Abstract
The authenticity determination of white rice is crucial to prevent deceptive origin labeling and dishonest trading. However, a non-destructive and comprehensive method for rapidly discriminating the geographical origins of white rice between countries is still lacking. In the current study, we developed a volatile organic compound based geographical discrimination method using headspace solid-phase microextraction coupled to gas chromatography–mass spectrometry (HS-SPME/GC–MS) to discriminate rice samples from Korea and China. A partial least squares discriminant analysis (PLS-DA) model exhibited a good classification of white rice between Korea and China (accuracy = 0.958, goodness of fit = 0.937, goodness of prediction = 0.831, and permutation test p-value = 0.043). Combining the PLS-DA based feature selection with the differentially expressed features from the unpaired t-test and significance analysis of microarrays, 12 discriminatory biomarkers were found. Among them, hexanal and 1-hexanol have been previously known to be associated with the cultivation environment and storage conditions. Other hydrocarbon biomarkers are novel, and their impact on rice production and storage remains to be elucidated. In conclusion, our findings highlight the ability to rapidly discriminate white rice from Korea and China. The developed method maybe useful for the authenticity and quality control of white rice.
Keywords: HS-SPME, GC-MS, Oryza sativa L., Origin discrimination, Volatile organic compound
1. Introduction
Rice (Oryza sativa L.), especially white rice, is the staple food of most Asian countries. In the modern era, rice has been cultivated in many countries around the world. This ultimately expands rice diversity in terms of genetics and rice field ecology [1]. Despite the significant divergence in rice quality and composition, it appears impossible to distinguish the geographical origins of white rice through its appearance or routine analyses [2]. Hence, illegal distribution and mislabeling for tax avoidance is a matter of concern for rice consuming countries [3]. To prevent the dishonest trading of white rice, systematic and high-throughput discrimination methods using modern analytical chemistry, which exhibit high sensitivity and precision, are now more crucial than ever.
Among analytical techniques, spectroscopy based techniques have been most commonly employed in rice research and have mainly been used for comparisons of rice constituents and derivatives. For instance, near infrared reflectance (NIR) spectroscopy has been applied in rice quality assessment and authentication because the sampling method is quite simple and the data acquisition time per sample is short [4]. On the other hand, nuclear magnetic resonance spectroscopy (NMR) has also been widely used for rice research [5]. Unlike NIR, NMR distinguishes only the differences of specific functional groups. NMR results consist of spectra in which the constituent elements make the quantitative comparison possible for each component. However, NMR possesses the major drawback that its sensitivity is relatively low [6]. Combinations of a separation technique and a spectroscopic method, such as liquid chromatography (LC) or gas chromatography (GC) coupled with mass spectrometry (MS), are currently the methods of choice for compound characterization in complex samples [7,8]. Especially, MS can profile a large number of components with high sensitivity and selectivity through mass to charge ratios (m/z) and fragment patterns of specific components. Nevertheless, one major drawback of MS is the relatively low reproducibility among laboratories. Overall, however, the above mentioned analytical methods have shown excellent performance in observing various biochemical phenotypes and the effects of environmental factors, such as rice cultivation conditions (e.g., temperature) and genetic factors (e.g., varieties) [9].
Although rice constituents have often been analyzed using spectroscopy based techniques, the geographical discrimination of white rice using volatile organic compounds (VOCs) has not been conducted. VOCs constitute the fragrance of rice, which is also an important phenotype [10]. Thus, the composition of VOCs in rice may be applied to differentiate rice samples with different origins. The analysis of volatile materials is mainly performed by integrating various methods for VOC extraction and GC–MS. Among these, headspace solid-phase microextraction (HS-SPME), a non-exhaustive and non-invasive extraction method, has been widely used prior to the analysis of VOCs [11]. The comparative advantages of HS-SPME include simplicity, the ability to reuse the sample, high sensitivity, and reproducibility [12,13].
In this study, HS-SPME coupled with GC–MS was utilized for the comprehensive profiling of various VOCs of white rice originating from different regions without any pre-analytical sample treatment. Our objective was to show that the volatiles can be used for geographical discrimination as well as flavor evaluation. Geographical discrimination is a major topic that include various and complex factors, from morphology to genetics, in which a universal method remains to be elucidated [14]. However, it seems more appropriate to find the discriminatory biomarkers that belong to small molecules whose concentrations vary depending on the environment. In particular, since white rice alters in flavor regarding different cultivation conditions, VOCs that constitute fragrance may be applied not only to the discrimination of cultivars but also to the differentiation of the country. Approaching from this view point, we first selected the optimal fiber and an efficient time for extracting volatile matters from white rice. Next, the aberrant VOCs of white rice originating from Korea and China were subject to advanced multivariate statistical analysis to find the discriminatory biomarkers. Our results suggest that the HS-SPME/GC–MS approach is suitable for the goal of identifying white rice from different geographical origins and evaluating white rice flavor.
2. Materials and methods
2.1. Materials
Twenty four different commercial white rice samples (12 from Korea and 12 from China) cultivated in 2016 were collected from local Korean and Chinese markets during the same period. All samples were stored unopened in a −70 °C frozen cabinet until completely used. The details of the white rice samples are shown in Table 1. In addition, the solid-phase microextraction (SPME) fibers and holder were purchased from Supelco (Bellefonte, PA, USA). Crimp type caps and polytetrafluoroethylene (PTFE)/silicone septa were obtained from Agilent (Avondale, PA, USA).
Table 1.
The origins of the 24 analyzed white rice samples from Korea and China.
| Country | No | Origin | Cultivar |
|---|---|---|---|
| Korea | K1 | Gunsan | Sindongjin |
| K2 | Gwangju | Choochung | |
| K3 | Ansung | Choochung | |
| K4 | Cheorwon | Ode | |
| K5 | Jinjoo | Samgwang | |
| K6 | Goheung | Unkwang | |
| K7 | Imsil | Sindongjin | |
| K8 | Yeoncheon | Daean | |
| K9 | Dangjin | Samgwang | |
| K10 | Ganghwa | Choochung | |
| K11 | Boseong | Hopyeong | |
| K12 | Uiseong | Ilpoom | |
| China | C1 | Heilongjiang | Daohuaxiang |
| C2 | Liaoning | Zhenshu | |
| C3 | Jilin | Baijinxiang | |
| C4 | Jilin | Shujing | |
| C5 | Heilongjiang | Wuchang | |
| C6 | Shandong | Zhanglixiang | |
| C7 | Heilongjiang | Zhanglixiang | |
| C8 | Heilongjiang | Zhonghuahe | |
| C9 | Heilongjiang | Daohuaxiang | |
| C10 | Jilin | Daohuaxiang | |
| C11 | Heilongjiang | Zhanglixiang | |
| C12 | Jiangsu | Ruanxiangdao |
2.2. Volatile compound extraction using HS-SPME
For the extraction, 1 g of white rice was stored in a 10 mL headspace vial with a crimp type cap and a PTFE/silicone septum to prevent the leakage of volatile compounds. Next, the vial with rice samples was heated using an oven at 80 °C for 20 min.
Five types of fiber coatings were tested, including carboxen/polydimethylsiloxane (CAR/PDMS) with 75 μm thickness, divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) with 50/30 μm thickness, polyacrylate (PA) with 85 μm thickness, polydimethylsiloxane (PDMS) with 100 μm thickness, and polydimethylsiloxane/divinylbenzene (PDMS/DVB) with 65 μm thickness for method optimization. Prior to the sampling, all the above fibers were conditioned using their recommended temperature and duration: CAR/PDMS, 300 °C for 60 min; DVB/CAR/PDMS, 270 °C for 60 min; PA, 280 °C for 60 min; PDMS 100 μm, 250 °C for 30 min; and PDMS/DVB, 250 °C for 30 min. Next, each conditioned fiber was sealed with a septum before being inserted into the vial containing rice samples for 40 min.
2.3. GC–MS analysis
The GC–MS analysis was performed using a Shimadzu GCMS-QP2010 system (Shimadzu, Kyoto, Japan). A DB-5 capillary column having a length of 30 m, an inner diameter of 0.25 mm, and a film thickness of 0.25 μm (Agilent, PA, USA) was utilized. The GC system was used in split mode (1:2 split) with the injector maintained at 270 °C. Helium as the carrier gas was maintained at a constant flow of 1 mL/min. The temperature conditions of the column were as follows: primary temperature of 40 °C increased to 100 °C at 5 °C/min, held for 3 min, and increased to 250 °C at 10 °C/min. The temperatures of the ion source and the transfer line were 200 °C and 270 °C, respectively. The MS detection proceeded in electron impact mode, and the ionization energy was 70 eV. The mass range scan was from m/z 40 to m/z 300 to look for the VOCs because of their low molecular weights.
2.4. Data processing and compound identification
Peak detection, isotopic peak grouping, peak list alignment, and gap filling were conducted using MZmine 2.5 [15]. Mass detection (centroid method, noise level of 5E3), chromatogram building (minimal peak height of 6E3 and m/z tolerance of 200 ppm), and deconvolution (Savitzky–Golay algorithm) were performed. Data alignment was conducted using the RANSAC algorithm (retention time tolerance of 0.1 min; retention time tolerance after correction of 0.05 min; iterations of 100,000; minimum number of points of 10%; and a threshold value of 4). The same retention time and m/z range gap filter were applied for gap filling. The processed data were exported to a *.csv file for further analysis. Compound identifications were carried out manually by matching the unknown spectra with two mass spectral libraries (NIST 08 and Wiley 7) and retention index (RI) values derived from n-alkane solution (Supelco) analysis. In addition, automatic compound identifications using the Golm library provided by the erah R package (version 1.05) were also conducted [16]. The match factor criterion for identification was ≥80%. The identities of the differentially expressed metabolites, when available, were further confirmed using standards.
2.5. Statistical analysis
Prior to the analysis, the processed data were further treated using log transformation and Pareto scaling. Significance analysis of microarray (SAM) and a t-test were applied for individual feature selection. A feature was considered to be differentially expressed when it has a p-value < 0.05 and an FDR < 0.1. Only the overlapping features between SAM and the t-test were considered to be significant. In addition, hierarchical cluster analysis (HCA) and k-means clustering were conducted to explore the variance tendency, examine the natural grouping structure, and visualize the samples. PLS-DA, 10-fold cross validation, and a 1000-time permutation test were performed to seek the best discrimination model for white rice samples from Korea and China. Finally, all the features with variable importance in the projection (VIP) score ≥ 1 and differentially expressed in the univariate analysis were considered to be potential candidates for the discrimination of white rice samples from Korea and China. The analyses were conducted using Metaboanalyst 3.0 [16].
3. Results and discussion
3.1. The impact of regional discrimination of white rice by VOCs
To prevent the illegal and dishonest distribution of white rice products in the global marketplace, it is indeed necessary to develop a comprehensive method for the discrimination of white rice geographical origins. One strategy is to focus on analyzing particular metabolites that are affected by cultivation and storage environment. From this angle, SPME, a nondestructive and rapid sample extraction method that does not require pretreatment of samples, can be utilized to develop a method for discriminating geographical origins of white rice between countries that is better than conventional LC and NMR based analysis methods [17]. There is considerable evidence that plant VOCs are altered by abiotic stress [18]. In addition, VOCs are considered to be an important factor related to white rice flavor that can be analyzed using GC–MS [10]. Therefore, it is objective and accurate to develop a VOC-based geographical discrimination method for white rice using SPME coupled with a GC–MS system.
3.2. SPME optimization: fiber selection, extraction temperature, and extraction time
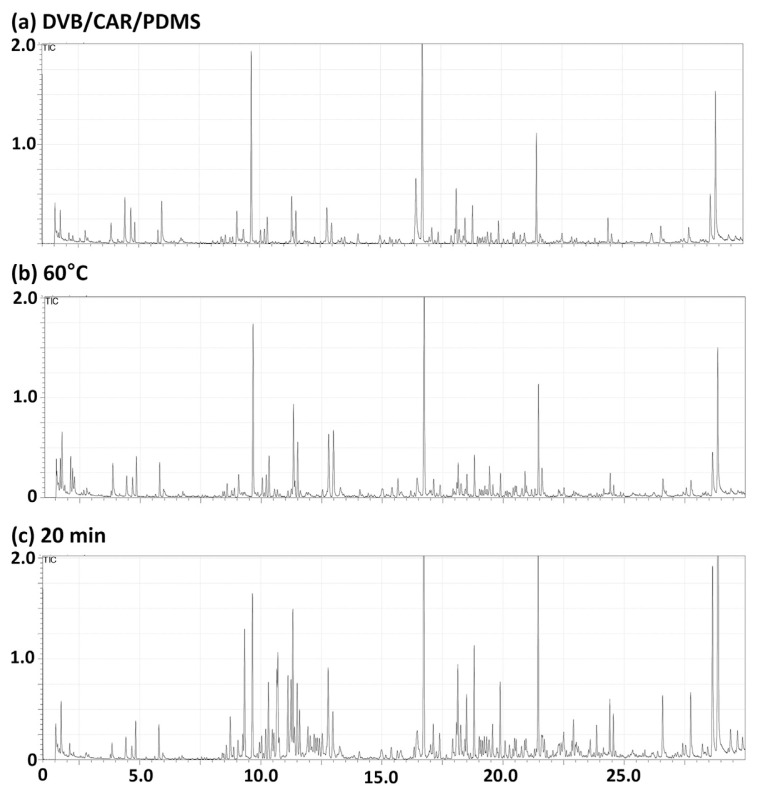
Prior to the discrimination analysis, the optimal SPME extraction conditions for white rice analysis were established. The number of detected peaks and identified compound classes were used to determine the efficiency of the extraction condition. The representative total ion chromatograms of the optimized extraction condition are shown in Fig. 1. The chromatograms of other suboptimal conditions can be found in Fig. S1. The fiber coating selection is the first step in SPME method optimization because the efficiency of the extraction process is critically dependent on the fiber coating and the sample matrix distribution constant [12]. Five different SPME fibers were tested using the same analysis conditions: extraction time = 30 min, extraction temperature = 80 °C. The results showed that PA and PDMS had poor extraction performance for VOCs in white rice. DVB/CAR/PDMS, PDMS/DVB, and CAR/PDMS, on the other hand, had better performances. However, only the DVB/CAR/PDMS fiber was selected because of its excellent sample absorption efficiency. As has been described previously, the DVB/CAR/PDMS fiber covers a wide range of volatiles and semi-volatiles. Thus, this fiber is appropriate for analyzing flavors and fragrances of white rice, which include many C2–C20 compounds [12]. Additionally, the optimal extraction temperature and extraction time of PDMS/DVB/CAR were determined for VOC analysis of white rice samples. For extraction temperature, five distinct temperatures, 20 °C, 40 °C, 60 °C, 80 °C, and 100 °C, were examined. A higher sample temperature tends to increase headspace capacity, which eventually increases the rate of extraction. However, the sample recovery decreases when the extraction temperature increases excessively [12,19]. In our experiment, the extraction at 60 °C showed the best VOC extraction performance. As one of the most crucial steps of SPME method development, the optimal extraction time was also determined by testing five distinct time periods: 10 min, 20 min, 30 min, 40 min, and 50 min. The extraction time determines the extraction efficiency, reproducibility, and analytical sensitivity. Broadly speaking, shorter extractions are more suitable for volatile substances, while longer extractions are favorable for less-volatile substances [19]. The optimization process revealed that when the extraction time increased, the number of peaks decreased comparatively. Notably, although the 10 min extraction exhibited more detectable peaks than the other time periods, the 20 min extraction showed better performance in terms of the diversity of detectable analytes. Collectively, the DVB/CAR/PDMS fiber at 60 °C for a 20 min extraction was confirmed to be the best condition for white rice VOC extraction.
Fig. 1.
Optimal SPME conditions for VOC profiling. (a) The GC–MS spectra of a representative sample using DVB/CAR/PDMS SPME extraction with extraction time = 30 min, extraction temperature = 80 °C. (b) The GC–MS spectra at the optimal DVB/CAR/PDMS SPME extraction temperature (60 °C). (c) The GC–MS spectra at the optimal DVB/CAR/PDMS SPME extraction time (20 min).
3.3. Chemometric analysis for discriminatory biomarker selection
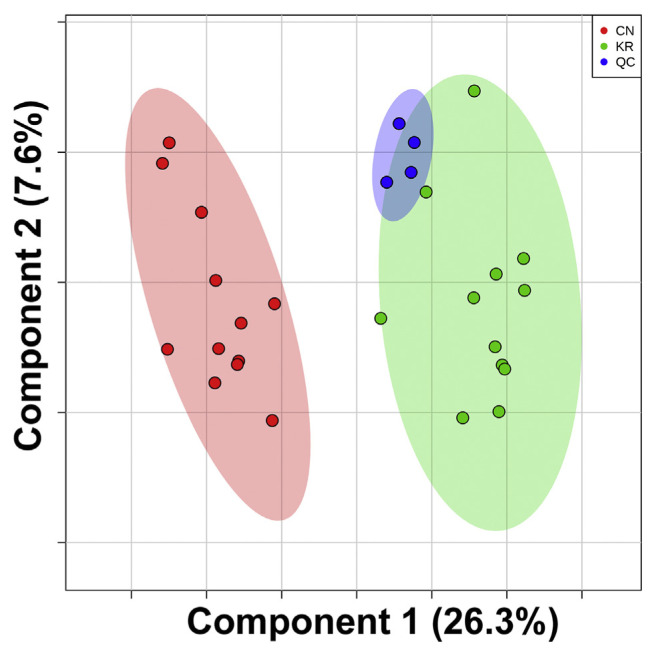
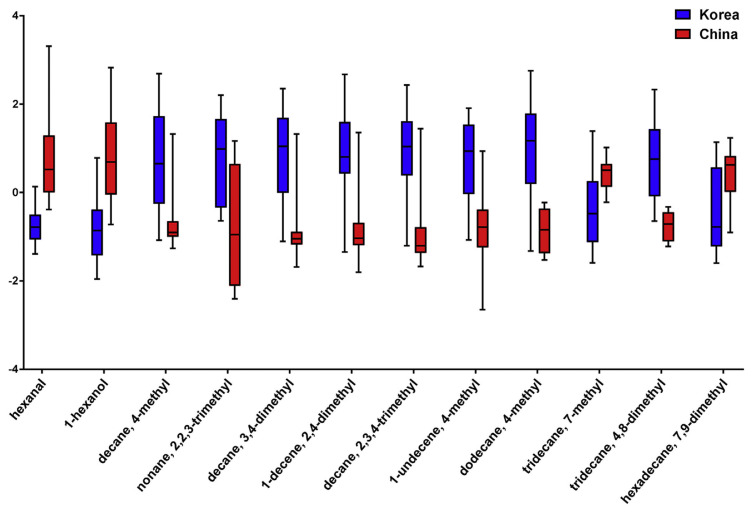
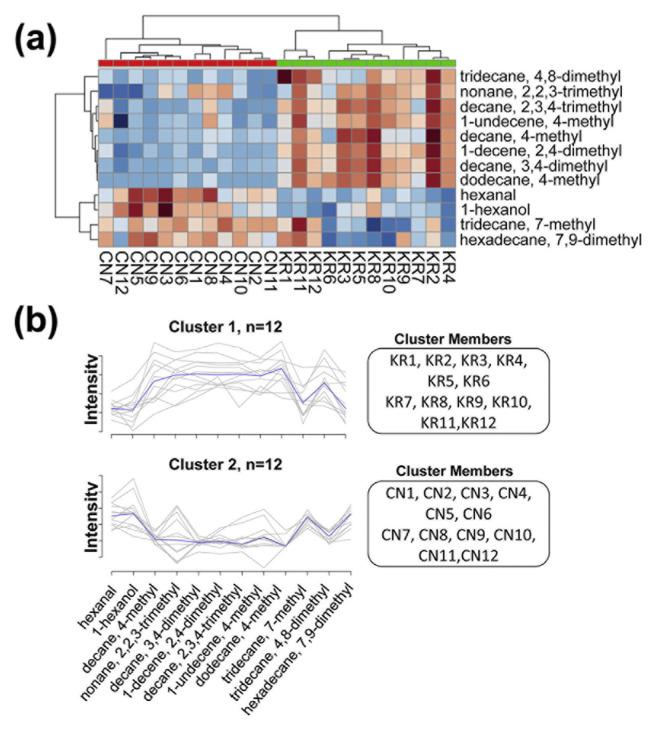
To guarantee the precision of the results, all features that had relative standard deviations (RSDs) larger than 30% were removed. The peak heights of the processed data were then introduced to univariate and multivariate analyses for discriminatory biomarker selection. There were 59 differentially expressed features from the t-test (p-value < 0.05, FDR < 0.1) and 44 differential expressed features from SAM (Fig. S2). Among them, there were 36 overlapped features between the t-test and SAM. These 36 overlapped features were considered to be the more robust differentially expressed features. Additionally, partial least squares discriminant analysis (PLS-DA) supervised classification and PLS-DA based feature selection were also conducted. As shown in Fig. 2, the white rice samples from Korea and China were well separated. The PLS-DA model was validated using a 10-fold cross-validation and permutation test. It revealed that the accuracy, goodness of fit (R2), goodness of prediction (Q2), and B/W based p-values were 0.958, 0.937, 0.831, and 0.043, respectively. Those parameters indicate a compact model for classification. PLS-DA based feature selection introduced 38 features that had VIP scores larger than 1. By overlapping the differentially expressed features of the t-test and SAM and the significant features of the PLS-DA based feature selection, 12 features were finally selected as potential discriminatory biomarkers for white rice samples from Korea and China. After compound identification, surprisingly, there were ten hydrocarbon compounds (8 alkanes and 2 alkenes), one aldehyde (hexanal), and one alcohol (1-hexanol). The details of the selected biomarkers can be found in Fig. 3 and Table 2. The concentrations of hexanal, 1-hexanol, 7-methyl-tridecane, and 7,9-dimethyl-hexadecane were higher in white rice from China, while the concentrations of the other eight biomarkers were higher in white rice from Korea. The unsupervised clustering and visualization using k-means and a heatmap of the 12 selected biomarkers showed two correct clusters of white rice from Korea and China without mislabeling (Fig. 4). The results suggested a 12-VOC discriminatory signature. However, further investigations are needed to guarantee our initial observation.
Fig. 2.
PLS-DA score plot of rice samples from Korea and China and QC samples.
Fig. 3.
The box plot of the 12 discriminatory biomarkers of rice samples from Korea and China. The mean concentrations of hexanal, 1-hexanol, 7-methyl-tridecane, and 7,9-dimethyl-hexadecane are higher in white rice from China, while the mean concentrations of the other eight biomarkers are higher in white rice from Korea.
Table 2.
The characteristics of the 12 discriminatory biomarkers from the statistical analyses.
| Retention time (min) | Compound name | Chemical formula | NIST match (%) | Retention index (RI) value | RSD (%) | VIP score | t-test | SAM | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Reference | Experiment | p-value | FDR | q-value | ||||||
| 4.42 | hexanala | C6H12O | 91 | 806 | 799 | 21.449 | 1.743 | <0.001 | 0.005 | 0.005 |
| 5.95 | 1-hexanola | C6H14O | 92 | 860 | 859 | 28.631 | 1.926 | <0.001 | 0.005 | 0.005 |
| 10.31 | decane, 4-methyla | C11H24 | 95 | 1051 | 1031 | 25.630 | 1.665 | <0.001 | 0.009 | 0.013 |
| 10.58 | nonane, 2,2,3-trimethyl | C12H26 | 92 | 1065 | 1039 | 13.765 | 1.852 | <0.001 | 0.007 | 0.021 |
| 11.24 | decane, 3,4-dimethyl | C12H26 | 91 | 1086 | 1069 | 12.606 | 2.106 | <0.001 | 0.005 | 0.005 |
| 12.01 | 1-decene, 2,4-dimethyl | C12H24 | 90 | 1117 | 1084 | 7.748 | 1.978 | <0.001 | 0.005 | 0.005 |
| 12.32 | decane, 2,3,4-trimethyl | C13H28 | 90 | 1121 | 1098 | 11.944 | 2.061 | <0.001 | 0.005 | 0.005 |
| 12.58 | 1-undecene, 4-methyl | C12H24 | 90 | 1140 | 1121 | 29.447 | 1.771 | <0.001 | 0.006 | 0.007 |
| 17.43 | dodecane, 4-methyla | C13H28 | 90 | 1249 | 1240 | 17.141 | 2.226 | <0.001 | 0.005 | 0.004 |
| 18.25 | tridecane, 7-methyla | C14H30 | 93 | 1349 | 1320 | 21.504 | 1.018 | <0.001 | 0.010 | 0.023 |
| 20.40 | tridecane, 4,8-dimethyl | C15H32 | 90 | 1384 | 1373 | 20.486 | 1.799 | <0.001 | 0.005 | 0.005 |
| 24.55 | hexadecane, 7,9-dimethyl | C18H38 | 93 | 1683 | 1642 | 23.236 | 1.005 | <0.001 | 0.010 | 0.077 |
Confirmed by standard.
Fig. 4.
The heatmap and the k-means clustering of the 12-VOC discriminatory signature.
3.4. The role of VOCs in rice flavor and regional discrimination of white rice
Flavor is a very important phenotype along with the texture of white rice, especially for aromatic rice, which includes basmati rice or jasmine rice. Currently, even for non-aromatic rice, various flavors have been added by genetic modifications or supplementation during white rice production [20]. Thus, several papers have analyzed VOCs related to specific flavors of white rice. However, most such studies have focused on VOC compositions of cooked rice or VOCs from different cultivars that were genetically improved for specific flavors [21]. VOCs such as alcohols, phenols, acids, amines, and aldehydes, which have particular flavors, have been well analyzed. However, from these studies, no distinctive patterns of VOCs being derived from different geographical origins of rice were observed [10]. In the current study, various cultivars originating from different regions of Korea and China were randomly gathered and analyzed. Our results showed that some hydrocarbons, especially alkanes, together with one alcohol and one aldehyde, were suggested as discriminatory biomarkers.
3.5. The role of hexanal and 1-hexanol in rice storage and regional discrimination
White rice deteriorates gradually when stored at elevated temperatures, and one of the most important characteristics of rice deterioration is the production of off-flavor compounds. In addition, it is well known that the off-flavor compounds of stored rice mainly result from the deterioration of polyunsaturated fatty acids (PUFAs), especially linoleic acid, which is one of the predominant PUFAs in white rice endosperm [22]. Colder cultivation environments results in higher concentrations of PUFAs [23]. In addition, increased carbonyl compound concentrations are associated with lipid deterioration, and hexanal concentration alters much faster than its companion aldehydes during rice storage, such that it ultimately becomes an indicator of rancidity [24]. Those facts suggest that hexanal concentration depends on three main factors: (1) storage condition and period, (2) fatty acid concentrations, and (3) the temperature alteration, especially during the rice ripening period, of the rice cultivation areas. The variation in hexanal concentration of white rice samples from Korea and China in our study is probably due to those factors.
1-hexanol, with a grass like flavor, has also been identified as a VOC of white rice in previous studies [10]. It occupies the highest proportion among alcohol species in rice [25]. Similar to hexanal, 1-hexanol concentration is also associated with rice storage [26]. For instance, 1-hexanol concentration has been found to be higher in 4 °C stored rice than in 40 °C stored rice [25]. Therefore, storage condition may have a considerable influence on the alteration of 1-hexanol concentration, which explains the significant difference in 1-hexanol in rice samples from Korea and China.
3.6. The role of hydrocarbons in rice storage and regional discrimination
Long-chain alkanes are found in the cuticle and epicuticular lipids in many plant species yet are rarely the major constituents [27]. The alkane composition of the lipids is not only species dependent but also highly sensitive to environmental factors such as lighting conditions, temperature, and humidity. Volatile short-chain alkanes are also synthesized and found in plant tissues and have also been detected at low levels [28]. In white rice, alkanes are thought to be associated with lipid breakdown products. Nevertheless, the role of alkanes on rice flavor has not been greatly investigated to date [10]. In this study, eight alkane compounds (Table 2) were identified as discriminatory biomarkers for white rice from Korea or China. Interestingly, our study revealed that most of the differentially expressed alkanes were enriched in the white rice from Korea. The two exceptions were 7-methyl-tridecane and 7,9-dimethyl-hexadecane; their concentrations were higher in white rice samples from China. Several studies about alkanes in plants have been carried out [29]. The alkane contents in irradiated cereals have also been investigated [30]. Nevertheless, most of the studies have only focused on long-chain alkanes with C14–C33 carbon atoms. The role of short-chain alkanes in rice is poorly understood, and their behavior with respect to different environmental factors remains to be elucidated.
Alkenes are known for grassy, fatty, or soapy flavors [10]. In this study, the concentrations of two alkenes, 2,4-dimethyl-1-decene and 4-methyl-1-undecene, were shown to be higher in white rice samples from Korea. 1-decene, which has a pleasant odor, is the first product in the microbial degradation of decane. Therefore, similarly to decane derived compounds, the 1-decene concentration was higher in Korean rice products. 1-undecene has a mild odor and is mostly found in green vegetables. The higher concentration of 1-undecene in white rice from Korea relative to that from China may result from a similar mechanism. However, a more sophisticated study targeted on alkenes and their corresponding alkanes is needed to clarify the association between environmental factors and hydrocarbon behaviors in white rice.
4. Conclusion
In this study, white rice cultivated in different regions of Korea and China was analyzed using DVB/CAR/PDMS-SPME coupled with GC–MS. Twelve discriminatory biomarkers that can be used to differentiate commercial white rice from the two countries were found. Of these, hexanal and 1-hexanol concentrations are known to correspond to environmental factors as well as storage conditions. On the other hand, the ten discriminatory hydrocarbons are novel, and their behaviors during white rice production and storage remain to be explored. In conclusion, the demonstrated method can be used to discriminate white rice from Korea and China. The method may also be applied to discriminate the origin of other food products.
Acknowledgments
This work was supported by the Rural Development Administration of Korea (PJ01164601) and BK21 Plus Program in 2016.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2017.04.005.
Funding Statement
This work was supported by the Rural Development Administration of Korea (PJ01164601) and BK21 Plus Program in 2016.
REFERENCES
- 1. Reig-Valiente JL, Viruel J, Sales E, Marqués L, Terol J, Gut M, et al. Genetic diversity and population structure of rice varieties cultivated in temperate regions. Rice. 2016;9:58. doi: 10.1186/s12284-016-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suzuki Y, Chikaraishi Y, Ogawa NO, Ohkouchi N, Korenaga T. Geographical origin of polished rice based on multiple element and stable isotope analyses. Food Chem. 2008;109:470–5. doi: 10.1016/j.foodchem.2007.12.063. [DOI] [PubMed] [Google Scholar]
- 3. Vemireddy LR, Satyavathi V, Siddiq E, Nagaraju J. Review of methods for the detection and quantification of adulteration of rice: basmati as a case study. J Food Sci Technol. 2015;52:3187–202. doi: 10.1007/s13197-014-1579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batten GD, Blakeney AB, Glennie-Holmes M, Henry RJ, McCaffery AC, Bacon PE, et al. Rapid determination of shoot nitrogen status in rice using near infrared reflectance spectroscopy. J Sci Food Agric. 1991;54:191–7. [Google Scholar]
- 5. Pramai P, Hamid NAA, Mediani A, Maulidiani M, Abas F, Jiamyangyuen S. Metabolite profiling, antioxidant, and α-glucosidase inhibitory activities of germinated rice: nuclear-magnetic-resonance-based metabolomics study. J Food Drug Anal. 2017 doi: 10.1016/j.jfda.2016.11.023. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emwas A-HM. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Metabonomics Methods Protoc. 2015:161–93. doi: 10.1007/978-1-4939-2377-9_13. [DOI] [PubMed] [Google Scholar]
- 7. De Vos RC, Moco S, Lommen A, Keurentjes JJ, Bino RJ, Hall RD. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat Protoc. 2007;2:778–91. doi: 10.1038/nprot.2007.95. [DOI] [PubMed] [Google Scholar]
- 8. Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc. 2006;1:387–96. doi: 10.1038/nprot.2006.59. [DOI] [PubMed] [Google Scholar]
- 9. Peres B, Barlet N, Loiseau G, Montet D. Review of the current methods of analytical traceability allowing determination of the origin of foodstuffs. Food Control. 2007;18:228–35. [Google Scholar]
- 10. Bryant R, McClung A. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC–MS. Food Chem. 2011;124:501–13. [Google Scholar]
- 11. Fries E, Püttmann W. Improvement of HS-SPME for analysis of volatile organic compounds (VOC) in water samples by simultaneous direct fiber cooling and freezing of analyte solution. Anal Bioanal Chem. 2006;386:1497–503. doi: 10.1007/s00216-006-0715-8. [DOI] [PubMed] [Google Scholar]
- 12. Risticevic S, Lord H, Gorecki T, Arthur CL, Pawliszyn J. Protocol for solid-phase microextraction method development. Nat Protoc. 2010;5:122–39. doi: 10.1038/nprot.2009.179. [DOI] [PubMed] [Google Scholar]
- 13. Lee D-K, Yi T, Park K-E, Lee H-J, Cho Y-K, Lee SJ, et al. Non-invasive characterization of the adipogenic differentiation of human bone marrow-derived mesenchymal stromal cells by HS-SPME/GC-MS. Sci Rep. 2014;4 doi: 10.1038/srep06550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chung IM, Kim JK, Prabakaran M, Yang JH, Kim SH. Authenticity of rice (Oryza sativa L.) geographical origin based on analysis of C, N, O and S stable isotope ratios: a preliminary case report in Korea, China and Philippine. J Sci Food Agric. 2016;96:2433–9. doi: 10.1002/jsfa.7363. [DOI] [PubMed] [Google Scholar]
- 15. Pluskal T, Castillo S, Villar-Briones A, Orešič M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinforma. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Domingo-Almenara X, Brezmes J, Vinaixa M, Samino S, Ramirez N, Ramon-Krauel M, et al. eRah: a computational tool integrating spectral deconvolution and alignment with quantification and identification of metabolites in GC/MS-based metabolomics. Anal Chem. 2016;88:9821–9. doi: 10.1021/acs.analchem.6b02927. [DOI] [PubMed] [Google Scholar]
- 17. Adiani V, Gupta S, Padole R, Variyar PS, Sharma A. SPME-GCMS integrated with chemometrics as a rapid nondestructive method for predicting microbial quality of minimally processed jackfruit (Artocarpus heterophyllus) bulbs. Postharvest Biol Technol. 2014;98:34–40. [Google Scholar]
- 18. Loreto F, Schnitzler J-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–66. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 19. Lord H, Pawliszyn J. Evolution of solid-phase microextraction technology. J Chromatogr A. 2000;885:153–93. doi: 10.1016/s0021-9673(00)00535-5. [DOI] [PubMed] [Google Scholar]
- 20. Champagne ET. Rice aroma and flavor: a literature review. Cereal Chem. 2008;85:445–54. [Google Scholar]
- 21. Zeng Z, Zhang H, Chen JY, Zhang T, Matsunaga R. Flavor volatiles of rice during cooking analyzed by modified headspace SPME/GC-MS. Cereal Chem. 2008;85:140–5. [Google Scholar]
- 22. Shin MG, Yoon SH, Rhee JS, Kwon TW. Correlation between oxidative deterioration of unsaturated lipid and n-hexanal during storage of brown rice. J Food Sci. 1986;51:460–3. [Google Scholar]
- 23. Mano Y, Kawaminami K, Kojima M, Ohnishi M, Ito S. Comparative composition of brown rice lipids (lipid fractions) of Indica and Japonica rices. Biosci Biotechnol Biochem. 1999;63:619. doi: 10.1271/bbb.63.619. [DOI] [PubMed] [Google Scholar]
- 24. Bergman C, Delgado J, Bryant R, Grimm C, Cadwallader K, Webb B. Rapid gas chromatographic technique for quantifying 2-acetyl-1-pyrroline and hexanal in rice (Oryza sativa, L.) Cereal Chem. 2000;77:454–8. [Google Scholar]
- 25. Tsugita T, Hiromichi K. Cooking flavor and texture of rice stored under different conditions. Agric Biol Chem. 1983;47:543–9. [Google Scholar]
- 26.Charalambous G. Off-flavors in foods and beverages. Elsevier; 2013. [Google Scholar]
- 27. Meusel I, Leistner E, Barthlott W. Chemistry and micromorphology of compound epicuticular wax crystalloids (Strelitzia type) Plant Syst Evol. 1994;193:115–23. [Google Scholar]
- 28. Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem. 1999;33:23–88. [Google Scholar]
- 29. Lamarque AL, Fortunato RH, Guzmán CA. Seed oil alkanes from leguminosae species: evencarbon number preference. Phytochemistry. 1998;49:731–6. [Google Scholar]
- 30. Hwang KT, Hong JS, Yang JS, Sohn HS, Weller CL. Detection of alkanes and alkenes for identifying irradiated cereals. J Am Oil Chem. 2001;78:1145–9. [Google Scholar]