [6]. Therefore, results on EGCG obtained from cell culture studies need to be interpreted with caution. In vivo, EGCG and other catechins can serve as antioxidants in general, but they may also cause the formation of ROS in the mitochondria under certain conditions [7,8]. The ROS may activate nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant and other cytoprotective enzymes [9–11].
An important biochemical property of catechins is their hydrogen-bonding to proteins, lipids and nucleic acids. Multiple hydrogen-bond formation provides high affinity binding of catechins to these biomolecules. As will be discussed subsequently, the binding of EGCG to many proteins has been proposed to be a key mechanism for its anti-cancer and other activities. Black tea polyphenols, with more phenolic groups, may bind to biomolecules with even higher affinity than EGCG in vitro.
2.3. Bioavailability and pharmacokinetics
The bioavailability of tea polyphenols is dependent on the molecular size and the number of phenolic groups (reviewed in Refs. [12,13]). Both human and animal studies have shown that the bioavailability of EC (molecular weight 290 with 5 phenolic groups) is much higher than that of EGCG (molecular weight 458 with 8 phenolic groups). In rats, following intragastric (i.g.) administration of decaffeinated green tea (200 mg/kg), the plasma bioavailabilities of EGCG, EGC and EC were 0.1, 14 and 31%, respectively. EGCG, EGC and EC in plasma had elimination half-lives of 165, 66 and 67 min, respectively [12]. However, the plasma bioavailability of EGCG in mice following i.g. administration of EGCG (75 mg/kg) was much higher, with more than 50% of EGCG present as glucuronide conjugates. In humans, following oral administration of the equivalent of two or three cups of green tea, the peak plasma levels of tea catechins (including the conjugated forms) were usually 0.2–0.3 μM. With high pharmacological oral doses of EGCG, peak plasma concentrations of 2–9 μM and 7.5 μM were reported in mice and humans, respectively [12]. Conversely, theaflavin and theaflavin-3,3′-digallate (molecular weights of 564 and 868 and containing 9 and 14 phenolic groups, respectively) were reported to have extremely low bioavailability when administered orally [14]. However, more studies in this area are needed.
Active efflux has been shown to limit the bioavailability of many polyphenolic compounds. The multidrug resistance-associated protein 2 (MRP2), located on the apical surface of the intestine and liver, mediates the transport of some polyphenolic compounds to the lumen and bile, respectively [15]. Therefore, EGCG and its metabolites are predominantly effluxed from the enterocytes into the intestinal lumen, or effluxed from the liver to the bile and excreted in the feces, with little or none of these compounds in the urine of humans and rats. However, urinary EGCG metabolites (in the conjugated forms) are detected in mice [5]. Several investigators have reported the pharmacokinetics of tea polyphenols in human volunteers. For example, after oral administration of 20 mg green tea extract per kg body weight, it took 1.4–1.6 h for the catechins to reach peak plasma concentrations [16]. The maximal plasma concentrations for EGCG, EGC and EC were 77.9, 223 and 124 ng/mL, respectively, with corresponding terminal half-lives of 3.4, 1.7 and 2 h. Plasma EGC and EC were present mainly in the conjugated forms, whereas 77% of the EGCG was in the free form. Methylated EGCG and EGC were also present in human plasma [12,16]. Chow et al. [17] demonstrated that, following 4 weeks of oral administration of EGCG (800 mg, once daily), there was an increase in the systemic bioavailability, but the molecular basis for this observation remains to be investigated.
2.4. Biotransformation of tea polyphenols
EGCG and other tea catechins undergo extensive biotrans-formation (reviewed in Ref. [5]). Because of the catechol structure, catechins can be oxidized to quinone and undergo redox cycling to produce ROS. As a detoxification mechanism, EGCG and other catechins are readily methylated by catechol-O-methyltransferase to form O-methylated EGCG. EGC is also readily methylated to form 4′-O-methyl-EGC. This metabolite as well as 4″-O-methyl-EGCG and 4′,4″-dimethyl-EGCG have been detected in both human and animal plasma and urine samples after the ingestion of tea. In addition to methylation, catechins are also glucuronidated by UDP-glucuronosyltransferases and sulfated by sulfotransferases. Multiple methylation and conjugation reactions can occur on the same molecule. For example, we have observed methyl-EGCG-glucuronide, EGCG-glucuronide-sulfate, dimethyl-EGCG-diglucuronide, and methyl-EGCG-glucuronide-sulfate, which have been found as urinary metabolites in mice [5].
Tea catechins can be degraded in the intestinal tract by microbiota. We have observed the formation of ring fission metabolites 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4), 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (M6), and 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone (M6′) in human urine and plasma samples several hours after the ingestion of tea [18,19]. These compounds can undergo further degradation to phenylacetic and phenylpropionic acids.
2.5. Caffeine and theanine
In contrast to the limited or low bioavailabilities of tea polyphenols, caffeine has bioavailability close to 100% and is mainly metabolized by cytochromes P450 1A2 to dimethylxanthines and theophylline [20]. The pharmacological and health effects of caffeine have been well studied. l-Theanine is a characteristic amino acid in tea and is composed of roughly 50% of the free amino acids in tea. It contributes to the flavor, especially relating to the umami taste of the tea (Narukawa et al., 2014). After administered orally, l-theanine is rapidly absorbed in the intestines through a methionine carrier transport system and then distributed in the major organs of the body. l-Theanine is also almost 100% bioavailable and is excreted through the kidney into the urine unmetabolized or after hydrolysis to glutamic acid and ethylamine [21,22]. Both caffeine and theanine readily cross the blood–brain barrier and are neurologically active. Because of this property, both compounds have been suggested to contribute to the neuroprotective function of tea [2,22].
3. Reduction of body weight, alleviation of metabolic syndrome and prevention of diabetes
Overweight, obesity and type 2 diabetes are emerging major health issues in many countries. MetS encompasses a complex of symptoms that include enlarged waist circumference and two or more of the following: elevated serum triglyceride, dysglycemia, high blood pressure and reduced high-density lipoprotein-associated cholesterol [23]. Studies on possible beneficial effects of tea consumption on body weight reduction and MetS alleviation are summarized below.
3.1. Studies in animal models
The effects of tea and tea constituents on body weight and MetS have been studied extensively in animal models (reviewed in Refs. [2,3]). Most of the studies showed that oral administration of green tea extracts (GTE) or EGCG significantly reduced the gaining of body weight and/or adipose tissue weight, lowered blood glucose or insulin levels, and increased insulin sensitivity. Most of these studies used high fat diets or genetically induced obese/diabetic rodent models. For example, in mice fed a high-fat (60% of the calories) diet, we found that EGCG treatment (0.32% in diet) for 16 weeks significantly reduced body weight gain, body fat and visceral fat weight [24]. These results were also reproduced in a second study using a high-fat/Western style diet [25]. EGCG treatment also attenuated insulin resistance, plasma cholesterol and monocyte chemoattractant protein levels [24,25]. Similar results were also observed in several recent studies [26–29]. Green tea polyphenols also alleviate MetS in other animal models including insulin-resistant rats [30], and insulin-resistant beagle dogs [31].
Diet-induced liver steatosis, which predisposes to liver cancer, is becoming a common disease, and its possible prevention by tea consumption warrants more investigation. We have shown that EGCG treatment markedly reduces the severity of hepatic steatosis, liver triglycerides and plasma alanine aminotransferase concentration in mice fed with a high-fat diet [24,25]. Tea catechins have been reported to also reduce hepatic steatosis and liver toxicity in rodents treated with ethanol, tamoxifen or endotoxins (reviewed in Ref. [32]). These findings have potential for practical applications.
3.2. Studies in humans
Systematic reviews and meta-analysis covering more than 26 earlier short-term randomized controlled trials (RCTs) indicated the beneficial effects of tea consumption in reducing body weight and alleviating MetS [33,34]. Most of these studies used green tea or green tea extracts with caffeine, in studies for 8–12 weeks, on normal weight or overweight subjects. Some RCTs also showed that daily intake of Polyphenon E capsules (tea catechin preparation containing 400 or 800 mg EGCG), for 2 months by postmenopausal American women, decreased blood levels of LDL-cholesterol, glucose and insulin [35]. GTE supplementation (379 mg daily) to obese patients for 3 months also decreased body weight, waist circumference and lipid profiles, as well as levels of total cholesterol, LDL-cholesterol, and triacylglycerols [36]. A recent RCT study in overweight subjects showed that supplementation with EGCG (282 mg/day) for 3 days decreased skeletal muscle lactate levels (suggesting a shift towards a more oxidative muscle phenotype), and decreased postprandial plasma glycerol concentration; but had no significant effects on skeletal muscle lipolysis and whole-body fat oxidation [37]. In a more recent RCT with hyperlipidomic subjects, Puer tea extract (38%) was shown to decrease body weight and BMI and improve lipid profiles, but did not affect fasting glucose levels [38]. A metabolomics study with healthy male subjects demonstrated that GTE supplementation (1200 mg catechins and 240 mg caffeine daily) for 7 days increased lipolysis, fat oxidation and citric acid cycle activity under resting conditions without enhancing adrenergic stimulation [39]. The role of caffeine in these studies was inconsistent among the different studies. A meta-analysis of metabolic studies showed that both catechins and caffeine dose-dependently stimulated daily energy expenditure, but only the catechin–caffeine combination significantly increased fat oxidation [40]. A more recent meta-analysis of 10 RCTs indicated that tea or tea extracts could alleviate the decrease of fasting insulin and reduce waist circumference after an 8-week intervention, but did not affect other parameters measured [41].
In contrast to the above described beneficial effects, two recent studies in British adults did not show such beneficial effects. The first study used a rather low dose of EGCG (200 mg daily) [42]. The second study used daily supplementation with green tea (>560 mg EGCG) plus caffeine (0.28–0.45 mg) for 12 weeks [43]. In an RCT with 237 overweight and obese post-menopausal women in the United States, daily supplementation of 1315 mg of GTE (843 mg EGCG) for 12 months had no effect on body weight, BMI or waist circumference; but decreased fasting insulin levels in those with elevated baseline levels [44]. Reasons for these negative results are unknown and remain for further investigation.
Two epidemiological studies suggested the beneficial effects of green tea consumption on MetS [45,46]. The one by Vernarelli and Lambert (2013), a cross-sectional study of U.S. adults, showed that intake of hot (brewed) tea, but not iced tea, was inversely associated with obesity and biomarkers of MetS and CVDs [46]. These results are exciting and need confirmation from additional studies. On the other hand, two recent cross-sectional studies in Japan did not find a preventive effect of green tea consumption against MetS [47,48].
The lowering of body weight and alleviation of MetS by tea should lead to the reduction of type 2 diabetes. Such an association was found in some, but not all, human studies (reviewed in Refs. [2,49]). For example, a prospective cross-sectional study with U.S. women showed that consumption of more than 4 cups of tea per day was associated with a 30% lower risk of developing type 2 diabetes [50]. A retrospective cohort study of 17,413 Japanese adults aged 40–65 years indicated that daily drinking of more than 6 cups of green tea (but not Oolong or black tea) lowered the morbidity of diabetes by 33% [51]. A meta-analysis based on 7 studies (286,701 total participants) showed that individuals who drank 3–4 or more cups of tea per day had a lower risk of type 2 diabetes than those consuming no tea [52].
3.3. Mechanistic considerations
There are many proposed mechanisms for the above described actions of tea polyphenols. They can be summarized into two major types of actions: 1) the action of tea polyphenols in the gastrointestinal tract, and 2) the systemic action of tea polyphenols in different organs. The combined effects would reduce body weight, alleviate MetS and reduce the risk of diabetes and CVDs.
3.3.1. Actions in the gastrointestinal tract
Ingestion of green tea polyphenols has been shown to increase fecal lipid and total nitrogen contents, suggesting that polyphenols can decrease digestion and absorption of lipids and proteins (reviewed in Ref. [3]). For example, in mice fed 13C-triglycerides enriched diet, EGCG supplementation increased 13C levels in the feces [53].
The possibility that tea may affect gut microbiome has been studied in animal models and humans. For example, green tea powder feeding affected gut microbiota and reduced the levels of body fat, hepatic triglyceride and hepatic cholesterol; the reduction was correlated with the amount of Akkermansia and/or the total amount of bacteria in the small intestine [54]. The abundance of Akkermansia muciniphila has been shown previously to be increased in prebiotic-treated ob/ob mice [55]. Changing gut microbiota, for example by the administration of Saccharomyces boulardii, has also been shown to reduce hepatic steatosis, low grade inflammation, and fat mass in obese and type 2 diabetic db/db mice [56]. A recent study in C57BL/6J Human Flora-Associated mice, found that a high-fat diet caused a significant increase in body weight and fat pad weights associated with reduction in microbial diversity, and these changes were blunted by 0.2% tea polyphenols in the diet, with an increased abundance of lactic acid bacteria [57]. In humans, green tea consumption has been reported to decrease the abundance of Clostridium species and increase the abundance of the Bifidobacterium species in fecal samples [58]. Increase of intestinal Bifidobacterium by a prebiotic (oligofructose) has been shown previously to decrease biomarkers for diabetes in mice [59]. These results suggest the possibility that tea may alleviate MetS by enriching the probiotic population in the intestine. However, a recent study in humans indicated that long-term green tea consumption did not change the gut microbiota [60]. More studies in this area with green and black tea preparations are needed.
3.3.2. Actions in internal organs
Many studies showed that ingestion of tea catechins suppressed gluconeogenesis and lipogenesis and enhanced lipolysis in a coordinated manner (reviewed in Ref. [3]). These results suggest that the actions of tea catechins are mediated by energy sensing molecules, possibly AMPK. In response to falling energy status, AMPK is activated to inhibit energy-consuming processes and promote catabolism to produce ATP [61–63]. We propose that the activation of AMPK is a key mechanism for EGCG and other catechins to influence energy metabolism and to alleviate MetS. The activation of AMPK by EGCG and green tea polyphenols has been demonstrated in vivo and in vitro [64–68]. There are also reports indicating that AMPK was activated in adipose tissues and skeletal muscle by green tea, black tea, Oolong tea and Puer teas [69–71]. The detailed mechanism by which EGCG activates AMPK is still unclear, although the involvement of ROS has been suggested based on studies in vitro [67]. The situation in vivo could be different due to the lack of auto-oxidation of EGCG. EGCG has been reported to inhibit mitochondrial oxidative phosphorylation to decrease ATP levels [72]. Another possibility is that EGCG may serve as an uncoupler of oxidative phosphorylation. Either action could increase the AMP (ADP) to ATP ratios and activate AMPK.
The downregulation of the two key enzymes in gluconeogenesis, PEPCK and G-6-Pase, and associated decrease of glucose production in the liver by EGCG has been shown to be mediated by AMPK activation [67]. The activated form, p-AMPK, is also known to phosphorylate and inactivate ACC, the rate limiting enzyme of fatty acid synthesis. The resulting lowered levels of malonyl-CoA can activate CPT-1, which facilitates long-chain fatty acyl CoA transport into mitochondria for β-oxidation [61]. The possible role of AMPK in mediating the actions of tea constituents in affecting other genes and proteins to increase catabolism and decrease anabolism has been reviewed [3].
We proposed that most of observed beneficial effects of tea polyphenols can be explained by the decreased absorption of macronutrients and the systemic effects of tea catechins in metabolic regulation, mostly mediated by the activation of AMPK. The relative importance of these two modes of action depends on the types and amounts of tea consumed as well as the dietary conditions. For example, with black tea, the decrease of nutrient absorption, especially with a high-fat diet, may play a more important role than its systemic effects, because of the low bioavailability of theaflavins and thearubigins. In addition, actions that are independent of AMPK may also be involved, and some of these actions have been discussed in reviews [49,73]. A recent report suggested that green tea reduced body fat via upregulation of neprilysin, an endopeptidase that regulates feeding behavior [74].
4. Lowering of blood cholesterol, blood pressure and incidence of cardiovascular diseases
4.1. Studies in humans
The alleviation of MetS by tea is expected to reduce the risk for CVDs (reviewed in Refs. [75–77]). Correlation between consumption of tea and decreased risk of stroke were reported by two studies from China and Japan [78,79]. A meta-analysis of 14 prospective studies, covering 513,804 participants with a median follow-up of 11.5 years, found an inverse association between tea consumption and risk of stroke, and the protective effect of green tea appeared to be stronger than that of black tea [80]. Many, but not all, studies in the U.S. and Europe demonstrated an inverse association between black tea consumption and CVD risk [75,81–83]. A meta-analysis, including 6 case–control and 12 cohort studies (5 measured green tea and 13 measured black tea as the exposure), found a reduced risk of coronary artery disease by 28% via green tea consumption; however, there was no significant protective effect from black tea [84].
Green tea has been shown to decrease plasma cholesterol levels and blood pressure as well as improve insulin sensitivity and endothelial function in humans (reviewed in Refs. [77,85]). A systematic review and meta-analysis of 10 trials (834 participants) on the effects of green tea on blood pressure in pre-hypertensive and hypertensive individuals showed significant reductions in systolic and diastolic blood pressure with tea consumption [86]. A similar meta-analysis of 14 RCTs also found that GTE supplementation caused a small but significant reduction in blood pressure among overweight and obese adults [87].
In a multiethnic study of men and women (6508 participants) in the U.S., over a median follow-up of 11.1 years for cardiovascular events, regular tea drinking was associated with a lower coronary artery calcification and lower incidence of cardiovascular events, while coffee drinking was associated with an increased incidence of cardiovascular events, compared with non-drinkers [88]. Similarly, in the Dongfeng-Tongji cohort study in China involving 19,471 participants followed for 3.3–5.1 years, green tea consumption was associated with a reduced risk of coronary heart disease incidence and related biomarkers in middle-aged and older Chinese population [89]. A prospective study using the China Kadorie Biobank involving 512,891 participants (aged 30–79) and a median follow-up of 7.2 years, daily tea consumption was associated with a reduced risk of ischaemic heart disease [90].
Reduction of mortality risk by the consumption of green tea was suggested earlier by large cohort studies in Japan. In the Ohsaki National Health Insurance Cohort Study (n = 40,530), deaths due to CVDs were decreased dose-dependently by tea consumption at quantities of 1 to >5 cups of tea per day [91]. In another study with 76,979 Japanese adults, the consumption of green tea was also associated with decreased CVD mortality, but daily consumption of >6 cups of tea was needed to manifest the effect [92]. Recent cohort studies in China also yielded similar results. The Chinese Prospective Smoking Study of 164,681 adult men, after a follow-up for 11 years, showed that tea consumption was negatively associated with lower risk of total deaths and deaths due to CVDs [93]. Similar results were also obtained in studies of middle-aged and elderly Chinese adults in urban Shanghai (Shanghai Women’s Health Study – followed up for 14.2 years and Shanghai Men’s Health Study – followed up for 8.3 years), 6517 deaths were documented. Green tea consumption was inversely associated with risk of CVDs mortality as well as all-cause mortality among non-smokers [94].
4.2. Possible mechanisms
Beneficial effects of tea catechins in lowering plasma cholesterol levels, preventing hypertension and improving endothelial function contribute to the prevention of CVDs. The cholesterol lowering effect is likely due to the decrease of cholesterol absorption or reabsorption by catechins as well as the decrease of cholesterol synthesis via the inhibition of HMGR (mediated by the activation of AMPK). Enhanced nitric oxide signaling has been suggested as a common mechanism for catechins to decrease blood pressure and the severity of myocardial infarction [77]. Several studies have shown that green tea or black tea polyphenols increased endothelial nitric oxide synthase (eNOS) activity in bovine aortic endothelial cells and rat aortic rings [95–97]. A recent study in rat skeletal muscle showed evidence that the EGCG-induced vasodilation was mediated by eNOS [98]. Tea catechins may also suppress the expression of caveolin-1, a negative regulator of eNOS, lower the expression of endothelin-1, and reduce vasoconstrictor tone; thus increasing bioavailability of nitric oxide to improve endothelial function [99,100]. EGCG has been shown to induce the expression of heme oxygenase 1 in aortic endothelial cells [101], and this may increase anti-inflammatory activity to benefit the cardiovascular system. While moderate doses of EGCG have yielded beneficial effects, a very high dose (1% in diet) has been shown to promote, rather than to attenuate, vascular inflammation in hyperglycemic mice [102]. In a recent cross-over RCT with 19 hypertensive patients, supplementation with black tea (150 mg polyphenols twice a daily for 8 days) increased functionally active circulating angiogenic cells and flow-mediated dilation [103]. These findings demonstrate that black tea also has vascular protective properties.
5. Cancer prevention by tea
5.1. Inhibition of carcinogenesis in animal models
Tea and its major constituents have been demonstrated to inhibit tumorigenesis in many animal models for different organ sites, including the lung, oral cavity, esophagus, stomach, small intestine, colon, skin, liver, pancreas, bladder, prostate and mammary glands (reviewed in Refs. [1,104]). For example, tea and tea preparations have been shown to inhibit tumorigenesis in the lung by at least 20 studies and tumorigenesis in the digestive tract by at least 30 studies (reviewed in Ref. [104]. Most of the studies were conducted with green tea, green tea polyphenol preparations, or pure EGCG, administered through the drinking water or diet. The overall assessment is that the laboratory evidence for cancer prevention by green tea is strong, with some exceptions, such as for mammary cancer prevention [105]).
5.2. Tea consumption and cancer risk in humans
5.2.1. Observational epidemiological studies
In contrast to the strong evidence for the cancer preventive activities of tea constituents in animal models, results from epidemiological studies have not been consistent. A large cohort study in Japan suggested that tea consumption decreased deaths due to CVDs, but not cancer [91]. However, a recent cohort study of 165,000 adult men in China indicated that tea consumption was associated with a significant reduction of deaths from cancer as well as CVDs [93]. However, another recent cohort study in Shanghai did not show an association between tea consumption and deaths from cancer, even though a decreased risk for CVDs was observed [94].
A comprehensive review by Yuan et al. [106] concluded that the consumption of green tea was frequently associated with a reduced risk of upper-gastrointestinal tract cancer, after adjusting for confounding factors, and limited data supported its protective effect of lung and hepatocellular carcinogenesis. However, intake of black tea in general was not associated with a lower risk of cancer [106]. Some recent studies are consistent with this conclusion. For example, a meta-analysis of perspective cohort studies in Asian populations (9 studies involving 465,274 participants and 3694 cases of liver cancer) found that protective effects of green tea for liver cancer was only observed in women (RR, 0.78), but not in men [107]. A population based cohort study in Japan also suggested that green tea consumption lowered the risk of biliary tract cancer [108]. In a recent systematic review and meta-analysis for endometrial cancer, a protective effect was found with green tea, but not black tea consumption [109]. There are also inconsistent results. For example, in a meta-analysis on the relation between green tea consumption and breast cancer published in 2010, an inverse association was found in the 4 case–control studies, but not in the 3 cohort studies [110]. A recent case–control study in Hong Kong also did not find an association [111]. These results are consistent with the rather weak evidence from animal studies on the prevention of mammary cancer by tea [105].
Smoking has been shown to be a strong interfering factor in studies on digestive tract cancers. In addition to the examples discussed above, a case–control study on the effect of green tea consumption on esophageal cancer in Shanghai showed a protective effect only in women, who were mostly non-smokers, but not in men who were mostly smokers [112]. Similarly, in a large-scale, population-based case–control study in urban Shanghai, regular green tea drinking was associated with a significant reduction of pancreatic cancer risk in women but not in men [113]. A systematic review of cohort studies in Japan on green tea consumption and gastric cancer also indicated a small consistent risk reduction in nonsmoking, nondrinking women, but not in the general cohort population [114].
5.2.2. Intervention studies
Many intervention trials have been conducted with green tea catechins. For example, in an RCT in Japan on patients with adenomas removed by polypectomy, supplementation with GTE (1.5 g/day) for one year significantly decreased the development of colorectal adenomas (Shimizu et al., 2008). In a similarly designed RCT in Korea, with 72 patients taking 0.9 g of GTE daily for 12 months (71 patients in the placebo group) showed that the incidence of metachronous colorectal polyps was significantly lower [115]. It is disappointing that in some other studies, some of the exciting results from earlier studies have not been reproduced in recent studies. For example, in a double blinded, Phase II trial in Italy, 30 men with high-grade prostate intraepithelial neoplasia (PIN) were given 300 mg of green tea catechins twice daily for 12 months [116]. Only 1 patient developed prostate cancer, whereas 9 of the 30 patients with high-grade PIN in the placebo group developed prostate cancer (highly statistically significant). However, a recent trial in Florida with a similar design using polyphenon E (containing 400 mg of EGCG) in 97 men with high-grade PIN and/or atypical small acinar proliferation, supplementation for 6–12 months did not prevent prostate cancer formation, although some biological effects and a decrease in serum prostate specific antigen levels were observed [117].
Some recent intervention studies on breast cancer and esophageal adenocarcinoma were limited to bioavailability and some biomarker studies [118,119]. At present, the earlier optimistic expectation of cancer preventive activity by tea polyphenols, based on laboratory results, has not materialized in RCTs. More than 10 human trials with green tea polyphenol preparations are ongoing at the U.S., China and Japan (NIH clinical trials website1). Some of these studies may yield clear conclusions concerning cancer preventive activities of tea polyphenols.
5.3. Mechanistic considerations
Based on the limited human data, actions of tea polyphenols in reducing oxidative stress and enhancing the elimination of carcinogens may be considered as important mechanisms. ROS have been shown to play key roles in carcinogenesis; the antioxidant actions of tea catechins could be an important, but not the only, mechanism for cancer prevention. Based on laboratory studies, many mechanisms have been proposed for cancer prevention by tea constituents, and this subject has been reviewed [1,104]. A common mechanism is through the binding of EGCG to target proteins, leading to the inhibition of enzyme activities or signal-transduction pathways. As reviewed previously, the 67-kDa laminin receptor, Bcl-2 proteins, vimentin, peptidyl prolyl cis/trans isomerase, and other proteins have been proposed as targets for EGCG [1,104]. It is reasonable to assume that the high affinity binding proteins reported in the literature could serve as initial targets, but this point remains to be substantiated in animal models. Some of the proposed mechanisms based on studies in cell lines, however, may not be relevant to cancer prevention. Apparently, mechanisms derived from cancer prevention studies in animal models are likely to be more relevant. These include the induction of apoptosis in different animal models, inhibition of the phosphorylation of c-Jun and Erk1/2 in lung tumorigenesis model, suppression of phospho-Akt and nuclear β-catenin levels in colon cancer models, inhibition of the IGF/IGF-1R axis in colon, prostate and other cancer models, and suppression of VEGF-dependent angiogenesis in lung and prostate cancer models [120]. It is still unclear whether these molecules are direct targets for EGCG or downstream events of the primary action.
6. Concluding remarks
Many laboratory studies strongly suggest the beneficial health effects of tea consumption in the prevention of chronic diseases. However, the results from epidemiological studies have not been consistent. Large cohort studies have shown beneficial effects in reducing the risk of deaths from all causes (combined) and CVDs, but that from cancer is inconsistent, and the protective effects are generally strong among non-smokers. Concerning human intervention studies, there are many successful examples that demonstrated the beneficial effects of tea constituents in weight reduction and MetS alleviation, even though there are still some inconsistencies. On the other hand, intervention trials for cancer prevention have not yielded convincing results, possibly due to the difficulties in conducting long-term studies in the appropriate populations.
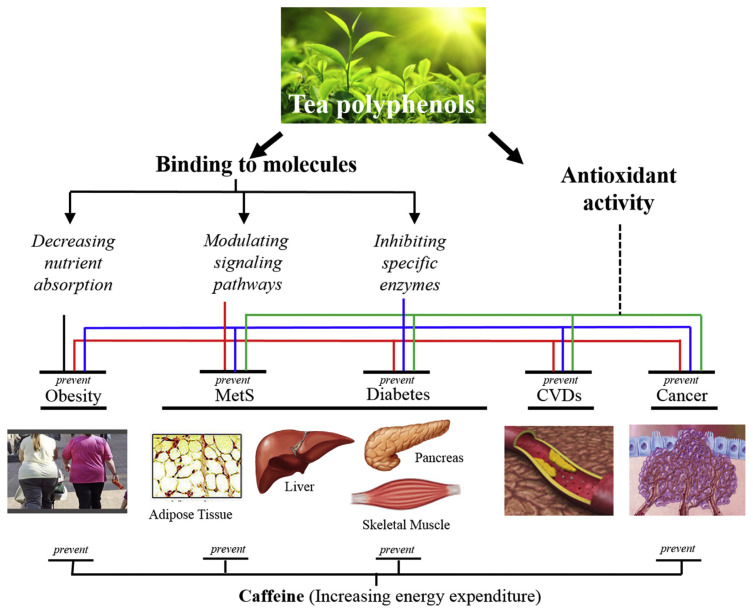
Tea constituents may lower body weight, alleviate MetS, and prevent diabetes, CVDs and cancer by some common and some specific molecular mechanisms (Fig. 2). The antioxidant activities of tea polyphenols appear to contribute to all these beneficial effects. These involve the direct antioxidant effect of polyphenols, their chelation of metal ions, and their activation of the Nrf2-mediated cellular defense system. The binding of tea polyphenols to lipids and proteins (including inhibitions of digestive enzymes) in the intestine results in decreased macronutrient absorption and could be a major mechanism for body weight reduction in animals and humans who ingest excessive amounts of calories. Caffeine also contributes to body weight reduction by increasing energy expenditure [121]. These body weight lowering effects provide beneficial effects to alleviate or prevent MetS, diabetes and CVDs, and perhaps also cancer. The modulation of specific signaling and metabolic pathways through the binding of tea polyphenols to specific regulatory molecules and enzymes could be important mechanisms for alleviating different metabolic abnormalities and preventing related diseases as shown in Fig. 2. In addition to decreasing body fat, caffeine may have direct actions to contribute to the inhibition of carcinogenesis [122].
Fig. 2.
Proposal of action of tea constituents. The antioxidant activities of tea polyphenols contribute to the prevention of obesity, MetS, diabetes, CVDs and cancer. The binding of tea polyphenols to lipid and proteins in the intestine decreases macronutrient absorption. Caffeine also contributes to body weight reduction by increasing energy expenditure. These body weight lowering effects help to prevent related diseases. The binding of tea polyphenols to specific regulatory proteins and enzymes are also important mechanisms for alleviating metabolic abnormalities and preventing related diseases, including cancer. Caffeine may prevent cancer via specific mechanisms.
Bioavailability is an important issue in determining the biological effects of tea polyphenols in internal organs. This factor could explain the results that the beneficial health effects were more clearly shown with green tea than with black tea. Tea polyphenols that are not absorbed into the blood, however, may exert their effects in the gastrointestinal tract; for example, in decreasing lipid absorption. This is probably why black tea is also effective in lowering body weight, body fat and cholesterol levels. The intestinal microbiota may degrade tea polyphenols [18,123], and this may affect the bioavailability of tea polyphenols. Some of the metabolites may have interesting biological activities. The microbial degradation of tea polyphenols and their effects on intestinal microbiota need to be further investigated.
In studies dealing with weight control, MetS and diabetes, beneficial effects have been observed in individuals consuming 3–4 cups of tea (600–900 mg catechins) daily. However, such cause-effect and dose–response relationship in the prevention of other diseases remain to be studied. Caution should be applied in the use of high doses of tea extracts for disease prevention. Many cases of hepatotoxicity due to the consumption of green tea extract-based dietary supplements have been reported (reviewed in Refs. [124,125]). In addition, because of the strong binding activities of tea polyphenols to minerals and biomolecules, ingestion of large quantities of tea extracts may cause nutritional and other problems, even though such problems are not expected to occur due to regular tea beverage consumption [2]. Even with a daily consumption lower than 3–4 cups, unsweetened tea beverages should still be beneficial considering the expected additive effects generated by the daily consumption of other plant materials, such as other flavonoids, anthocyamins, resveratrol, curcumin and leuteolin. These compounds have shown similar beneficial health activities in laboratory studies [49] as we discussed for tea polyphenols.
Acknowledgements
This work was supported by NIH grants CA120915, CA122474, and CA133021. We thank Ms. Vi P. Dan for her assistance in the preparation of this manuscript.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- BTE
black tea extract
- CPT-1
carnitine palmi-toyltransferase 1
- COMT
catechol-O-methyltransferase
- CVDs
cardiovascular diseases
- EC
epicatechin
- ECG
epicatechin-3-gallate
- EGC
epigallocatechin
- EGCG
(−)-epigallocatechin gallate
- eNOS
endothelial nitric oxide synthase
- FAS
fatty acid synthase
- G-6-Pase
glucose-6-phosphatase
- GLUT4
glucose transporter type 4
- GTE
green tea extract
- HMGR
3-hydroxy-3-methylglutaryl-CoA reductase
- i.g.
intragastric
- IRS
insulin receptor substrate
- MetS
metabolic syndrome
- PEPCK
phosphoenolpyruvate carboxykinase
- PPAR
peroxisome proliferator activated receptor
- RCTs
randomized controlled trials
- ROS
reactive oxygen species
Funding Statement
This work was supported by NIH grants CA120915, CA122474, and CA133021.
Footnotes
http://clinicaltrials.gov search on July 19, 2017.
REFERENCES
- 1. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang CS, Hong J. Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu Rev Nutr. 2013;33:161–81. doi: 10.1146/annurev-nutr-071811-150717. [DOI] [PubMed] [Google Scholar]
- 3. Yang CS, Zhang J, Zhang L, Huang J, Wang Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol Nutr Food Res. 2016;60:160–74. doi: 10.1002/mnfr.201500428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [in Eng] [DOI] [PubMed] [Google Scholar]
- 5. Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [in Eng] [DOI] [PubMed] [Google Scholar]
- 6. Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, et al. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–56. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 7. Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, et al. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31:902–10. doi: 10.1093/carcin/bgq039. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tao L, Forester SC, Lambert JD. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (−)-epigallocatechin-3-gallate, in oral cells. Mol Nutr Food Res. 2014;58:665–76. doi: 10.1002/mnfr.201300427. [DOI] [PubMed] [Google Scholar]
- 9. Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, et al. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22:1805–20. doi: 10.1007/s11095-005-7546-8. [in Eng] [DOI] [PubMed] [Google Scholar]
- 10. Wang D, Wang Y, Wan X, Yang CS, Zhang J. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol Appl Pharmacol. 2015;283:65–74. doi: 10.1016/j.taap.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 11. James KD, Forester SC, Lambert JD. Dietary pretreatment with green tea polyphenol, (−)-epigallocatechin-3-gallate reduces the bioavailability and hepatotoxicity of subsequent oral bolus doses of (−)-epigallocatechin-3-gallate. Food Chem Toxicol. 2015;76:103–8. doi: 10.1016/j.fct.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–51. doi: 10.1002/mnfr.200700234. [in Eng] [DOI] [PubMed] [Google Scholar]
- 13. Chow HH, Hakim IA. Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol Res. 2011;64:105–12. doi: 10.1016/j.phrs.2011.05.007. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mulder TP, van Platerink CJ, Wijnand Schuyl PJ, van Amelsvoort JM. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;760:271–9. doi: 10.1016/s0378-4347(01)00285-7. [in Eng] [DOI] [PubMed] [Google Scholar]
- 15. Jemnitz K, Heredi-Szabo K, Janossy J, Ioja E, Vereczkey L, Krajcsi P. ABCC2/Abcc2: a multispecific transporter with dominant excretory functions. Drug Metab Rev. 2010;42:402–36. doi: 10.3109/03602530903491741. [in Eng] [DOI] [PubMed] [Google Scholar]
- 16. Lee M-J, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomark Prev. 2002;11:1025–32. [PubMed] [Google Scholar]
- 17. Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [in Eng] [PubMed] [Google Scholar]
- 18. Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol. 2000;13:177–84. doi: 10.1021/tx9901837. [in Eng] [DOI] [PubMed] [Google Scholar]
- 19. Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–32. [PubMed] [Google Scholar]
- 20.Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Methylxanthines. In: Fredholm BB, editor. Handbook of experimental pharmacology. Vol. 200. Berlin Heidelberg: Springer-Verlag; 2011. [DOI] [PubMed] [Google Scholar]
- 21. van der Pijl PC, Chen L, Mulder TPJ. Human disposition of L-theanine in tea or aqueous solution. J Funct Food. 2010;2:239–44. [in Eng] [Google Scholar]
- 22. Vuong QV, Bowyer MC, Roach PD. L-Theanine: properties, synthesis and isolation from tea. J Sci Food Agric. 2011;91:1931–9. doi: 10.1002/jsfa.4373. [DOI] [PubMed] [Google Scholar]
- 23. Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–9. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 24. Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–83. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, et al. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J Agric Food Chem. 2011;59:11862–71. doi: 10.1021/jf2029016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okuda MH, Zemdegs JC, de Santana AA, Santamarina AB, Moreno MF, Hachul AC, et al. Green tea extract improves high fat diet-induced hypothalamic inflammation, without affecting the serotoninergic system. J Nutr Biochem. 2014;25:1084–9. doi: 10.1016/j.jnutbio.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 27. Byun JK, Yoon BY, Jhun JY, Oh HJ, Kim EK, Min JK, et al. Epigallocatechin-3-gallate ameliorates both obesity and autoinflammatory arthritis aggravated by obesity by altering the balance among CD4+ T-cell subsets. Immunol Lett. 2014;157:51–9. doi: 10.1016/j.imlet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 28. Ortsater H, Grankvist N, Wolfram S, Kuehn N, Sjoholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr Metab. 2012;9:11. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee LS, Choi JH, Sung MJ, Hur JY, Hur HJ, Park JD, et al. Green tea changes serum and liver metabolomic profiles in mice with high-fat diet-induced obesity. Mol Nutr Food Res. 2015;59:784–94. doi: 10.1002/mnfr.201400470. [DOI] [PubMed] [Google Scholar]
- 30. Qin B, Polansky MM, Harry D, Anderson RA. Green tea polyphenols improve cardiac muscle mRNA and protein levels of signal pathways related to insulin and lipid metabolism and inflammation in insulin-resistant rats. Mol Nutr Food Res. 2010;54(Suppl 1):S14–23. doi: 10.1002/mnfr.200900306. [in Eng] [DOI] [PubMed] [Google Scholar]
- 31. Serisier S, Leray V, Poudroux W, Magot T, Ouguerram K, Nguyen P. Effects of green tea on insulin sensitivity, lipid profile and expression of PPARalpha and PPARgamma and their target genes in obese dogs. Br J Nutr. 2008;99:1208–16. doi: 10.1017/S0007114507862386. [DOI] [PubMed] [Google Scholar]
- 32. Sae-tan S, Grove KA, Lambert JD. Weight control and prevention of metabolic syndrome by green tea. Pharmacol Res. 2011;64:146–54. doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes. 2009;33:956–61. doi: 10.1038/ijo.2009.135. [in Eng] [DOI] [PubMed] [Google Scholar]
- 34. Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A, Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta-analysis. J Clin Nutr. 2010;91:73–81. doi: 10.3945/ajcn.2009.28157. [in Eng] [DOI] [PubMed] [Google Scholar]
- 35. Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res. 2012;5:393–402. doi: 10.1158/1940-6207.CAPR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. 2012;149:315–22. doi: 10.1007/s12011-012-9448-z. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Most J, van Can JG, van Dijk JW, Goossens GH, Jocken J, Hospers JJ, et al. A 3-day EGCG-supplementation reduces interstitial lactate concentration in skeletal muscle of overweight subjects. Sci Rep. 2015;5:17896. doi: 10.1038/srep17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jensen GS, Beaman JL, He Y, Guo Z, Sun H. Reduction of body fat and improved lipid profile associated with daily consumption of a Puer tea extract in a hyperlipidemic population: a randomized placebo-controlled trial. Clin Interv Aging. 2016;11:367–76. doi: 10.2147/CIA.S94881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hodgson AB, Randell RK, Boon N, Garczarek U, Mela DJ, Jeukendrup AE, et al. Metabolic response to green tea extract during rest and moderate-intensity exercise. J Nutr Biochem. 2013;24:325–34. doi: 10.1016/j.jnutbio.2012.06.017. [in Eng] [DOI] [PubMed] [Google Scholar]
- 40. Hursel R, Viechtbauer W, Dulloo AG, Tremblay A, Tappy L, Rumpler W, et al. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obes Rev. 2011;12:e573–81. doi: 10.1111/j.1467-789X.2011.00862.x. [in Eng] [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Wang C, Huai Q, Guo F, Liu L, Feng R, et al. Effects of tea or tea extract on metabolic profiles in patients with type 2 diabetes mellitus: a meta-analysis of ten randomized controlled trials. Diabetes Metab Res Rev. 2016;32:2–10. doi: 10.1002/dmrr.2641. [DOI] [PubMed] [Google Scholar]
- 42. Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrarte E, Margareto J, Labayen I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br J Nutr. 2014;111:1263–71. doi: 10.1017/S0007114513003784. [DOI] [PubMed] [Google Scholar]
- 43. Janssens PL, Hursel R, Westerterp-Plantenga MS. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J Nutr. 2015;145:864–70. doi: 10.3945/jn.114.207829. [DOI] [PubMed] [Google Scholar]
- 44. Dostal AM, Samavat H, Espejo L, Arikawa AY, Stendell-Hollis NR, Kurzer MS. Green tea extract and catechol-O-methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J Nutr. 2016;146:38–45. doi: 10.3945/jn.115.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang CS, Chang YF, Liu PY, Chen CY, Tsai YS, Wu CH. Smoking, habitual tea drinking and metabolic syndrome in elderly men living in rural community: the Tianliao old people (TOP) study 02. PLoS One. 2012;7:e38874. doi: 10.1371/journal.pone.0038874. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vernarelli JA, Lambert JD. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur J Nutr. 2013;52:1039–48. doi: 10.1007/s00394-012-0410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takami H, Nakamoto M, Uemura H, Katsuura S, Yamaguchi M, Hiyoshi M, et al. Inverse correlation between coffee consumption and prevalence of metabolic syndrome: baseline survey of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study in Tokushima, Japan. J Epidemiol. 2013;23:12–20. doi: 10.2188/jea.JE20120053. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pham NM, Nanri A, Kochi T, Kuwahara K, Tsuruoka H, Kurotani K, et al. Coffee and green tea consumption is associated with insulin resistance in Japanese adults. Metabolism. 2014;63:400–8. doi: 10.1016/j.metabol.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 49. Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, et al. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr. 2005;24:376–84. doi: 10.1080/07315724.2005.10719488. [in Eng] [DOI] [PubMed] [Google Scholar]
- 51. Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554–62. doi: 10.7326/0003-4819-144-8-200604180-00005. [in Eng] [DOI] [PubMed] [Google Scholar]
- 52. Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–63. doi: 10.1001/archinternmed.2009.439. [in Eng] [DOI] [PubMed] [Google Scholar]
- 53. Friedrich M, Petzke KJ, Raederstorff D, Wolfram S, Klaus S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int J Obes (Lond) 2012;36:735–43. doi: 10.1038/ijo.2011.136. [in Eng] [DOI] [PubMed] [Google Scholar]
- 54. Axling U, Olsson C, Xu J, Fernandez C, Larsson S, Strom K, et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab. 2012;9:105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–86. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Everard A, Matamoros S, Geurts L, Delzenne NM, Cani PD. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio. 2014;5:e01011–4. doi: 10.1128/mBio.01011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang L, Zeng B, Zhang X, Liao Z, Gu L, Liu Z, et al. The effect of green tea polyphenols on gut microbial diversity and fat deposition in C57BL/6J HFA mice. Food Funct. 2016;7:4956–66. doi: 10.1039/c6fo01150k. [DOI] [PubMed] [Google Scholar]
- 58. Jin JS, Touyama M, Hisada T, Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol Immunol. 2012;56:729–39. doi: 10.1111/j.1348-0421.2012.00502.x. [DOI] [PubMed] [Google Scholar]
- 59. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 60. Janssens PL, Penders J, Hursel R, Budding AE, Savelkoul PH, Westerterp-Plantenga MS. Long-term green tea supplementation does not change the human gut microbiota. PLoS One. 2016;11:e0153134. doi: 10.1371/journal.pone.0153134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Investig. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 64. Murase T, Misawa K, Haramizu S, Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem Pharmacol. 2009;78:78–84. doi: 10.1016/j.bcp.2009.03.021. [in Eng] [DOI] [PubMed] [Google Scholar]
- 65. Banerjee S, Ghoshal S, Porter TD. Phosphorylation of hepatic AMP-activated protein kinase and liver kinase B1 is increased after a single oral dose of green tea extract to mice. Nutr Res. 2012;32:985–90. doi: 10.1016/j.nutres.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou J, Farah BL, Sinha RA, Wu Y, Singh BK, Bay BH, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS One. 2014;9:e87161. doi: 10.1371/journal.pone.0087161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem. 2007;282:30143–9. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Serrano JC, Gonzalo-Benito H, Jove M, Fourcade S, Cassanye A, Boada J, et al. Dietary intake of green tea polyphenols regulates insulin sensitivity with an increase in AMP-activated protein kinase alpha content and changes in mitochondrial respiratory complexes. Mol Nutr Food Res. 2013;57:459–70. doi: 10.1002/mnfr.201200513. [DOI] [PubMed] [Google Scholar]
- 69. Yamashita Y, Wang L, Wang L, Tanaka Y, Zhang T, Ashida H. Oolong, black and pu-erh tea suppresses adiposity in mice via activation of AMP-activated protein kinase. Food Funct. 2014;5:2420–9. doi: 10.1039/c4fo00095a. [DOI] [PubMed] [Google Scholar]
- 70. Yamashita Y, Wang L, Tinshun Z, Nakamura T, Ashida H. Fermented tea improves glucose intolerance in mice by enhancing translocation of glucose transporter 4 in skeletal muscle. J Agric Food Chem. 2012;60:11366–71. doi: 10.1021/jf303597c. [DOI] [PubMed] [Google Scholar]
- 71. Rocha A, Bolin AP, Cardoso CA, Otton R. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur J Nutr. 2016;55:2231–44. doi: 10.1007/s00394-015-1033-8. [DOI] [PubMed] [Google Scholar]
- 72. Valenti D, de Bari L, Manente GA, Rossi L, Mutti L, Moro L, et al. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim Biophys Acta. 2013;1832:2085–96. doi: 10.1016/j.bbadis.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 73. Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–95. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Muenzner M, Tappenbeck N, Gembardt F, Rulke R, Furkert J, Melzig MF, et al. Green tea reduces body fat via upregulation of neprilysin. Int J Obes. 2016;40:1850–5. doi: 10.1038/ijo.2016.172. [DOI] [PubMed] [Google Scholar]
- 75. Deka A, Vita JA. Tea and cardiovascular disease. Pharmacol Res. 2011;64:136–45. doi: 10.1016/j.phrs.2011.03.009. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Di Castelnuovo A, di Giuseppe R, Iacoviello L, de Gaetano G. Consumption of cocoa, tea and coffee and risk of cardiovascular disease. Eur J Intern Med. 2012;23:15–25. doi: 10.1016/j.ejim.2011.07.014. [in Eng] [DOI] [PubMed] [Google Scholar]
- 77. Munir KM, Chandrasekaran S, Gao F, Quon MJ. Mechanisms for food polyphenols to ameliorate insulin resistance and endothelial dysfunction: therapeutic implications for diabetes and its cardiovascular complications. Am J Physiol Endocrinol Metab. 2013;305:E679–86. doi: 10.1152/ajpendo.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liang W, Lee AH, Binns CW, Huang R, Hu D, Zhou Q. Tea consumption and ischemic stroke risk: a case-control study in southern China. Stroke. 2009;40:2480–5. doi: 10.1161/STROKEAHA.109.548586. [in Eng] [DOI] [PubMed] [Google Scholar]
- 79. Kokubo Y, Iso H, Saito I, Yamagishi K, Yatsuya H, Ishihara J, et al. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in Japanese population: the Japan public health center-based study cohort. Stroke. 2013;44:1369–74. doi: 10.1161/STROKEAHA.111.677500. [DOI] [PubMed] [Google Scholar]
- 80. Shen L, Song LG, Ma H, Jin CN, Wang JA, Xiang MX. Tea consumption and risk of stroke: a dose-response meta-analysis of prospective studies. J Zhejiang Univ Sci B. 2012;13:652–62. doi: 10.1631/jzus.B1201001. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. de Koning Gans JM, Uiterwaal CS, van der Schouw YT, Boer JM, Grobbee DE, Verschuren WM, et al. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler Thromb Vasc Biol. 2010;30:1665–71. doi: 10.1161/ATVBAHA.109.201939. [in Eng] [DOI] [PubMed] [Google Scholar]
- 82. Mukamal KJ, Alert M, Maclure M, Muller JE, Mittleman MA. Tea consumption and infarct-related ventricular arrhythmias: the determinants of myocardial infarction onset study. J Am Coll Nutr. 2006;25:472–9. doi: 10.1080/07315724.2006.10719561. [in Eng] [DOI] [PubMed] [Google Scholar]
- 83. Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr. 2003;77:1400–8. doi: 10.1093/ajcn/77.6.1400. [in Eng] [DOI] [PubMed] [Google Scholar]
- 84. Wang ZM, Zhou B, Wang YS, Gong QY, Wang QM, Yan JJ, et al. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. J Clin Nutr. 2011;93:506–15. doi: 10.3945/ajcn.110.005363. [in Eng] [DOI] [PubMed] [Google Scholar]
- 85. Hartley L, Flowers N, Holmes J, Clarke A, Stranges S, Hooper L, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;6:CD009934. doi: 10.1002/14651858.CD009934.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yarmolinsky J, Gon G, Edwards P. Effect of tea on blood pressure for secondary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:236–46. doi: 10.1093/nutrit/nuv001. [DOI] [PubMed] [Google Scholar]
- 87. Li G, Zhang Y, Thabane L, Mbuagbaw L, Liu A, Levine MA, et al. Effect of green tea supplementation on blood pressure among overweight and obese adults: a systematic review and meta-analysis. J Hypertens. 2015;33:243–54. doi: 10.1097/HJH.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 88. Miller PE, Zhao D, Frazier-Wood AC, Michos ED, Averill M, Sandfort V, et al. Associations of coffee, tea, and caffeine intake with coronary artery calcification and cardiovascular events. Am J Med. 2017;130:188–97e5. doi: 10.1016/j.amjmed.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tian C, Huang Q, Yang L, Legare S, Angileri F, Yang H, et al. Green tea consumption is associated with reduced incident CHD and improved CHD-related biomarkers in the Dongfeng-Tongji cohort. Sci Rep. 2016;6:24353. doi: 10.1038/srep24353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li X, Yu C, Guo Y, Bian Z, Si J, Yang L, et al. China Kadoorie Biobank Collaborative G. Tea consumption and risk of ischaemic heart disease. Heart. 2017;103:783–9. doi: 10.1136/heartjnl-2016-310462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. Jama. 2006;296:1255–65. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 92. Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. 2011;65:230–40. doi: 10.1136/jech.2009.097311. [in Eng] [DOI] [PubMed] [Google Scholar]
- 93. Liu J, Liu S, Zhou H, Hanson T, Yang L, Chen Z, et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol. 2016;31:853–65. doi: 10.1007/s10654-016-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao LG, Li HL, Sun JW, Yang Y, Ma X, Shu XO, et al. Green tea consumption and cause-specific mortality: results from two prospective cohort studies in China. J Epidemiol. 2017;27:36–41. doi: 10.1016/j.je.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jochmann N, Lorenz M, Krosigk A, Martus P, Bohm V, Baumann G, et al. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br J Nutr. 2008;99:863–8. doi: 10.1017/S0007114507838992. [DOI] [PubMed] [Google Scholar]
- 96. Lorenz M, Wessler S, Follmann E, Michaelis W, Dusterhoft T, Baumann G, et al. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279:6190–5. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 97. Aggio A, Grassi D, Onori E, D’Alessandro A, Masedu F, Valenti M, et al. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur J Nutr. 2013;52:263–72. doi: 10.1007/s00394-012-0320-x. [DOI] [PubMed] [Google Scholar]
- 98. Ng HL, Premilovac D, Rattigan S, Richards SM, Muniyappa R, Quon MJ, et al. Acute vascular and metabolic actions of the green tea polyphenol epigallocatechin 3-gallate in rat skeletal muscle. J Nutr Biochem. 2017;40:23–31. doi: 10.1016/j.jnutbio.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 99. Li Y, Ying C, Zuo X, Yi H, Yi W, Meng Y, et al. Green tea polyphenols down-regulate caveolin-1 expression via ERK1/2 and p38MAPK in endothelial cells. J Nutr Biochem. 2009;20:1021–7. doi: 10.1016/j.jnutbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 100. Akiyama S, Katsumata S, Suzuki K, Nakaya Y, Ishimi Y, Uehara M. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci Biotechnol Biochem. 2009;73:2779–82. doi: 10.1271/bbb.90576. [DOI] [PubMed] [Google Scholar]
- 101. Pullikotil P, Chen H, Muniyappa R, Greenberg CC, Yang S, Reiter CE, et al. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-alpha. J Nutr Biochem. 2012;23:1134–45. doi: 10.1016/j.jnutbio.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pae M, Ren Z, Meydani M, Shang F, Smith D, Meydani SN, et al. Dietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in mice. J Nutr Biochem. 2012;23:526–31. doi: 10.1016/j.jnutbio.2011.02.006. [in Eng] [DOI] [PubMed] [Google Scholar]
- 103. Grassi D, Draijer R, Schalkwijk C, Desideri G, D’Angeli A, Francavilla S, et al. Black tea increases circulating endothelial progenitor cells and improves flow mediated dilatation counteracting deleterious effects from a fat load in hypertensive patients: a randomized controlled study. Nutrients. 2016;8 doi: 10.3390/nu8110727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yang CS, Wang H. Cancer preventive activities of tea catechins. Molecules. 2016;21 doi: 10.3390/molecules21121679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. 2007;17:395–402. doi: 10.1016/j.semcancer.2007.06.013. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yuan JM, Sun C, Butler LM. Tea and cancer prevention: epidemiological studies. Pharmacol Res. 2011;64:123–35. doi: 10.1016/j.phrs.2011.03.002. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Huang YQ, Lu X, Min H, Wu QQ, Shi XT, Bian KQ, et al. Green tea and liver cancer risk: a meta-analysis of prospective cohort studies in Asian populations. Nutrition. 2016;32:3–8. doi: 10.1016/j.nut.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 108. Makiuchi T, Sobue T, Kitamura T, Ishihara J, Sawada N, Iwasaki M, et al. Association between green tea/coffee consumption and biliary tract cancer: a population-based cohort study in Japan. Cancer Sci. 2016;107:76–83. doi: 10.1111/cas.12843. [in English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhou Q, Li H, Zhou JG, Ma Y, Wu T, Ma H. Green tea, black tea consumption and risk of endometrial cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2016;293:143–55. doi: 10.1007/s00404-015-3811-1. [DOI] [PubMed] [Google Scholar]
- 110. Ogunleye AA, Xue F, Michels KB. Green tea consumption and breast cancer risk or recurrence: a meta-analysis. Breast Cancer Res Treat. 2010;119:477–84. doi: 10.1007/s10549-009-0415-0. [in Eng] [DOI] [PubMed] [Google Scholar]
- 111. Li M, Tse LA, Chan WC, Kwok CH, Leung SL, Wu C, et al. Evaluation of breast cancer risk associated with tea consumption by menopausal and estrogen receptor status among Chinese women in Hong Kong. Cancer Epidemiol. 2016;40:73–8. doi: 10.1016/j.canep.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–8. doi: 10.1093/jnci/86.11.855. [in Eng] [DOI] [PubMed] [Google Scholar]
- 113. Wang J, Zhang W, Sun L, Yu H, Ni QX, Risch HA, et al. Green tea drinking and risk of pancreatic cancer: a large-scale, population-based case-control study in urban Shanghai. Cancer Epidemiol. 2012;36(6):e354–8. doi: 10.1016/j.canep.2012.08.004. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sasazuki S, Tamakoshi A, Matsuo K, Ito H, Wakai K, Nagata C, et al. Green tea consumption and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:335–46. doi: 10.1093/jjco/hys009. [in Eng] [DOI] [PubMed] [Google Scholar]
- 115. Shin CM, Lee DH, Seo AY, Lee HJ, Kim SB, Son WC, et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: a randomized clinical trial. Clin Nutr. 2017 doi: 10.1016/j.clnu.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 116. Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [in Eng] [DOI] [PubMed] [Google Scholar]
- 117. Kumar NB, Pow-Sang J, Egan KM, Spiess PE, Dickinson S, Salup R, et al. Randomized, placebo-controlled trial of green tea catechins for prostate cancer prevention. Cancer Prev Res (Phila) 2015;8(10):879–87. doi: 10.1158/1940-6207.CAPR-14-0324. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Crew KD, Brown P, Greenlee H, Bevers TB, Arun B, Hudis C, et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev Res. 2012;5:1144–54. doi: 10.1158/1940-6207.CAPR-12-0117. [in Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Joe AK, Schnoll-Sussman F, Bresalier RS, Abrams JA, Hibshoosh H, Cheung K, et al. Phase Ib randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in patients with Barrett’s Esophagus. Cancer Prev Res. 2015;8:1131–7. doi: 10.1158/1940-6207.CAPR-14-0274-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res. 2011;64:113–22. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 121. Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70:1040–5. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 122. Lu YP, Lou YR, Peng QY, Nghiem P, Conney AH. Caffeine decreases phospho-Chk1 (Ser317) and increases mitotic cells with cyclin B1 and caspase 3 in tumors from UVB-treated mice. Cancer Prev Res. 2011;4:1118–25. doi: 10.1158/1940-6207.CAPR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chen H, Hayek S, Rivera Guzman J, Gillitt ND, Ibrahim SA, Jobin C, et al. The microbiota is essential for the generation of black tea theaflavins-derived metabolites. PLoS One. 2012;7:e51001. doi: 10.1371/journal.pone.0051001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48:409–16. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Schonthal AH. Adverse effects of concentrated green tea extracts. Mol Nutr Food Res. 2011;55:874–85. doi: 10.1002/mnfr.201000644. [DOI] [PubMed] [Google Scholar]