Abstract
The aim of this study was to investigate probiotic attributes of Saccharomyces cerevisiae ARDMC1 isolated from traditional rice beer starter cake and its hypocholesterolemic effects on Wistar rats fed a high-cholesterol diet. The indigenous isolate ARDMC1 showed potential probiotic characteristics such as tolerance to simulated gastrointestinal stress conditions, autoaggregation properties, and adhesion to intestinal epithelium Caco-2 cell line. In addition, ARDMC1 isolate exhibited in vitro cholesterol assimilation properties in media supplemented with cholesterol. Furthermore, administration of probiotic isolate to rats fed a hypercholesterolemic diet resulted in significant reduction of serum total cholesterol, low-density lipoprotein cholesterol, and triglyceride at the end of 42 days. The present study envisages ARDMC1 as a promising starter culture for the preparation of functional foods with properties to combat cardiovascular diseases.
Keywords: atherogenic index, cholesterol lowering, in vivo model, Probiotic yeast
1. Introduction
Cardiovascular disease (CVD) and its related complications are triggered by elevated serum cholesterol levels and are considered as the leading causes of death worldwide. According to a report published by the World Health Organization, 17.5 million people died from CVDs in 2012, representing 31% of all global deaths and morbidity is expected to increase to 23.3 million by 2030 [1,2].
Several forms of therapy have been reported to prevent CVD; however, the resources available for its management in low and middle-income countries are limited. Statins are the most well-known hypolipidemic drugs, which act as inhibitors of the enzyme 3-hydroxy-3-methylglutaryl-coen-zyme reductase essential for the metabolic pathway producing cholesterol and other isoprenoids in the body [3]. However, side effects associated with statins, such as myalgia and muscle weakness, increased fatigue, reduced energy, deteriorating hyperglycemia, and risk of new-onset diabetes pose a greater threat to human health [4,5].
Many studies have shown that probiotics or products containing them impart various health benefits that include prevention of CVDs and enhancing general wellness of consumers [6,7]. Probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [8]. Recent studies also have reported that the microbial communities for probiotics have revealed some interesting attributes of yeasts, and functional foods prepared with probiotic yeasts reduce the levels of lipids in the serum of rats fed a high-cholesterol diet [9]. Yeast cells from food and of animal origin are also reported to remove cholesterol from media, under simulated conditions that mimics the gastrointestinal tracts of monogastric animals [10,11].
To address the alarming burden of CVDs, a cost-effective and affordable alternative strategy is required to reduce the CVD-related risk factors. A recent approach for lowering cholesterol and hence minimizing the risk of CVDs is the use of probiotic-and prebiotic-based functional or health foods that modulate the gut microbial ecosystem or their metabolic products [12,13]. In recent studies, antiatherosclerotic effects of traditional fermented foods from Asia have gained much attention [14]. Keeping this in view, Saccharomyces cerevisiae ARDMC1 was investigated for in vitro probiotic attributes and cholesterol-lowering properties in rat fed a high-cholesterol diet. In the present study, S. cerevisiae ARDMC1 was isolated from starter culture cake of Apong, a traditional rice beer of the Mishing tribe of Assam, India. Apong has a sociocultural status as a popular alcoholic beverage inimitable to the Mishing community and reported to have various health promoting benefits [15].
2. Materials and methods
2.1. Yeast and bacterial strains, culture media, and growth conditions
In this study, S. cerevisiae ARDMC1 (out of 23 isolates) was isolated from rice beer starter culture of Assam, India and selected based upon promising probiotic attributes and their in vitro cholesterol-removal properties. The probiotic reference strain Saccharomyces boulardii was isolated from marketed probiotic drug (Lupin Laboratories, India). Salmonella enterica typhimurium MTCC 1252 was procured from Microbial Type Culture Collection (Chandigarh, India). The yeast strains, S. cerevisiae ARDMC1 and S. boulardii were grown in Yeast and Mould Broth (YMB; HiMedia, India) medium under shaking conditions at 30°C for 48 hours, whereas S. enterica typhimurium was grown in nutrient agar medium (HiMedia) at 37°C for 24 hours.
2.2. Molecular identification of isolates
The 5.8S internal transcribed spacer (ITS) rDNAs of yeast isolates ARDMC1 and S. boulardii were amplified using the primers ITS-1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) [16]. The D1/D2 domain of the 26S rDNA gene was amplified using the primer pair NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) [17]. The polymerase chain reaction (PCR) amplification was performed in an Eppendorf Thermocycler according to a protocol developed in our laboratory with slight modifications [18]. Briefly, amplification parameters consisted of an initial denaturation step of 3 minutes at 95°C, followed by 30 cycles of 95°C for 30 seconds, primer annealing for 30 seconds at 58°C, elongation for 1 minute at 72°C, and final extension of 10 minutes at 72°C for one cycle. The amplified PCR product was purified and subjected to automated DNA sequencing using a 3130 Genetic Analyzer (Applied Biosystems, Rotkreuz, Switzerland). The phylogenetic tree was generated by the neighbor-joining method using MEGA version 5.05. The sequences obtained were submitted to Genbank (http://www.ncbi.nlm.nih.gov/genbank).
2.3. In vitro gastrointestinal stress tolerance test
Tolerance to simulated gastrointestinal conditions was evaluated according to a method developed by Maragkoudakis et al [19] with some modifications. Briefly, cells from a 48-hour culture were harvested by centrifugation at 6000 × g for 5 minutes at 4°C, washed once with phosphate-buffered saline (PBS) solution with pH 7.4, before being resuspended in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) at 108 colony forming units (CFU)/mL. SGF was prepared by supplementing sterilized PBS, pH 2, pH 3, and pH 4 with pepsin to a final concentration of 3 g/L. SIF was prepared by supplementing sterilized PBS, pH 6.8 and pH 8 with pancreatin (Sigma Aldrich, St. Louis, MO, USA) to a final concentration of 1 g/L and 0.3 g/L bile salt mixtures. The resistance of isolates was evaluated by counting viable colony on YMB agar plates after 0 hours, 1 hour, 2 hours, and 3 hours for SGF and 0 hours, 1 hour, 2 hours, 3 hours, and 4 hours for SIF experiments, respectively.
2.4. Hydrophobicity
The hydrophobicity of the isolates was assessed by following the method of Rosenberg [20]. Cells from a previously grown culture were harvested and washed twice with PBS, pH 7.4. Cell count was adjusted to ~109 CFU/mL. Two milliliters of cell suspension was mixed with an equal volume of n-hexadecane by vortexing for 2 minutes. The aqueous and the organic phases were allowed to separate by keeping the mixture undisturbed for 1 hour. After that, the aqueous layer was gently pipetted out and optical density at 600 nm (OD600) was measured. The cell surface hydrophobicity was calculated as:
| (1) |
Where Absinitial represents initial absorption before mixing and Absfinal represents final absorption after mixing with n-hexadecane.
2.5. Autoaggregation and coaggregation
Autoaggregation capacity of the isolate was evaluated using previously described methods [21]. Briefly, 4 mL of cell suspension (109 CFU/mL) was vortexed for 10 seconds and incubated at 37°C. After 4 hours, a 100-μL aliquot was taken out from the upper surface, mixed with 900 μL PBS (pH 7.4) and OD600 was measured. Autoaggregation percentage was calculated as:
| (2) |
Where At = absorbance at 4 hours and A0 = absorbance at 0 hours.
For the coaggregation assay, a mixed culture was prepared by mixing equal volume (2 mL) of the isolate and pathogenic indicator (S. enterica typhimurium MTCC 1252) cell suspension (~109 CFU/mL) by vortexing for 10 seconds. Control tubes were set up at the same time, containing 4 mL each bacterial suspension. The absorbance at 600 nm of the suspensions was measured followed by mixing and 4 hours of incubation at room temperature. Samples were taken in the same way as in the autoaggregation assay. The percentage of coaggregation was calculated using the equation of Handley et al [22].
| (3) |
Where x and y represent each of the two strains in the control tubes, and (x + y) represents the mixture.
2.6. Probiotic adhesion to Caco-2 cells
The human colorectal adenocarcinoma Caco-2 cell line was procured from the National Centre for Cell Science (Pune, India). The cell line was routinely grown and maintained in minimal essential medium supplemented with 20% heat-inactivated fetal bovine serum [23]. Media and reagents were purchased from Sigma (India) and Gibco Life Technologies (Waltham, MA, USA).
The adhesion study was performed using previously described method of García-Cayuela et al [24]. Briefly, Caco-2 cells were seeded at a concentration of 104 cells/mL in 24-well tissue culture plates (NEST Biotechnology, New Jessey, 07065, USA) and grown for 14 days to achieve about 80% confluence at 37°C in a humidified atmosphere containing 5% CO2. The yeast cells grown previously were harvested by centrifugation (2000×g for 5 minutes), washed twice with PBS 1× and resuspended in minimal essential medium without antibiotic supplementation at a concentration of ~108 CFU/mL. For adhesion assay, Caco-2 cell monolayers were washed to remove medium containing antibiotic and inoculated with fresh yeast cell suspensions (yeast cells: Caco-2 cells at a ratio of 10: 1). After an incubation period of 1 hour, the medium was discarded and the wells were gently washed three times with PBS buffer to remove nonadhering probiotic cells. Finally, Caco-2 monolayers were trypsinized with 0.25% trypsin–EDTA solution (Sigma) and the number of adherent isolates was determined by serial dilution plating on yeast and mould agar (YMA). All the experiments were performed in triplicate. Adhesion data were expressed as the percentage of yeast cells adhered compared to the total inoculum added (CFU yeast cells adhered/CFU yeast cells added). For visualization of adhesion, Caco-2 cell monolayers were washed three times with PBS, dried in air, and adherent yeast cells were observed in microscope (EVOS FL Cell Imaging System; ThermoFisher, USA) under 20× magnification after fixing with 3% paraformaldehyde.
2.7. Assimilation of cholesterol by isolates
The ability to assimilate cholesterol was determined following the method of Lye et al [25] with some modifications. Yeast isolates were inoculated into YMB supplemented with water-soluble cholesterol (polyoxyethanyl-cholesterylsebacate; Sigma) at a concentration of 50 μg/mL and 0.3% ox bile, followed by incubation at 37°C. The presence of cholesterol in the spent broth was determined using a colorimetric method [26]. The attachment of cholesterol particles onto the cell surface was visualized by scanning electron microscopy. The cell pellet obtained after centrifugation in the previous step was fixed with 2.5% glutaraldehyde for 6 hours. The samples were then centrifuged and the pellet was resuspendedin 1× PBS, pH 7.4 for 1 hour containing 1% osmium tetroxide (Sigma–Aldrich, St. Louis, MO, USA). Further cells were dehydrated in graded concentrations of ethanol. Then, specimens were platinum coated using a Jeol JFC-1600 auto-fine coater and observed under scanning electron microscopy (JSM-6390 LV; Jeol, Akishima, Tokyo, Japan) at 20 kV.
2.8. Cholesterol-lowering ability of isolates in Wistar rat model
Cholesterol-lowering ability was tested following a method reported by Kumar et al [27], with some modifications. Thirty adult male Wistar rats (mean body weight 150 g) used in this study were maintained at the Defence Research Laboratory, Tezpur, India (Animal Ethics Approval No: 1AEC/01/2015). We housed six animals per cage under a constant 12-hour light–12-hour dark cycle with a controlled temperature at 25°C and a relative humidity of 56–60%. Both S. cerevisiae ARDMC1 and S. boulardii were separately mixed with high-fat diet (HFD) to achieve a final concentration of ~ 108 CFU/g. The CFU/g of the diet was counted by suspending 1 g diet in 9 mL PBS, and then the appropriate dilutions were made and plated on YMA to determine the exact CFU/g of the diet. All animals were acclimatized by feeding on a basal diet for 1 week prior to experiment. The animals were divided into five experimental groups: (1) normal diet, (2) HFD, (3) HFD with statin (atorvastatin, Macleods Pharmaceuticals Ltd., Mumbai, India), (4) HFD with S. boulardii, and (5) HFD with S. cerevisiae ARDMC1. All rats had free access to water and their specific diets (20 g/100 g of body weight/day). The body weights of the animals were measured weekly. The experimental HFD was formulated and fed to the animals for 42 days with slight modification of the method reported earlier [27]. The HFD contained cholesterol (0.5%), dalda (30%), refined soy oil (10%), and wheat flour (50%) as major constituents.
2.9. Analysis of serum lipid profile
For the collection of blood samples, animals were fasted overnight and on the next day, blood was collected from the retro-orbital sinus and preserved in prechilled tubes at −20°C. For serum lipid analysis, blood samples were collected at 21 and 42 days of feeding trial. The collected blood samples were centrifuged at 2000 × g for 15 minutes at 4°C. The serum obtained was analyzed for total cholesterol (TC), triglyceride (TG), and high-density lipoprotein (HDL)-cholesterol using commercial enzymatic kits (Autopak; M/s Siemens Diagnostics, Mumbai, India). Friedewald’s equation [28] was applied to analyze the following plasma lipid fractions: (1) low-density lipoprotein (LDL)-cholesterol = TC – HDL-cholesterol – (TG/5) and (2) very low-density lipoprotein (VLDL)-cholesterol = TG/5. Atherogenic index (AI) was calculated according to the formula AI = (TC – HDL-C)/HDL-C and LDL-C/HDL-C ratio was calculated as the ratio between plasma LDL-C and HDL-C as proposed by Harnafi et al [29]. All concentrations were expressed in mg/dL.
2.10. Statistical analysis
Results were expressed as mean ± standard deviation and the data were analyzed by GraphPad version 5.00 (San Diego, CA, USA). One-way analysis of variance with Tukey’s multiple comparison tests was used to compare the differences among various groups. A value of p < 0.05 was considered to be statistically significant.
2.11. Ethical approval
All rat model experiments were performed according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India with institutional approval no: 1AEC/01/2015.
3. Results
3.1. Molecular identification of isolates
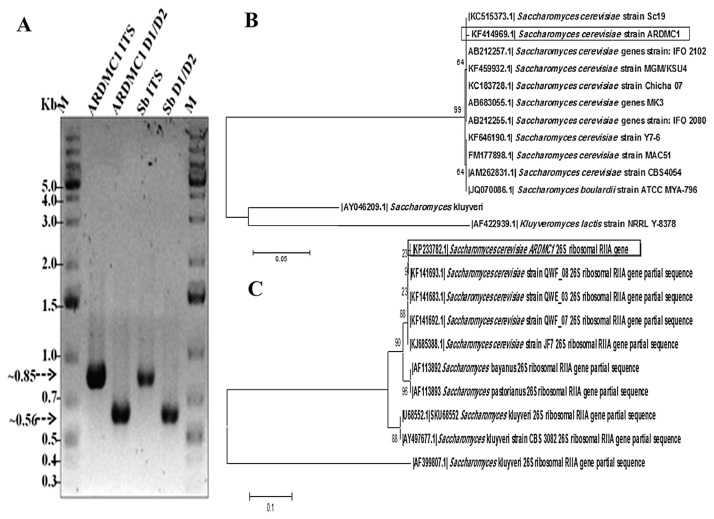
Figure 1A depicts the amplicons obtained from PCRs. The sizes of the amplicons were around 850 bp (5.8S-ITS) and 560 bp (D1/D2 domain) for ARDMC1, which corroborate with the expected amplicon size of Saccharomyces strain reported previously [30,31]. The sequences obtained from 5.8S-ITS rDNA and domain D1/D2 were used to construct the phylogenetic tree (Figures 1B and 1C) using the neighbor-joining method and showed maximum similarity to S. cerevisiae Sc19 and S. cerevisiae QWF, respectively. The gene sequences of 5.8S-ITS rDNA (KF414969) and the D1/D2 domain of the large subunit of 26S rDNA (KP233782) were submitted to NCBI GenBank. The probiotic reference strain S. boulardii used in this study was confirmed based on sequencingof 5.8S-ITS rDNA and 26S rDNA.
Figure 1.
(A) Molecular typing of Saccharomyces cerevisiae ARDMC1 and Saccharomyces boulardii by using ITS primer (Lanes 2 and 4) and 26S rRNA gene D1/D2 region (Lanes 3 and 5); Lanes 1 and 6 (M) indicate GeneRuler 1 Kb Plus DNA ladder (Fermentas, Thermo Scientific, USA). Phylogenetic tree showing S. cerevisiae ARDMC1 with closely related species based upon (B) 5.8S ITS rRNA and (C) D1/D2 26S rRNA sequences. Bootstrap values (1000 replicates) are indicated at branch nodes. ITS =internal transcribed spacer.
3.2. In vitro gastrointestinal stress tolerance test
Table 1 represents the survivability of ARDMC1 and probiotic reference strain S. boulardii after exposure to SGF and SIF conditions. When exposed to pepsin supplemented SGF of pH 2 for 3 hours, the yeast cell (ARDMC1) count was reduced to ~1.5 log units or 20.79%, whereas S. boulardii showed a decrease of 2.16 log units or 26.6% after exposure to the same conditions. The viability of ARDMC1 at pH 3 and pH 4 was found to be more than 6 log units which is comparable to that of S. boulardii. However, survivability of ARDMC1 was not inhibited significantly (p < 0.05) and showed considerable resistance under SIF conditions at pH 8.0, although its viability decreased from log 8.2 CFU/mL to about log 6.4 CFU/mL.
Table 1.
Simulated gastric fluid tolerance test and simulated intestinal fluid tolerance test
| Gastric fluid tolerance | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Time (h) | pH 2.0 (SGF) | pH 3.0 (SGF) | pH 4.0 (SGF) | |||
|
|
|
|
||||
| ARDMC1 | Sb | ARDMC1 | Sb | ARDMC1 | Sb | |
| 0 | 7.63 ± 0.405a | 8.10 ± 0.54a | 7.86 ± 0.169a | 7.79 ± 0.34a | 7.78 ± 0.34a | 7.91 ± 0.172a |
| 1 | 6.13 ± 0.721b | 6.62 ± 0.359b | 6.06 ± 0.015bd | 6.176 ± 0.28b | 6.69 ± 0.42b | 6.24 ± 0.51b |
| 2 | 6.321 ± 0.45b | 6.28 ± 0.55c | 6.56 ± 0.412c | 6.193 ± 0.151b | 7.052 ± 0.6b | 6.68 ± 0.43b |
| 3 | 6.043 ± 0.06b | 5.94 ± 0.084c | 6.18 ± 0.413cd | 6.26 ± 0.345b | 6.27 ± 0.042b | 5.84 ± 0.34b |
|
| ||||||
| Intestinal fluid tolerance | ||||||
|
| ||||||
| Time (h) | pH 6.8 (SIF) | pH 8.0 (SIF) | ||||
|
|
|
|||||
| ARDMC1 | Sb | ARDMC1 | Sb | |||
|
| ||||||
| 0 | 7.92 ± 1.43 | 7.48 ± 0.44 | 8.24 ± 0.75 | 8.07 ± 0.94a | ||
| 1 | 6.62 ± 0.40 | 6.20 ± 0.20 | 6.27 ± 0.15 | 5.96 ± 0.26bc | ||
| 2 | 6.90 ± 0.48 | 6.0 ± 0.19 | 6.77 ± 0.64 | 6.31 ± 0.34ac | ||
| 3 | 6.49 ± 0.25 | 6.10 ± 0.2 | 6.12 ± 0.36 | 6.20 ± 0.19ac | ||
| 4 | 6.58 ± 0.26 | 6.59 ± 0.17 | 6.47 ± 6.47 | 6.57 ± 0.47ac | ||
Viability of isolates under simulated gastric fluid at pH 2.0, pH 3.0, and pH 4.0 and SIF simulated intestinal fluid at pH 6.8 and pH 8.0. Values are represented as mean ± standard deviation, n = 3. Different letters along the same column represent significant differences (p < 0.05). ARDMC1 = Saccharomyces cerevisiae ARDMC1; Sb = Saccharomyces boulardii; SGF = simulated gastric fluid; SIF = simulated intestinal fluid.
3.3. Hydrophobicity, autoaggregation, and coaggregation
Hydrophobicity indices of ARDMC1 and S. boulardii were found to be 61.4 ± 0.10% and 58.59 ± 0.2.25%, respectively. The autoaggregation capability of ARDMC1 and S. boulardii did not differ significantly (p < 0.05) and were 43.19 ± 0.90% and 40.40 ± 0.93%, respectively. Both ARDMC1 and S. boulardii could coaggreagate S. enterica typhimurium up to 54.47 ± 0.02 (Table 2).
Table 2.
Autoaggrgation, coaggregation, and hydrophobicity of ARDMC1 and Sb
| Isolates | Autoaggregation (%) | Coaggregation (%) | Hydrophobicity (%) |
|---|---|---|---|
| ARDMC1 | 43.19 ± 0.90 | 44.41 ± 0.005 | 61.4 ± 0.1 |
| Sb | 40.40 ± 0.93 | 54.47 ± 0.024 | 58.59 ± 0.225 |
Values are represented as mean ± standard deviation, n = 3. ARDMC1 = Saccharomyces cerevisiae ARDMC1; Sb = Saccharomyces boulardii.
3.4. Microbial adhesion to Caco-2 cells
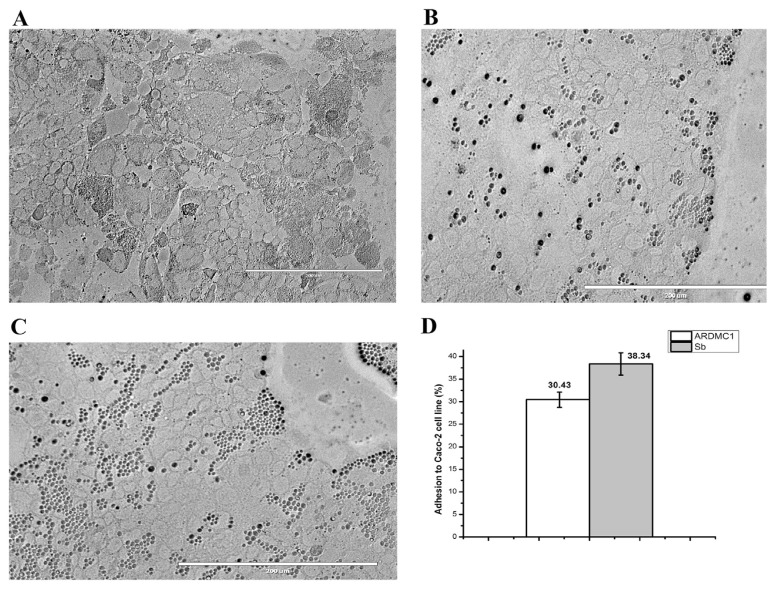
Microscopic observation showed adhesion between Caco-2 cell culture and the isolated strain as depicted in Figures 2A–C. The ARDMC1 strain was also examined quantitatively for its capability to adhere to Caco-2 cells using S. boulardii as a reference strain. The adhesion ability of probiotic reference strain S. boulardii (38.34%) was found to be higher as compared with ARDMC1 (30.43%) as shown in Figure 2D.
Figure 2.
Adhesion to Caco-2 cell line as observed under inverted microscope (20×). (A) Control Caco-2 cells before addition of yeast cells, (B) adhesion of Saccharomyces cerevisiae ARDMC1, (C) adhesion of Saccharomyces boulardii and (D) percentage adhesion as calculated by plate count method.
3.5. In vitro cholesterol assimilation and SEM of cholesterol binding to cellular surface
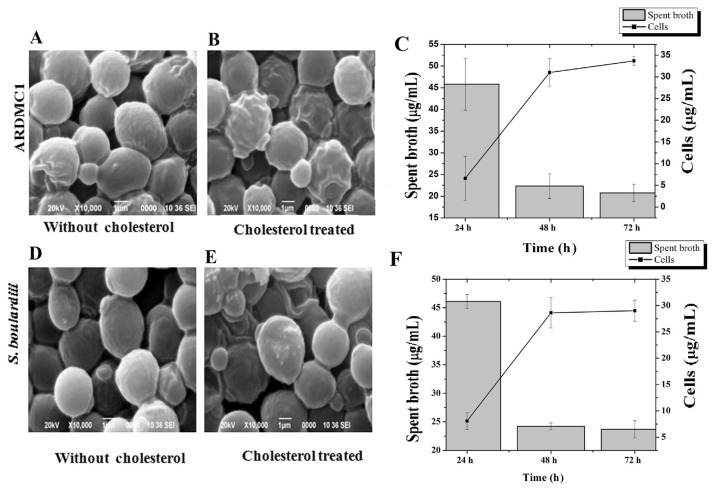
The cholesterol assimilation efficiency of spent broth and cells of ARDMC1 and S. boulardii from culture media is presented in Figure 3. The concentration of cholesterol determined by spent broth decreased up to 20.76 ± 2 μg/mL that have been associated with the significantly (p < 0.05) increased amount of cholesterol (33.69 ± 1.0 μg/mL) detected in the resuspended cells of ARDMC1 after 72 hours incubation (Figure 3C). Contrary to this, S. boulardii could assimilate a small amount of cholesterol (8.62 μg/mL) after 72 hours incubation (Figure 3F). The cholesterol assimilation of ARDMC1 yeast cells was found to be higher as compared with S. boulardii isolated from the marketed probiotic drug, which is in accordance with the findings of Psomas et al [11]. Scanning micrographs also showed that cholesterol particles adhered to the cellular surface of yeast cells (Figures 3A, 3B, 3D, and 3E). The bounded cholesterol particles on the cell surfaces resulted in the roughness of cell wall. The capability of cholesterol-binding appeared to be strain specific [32].
Figure 3.
(A) SEM image of Saccharomyces cerevisiae ARDMC1 grown in YMB without cholesterol. (B) S. cerevisiae ARDMC1 grown with cholesterol. (C) Cholesterol assimilation by S. cerevisiae ARDMC1 spent broth and resting cells. (D) SEM image of Saccharomyces boulardii grown in YM broth without cholesterol, (E) S. boulardii grown with cholesterol, (F) cholesterol assimilation by S. boulardii spent broth and resting cells. SEM =scanning electron microscopy; YMB =Yeast and Mold Broth.
3.6. Cholesterol-lowering ability of probiotic strains in Wistar rat model
S. cerevisiae ARDMC1 and S. boulardii were evaluated for their cholesterol-lowering capability under in vivo conditions using a Wistar rat model system. As shown in Table 3, the levels of TG, LDL-C, VLDL-C, and TC of the HFD group were found to be significantly higher (p < 0.05) than those of the HFD + statin, HFD + ARDMC1 and HFD + S. boulardii groups after 42-days feeding trial. In contrast to the control group (HFD), the probiotic supplemented group (HFD + ARDMC1) showed lower levels of TC (62.75 ± 1.34 mg/dL), TG (122.70 ± 10.04 mg/dL), and LDL-C (21.71 ± 0.18 mg/dL) at the end of 42 days. As anticipated, the HFD + statin group had significantly lower levels of serum TC, TG, and LDL-C levels of 65.83 mg/dL, 112.90 mg/dL, and 27.95 mg/dL, respectively. These values were not significantly different from those of the HFD + ARDMC1 and HFD + S. boulardii groups. Moreover, in probiotic-supplemented groups, AI and LDL-C/HDL-C ratios were significantly lower than in the HFD group. There is a dearth of published information on hypocholesterolemic effects of yeast cells and few attempts have been made to assess the possible cholesterol-lowering mechanisms based on in vivo experiments [27,33]. At the end of the study, the mean body weight of the normal diet group was found to be significantly lower than the other treatment groups. The mean body weights of probiotic-treated groups were similar to the statin-treated group.
Table 3.
Effect of probiotic isolates on serum lipids level (mg/dL) and body weight (g)
| ND | HFD | HFD + statin | HFD + ARDMC1 | HFD + Sb | p | |
|---|---|---|---|---|---|---|
| 21 days | ||||||
| HDL-C | 12.16 ± 2.14 | 16.50 ± 2.03 | 13.00 ± 3.52 | 12.80 ± 2.17 | 14.25 ± 2.87 | 0.6644 |
| TG | 74.27 ± 6.41a | 108.12 ± 11.44b | 87.87 ± 6.94c | 80.36 ± 6.35a | 85.63 ± 4.19a | < 0.0001 |
| TC | 59.60 ± 5.37a | 98.88 ± 9.55b | 75.06 ± 7.73c | 81.42 ± 7.78c | 74.70 ± 3.76c | < 0.0001 |
| LDL-C | 32.58 ± 5.84a | 62.86 ± 11.27b | 44.49 ± 9.15ac | 52.55 ± 7.84ac | 43.33 ± 7.19bc | 0.0001 |
| VLDL-C | 14.85 ± 1.28a | 21.62 ± 2.29b | 17.57 ± 1.39c | 16.07 ± 1.27ac | 17.13 ± 0.84ac | < 0.0001 |
| AI | 4.02 ± 0.92 | 6.11 ± 1.48 | 5.35 ± 2.64 | 5.52 ± 1.29 | 4.43 ± 1.27 | 0.2905 |
| LDL/HDL | 2.78 ± 0.78 | 4.52 ± 1.13 | 3.88 ± 2.07 | 4.24 ± 1.11 | 3.20 ± 1.10 | 0.2296 |
| Body weight | 234.16 ± 7.36ab | 245 ± 8.94b | 229.16 ± 8.01a | 240.0 ± 6.12ab | 234.16 ± 3.76ab | 0.0088 |
| 42 d | ||||||
| HDL-C | 14.33 ± 2.08 | 16.33 ± 1.26 | 13.33 ± 4.16 | 15.50 ± 3.54 | 16.33 ± 1.53 | 0.4881 |
| TG | 93.37 ± 6.70a | 260.20 ± 14.32b | 119.43 ± 3.00a | 122.70 ± 10.04a | 108.73 ± 10.24a | < 0.0001 |
| TC | 59.13 ± 1.45a | 173.97 ± 8.86b | 66.50 ± 0.44a | 62.75 ± 1.34a | 65.63 ± 3.30a | < 0.0001 |
| LDL-C | 26.13 ± 2.04a | 110.37 ± 3.82b | 29.28 ± 3.43a | 21.71 ± 0.18a | 27.55 ± 6.24a | < 0.0001 |
| VLDL-C | 18.67 ± 1.34a | 52.04 ± 2.82b | 23.89 ± 0.60a | 24.54 ± 2.01a | 21.75 ± 2.05a | < 0.0001 |
| AI | 3.18 ± 0.57a | 9.67 ± 0.33b | 4.29 ± 1.49a | 2.88 ± 0.75a | 3.05 ± 0.48a | < 0.0001 |
| LDL/HDL | 1.87 ± 0.44a | 6.82 ± 0.64b | 2.38 ± 0.91a | 1.35 ± 0.30a | 1.64 ± 0.48a | < 0.0001 |
| Body weight | 268.33 ± 5.16a | 314.16 ± 5.84b | 295.83 ± 9.70c | 306.0 ± 6.51bc | 300.83 ± 7.35c | < 0.0001 |
Values are represented as mean ± standard deviation, n = 6. Means with different letters in the same row are significantly different (p < 0.05), checked by Tukey’s multiple comparison test, GraphPad Prism, version 5.0. AI = atherogenic index; ARDMC1 = Saccharomyces cerevisiae ARDMC1; HDL = high-density lipoprotein; HFD: high-fat diet; LDL = low-density lipoprotein; ND = normal diet; Sb = Saccharomyces boulardii; TC = total cholesterol; TG = triglyceride; VLDL = very low-density lipoprotein.
4. Discussion
The use of yeast as potential probiotics has gained interest in the food pharmaceutical industry. Yeast contributes a significant role in the production of some cheeses and fermented milk [34,35]. They have the capability to significantly enhance the aroma of the final product through the generation of free amino acids and free fatty acids [36]. The low pH of the stomach and intestinal fluids (e.g., bile and pancreatic juice) towards the distal part of the gastrointestinal tract is inhibitory to most of the microbes. The probiotic candidate must withstand these harsh conditions to exhibit health benefits on the host. In the present study, ARDMC1 yeast isolate exhibited considerable tolerance to gastrointestinal stress conditions, proving its suitability as putative probiotics. Previous studies also have reported that probiotic yeasts can survive under gastrointestinal stress conditions [37].
The adhesion to and colonization of the intestinal mucosa is an essential criterion for probiotic microorganisms to enhance the immune system and impart health benefits on the host. The adhesion assay was performed using the well-established Caco-2 intestinal cell line from human colonic adenocarcinoma. The differences in the adhesion abilities of yeast cells might be strain specific as reported earlier [38]. Our results suggest that both the yeast strains have significant adhesive properties, but adhesion of S. boulardii to Caco-2 cells was found to be similar to S. cerevisiae ARDMC1, in spite of showing less autoaggregation and hydrophobic properties than the latter. These findings imply that the adhesion properties of probiotic yeasts are not correlated to hydrophobicity. This finding is in accordance with the work of Martins et al [39].
Low HDL-C and elevated levels of LDL-C, VLDL-C, TC, and TG are associated with the inception of CVD [40] and it is important to keep them at the threshold level. Cholesterol lowering by yeasts in in vitro conditions is due to the uptake of cholesterol as in growing yeast cells [11]. Yeasts also contain β-glucans that are reported to bind to bile acids in the intestine, resulting in a decrease in bile acid pool and enhanced cholesterol breakdown. Moreover, yeasts also enhance the production of short-chain fatty acids, which in turn reduce the synthesis of hepatic cholesterol [41]. In our study, the strain ARDMC1 that showed promising in vitro cholesterol assimilation activity also showed significant lowering (p < 0.05) of “bad cholesterols” LDL-C, VLDL-C, TC, and TG compared to the HFD group, without affecting the concentration of “good cholesterol” HDL-C in serum. Rats fed with yeast cells or yeast-supplemented functional food showed similar results in previous studies. Both AI and LDL-C/HDL-C ratio, which are two important risk factors for atherosclerosis and other CVDs [42], were found to be decreased in the probiotic-treated groups. The body weight increase of the rats with probiotic feed supplements may be attributed to the growth-promoting effects of yeast [9].
5. Conclusion
Our isolate S. cerevisiae ARDMC1 showed potential in vitro probiotic attributes and hypocholesterolemic activity. Supplementation of ARDMC1 to rats fed a HFD resulted in a significant decrease in serum cholesterol levels in rats. Therefore, it can be concluded that S. cerevisiae ARDMC1 has the capability to play an important role in the preparation of functional foods with health-promoting effects.
Acknowledgments
This work was financially supported by Department of Biotechnology (DBT), India (Grant No. BT/219/NE/TBP/2011). The authors also acknowledge the help of Manash Pratim Pathak and Johirul Islam of DRL, Tezpur during in vivo experiments.
Funding Statement
This work was financially supported by Department of Biotechnology (DBT), India (Grant No. BT/219/NE/TBP/2011).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCES
- 1.World Health Organization. Cardiovascular diseases (CVDs) Fact sheet No 355 volume 2015. Geneva: WHO; 2015. [Accessed 14 September, 2016]. Updated January 2015. Available at: http://www.who.int/mediacentre/factsheets/fs355/en. [Google Scholar]
- 2. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liang Y, Chen J, Zuo Y, Ma KY, Jiang Y, Huang Y, Chen ZY. Blueberry anthocyanins at doses of 0.5 and 1% lowered plasma cholesterol by increasing fecal excretion of acidic and neutral sterols in hamsters fed a cholesterol-enriched diet. Eur J Nutr. 2012;52:869–75. doi: 10.1007/s00394-012-0393-6. [DOI] [PubMed] [Google Scholar]
- 4. Gotto AM. Statins, cardiovascular disease, and drug safety. Am J Cardiol. 2006;97:S3–5. doi: 10.1016/j.amjcard.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 5. Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–90. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 6. Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, Yadav H. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res. 2012;2012:902917. doi: 10.1155/2012/902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Plessas S, Bosnea L, Alexopoulos A, Bezirtzoglou E. Potential effects of probiotics in cheese and yogurt production: a review. Eng Life Sci. 2012;12:433–40. [Google Scholar]
- 8. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 9. Ogunremi OR, Sanni AI, Agrawal R. Hypolipidaemic and antioxidant effects of functional cereal-mix produced with probiotic yeast in rats fed high cholesterol diet. J Funct Foods. 2015;17:742–8. [Google Scholar]
- 10. Chen LS, Ma Y, Maubois JL, He SH, Chen LJ, Li HM. Screening for the potential probiotic yeast strains from raw milk to assimilate cholesterol. Dairy Sci Technol. 2010;90:537–48. [Google Scholar]
- 11. Psomas EI, Fletouris DJ, Litopoulou TE, Tzanetakis N. Assimilation of cholesterol by yeast strains isolated from infant feces and Feta cheese. J Dairy Sci. 2003;86:3416–22. doi: 10.3168/jds.S0022-0302(03)73945-9. [DOI] [PubMed] [Google Scholar]
- 12. Wu PW. A review on the analysis of ingredients with health care effects in health food in Taiwan. J Food Drug Anal. 2015;23:343–50. doi: 10.1016/j.jfda.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conterno L, Fava F, Viola R, Tuohy KM. Obesity and the gut microbiota: does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011;6:241–60. doi: 10.1007/s12263-011-0230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee BH, Lai YS, Wu SC. Antioxidation, angiotensin converting enzyme inhibition activity, nattokinase, and antihypertension of Bacillus subtilis (natto)-fermented pigeon pea. J Food Drug Anal. 2015;23:750–7. doi: 10.1016/j.jfda.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kardong D, Deori K, Sood K, Yadav RNS, Bora TC, Gogoi BK. Evaluation of nutritional and biochemical aspects of Poro apong (Saimod) – a homemade alcoholic rice beverage of Mising tribe of Assam, India. Ind J Trad Knowl. 2012;11:499–504. [Google Scholar]
- 16.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–22. [Google Scholar]
- 17. Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek. 1998;73:331–71. doi: 10.1023/a:1001761008817. [DOI] [PubMed] [Google Scholar]
- 18. Manhar AK, Saikia D, Bashir Y, Mech RK, Nath D, Konwar BK, Mandal M. In vitro evaluation of celluloytic Bacillus amyloliquefaciens AMS1 isolated from traditional fermented soybean (Churpi) as an animal probiotic. Res Vet Sci. 2015;99:149–56. doi: 10.1016/j.rvsc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 19. Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int Dairy J. 2006;16:189–99. [Google Scholar]
- 20. Rosenberg M. Microbial adhesion to hydrocarbons: twenty-five years of doing MATH. FEMS Microbiol Lett. 2006;262:129–34. doi: 10.1111/j.1574-6968.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 21. Del Re B, Sgorbati B, Miglioli M, Palenzona D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol. 2000;31:438–42. doi: 10.1046/j.1365-2672.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 22. Handley PS, Harty DW, Wyatt JE, Brown CR, Doran JP, Gibbs AC. A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. J Gen Microbiol. 1987;133:3207–17. doi: 10.1099/00221287-133-11-3207. [DOI] [PubMed] [Google Scholar]
- 23. Manhar AK, Saikia D, Borah A, Das AS, Gupta K, Roy R, Mahanta CL, Mukhopadhyay R, Mandal M. Assessment of goat milk-derived potential probiotic L. lactis AMD17 and its application for preparation of dahi using honey. Ann Microbiol. 2016;66:1217–28. [Google Scholar]
- 24. García-Cayuela T, Korany AM, Bustos IP, Gómez de Cadinaños L, Peláez RT, Martínez-Cuesta MC. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res Int. 2014;57:44–50. [Google Scholar]
- 25. Lye HS, Rahmat-Ali GR, Liong MT. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J. 2010;20:169–75. [Google Scholar]
- 26. Rudel LL, Morris MD. Determination of cholesterol using o-phtaldealdehyde. J Lipid Res. 1973;14:364–6. [PubMed] [Google Scholar]
- 27. Kumar R, Grover S, Batish VK. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague-Dawley rats. Br J Nutr. 2011;105:561–73. doi: 10.1017/S0007114510003740. [DOI] [PubMed] [Google Scholar]
- 28. Friedewald WRR, Levy I, Frederickson DS. Lipoproteins in serum. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29. Harnafi H, Serghini CH, el Houda BN, Aziz M, Amrani S. Hypolipidemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem. 2008;108:205–12. [Google Scholar]
- 30. Martorell P, Querol A, Fernandez-Espinar MT. Rapid identification and enumeration of Saccharomyces cerevisiae cells in wine by real-time PCR. Appl Env Microbiol. 2005;71:6823–30. doi: 10.1128/AEM.71.11.6823-6830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van der Aa Kühle A, Jespersen L. The taxonomic position of Saccharomyces boulardii as evaluated by sequence analysis of the D1/D2 domain of 26S rDNA, the ITS1-5.8S rDNA-ITS2 region and the mitochondrial cytochrome-c oxidase II gene. Syst Appl Microbiol. 2003;26:564–71. doi: 10.1078/072320203770865873. [DOI] [PubMed] [Google Scholar]
- 32. Ooi LG, Liong MT. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 2010;11:2499–522. doi: 10.3390/ijms11062499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alfitori AM, Bashir A, Bahriz AF. Brewer’s yeast (Saccharamyces cervisiae) has hypolipidemic effect in hyperlipidemic model. J Yeast Fungal Res. 2013;4:33–7. [Google Scholar]
- 34. Fleet GH. Yeasts in dairy products. J Appl Bacteriol. 1990;68:199–211. doi: 10.1111/j.1365-2672.1990.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 35. Pereira-Dias S, Potes M, Marinho A, Malfeito-Ferreira M, Loureiro V. Characterisation of yeast flora isolated from an artisanal Portuguese ewes’ cheese. Int J Food Microbiol. 2000;60:55–63. doi: 10.1016/s0168-1605(00)00323-8. [DOI] [PubMed] [Google Scholar]
- 36. Ferreira A. Yeasts as adjunct starters in matured Cheddar cheese. Int J Food Microbiol. 2003;86:131–40. doi: 10.1016/s0168-1605(03)00252-6. [DOI] [PubMed] [Google Scholar]
- 37. Czerucka D, Piche T, Rampal P. Yeast as probiotics – Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26:67–78. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- 38. Zivkovic M, Cadez N, Uroic K, Miljkovic M, Tolinacki M, Dousova P, Kos B, Raspor P, Topisirovic L, Golic N. Evaluation of probiotic potential of yeasts isolated from traditional cheeses manufactured in Serbia and Croatia. J Intercult Ethnopharmacol. 2015;4:12. doi: 10.5455/jice.20141128051842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martins FS, Nardi RMD, Arantes RME, Rosa CA, Neves MJ, Nicoli JR. Screening of yeasts as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J Gen Appl Microbiol. 2005;51:83–92. doi: 10.2323/jgam.51.83. [DOI] [PubMed] [Google Scholar]
- 40. Weissglas-Volkov D, Pajukanta P. Genetic causes of high and low serum HDL cholesterol. J Lipid Res. 2010;51:2032–57. doi: 10.1194/jlr.R004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bell S, Goldman VM, Bistrian BR, Arnold AH, Ostroff G, Forse RA. Effect of β-glucan from oats and yeast on serum lipids. Crit Rev Food Sci Nutr. 1999;39:189–202. doi: 10.1080/10408399908500493. [DOI] [PubMed] [Google Scholar]
- 42. Munoz Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]