Abstract
Cordycepin is one of the most crucial bioactive compounds produced by Cordyceps militaris and has exhibited antitumor activity in various cancers. However, industrial production of large amounts of cordycepin is difficult. The porcine liver is abundant in proteins, vitamins, and adenosine, and these ingredients may increase cordycepin production and bioconversion during C. militaris fermentation. We observed that porcine liver extracts increased cordycepin production. In addition, air supply (2 h/d) significantly increased the cordycepin level in surface liquid-cultured C. militaris after 14 days. Moreover, blue light light-emitting diode irradiation (16 h/d) increased cordycepin production. These findings indicated that these conditions are suitable for increasing cordycepin production. We used these conditions to obtain water extract from the mycelia of surface liquid-cultured C. militaris (WECM) and evaluated the anti-oral cancer activity of this extract in vitro and in vivo. The results revealed that WECM inhibited the cell viability of SCC-4 oral cancer cells and arrested the cell cycle in the G2/M phase. Oxidative stress and mitochondrial dysfunction (mitochondrial fission) were observed in SCC-4 cells treated with WECM for 12 hours. Furthermore, WECM reduced tumor formation in 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis through the downregulation of proliferating cell nuclear antigen, vascular endothelial growth factor, and c-fos expression. The results indicated that porcine liver extracts irradiated with blue light light-emitting diode and supplied with air can be used as a suitable medium for the growth of mycelia and production of cordycepin, which can be used in the treatment of oral cancer.
Keywords: 7, 12-dimethylbenz[a]anthracene, anticancer activity, cordycepin, Cordyceps militaris, mycelial extract, oral cancer, porcine liver extracts, water extract
1. Introduction
Oral cancer is one of the most common cancers worldwide and causes 135,000 deaths annually [1]. Oral squamous cell carcinoma (OSCC) accounts for 90% of oral malignant tumors. Therapeutic targets for cancer, such as gain-of-function mutations in oncogenes and loss-of-function mutations in tumor suppressor genes, are key factors for head and neck carcinogenesis. Tobacco and alcohol consumption have been reported to be major factors contributing to oral cancer development [2]. However, betel quid chewing is one of the major causes of oral cancer in Taiwan, which has high mortality and poor prognosis. Therefore, new therapeutic approaches focusing on molecular targets and mechanisms mediating tumor cell growth or cell death have gained increasing attention for improving patients’ survival and quality of life.
The hamster buccal pouch (HBP) carcinogenesis model is the most well-characterized animal model for investigating the development of oral cancer and the effect of intervention with chemopreventive agents. The development of both OSCC and HBP carcinoma is associated with sustained genetic mutations leading to excessive cell proliferation, prolonged cell survival, and apoptosis evasion [3]. In addition, various morphological and histological similarities are present between human OSCC and HBP carcinoma [4,5], providing a rationale for analyzing the effect of putative chemopreventive agents on the HBP model. Moreover, the HBP model has been used in several studies on oral cancer [6–10].
Chemoprevention through dietary agents has evolved as an effective strategy for controlling the incidence of oral cancer. In recent years, natural food products have received increasing attention because of their potential role in the prevention of and intervention in carcinogenesis and neoplastic progression [11,12]. Therefore, chemotherapy by using functional foods may aid in cancer prevention.
The fungus Cordyceps militaris has been used as a herbal tonic in traditional Chinese medicine for > 300 years. Numerous studies have recently reported that the anticancer activity of Cordyceps sinensis, including the inhibition of B16 melanoma [13], leukemia [14], thyroid carcinoma [15], hepatocellular carcinoma [16], and renal cancer [17] cells, is due to cordycepin, a crucial bioactive compound produced by C. militaris. However, tumor inhibition by cordycepin is attenuated by adenosine deaminase catalysis in vivo [18]. In addition, the inhibition of oral cancer cells by cordycepin and C. militaris remains unclear.
A study reported the use of a biochemical medium as a fermentation medium in the surface liquid cultivation of C. militaris [19]. In addition, another study indicated that natural materials can be used as the components of a fungal medium [20]. These studies suggested that natural materials can be used to cultivate C. militaris. In addition, recent studies have investigated optimal carbon and nitrogen resources and other nutrients required for C. militaris cultivation and cordycepin production [21]. Extracts from the porcine liver contain a high amount of vitamins, proteins, adenosine, and Mg2+; however, the effect of porcine liver water extracts on the surface liquid cultivation of C. militaris for cordycepin production remains unknown. Therefore, in this study, we investigated the effect of porcine liver extracts on the surface liquid cultivation of C. militaris for cordycepin production. In addition, we evaluated the anti-oral-cancer activity of water extract isolated from the mycelia of surface liquid-cultured C. militaris (WECM). Furthermore, we investigated whether WECM inhibits tumor growth in 7,12-dimethylbenz[a]anthracene (DMBA)-induced HBP carcinogenesis.
2. Materials and methods
2.1. Materials
Crystal violet, propidium iodide, DMBA, sodium dodecyl sulfate (SDS), cordycepin, Triton X-100, trypsin, and trypan blue were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fetal bovine serum was purchased from Life Technologies (Auckland, New Zealand). MitoSOX Red and Mitotracker Deep-Red FM were purchased from Invitrogen (Carlsbad, CA, USA). Porcine liver (500 g) extract was prepared through treatment in water (5 L) at 4°C for 24 hours. The liquid extract was filtered and freeze dried, and the porcine liver powder extract was stored at −80°C.
2.2. Sample preparation
C. militaris (BCRC34380) was obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). The liquid seed culture was inoculated with a mycelial mat from a stock slant and cultured in a 500-mL conical flask containing 200 mL medium (10 g/L peptone, 30 g/L glucose, 0.5 g/L MgSO4, 0.5 g/L K2HPO4, and 0.5 g/L KH2PO4) at 25°C on a rotary shaker at 150 rpm for 7 days for maintenance. To evaluate the effects of porcine liver powder extracts on cordycepin production in the mycelia of surface liquid-cultured C. militaris, porcine liver powder extracts at 0.5 g/L, 1 g/L, 5 g/L, 7.5 g/L, and 10 g/L were added into the basal medium (10 g/L peptone, 30 g/L glucose, 0.5 g/L MgSO4, 0.5 g/L K2HPO4, and 0.5 g/L KH2PO4), and the surface liquid cultivation of C. militaris was performed at 25°C for 14 days for cordycepin production. A blue light light-emitting diode (LED; wavelength: 440–450 nm; 1.5 W) was used in a light irradiation experiment (8 h/d, 16 h/d, and 24 h/d). The cultured mycelia were collected and freeze dried, and the mycelium powder was dissolved in 50% ethanol and heated for 60 minutes at 60°C to extract intracellular cordycepin. Finally, the extracts were filtered using a 0.22-μm membrane, and the cordycepin level was assayed through high-performance liquid chromatography. In the high-performance liquid chromatography cordycepin assay, the mobile phase was a mixture of methanol and 0.02 M potassium dihydrogenphosphate (15:85), the column temperature was 40°C, and the flow rate was 1.0 mL/min. The detector was set at 260 nm [20]. In addition, WECM was freeze-dried for use in cell and animal models.
2.3. Cell culture
Human SCC-4 cells were obtained from the Bioresource Collection and Research Center. SCC-4 cells were maintained in Dulbecco’s modified Eagle’s medium/Ham’s F-12 (1:1 v/v) medium supplemented with 100 mL/L fetal bovine serum, 1.5 g/L sodium bicarbonate, 400 ng/mL hydrocortisone, and 10 mL/L antibiotic solution. The cells were incubated in 5% CO2 and a 95% humidified atmosphere at 37°C.
2.4. Cell viability
The cell-killing effect of WECM on oral cancer cells was evaluated using the crystal violet staining assay. The cells were seeded on 24 well plates (3 × 104 cells/well) and treated with various concentrations of the water extracts for 24 hours and 48 hours. The medium was then removed, washed with phosphate-buffered saline (PBS), and stained with 2 g/L crystal violet in 100 mL/L phosphate-buffered formaldehyde for 20 minutes before being washed with water. The crystal violet bound to the cells was dissolved using 20 g/L SDS solution, and its absorbance was measured at 600 nm.
2.5. Cell cycle
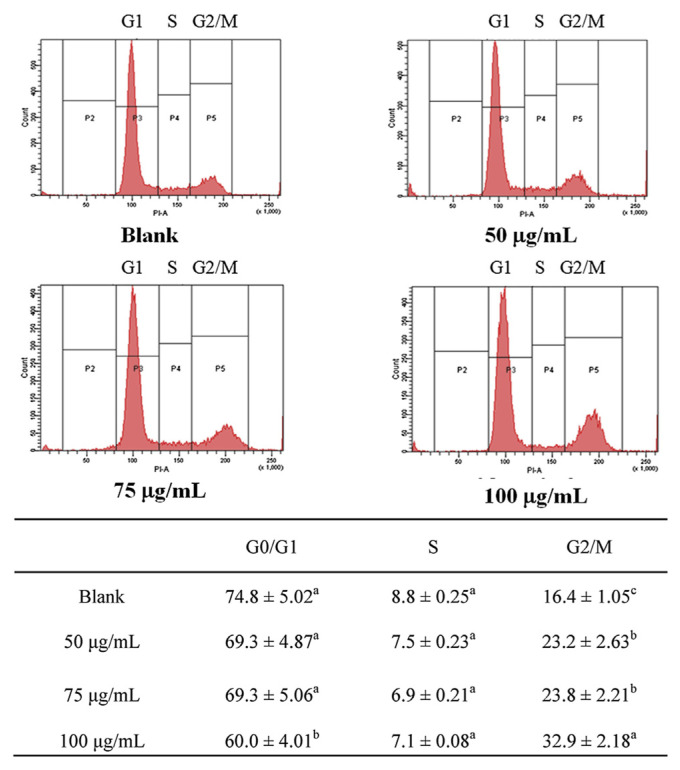
After treatment with WECM for 12 hours, the medium was aspirated and adherent cells were harvested and centrifuged at 300 × g for 5 minutes. The cells were washed with PBS, fixed with ice-cold ethanol at −20°C overnight, and then stained with propidium iodide at room temperature for 30 minutes. The cell cycle distribution was analyzed through flow cytometry by using a FACScan-LSR flow cytometer equipped with the CellQuest software (BD Biosciences, San Jose, CA, USA).
2.6. Measurements of superoxide level and oxidative stress
The assumption that mitochondria serve as the major intra-cellular source of reactive oxygen species (ROS) has been largely based on experiments with isolated mitochondria rather than on direct measurements in living cells. The MitoSOX Red mitochondrial superoxide indicator is a novel fluorogenic dye for highly selective detection of superoxide in the mitochondria of live cells. The MitoSOX Red reagent is a live-cell permeant and rapidly and selectively targets the mitochondria. Once in the mitochondria, the MitoSOX Red reagent is oxidized by superoxide and exhibits red fluorescence. The MitoSOX Red reagent is readily oxidized by superoxide but not by other ROS- or reactive-nitrogen-species-generating systems, and the oxidation of the probe is prevented by superoxide dismutase. The oxidation product becomes highly fluorescent upon binding to nucleic acids. Mitochondrial superoxide is generated as a byproduct of oxidative phosphorylation. The assumption that mitochondria serve as the major intracellular source of ROS has been largely based on experiments with isolated mitochondria rather than on direct measurements in living cells. MitoSOX Red mitochondrial superoxide indicator is a novel fluorogenic dye for highly selective detection of superoxide in the mitochondria of live cells by using a fluorescence microscope. The oxidative stress was monitored through the measurement of ROS. Collected cells were suspended in 500 μL PBS and mixed with 10 μM (final concentration) dichlorodihydrofluorescein diacetate (DCFH-DA) for incubation for 20 minutes at 37°C. The cells were washed three times with PBS to remove redundant DCFH-DA. The cell pellet was mixed with 500 μL PBS, and the ROS level was assayed through flow cytometry (BD Biosciences).
2.7. Mitochondrial morphology
Cells were treated with 250 nM Mitotracker Deep-Red FM (Invitrogen) for 30 minutes in a serum-free culture medium. After being washed twice with PBS, nuclei were stained with Hochest 33342 for 10 minutes. The mitochondrial morphology was observed using a confocal microscope [22].
2.8. Animal model
An experiment was performed using 5-week-old male Syrian hamsters (n = 6) obtained from the National Laboratory Animal Center (Taipei, Taiwan). The hamsters were subjected to a 12-hour light/dark cycle with maintained relative humidity of 60% and temperature of 25°C. The protocol complied with guidelines described in the Animal Protection Law, amended on January 17, 2001, Hua-Zong-(1)-Yi-Tzi-9000007530, Council of Agriculture, Executive Yuan, Taiwan, R.O.C. The model of DMBA-induced HBP carcinogenesis was modified [1,12]. A 0.5% solution of DMBA dissolved in mineral oil was applied to the entire mucosal surface of the left buccal pouch three times a week for 8 consecutive weeks, and WECM was applied for a further 4 weeks. Mineral oil was applied in the control group. The hamsters were killed, and the tumor volume and burden were determined [1,12]. The tumor volume was measured using the V = 4/3 (D1/2) (D2/2) (D3/2), where D1, D2, and D3 are the three diameters (mm) of the tumor. The tumor burden was measured using the following formula: tumor burden = total number of tumors × mean volume.
2.9. Western blotting
The cells were lysed in an ice-cold lysis buffer containing 20 mM Tris–HCl (pH 7.4), 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 500 μM sodium vanadate overnight. The cell extract was then centrifuged (12,000 × g for 10 minutes) to recover the supernatant. The supernatant was used as the cell extract. The cell protein was resolved on 10% SDS–polyacrylamide gel electrophoresis and transferred onto polyvinyldiene fluoride membranes. The membranes were blocked with 5% nonfat dry milk solution for 1 hour and incubated overnight with primary antibodies for 4 hours. Subsequently, each membrane was washed three times for 5 minutes in PBS with Tween 20, shaken in a solution of a horseradish-peroxidase-linked secondary antibody for 1 hour, and washed three times for 5 minutes in PBS with Tween 20. The expression of proteins was detected using an enhanced chemiluminescence reagent (Millipore, Billerica, MA, USA).
2.10. Real-time polymerase chain reaction
Total RNA was obtained using the TRIzol reagent (Gibco BRL Life Technologies, Gaithersburg, MD, USA). Primers were synthesized by MD-Bio (Taipei, Taiwan). The gene expression level was determined through relative quantitative real-time polymerase chain reaction (PCR) (CFX Cycler System; Bio-Rad Laboratories, Hercules, CA, USA). The primers used were as follows: F: 5′GACATCAAGAAGGTGGTGAAGCA′3 and R: 5′CATCAAAGGTG-GAAGAGTGGGA′3 for GAPDH; F: 5′GGTGCTTGGCGGGAGC′3 and R: 5′ATCGCTTGAGCCCAGAAGT′5 for proliferating cell nuclear antigen (PCNA); F: 5′ATGAACTTTCTGCTGTCTTGG′3 and R: 5′TCACCGCCTCGGCTTGTCACA′3 for vascular endothelial growth factor (VEGF); F: 5′GGAGCAGCTTTGTGTGTGTGATTC′3 and R: 5′TCCATCCTTTG-ACTCTGCTCATGG′3 for caspase-3; and F: 5′TTCAGTAGATTGGCAATCTCGGTC′3 and R: 5′TTGCGCA-GATCTGTCCGTCTCTAG′3 for c-fos.
2.11. Statistical analysis
Experimental results were analyzed in triplicate and expressed as the mean ± standard deviation. The results were examined using one-way analysis of variance and Duncan’s multiple range tests, and the significance of differences between sample means was calculated. A p value ≤ 0.05 was considered significant.
3. Results
3.1. Optimal conditions for cordycepin production
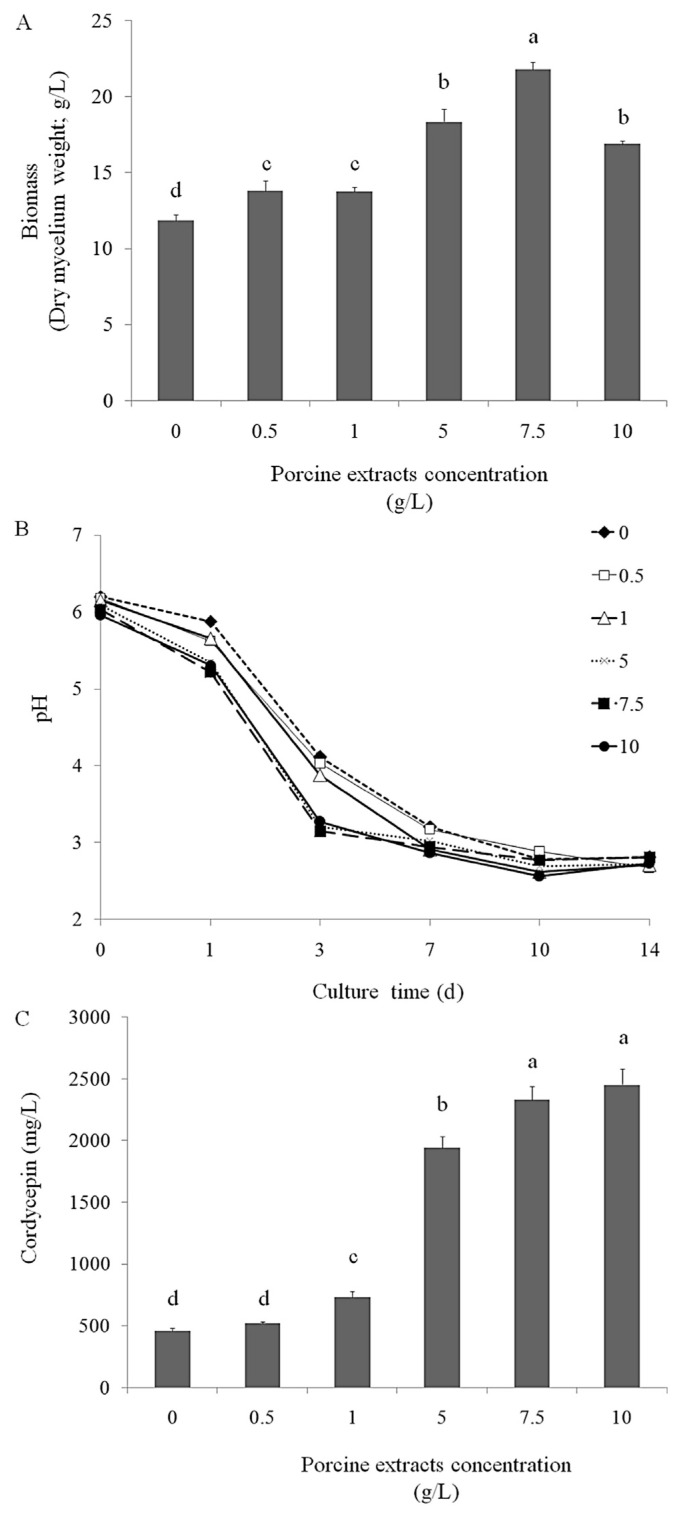
Cordycepin is an anticancer compound and has potential for development as a therapeutic agent [22–25] and functional food. Porcine liver extracts have a high amount of adenosine and vitamins, which are the precursors of cordycepin and act as cofactors during cordycepin bioconversion. This study investigated the optimal condition required for cordycepin production from surface liquid-cultured C. militaris in a medium containing porcine liver extracts. We observed that at 5 g/L, 7.5 g/L, and 10 g/L, porcine liver extracts significantly increased biomass production (mycelium weight) in surface liquid-cultured C. militaris (Figure 1A) and reduced the pH in the surface liquid culture of C. militaris during biomass production (Figure 1B). In addition, we assayed the cordycepin level in the mycelia of surface liquid-cultured C. militaris and observed that at 5 g/L (1942 mg/L), 7.5 g/L (2331 mg/L), and 10 g/L (2452 mg/L), porcine liver extracts significantly increased the production of cordycepin (isolated from the dry mycelia of surface liquid-cultured C. militaris; Figure 1C).
Figure 1.
Effects of various concentrations of porcine liver powder extracts (0.5 g/L, 1 g/L, 5 g/L, 7.5 g/L, and 10 g/L in the medium) on (A) biomass production (g/L in medium), (B) pH, and (C) cordycepin levels (cordycepin mg/dry mycelia g/medium L) of the mycelia of surface liquid-cultured Cordyceps militaris in 200 mL medium at 25°C for 14 days. Data are represented as mean ± standard deviation (n = 3). Significant differences are shown by different letters (p < 0.05).
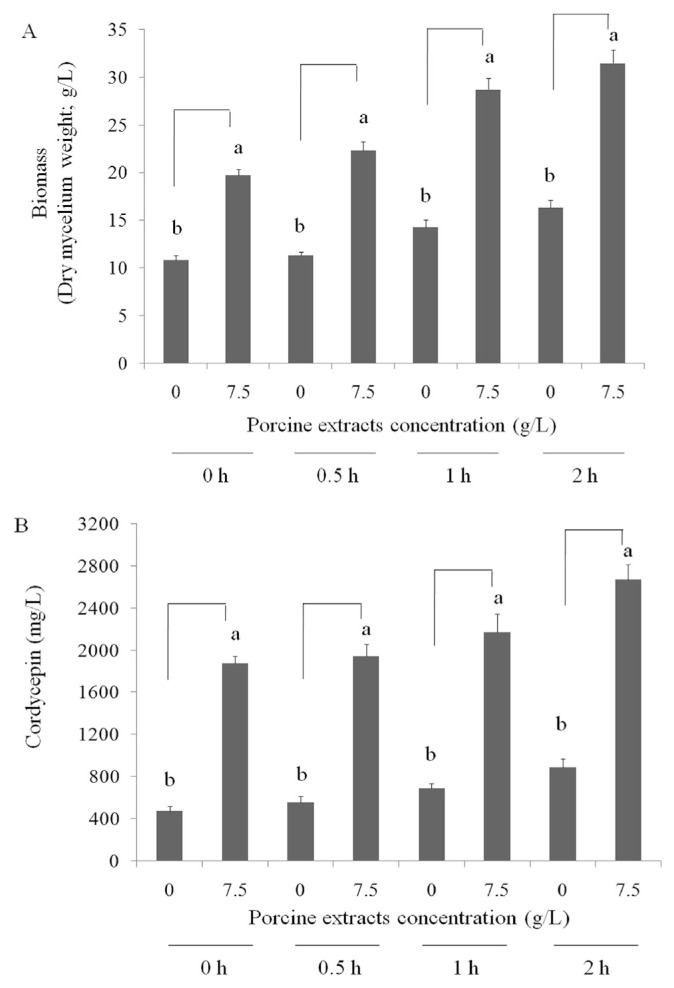
As presented in Figure 2, air was pumped several times into the surface liquid culture of C. militaris (0, 30 min/d, 1 h/d, or 2 h/d). The biomass production and cordycepin bioconversion of the mycelia of surface liquid-cultured C. militaris were high when porcine liver extracts were applied at 10 g/L. However, the cordycepin production by 10 g/L supply was similar to 7.5 g/L, thereby the 7.5 g/L porcine liver extracts was chosen and air was supplied for 2 h/d for investigation of cordycepin production. These results indicated that porcine liver extracts have potential for cordycepin production in surface liquid-cultured C. militaris for 14 days.
Figure 2.
Effects of air supply for various times (0, 30 min/d, 1 h/d, or 2 h/d) on (A) biomass production (g/L in medium) and (B) cordycepin level (cordycepin mg/dry mycelium g/medium L) of the mycelia of surface liquid-cultured Cordyceps militaris in 200 mL medium with or without porcine liver powder extracts (7.5 g/L) at 25°C for 14 days. Data are represented as the mean ± standard deviation (n = 3). Significant differences are shown by different letters (p < 0.05).
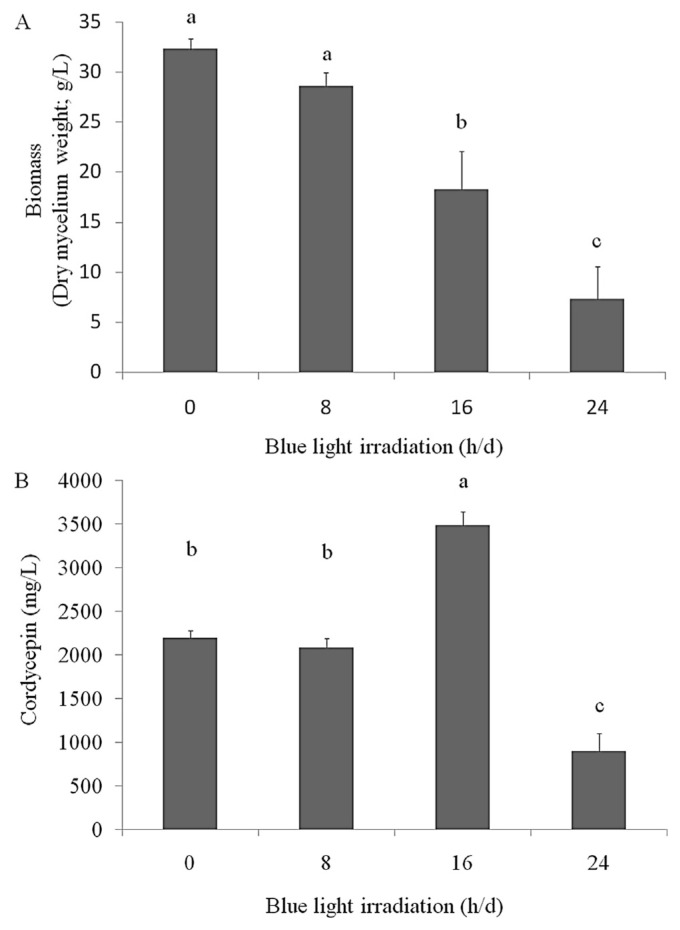
A recent study reported that blue light LED irradiation increases cordycepin production during the liquid cultivation of C. militaris [26–28]. In this study, we evaluated the effects of blue light LED irradiation on cordycepin production by the mycelia of surface liquid-cultured C. militaris under the optimal air supply and porcine liver extract conditions. The results revealed that the weight of the mycelia of surface liquid-cultured C. militaris irradiated with blue light was lower (both for 16 h/d and 24 h/d) than that of mycelia of nonirradiated surface liquid-cultured C. militaris. However, blue light LED irradiation (16 h/d) significantly increased the cordycepin level (3483 mg cordycepin in 18.3 g dry mycelia in 1 L medium; Figure 3). These results indicated that porcine liver extracts at 7.5 g/L (enriched with essential components such as selenium, vitamins, proteins, and adenosine) can increase cordycepin production when supplied with air for 2 h/d and irradiated with blue light for 16 h/d. In addition, WECM was obtained using these conditions.
Figure 3.
Effects of blue light light-emitting diode irradiation (8 h/d, 16 h/d, and 24 h/d) on (A) biomass production (g/L in medium) and (B) cordycepin level (cordycepin mg/dry mycelium g/medium L) of the mycelium of surface liquid-cultured Cordyceps militaris in 200 mL medium with porcine liver powder extracts (7.5 g/L) and air supply (2 h/d) at 25°C for 14 days. Data are represented as the mean ± standard deviation (n = 3). Significant differences are shown by different letters (p < 0.05).
3.2. Suppression of oral cancer cells by WECM in vitro
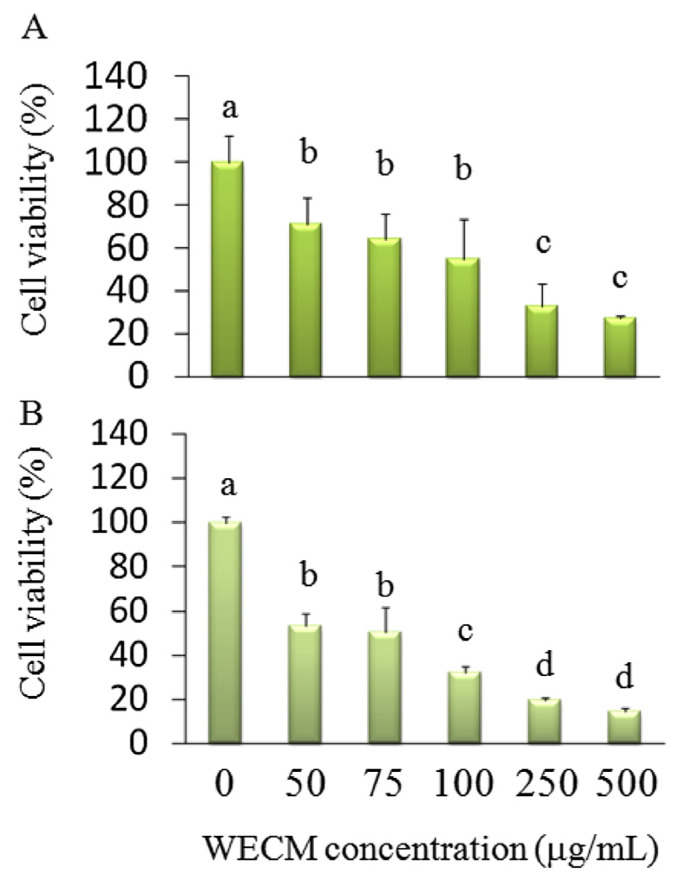
We used WECM from the mycelia of surface liquid-cultured C. militaris treated with porcine liver extracts at 7.5 g/L, an air supply of 2 h/d, and blue light LED irradiation to investigate the anti-oral-cancer activity of WECM in vitro and in vivo. Cordycepin is a potential anticancer compound. The mechanisms underlying the inhibition of OSCC by cordycepin remain unknown. We investigated whether WECM (18.3 g mycelia contained 3483 mg cordycepin) inhibited the growth of SCC-4 oral cancer cells. The results revealed that the isolated WECM (50–500 μg/mL) inhibited SCC-4 cell viability after 24 hours (50% inhibitory concentration: 127.6 μg/mL) and 48 hours (50% inhibitory concentration: 74.2 μg/mL) treatment. The cell viability significantly differed between the control group (without treatment) and treatment group (treated with 50 μg/mL and higher WECM; Figure 4).
Figure 4.
Inhibition of the cell viability of SCC-4 oral cancer cells after treatment with water extracts isolated from the mycelia of surface liquid-cultured Cordyceps militaris (WECM) for (A) 24 hours and (B) 48 hours. Data are represented as the mean ± standard deviation (n = 3). Significant differences are shown by different letters (p < 0.05).
We investigated the effects of treatment with 50 μg/mL, 75 μg/mL, and 100 μg/mL WECM for 12 hours on the cell cycle of SCC-4 oral cancer cells. As presented in Figure 5, WECM significantly arrested the cell cycle of SCC-4 oral cancer cells in the G2/M phase after 12 hours treatment.
Figure 5.
Regulation of the cell cycle of SCC-4 cells after treatment with water extracts isolated from the mycelia of surface liquid-cultured Cordyceps militaris for 12 hours. Data are represented as the mean ± standard deviation (n = 3). Significant differences are shown by different letters (p < 0.05).
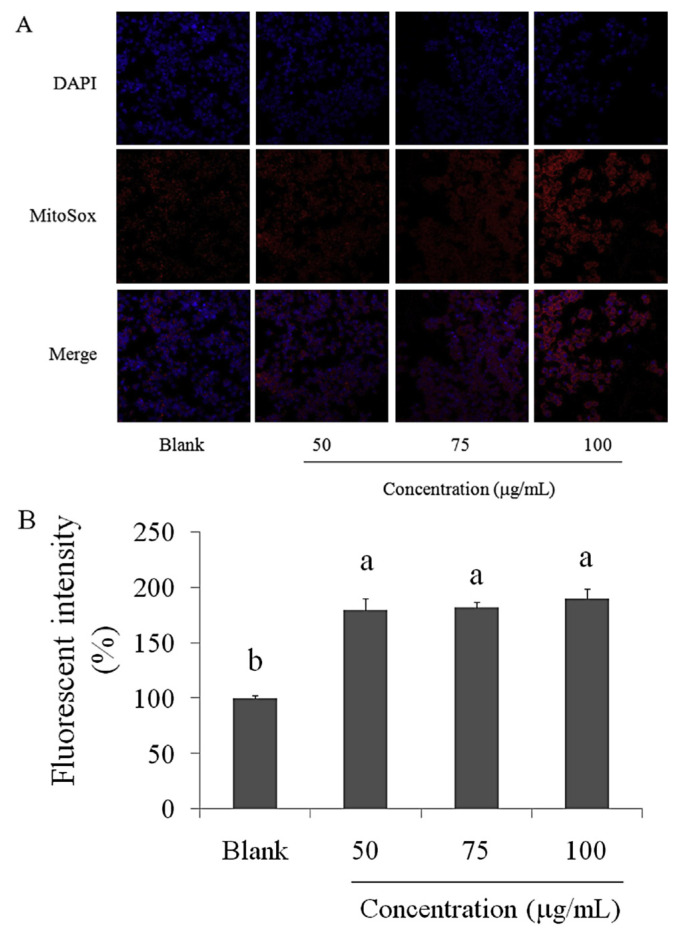
Mitochondrial superoxide is generated as a byproduct of oxidative phosphorylation. In an otherwise tightly coupled electron transport chain, 1–3% of mitochondrial oxygen consumed is incompletely reduced; these “leaky” electrons can rapidly interact with molecular oxygen to form superoxide anions; the predominant ROS in mitochondria [23,24]. The results of the staining of SCC-4 oral cancer cells with the MitoSOX Red reagent after treatment with WECM for 12 hours are presented in Figure 6A. The superoxide levels in SCC-4 oral cancer cells were markedly increased after treatment with WECM. DCFH-DA reacts with hydroxyl, peroxyl, or ROS, and DCFH-DA is diffused into cells and is deacetylated by cellular esterases to nonfluorescent DCFH, which is rapidly oxidized to highly fluorescent 2, 7-dichlorodihydrofluorescein by free radicals. As presented in Figure 6B, the fluorescence intensity of DCFH-DA was significantly increased in SCC-4 oral cancer cells treated with WECM for 12 hours.
Figure 6.
(A) Superoxide level (MitoSOX Red reagent) and (B) oxidative stress (dichlorodihydrofluorescein diacetate) induced in SCC-4 oral cancer cells after treatment with water extracts isolated from the mycelia of surface liquid-cultured Cordyceps militaris for 12 hours. Data are represented as the mean ± standard deviation (n = 3). Significant differences are shown by different letters (p < 0.05). DAPI = 4′,6-diamidino-2-phenylindole.
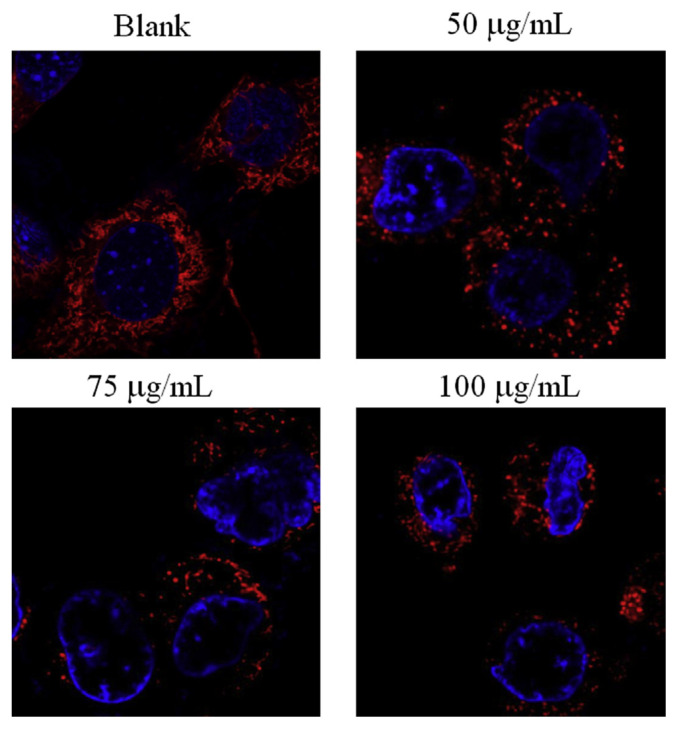
Fission and fusion are crucial for growth, mitochondrial redistribution, and maintenance of a healthy mitochondrial network. In addition, mitochondrial fission and fusion have prominent roles in disease-related processes such as apoptosis and mitophagy. Recent studies have reported that mitochondrial fission can be detected through staining [22,25]. We observed that mitochondrial fission was induced in SCC-4 oral cancer cells after treatment with WECM for 12 hours (Figure 7).
Figure 7.
Mitochondrial morphology of SCC-4 oral cancer cells after treatment with water extracts isolated from the mycelia of surface liquid-cultured Cordyceps militaris for 12 hours. Mitochondrial fission was observed through confocal microscopy.
3.3. Effects of WECM on oral tumor cells in vivo
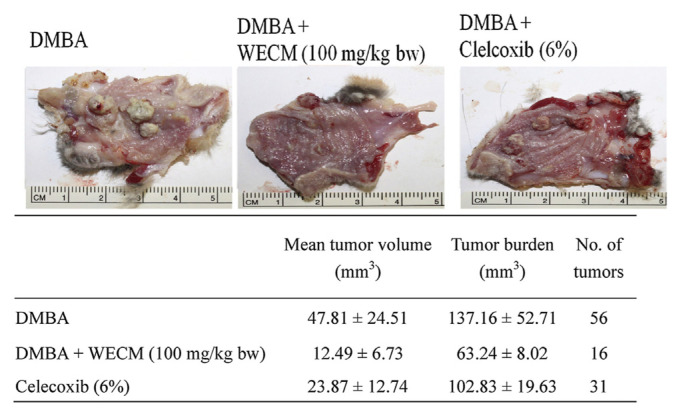
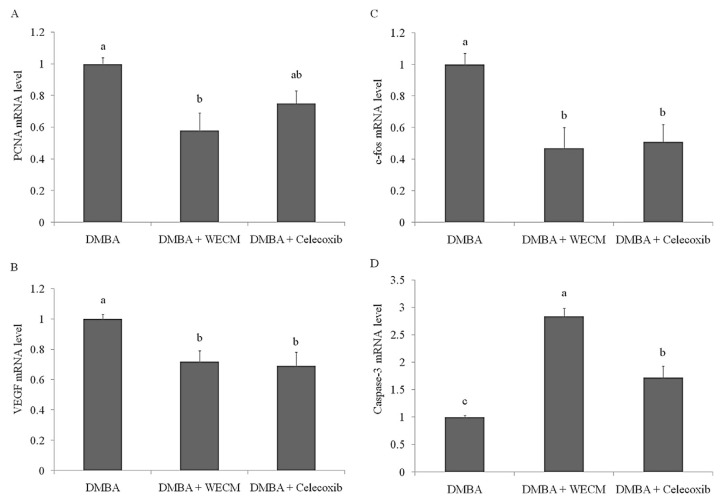
The HBP carcinogenesis model was used to investigate the anti-oral-cancer activity of WECM. HBP carcinogenesis was induced through treatment with DMBA for 8 weeks, and oral carcinoma was observed in the hamsters. Subsequently, WECM or celecoxib (an anti-inflammatory drug) was applied for 4 weeks for the inhibition of oral carcinoma. The results revealed that WECM (100 mg/kg body weight) efficiently reduced the tumor volume, tumor number, and tumor burden, and this decrease was more substantial compared with that caused by celecoxib treatment (Figure 8). Moreover, we measured the mRNA levels of PCNA, VEGF, caspase-3, and c-fos in DMBA-induced oral tumors through real-time PCR (Figure 9). Our results demonstrated that the DMBA-induced increase in the levels of PCNA, VEGF, and c-fos was inhibited by treatment with WECM. In addition, expression of the pro-apoptotic marker caspase-3 significantly increased after treatment with WECM. These results indicated that WECM has potential for inhibiting the growth of oral cancer cells through triggering mitochondrial dysfunction involved in the proapoptosis pathway (Figure 10).
Figure 8.
Inhibition of oral tumor formation in hamsters after treatment with water extracts isolated from the mycelia of surface liquid-cultured Cordyceps militaris (WECM). The hamsters (n = 6) were treated with DMBA for 8 weeks and with WECM or celecoxib for 4 weeks. Significant differences are shown by different letters (p < 0.05). bw = body weight; DMBA = 7,12-dimethylbenz [a]anthracene.
Figure 9.
Regulation of the mRNA levels of (A) proliferating cell nuclear antigen (PCNA), (B) vascular endothelial growth factor (VEGF), (C) c-fos, and (D) caspase-3 in DMBA-induced oral carcinogenesis in hamsters after treatment with water extracts isolated from the mycelia of surface liquid-cultured Cordyceps militaris (WECM). The hamsters (n = 6) were treated with DMBA for 8 weeks and then with WECM or clelcoxib for 4 weeks. Significant differences are shown by different letters (p < 0.05). DMBA = 7,12-dimethylbenz[a]anthracene.
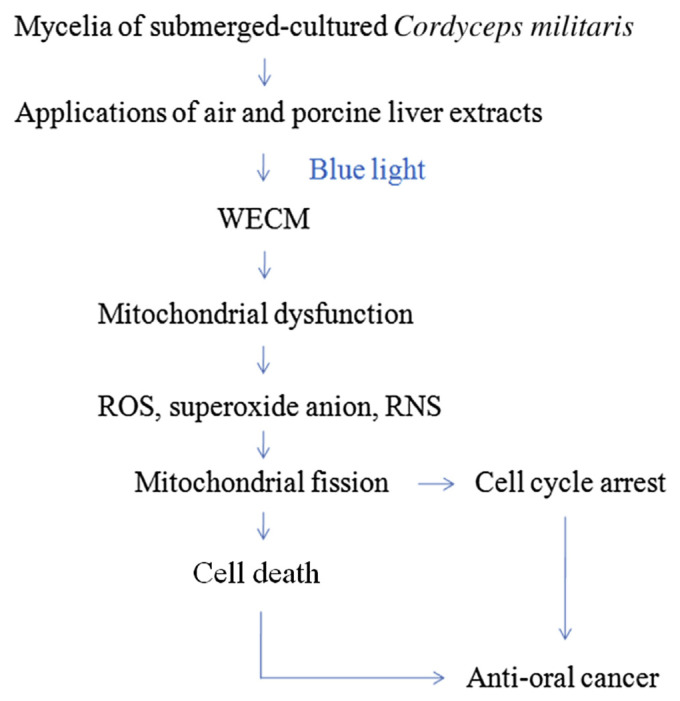
Figure 10.
Water extracts isolated from the mycelia of surface liquid-cultured Cordyceps militaris containing cordycepin inhibited oral cancer cell growth through the downregulation of mitochondrial activity. RNS = reactive nitrogen species; ROS = reactive oxygen species; WECM = water extract from the mycelia of surface liquid-cultured C. militaris.
4. Discussion
Different cultivation conditions, such as submerged culture, surface liquid culture, and static liquid culture, affect cordycepin production during C. militaris fermentation. Moreover, optimal conditions required for cordycepin production during surface liquid culture were investigated [28]. Therefore, we used the surface liquid culture of C. militaris for cordycepin production. Selenium has been found to promote cordycepin production under blue light irradiation [29]. Because the porcine liver contains an abundant amount of selenium [30], it can be used in C. militaris culture for cordycepin production. In our study, we observed that porcine liver extracts increased cordycepin production (Figure 1). A study reported that oxygen input increases the cordycepin level in surface liquid-cultured C. militaris [31]. This result was consistent with that of our study. We observed that compared with surface liquid-cultured C. militaris without air supply, the cordycepin level was markedly increased in those supplied with air for 2 h/d (Figure 2). We observed that 14-day fermentation is suitable for cordycepin and mycelial production in surface liquid-cultured C. militaris.
Light significantly affects mycelial growth. In the plant research field, red light is considered optimal for cell proliferation and blue–purple light for secondary product accumulation [32]. However, in the traditional culture of microorganisms for bioactive compound production, darkness or daylight has been considered a suitable light condition. In the cell cultures of microorganisms, the effect of light at different wavelengths on mycelial growth and secondary metabolite production deserves thorough study. In recent years, a few studies evaluated the effect of light wavelengths on fungal metabolism-related factors, such as biomass production, exopolysaccharides and endopolysaccharides, polysaccharide composition, and mycelial sensitivity [33,34]. In this study, blue light (440–450 nm) significantly affected mycelial and cordycepin production in surface liquid-cultured C. militaris (Figure 3). These results were consistent with those of recent studies, which showed that blue light promotes cordycepin production in biomass (mycelium) of liquid-cultured C. militaris, however, the over irradiation with blue light is able to suppress growth of C. militaris and biomass production, thereby lowering the total level of cordycepin [26–29]. Moreover, the amount of cordycepin produced under these conditions through surface liquid cultivation in our study was higher (3483 mg/L) than that reported in the studies of Masuda et al [35] (640 mg/L), Masuda et al [36] (2500 mg/L), and Das et al [37] (3100 mg/L).
The toxicity and safety of cordycepin have been examined. Tsai et al [38] reported that cordycepin did not cause acute toxicity at 10 mg/kg body weight in rats; however, cordycepin was metabolized and lost its function in vivo. Therefore, we considered the therapeutic potential of WECM in cancer cell suppression and evaluated its effects on oral carcinoma. The results revealed that WECM inhibited the cell viability of SCC-4 oral cancer cells (Figure 4) and arrested the cell cycle in the G2/M phase (Figure 5).
Mitochondria undergo fission and fusion change, called mitochondrial dynamics, which have a critical role in regulating cell metabolism, survival, and proliferation [39]. Molecular mechanisms involved in mitochondrial dynamics have been partially elucidated. Fusion unifies mitochondrial compartments, whereas fission generates morphologically and functionally distinct mitochondria. Mitochondrial fission often occurs early in an apoptotic event [39]. Oxidative stress induction (Figure 6) and mitochondrial fission were observed in SCC-4 oral cancer cells treated with WECM for 12 hours (Figure 7).
PCNA is an acidic nuclear protein that fluctuates during the cell cycle. The synthesis of PCNA is regulated during the cell cycle and increases during the DNA replication period [40]. VEGF is a secreted protein that promotes angiogenesis in tumors, chronic inflammation, and wound healing. VEGF has also been reported to cause metastasis during cancer development [41]. Studies have reported that the c-fos has a crucial role in the G0/G1 transition [42], which is a transcriptional regulator that can elevate expression of many genes associated with cell proliferation. Moreover, studies have reported that c-fos can be activated by different kinase (such as extracellular signal-regulated and Akt) to promote cell proliferation and invasion [43–45]. These findings suggested that suppression of c-fos level could attenuate cell proliferation. Different caspases mediate two apoptotic signaling pathways. The initiation of the Fas signaling pathway by Fas/death receptors or tumor necrosis factor receptors causes the recruitment of Fas-associated protein with death domain (FADD) through interactions between the death domains of Fas and FADD. The binding of procaspase-8 to the Fas/FADD complex in turn activates caspase-3 and induces apoptosis. The second apoptotic pathway is regulated by the mitochondrial release of cytochrome c, resulting in the activation of caspase-9 and caspase-3 [1]. Moreover, we investigated the anti-oral-cancer activity of WECM in a DMBA-induced HBP model. As presented in Figures 8 and 9, WECM markedly inhibited oral carcinogenesis in vivo through inhibiting VEGF, PCNA, and c-fos expression and increasing caspase-3 expression.
Acknowledgements
This research work and subsidiary spending were supported by Taipei Medical University and Taipei Medical University Hospital (Taiwan) (104TMU-TMUH-02).
Funding Statement
This research work and subsidiary spending were supported by Taipei Medical University and Taipei Medical University Hospital (Taiwan) (104TMU-TMUH-02).
Footnotes
Conflicts of interest
The authors declare no competing financial interest.
REFERENCES
- 1. Hsu WH, Lee BH, Pan TM. Protection of Monascus-fermented dioscorea against DMBA-induced oral injury in hamster by anti-inflammatory and antioxidative potentials. J Agric Food Chem. 2010;58:6715–20. doi: 10.1021/jf100889w. [DOI] [PubMed] [Google Scholar]
- 2. Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–7. [PubMed] [Google Scholar]
- 3. Shklar G. Development of experimental oral carcinogenesis and its impact on current oral cancer research. J Dent Res. 1999;78:1768–72. doi: 10.1177/00220345990780120101. [DOI] [PubMed] [Google Scholar]
- 4. Gimenez-Conti IB, Slaga TJ. The hamster cheek pouch carcinogenesis model. J Cell Biochem. 1993;17:83–90. doi: 10.1002/jcb.240531012. [DOI] [PubMed] [Google Scholar]
- 5. Han Y, Cui Z, Li YH, Hsu WH, Lee BH. In vitro and in vivo anticancer activity of pardaxin against proliferation and growth of oral squamous cell carcinoma. Mar Drugs. 2015;14:2. doi: 10.3390/md14010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balakrishnan S, Manoharan S, Alias LM, Nirmal MR. Effect of curcumin and ferulic acid on modulation of expression pattern of p53 and bcl-2 proteins in 7,12-dimethylbenz[a] anthracene-induced hamster buccal pouch carcinogenesis. Indian J Biochem Biophys. 2010;47:7–12. [PubMed] [Google Scholar]
- 7. Rajkamal G, Suresh K, Sugunadevi G, Vijayaanand MA, Rajalingam K. Evaluation of chemopreventive effects of thymoquinone on cell surface glycoconjugates and cytokeratin expression during DMBA induced hamster buccal pouch carcinogenesis. BMC Reprod. 2010;43:664–9. doi: 10.5483/BMBRep.2010.43.10.664. [DOI] [PubMed] [Google Scholar]
- 8. Thanusu J, Kanagarajan V, Nagini S, Gopalakrishnan M. Chemopreventive potential of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one on 7,12-dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinogenesis. J Enzyme Inhib Med Chem. 2010;25:836–43. doi: 10.3109/14756361003724786. [DOI] [PubMed] [Google Scholar]
- 9. Chen YK, Lin LM. DMBA-induced hamster buccal pouch carcinoma and VX2-induced rabbit cancer as a model for human oral carcinogenesis. Exp Rev Anticancer Ther. 2010;10:1485–96. doi: 10.1586/era.10.108. [DOI] [PubMed] [Google Scholar]
- 10. Yang K, Zhang G, Mei J, Chen D, Wu M. Screening and analysis of pathogenic genes during DMBA-induced buccal mucosa carcinogenesis in golden hamsters. Oncol Report. 2010;23:1619–24. doi: 10.3892/or_00000803. [DOI] [PubMed] [Google Scholar]
- 11. Hsu WH, Lee BH, Pan TM. Red mold dioscorea-induced G2/M arrest and apoptosis in human oral cancer cells. J Sci Food Agric. 2010;90:2709–15. doi: 10.1002/jsfa.4144. [DOI] [PubMed] [Google Scholar]
- 12. Hsu WH, Lee BH, Pan TM. Effects of red mold dioscorea on oral carcinogenesis in DMBA-induced hamster animal model. Food Chem Toxicol. 2011;49:1292–7. doi: 10.1016/j.fct.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 13. Yoshikaw N, Nakamura K, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumour activity of cordycepin in mice. Clin Exp Pharmacol Physiol. 2004;31:S51–3. doi: 10.1111/j.1440-1681.2004.04108.x. [DOI] [PubMed] [Google Scholar]
- 14. Wehbe-Janek H, Shi Q, Kearney CM. Cordycepin/hydroxyurea synergy allows low dosage efficacy of cordycepin in MOLT-4 leukemia cells. Anticancer Res. 2007;27:3143–6. [PubMed] [Google Scholar]
- 15. Chen Y, Chen YC, Lin YT, Huang SH, Wang SM. Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma cells through the calcium-calpain-caspase 7-PARP pathway. J Agric Food Chem. 2010;58:11645–52. doi: 10.1021/jf1028976. [DOI] [PubMed] [Google Scholar]
- 16. Xu HL, Zhang LJ, Shi H, Zhu X, He X. Effects of cordycepin on Hep G2 and EA.hy926 cells: potential antiproliferative, antimetastatic and anti-angiogenic effects on hepatocellular carcinoma. Oncol Lett. 2014;7:1556–62. doi: 10.3892/ol.2014.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto K, Shichiri H, Uda A, Yamashita K, Nishioka T, Kume M, Makimoto H, Nakagawa T, Hirano T, Hirai M. Apoptotic effects of the extracts of Cordyceps militaris via Erk phosphorylation in a renal cell carcinoma cells line. Phytother Res. 2015;29:707–13. doi: 10.1002/ptr.5305. [DOI] [PubMed] [Google Scholar]
- 18. Li G, Nakagome I, Hirono S, Itoh T, Fujiwara R. Inhibition of adenosine deaminase (ADA)-mediated metabolism of cordycepin by natural substances. Pharmacol Res Per. 2015;3:e00121. doi: 10.1002/prp2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shih IL, Tsai KL, Hsieh CY. Effects of culture conditions on the mycelia growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem Engineer J. 2007;33:193–201. [Google Scholar]
- 20. Xie CY, Gu ZX, Fan GJ, Gu FR, Han YB, Chen ZG. Production of cordycepin and mycelia by submerged fermentation of Cordyceps militaris in mixture natural culture. Appl Biochem Biotechnol. 2009;158:483–92. doi: 10.1007/s12010-009-8567-2. [DOI] [PubMed] [Google Scholar]
- 21. Masuda M, Das SK, Fujihara S, Hatashita M, Sakurai A. Production of cordycepin by a repeated batch culture of Cordyceps militaris mutant obtained by proton beam irradiation. J Biosci Bioengineer. 2011;111:55–60. doi: 10.1016/j.jbiosc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 22. Hsu WH, Lee BH, Pan TM. Leptin-induced mitochondrial fusion mediates hepatic lipid accumulation. Int J Obesity. 2015;39:1750–6. doi: 10.1038/ijo.2015.120. [DOI] [PubMed] [Google Scholar]
- 23. Batandier C, Fontaine E, Keriel C, Leverve XM. Determination of mitochondrial reactive oxygen species: methodological aspects. J Cell Mol Med. 2002;6:175–87. doi: 10.1111/j.1582-4934.2002.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–35. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 25. Meyer JE, Harder J. Antimicrobial peptides in oral cancer. Curr Pharm Des. 2007;13:3119–30. doi: 10.2174/138161207782110372. [DOI] [PubMed] [Google Scholar]
- 26. Wang JF, Yang DD, Li HM, Han WJ. Study on monosaccharide composition of intracellular polysaccharide and contents of cordycepin and Cordyceps polysaccharide produced by Cordyceps militaris induced by blue light. Zhong Yao Cai. 2014;37:1395–9. [PubMed] [Google Scholar]
- 27. Yang T, Guo M, Yang H, Guo S, Dong C. The blue-light receptor CmWC-1 mediates fruit body development and secondary metabolism in Cordyceps militaris. Appl Microbiol Biotechnol. 2016;100:743–55. doi: 10.1007/s00253-015-7047-6. [DOI] [PubMed] [Google Scholar]
- 28. Kang C, Wen TC, Kang JC, Meng ZB, Li GR, Hyde KD. Optimization of large-scale culture conditions for the production of cordycepin with Cordyceps militaris by liquid static culture. Scientific World Journal. 2014;2014:510627. doi: 10.1155/2014/510627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong JZ, Liu MR, Lei C, Zheng XJ, Wang Y. Effects of selenium and light wavelengths on liquid culture of Cordyceps militaris Link. Appl Biochem Biotechnol. 2012;166:2030–6. doi: 10.1007/s12010-012-9628-5. [DOI] [PubMed] [Google Scholar]
- 30. Daun C, Akesson B. Glutathione peroxidase activity, and content of total and soluble selenium in five bovine and porcine organs used in meat production. Meat Sci. 2004;66:801–7. doi: 10.1016/S0309-1740(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 31. Mao XB, Zhong JJ. Hyperproduction of cordycepin by two-stage dissolved oxygen control in submerged cultivation of medicinal mushroom Cordyceps militaris in bioreactors. Biotechnol Prog. 2004;20:1408–13. doi: 10.1021/bp049765r. [DOI] [PubMed] [Google Scholar]
- 32. Idnurm A, Rodríguez-Romero J, Corrochano LM, Sanz C, Iturriaga EA, Eslava AP, Heitman J. The phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses. Proc Natl Acad Sci U S A. 2006;103:4546–51. doi: 10.1073/pnas.0600633103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smirnov DA, Shcherba VV, Bisko NA, Poyedinok NL. Some biologically active substances from a mycelial biomass of medicinal caterpillar fungus Cordyceps sinensis (Berk.) Sacc. (Ascomycetes) Int J Med Mushrooms. 2009;1:69–76. [Google Scholar]
- 34. Puchkova TA, Babitskaya VG, Scherba V, Bisko NA, Poyedinok NL. Polysaccharides of medicinal caterpillar fungus, Cordyceps militaris Link (Ascomycetes): production and composition. Int J Med Mushrooms. 2010;4:419–25. [Google Scholar]
- 35. Masuda M, Urabe E, Sakurai A, Sakakibara M. Production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb Technol. 2006;39:641–6. [Google Scholar]
- 36. Masuda M, Urabe E, Honda H, Sakurai A, Sakakibara M. Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb Technol. 2007;40:1199–205. [Google Scholar]
- 37. Das SK, Masuda M, Hatashita M, Sakurai A, Sakakibara M. A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high energy ion beam irradiation. Lett Appl Microbiol. 2008;47:534–8. doi: 10.1111/j.1472-765X.2008.02456.x. [DOI] [PubMed] [Google Scholar]
- 38. Tsai YJ, Lin LC, Tsai TH. Pharmacokinetics of adenosine and cordycepin, a bioactive constituent of Cordyceps sinensis in rat. J Agric Food Chem. 2010;58:4638–43. doi: 10.1021/jf100269g. [DOI] [PubMed] [Google Scholar]
- 39. Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–84. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 40. Poosarla C, Ramesh M, Ramesh K, Gudiseva S, Bala S, Sundar M. Proliferating cell nuclear antigen in premaliganacy and oral squamous cell carcinoma. J Clin Diagn Res. 2015;9:ZC39–41. doi: 10.7860/JCDR/2015/12645.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yi BR, Kim SU, Choi KC. Synergistic effect of therapeutic stem cells expressing cytosine deaminase and interferon-beta via apoptotic pathway in the metastatic mouse model of breast cancer. Oncotarget. 2016;7:5985–99. doi: 10.18632/oncotarget.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang C, Niu Z, Gu N. Analysis of the ways and methods of signaling pathways in regulating cell cycle of NIH3T3 at transcriptional level. BMC Cell Biol. 2015;16:85. doi: 10.1186/s12860-015-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ho BY, Wu YM, Chang KJ, Pan TM. Dimerumic acid inhibits SW620 cell invasion by attenuaing H2O2-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1 dependent manner. Int J Biol Sci. 2011;7:869–80. doi: 10.7150/ijbs.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 45. Habib GM. Arsenite causes down-regulation of Akt and c-Fos, cell cycle dysfunction and apoptosis in glutathione-deficient cells. J Cell Biochem. 2010;110:363–71. doi: 10.1002/jcb.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]