Abstract
Hyperlipidemia and inflammation play important roles in the development and progression of atherosclerosis. Atherosclerosis is regarded as an inflammatory response of blood vessels to injury at the start of atherosclerotic plaque formation, which then leads to cardiovascular events. Edible fungi of the Monascus species have been used as traditional Chinese medicines in East Asia for several centuries. The fermented products of Monascus purpureus NTU 568 possess a number of functional secondary metabolites including the anti-inflammatory pigments monascin and ankaflavin. Compounds derived from M. purpureus have been shown to have hypolipidemic effects. We aimed to evaluate the effects of M. purpureus NTU 568 fermentation product an extract (Ankascin 568 plus) containing monascin and ankaflavin on blood lipids in volunteers with borderline high levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) by conducting a 12-week randomized, double-blind, placebo-controlled, adaptive-design study. This study enrolled 40 subjects aged 18–65 years from a population of patients with TC and LDL-C levels of ≥ 180 mg/dL and 130–190 mg/dL, respectively. Measured endpoints included lipid profile, liver, kidney and thyroid function, electrolyte balance, creatinine phosphokinase, and fasting blood glucose. After 4 weeks of treatment (500 mg Ankascin 568 plus/day), the changes in the lipid levels showed that the active products had a more favorable effect than the placebo. Compared to the baseline, statistically significant decreases of 11.9% and 19.0% were observed in TC and LDL-C levels, respectively (p < 0.05 for all pairs). This study demonstrated that subjects administered one 500 mg capsule of Ankascin 568 plus for more than 4 weeks exhibited a significant reduction in serum TC and LDL-C levels. Therefore, Ankascin 568 plus may be a potentially useful agent for the regulation of blood lipids and the treatment of coronary artery diseases.
Keywords: Ankaflavin, Ankascin 568 plus, Hyperlipidemia, Monascin, Monascus purpureus NTU 568
1. Introduction
Hyperlipidemia refers to increased blood plasma levels of lipids and lipoproteins, including cholesterol and triglycerides. Although hyperlipidemia in itself does not cause symptoms, the lipids can enter the walls of arteries and increase the risk of developing atherosclerosis (hardening of the arteries), which can lead to stroke, heart attack, and amputation. Moreover, it significantly increases the risk of developing cardiovascular diseases, including disease of the blood vessels supplying the heart (coronary artery disease), brain (cerebrovascular disease), and limbs (peripheral vascular disease). These conditions can in turn lead to chest pain, heart attacks, strokes, and other problems. Because of these risks, treatment is often recommended for people with hyperlipidemia. Cholesterol-lowering drugs decrease cardiovascular disease progression by halting the development of cholesterol-laden plaques in vessel linings [1,2].
Statins are widely used cholesterol-lowering drugs [3]. They inhibit the enzyme 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, which plays a central role in cholesterol production in the liver. The most common adverse effects of statins are liver and muscle damage including elevated liver enzyme levels in the serum, myopathy, myositis, and rhabdomyolysis [4–6]. The statin drug lovastatin is a hypolipidemic agent that is used to lower circulating levels of cholesterol in patients with hypercholesterolemia, thereby aiding in the prevention of cardiovascular disease [7]. Despite their therapeutic effects, statins have the propensity to cause rhabdomyolysis via a number of mechanisms [8].
In our previous study, we demonstrated the effects of a combination of Monascus-fermented products and lovastatin on increased risk of rhabdomyolysis in hyperlipidemic hamsters [9]. Monascus species produce several kinds of pigments as functional secondary metabolites. These include the yellow pigments ankaflavin and monascin, the orange pigments monascorubrin and rubropunctanin, and the red pigments monascorubramine and rubropuctamine [10]. Monascin and ankaflavin have numerous biological effects such as inhibition of non-alcoholic fatty liver, amelioration of pancreatic damage and hyperglycemia in diabetes, and antioxidant and anti-inflammatory effects [11,12]. The mechanisms of action of monascin and ankaflavin show that they are peroxisome proliferator-activated receptor (PPAR)-γ agonists that initiate the transcription of downstream genes [13–15]. In view of the reported health benefits of monascin and ankaflavin, which are major constituents of Ankascin 568 plus, this clinical trial was conducted per the recommendations of the health food to evaluate the effects of Ankascin 568 plus on blood lipid regulation.
2. Materials and methods
2.1. Materials
The study material consisted of ANKASCIN 568 plus product fermented by Monascus purpureus NTU 568, which was obtained from SunWay Biotech., Co., LTD. (Taipei, ROC). One capsule (500 mg/capsule) of ANKASCIN 568 plus powder contained 3 mg of monascin (MS) and 1.5 mg ankaflavin (AK). The material made of maltodextrin was also used as the placebo.
2.2. Study population
The clinical study was conducted from September 2012 to November 2014 at the Chung Shan Medical University following acquisition of the proof of the approval of the Institutional Review Board (IRB) of the Taichung Chung Shan Medical University Hospital (IRB proof document CHMUH No: CS12121). Written informed consent was obtained from each participant prior to enrollment. We screened 377 patients in a series of stages. In the first stage, patients were screened for serum low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC) levels in the range of 130–190 and >180 mg/dL, respectively. After a 2-week stabilization period, blood samples were collected from the subjects for the second phase of the screening. Fifty-seven subjects that showed acceptable lipid levels based on the values observed 2 weeks previously during the initial screening were screened. The subjects that exhibited a reasonable range of variation (TC and LDL-C original value ± 20%) were included in the main study, which commenced with 40 participants: 20 in each of the treatment and placebo groups. The treatment group consisted of 6 males and 14 females and the placebo group contained 4 males and 16 females. The average age was 44.9 ± 9.5 and 44.7 ± 12.5 years, in the test and placebo groups, respectively (total range 22–65 years).
During the treatment, patients with hypertension were not allowed to use depressor drugs unless their blood pressure was suddenly elevated. Patients with coronary heart disease were not allowed to use analgesics unless they were experiencing symptoms of angina pectoris. All patients were on a low-cholesterol diet throughout the entire treatment period. Carotid ultrasound examinations and plasma biochemical assays were performed at the end of the treatment.
At the initial screening visit, all potential subjects received a written Informed Consent Document (ICD) to review and discuss with the clinic staff and their families. Prior to any screening procedures, all potential subjects had to sign the ICD and were given a copy of the form. Subjects were reminded that study participation was voluntary. They were judged by the Investigator to be in general good health on the basis of their medical history. They agreed not to initiate any new exercise or diet programs during the entire study period, understood the study procedures, and signed the forms providing both informed consent to participate in the study and authorization for the release of relevant protected health information to the study investigator.
2.2.1. Inclusion and exclusion criteria
Inclusion criteria indicated that the patients had metabolic syndrome and would use the drug from sub-healthy to maintain healthy. Patients who fulfilled the following inclusion criteria at registration were included in this study: (i) age 20–65 years; (ii) LDL-C range and TC levels 130–190 and >180 mg/dL, respectively; (iii) body mass index (BMI) 23–30 kg/m2; (iv) when used, administration of hypolipidemic or anti-hypertensive drugs stabilized for at least 3 months. Exclusion criteria for all participants were: (i) administration of antidiabetic drugs; (ii) inconsistent or unstable administration of drugs that may interfere with lipid or glucose metabolism; (iii) chronic gastrointestinal diseases and administration of drugs for treatment; (iv) confirmation of thyroid, liver, renal, or muscular diseases; (v) known allergy or intolerance to a component of the test product; and (vi) any medical or surgical condition that could lead to non-adherence to the study protocol.
2.3. Methods
2.3.1. Randomization, treatment, and follow-up
Regardless of the study-group assignment in the parent study, eligible patients were randomly assigned during the last visit of the parent study or as soon as possible after that, to receive either Ankascin 568 plus (treatment group) or placebo (control group) at a ratio of 1:1. Randomization was executed centrally using an interactive voice- or web response system. Treatments were stratified based on the study-group assignment in the parent trial, and the placebo was based on the drug dose frequency in the parent trial.
2.3.2. Outcome measures
The designated study endpoint of both trials was the incidence of adverse events. Additional safety endpoints included serious adverse events, adverse events leading to the discontinuation of the study health food (for patients in the Ankascin 568 plus group), and abnormalities in creatine kinase levels, liver and kidney function, and electrolyte balance. A prespecified exploratory outcome was defined as the incidence of confirmed cardiovascular events over the course of the study.
2.4. Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The statistical significance of the biochemical analyses was determined by one-way analysis of variance (ANOVA) using the general linear model procedure of the statistical product and service solutions software (SPSS Institute, Inc., Chicago, IL, USA). This was followed by ANOVA with a paired t-test to evaluate the differences before and after sample and placebo administration while the Student t-test was used to compare the difference between test and placebo groups (p < 0.05).
3. Results
3.1. Anthropometric measurements
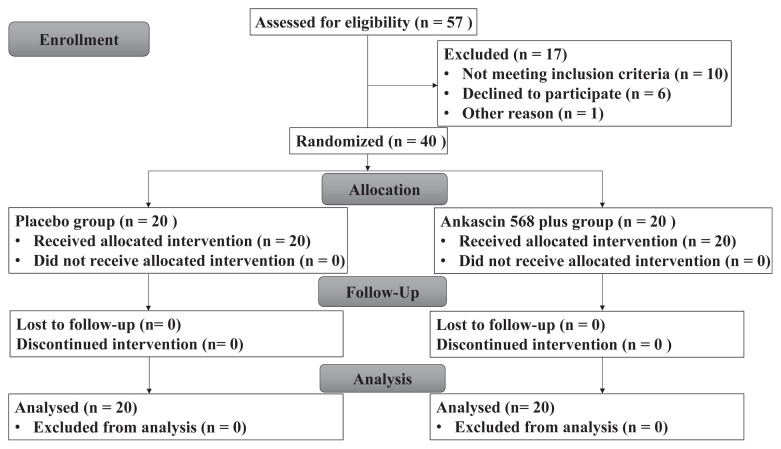
In total, 40 sub-healthy patients meeting the inclusion criteria were assigned randomly to either the Ankascin 568 plus or the placebo group (n = 20 each). The treatment group consisted of 6 males and 14 females and the placebo group contained 4 males and 16 females. All of the subjects completed the trial (Fig. 1). The body weight, BMI, waistline, and blood pressure of participants in this trial were shown in Table 1. There were no differences in these values within the groups during the course of the experiment. Therefore, the appearance and health condition of participants were maintained during the trial.
Fig. 1.
CONSORT flow diagram of patients with hyperlipidemia.
Table 1.
Effect of chronic administration of Ankascin 568 plus or placebo on anthropometric measurements of subjects.
| Treatment | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0 (Initial) | 4 | 8 | 12 (Follow-up) | 0 (Initial) | 4 | 8 | 12 (Follow-up) | |
|
|
|
|||||||
| Week | Week | |||||||
| Age (years) | 44.9 ± 9.5 | 44.7 ± 12.5 | ||||||
| Male | 6 | 4 | ||||||
| Female | 14 | 16 | ||||||
| Weight (kg) | 67.9 ± 13.4 | 67.0 ± 12.9 | 67.0 ± 12.8 | 66.6 ± 13.0 | 62.7 ± 13.7 | 62.5 ± 13.7 | 63.4 ± 13.1 | 62.4 ± 13.4 |
| Body fat (%) | 30.9 ± 6.1 | 31.0 ± 5.4 | 30.6 ± 5.1 | 31.5 ± 5.7 | 29.3 ± 6.1 | 30.2 ± 5.8 | 30.7 ± 6.4 | 31.0 ± 6.3 |
| BMI | 25.3 ± 4.0 | 25.2 ± 3.7 | 25.0 ± 3.8 | 25.1 ± 3.7 | 24.7 ± 3.9 | 25.0 ± 3.8 | 24.8 ± 4.1 | 24.9 ± 4.2 |
| Waistline (cm) | 81.7 ± 9.1 | 80.6 ± 5.4 | 81.7 ± 9.1 | 81.1 ± 9.9 | 80.5 ± 10.9 | 76.7 ± 12.7 | 79.5 ± 10.4 | 78.4 ± 10.0 |
| Blood pressure | ||||||||
| SBP (mmHg) | 124.8 ± 17.2 | 122.7 ± 16.6 | 122.7 ± 11.7 | 123.5 ± 16.5 | 119.9 ± 11.5 | 117.8 ± 12.5 | 123.3 ± 11.1 | 122.1 ± 17.0 |
| DBP (mmHg) | 81.2 ± 14.0 | 78.0 ± 11.6 | 75.9 ± 9.6 | 74.3 ± 11.7 | 74.2 ± 6.5 | 71.5 ± 7.8 | 74.5 ± 7.8 | 72.8 ± 8.0 |
Student’s t-test, no significant difference between placebo and treatment group at week 0;
p < 0.05 vs. week 0 for each group, data are the mean ± standard deviation (SD).
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
3.2. Hypolipidemic effect of Ankascin 568 plus
In this study, we used Ankascin 568 plus capsules fermented by M. purpureus NTU 568 as the study material. Table 2 shows that TC levels at week 0 showed no significant difference between the placebo and treatment groups. Patients were administered Ankascin 568 plus for 4 and 8 weeks followed by a 4-week washout (12 weeks total). TC levels for the treatment and placebo groups at weeks 0, 4, 8, and 12 are shown in Table 2; after 8 weeks of treatment with Ankascin 568 plus, a significant decrease of 11.1% compared to week 0 was observed (p < 0.05). Next, we compared the serum TG levels of the treatment and placebo groups at weeks 0, 4, 8, and 12 (Table 2). The results revealed no differences between the groups.
Table 2.
Effect of chronic administration of Ankascin 568 plus or placebo on blood lipid profiles of subjects.
| Treatment | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0 (Initial) | 4 | 8 | 12 (Follow-up) | 0 (Initial) | 4 | 8 | 12 (Follow-up) | |
|
|
|
|||||||
| Week | Week | |||||||
| TG (mg/dL) | 118.1 ± 59.3 | 110.0 ± 61.3 | 119.0 ± 71.7 | 118.0 ± 60.1 | 107.7 ± 54.8 | 109.2 ± 60.3 | 120.5 ± 72.5 | 107.4 ± 45.9 |
| TC (mg/dL) | 228.7 ± 26.3 | 201.4 ± 32.1* | 203.4 ± 31.6* | 233.0 ± 24.9 | 226.7 ± 22.7 | 226.5 ± 23.1 | 225.1 ± 23.1 | 229.9 ± 29.4 |
| HDL-C (mg/dL) | 54.8 ± 20.5 | 57.6 ± 15.2 | 59.4 ± 14.9 | 59.9 ± 12.7 | 55.4 ± 21.8 | 56.4 ± 15.2 | 58.3 ± 14.9 | 57.4 ± 9.3 |
| LDL-C (mg/dL) | 153.7 ± 15.6 | 124.5 ± 21.9* | 122.3 ± 19.5* | 148.2 ± 17.1 | 155.5 ± 17.0 | 152.5 ± 17.7 | 149.0 ± 19.7 | 154.1 ± 21.3 |
| LDL-C/HDL-C | 2.8 ± 0.6 | 2.2 ± 0.6* | 2.1 ± 0.5* | 2.5 ± 0.6* | 2.8 ± 0.6 | 2.7 ± 0.6 | 2.6 ± 0.5 | 2.7 ± 0.5 |
| TC/HDL-C | 4.2 ± 0.6 | 3.5 ± 0.5* | 3.4 ± 0.6* | 3.9 ± 0.5* | 4.1 ± 0.6 | 4.0 ± 0.5 | 3.9 ± 0.5 | 4.0 ± 0.5 |
Student’s t-test, no significant difference between placebo and treatment group at week 0;
p < 0.05 vs. week 0 for each group, data are the mean ± standard deviation (SD).
TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipo-protein cholesterol.
3.3. Effect of Ankascin 568 plus on serum lipid profile
LDL-C and HDL-C levels can be used to determine lipid metabolic status and the human standards are <130 mg/dL and >40 mg/dL, respectively. Extremely low HDL-C or high LDL-C levels are thought to have a considerable impact on cardiovascular health. The LDL-C level of the placebo group in this study showed no significant difference from that of the treatment group at week 0.
We compared the LDL-C of the Ankascin 568 plus-treated and placebo groups at weeks 0, 4, 8, and 12 (Table 2); the level in the treated group decreased by 19.0 and 20.4% at weeks 4 and 8, respectively (p < 0.05 for all pairs). HDL-C levels showed no significant changes over the course of the study for both the Ankascin 568 plus-treated and placebo groups (p > 0.05; Table 2). These results indicate that the treatment group showed a significant improvement in LDL-C.
LDL-C and HDL-C levels >130 mg/dL and <30 mg/dL and LDL-C/HDL-C and TC/HDL-C ratios that are >3.5 and >5.0, respectively, are considered risk factors for cardiovascular diseases (including heart disease and stroke) and atherosclerosis. Therefore, we determined the changes in LDL-C/HDL-C and TC/HDL-C ratios. The results (Table 2) showed significant differences between the LDL-C/HDL-C ratios of the treatment and placebo groups at weeks 4, 8, and 12 (p < 0.05). In addition, changes in TC/HDL-C were significantly different (p < 0.05) between the treatment and placebo groups. This was in contrast to the TG outcome, which was not significantly different, and may be explained by the fact that the ratios are easily affected by various factors including the subject’s blood and diet consumed the day before testing. These factors are typically harder to control in human testing than in animal testing. Regarding the lipids, the results also suggested that Ankascin 568 plus capsules may effectively improve lipid metabolism in subjects as well as reduce the risk of hardening of the arteries and the probability of developing coronary atherosclerosis and heart disease. It has been previously reported that increased LDL-C levels cause atherosclerosis and other cardiovascular diseases. These results suggest that the functional components of Ankascin 568 plus can effectively reduce blood LDL-C levels and, thereby, possibly reduce the incidence of cardiovascular diseases.
3.4. Effects on liver function
The majority of commercial red mold products contain citrinin, which is toxic to the liver and kidney. Therefore, the food safety risks associated with red mold-fermented products should be evaluated. To assess the safety of the product, we assayed the liver levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). As shown in Table 3, there was no significant difference in the AST and ALT levels between the placebo and Ankascin 568 plus groups. Gamma-glutamyltransferase (γ-GT), a membrane-bound heterodimeric glycoprotein, is abundant in various tissues including the kidney, intestine, and liver. Under pathological conditions, when liver cells are damaged, γ-GT is released in the serum. Table 3 shows that the γ-GT levels were not significantly different between groups (p > 0.05). Blood urea nitrogen (BUN) is a protein metabolite, a high concentration of which is indicative of a weak renal excretory mechanism. In this study, BUN and creatinine levels (Table 3) were not significantly different between both groups (p > 0.05). Furthermore, there were no significant differences in the serum calcium, sodium, potassium, and chloride concentrations between groups (p > 0.05, Table 3). These results imply that there was no significant effect on renal metabolism and physiological function.
Table 3.
Effect of chronic administration of Ankascin 568 plus or placebo on liver and kidney function and electrolyte balance of subjects.
| Treatment | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0 (Initial) | 4 | 8 | 12 (Follow-up) | 0 (Initial) | 4 | 8 | 12 (Follow-up) | |
|
|
|
|||||||
| Week | Week | |||||||
| Liver function | ||||||||
| AST (IU/L) | 22.4 ± 12.7 | 23.5 ± 14.1 | 23.7 ± 12.4 | 21.3 ± 9.2 | 23.3 ± 11.8 | 24.6 ± 15.2 | 24.7 ± 13.8 | 21.5 ± 9.6 |
| ALT (IU/L) | 21.6 ± 10.5 | 21.1 ± 9.1 | 21.0 ± 6.0 | 23.2 ± 10.7 | 20.7 ± 9.8 | 21.1 ± 9.2 | 20.9 ± 6.0 | 23.2 ± 10.8 |
| γ-GTP (IU/L) | 17.7 ± 10.6 | 20.6 ± 20.0 | 21.3 ± 18.1 | 20.9 ± 19.6 | 19.4 ± 16.3 | 20.9 ± 13.4 | 22.1 ± 14.5 | 20.5 ± 12.2 |
| Kidney function | ||||||||
| Creatinine (mg/dL) | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| BUN (mg/dL) | 13.0 ± 2.9 | 11.5 ± 2.8 | 11.7 ± 2.4 | 12.1 ± 2.6 | 11.3 ± 3.7 | 10.4 ± 2.9 | 11.4 ± 3.5 | 12.1 ± 3.5 |
| pH in urine | 6.4 ± 0.8 | 6.5 ± 0.6 | 6.4 ± 0.8 | 6.4 ± 0.8 | 6.1 ± 0.7 | 6.3 ± 0.8 | 6.6 ± 0.6 | 6.3 ± 0.9 |
| Electrolyte balance | ||||||||
| Ca (mg/dL) | 9.7 ± 0.03 | 9.5 ± 0.3 | 9.5 ± 0.2 | 9.5 ± 0.2 | 9.7 ± 0.3 | 9.5 ± 0.3 | 9.4 ± 0.3 | 9.4 ± 0.3 |
| Na (mmol/L) | 138.4 ± 2.5 | 139.3 ± 2.2 | 139.7 ± 1.6 | 138.8 ± 2.0 | 138.8 ± 2.8 | 139.8 ± 2.6 | 139.1 ± 2.1 | 138.0 ± 1.8 |
| K (mmol/L) | 4.3 ± 0.3 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.3 ± 0.4 | 4.28 ± 0.44 | 4.36 ± 0.58 | 4.22 ± 0.31 | 4.40 ± 0.70 |
| Cl (mmol/L) | 103.6 ± 1.8 | 104.5 ± 1.8 | 103.8 ± 1.7 | 103.9 ± 2.0 | 104.0 ± 2.7 | 105.2 ± 2.3 | 103.8 ± 2.3 | 104.0 ± 2.8 |
Student’s t-test, no significant difference between placebo and treatment group at week 0,
p < 0.05 vs. week 0 for each group, data are the mean ± standard deviation (SD).
AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; BUN, blood urea nitrogen.
3.5. Effect of Ankascin 568 plus on serum thyroid function, CPK, and fasting blood glucose levels
Thyroid-stimulating hormone (TSH, also known as thyrotropin, or hTSH for human TSH) is a pituitary hormone that stimulates the thyroid gland to produce thyroxine (T4), and then triiodothyronine (T3), which stimulates metabolism in almost every body tissue. It is a glycoprotein hormone synthesized and secreted by thyrotrope cells in the anterior pituitary gland, and it regulates the endocrine function of the thyroid. Serum TSH thyroid function is often interpreted in conjunction with free T4. TSH levels decrease and increase in hyperthyroidism and hypothyroidism, respectively, and high levels of free thyroid-stimulating hormone (free T4) are indicative of hyperthyroidism and low organic energy. The results shown in Table 4 revealed no significant differences in free T4 and TSH between the groups, with values remaining in the normal range, indicating that administration of Ankascin 568 plus did not affect thyroid function. During rhabdomyol-ysis, damaged muscle cells release creatine phosphate kinase (CPK), which is also often used in the diagnosis and monitoring of clinical myocardial infarction and muscle diseases. Table 4 shows that administration of Ankascin 568 plus did not increase CPK levels of the treated patients. Lipid peroxidation and oxidative modification of LDL have been implicated as causal factors in the pathogenesis of atherosclerosis. Therefore, prevention of LDL oxidation by antioxidants may be an effective strategy for inhibiting disease progression. Furthermore, oxygen-derived radicals impair endothelial function and have been implicated as mediators of this process. Oxidative susceptibility of LDL was determined by lag-time assay in vitro and by using a human umbilical vein endothelial cell-mediated oxidation model. After the monocyte investigations, we further examined the antioxidant effect after the intake of Ankascin 568 plus-treated group. The LDL lag-time measurement results in Table 4 indicate that Ankascin 568 plus induced a 78.7% increase in treated subjects. Ankascin 568 plus significantly prolonged the lag time of LDL oxidation. Based on our findings, it appears that Ankascin 568 plus enhances the antioxidant defense capacity of LDL and may play a preventive role in atherosclerosis progression.
Table 4.
Effect of chronic administration of Ankascin 568 plus or placebo on thyroid function, creatinine phosphokinase (CPK), and fasting blood glucose (AC) of subjects.
| Treatment | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0 (Initial) | 4 | 8 | 12 (Follow-up) | 0 (Initial) | 4 | 8 | 12 (Follow-up) | |
|
|
|
|||||||
| Week | Week | |||||||
| Free T4 (ng/dL) | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 |
| TSH (μIU/mL) | 1.8 ± 0.8 | 1.8 ± 1.2 | 1.9 ± 0.9 | 1.83 ± 1.0 | 1.9 ± 1.1 | 1.9 ± 0.9 | 2.1 ± 2.1 | 1.8 ± 1.1 |
| CPK (IU/L) | 81.2 ± 33.0 | 84.5 ± 53.1 | 83.7 ± 26.9 | 81.8 ± 35.5 | 84.5 ± 32.5 | 87.8 ± 26.9 | 83.1 ± 32.6 | 85.6 ± 31.3 |
| AC (mg/dL) | 88.3 ± 6.8 | 89.9 ± 7.5 | 88.6 ± 7.7 | 85.7 ± 11.0 | 88.5 ± 7.6 | 90.4 ± 9.1 | 91.0 ± 8.5 | 87.6 ± 8.6 |
| LDL lag-time | ||||||||
| Lag-time (min) | 38.5 ± 6.9 | n/a | 68.8 ± 9.7* | n/a | 39.0 ± 7.5 | n/a | 37.5 ± 6.8 | n/a |
Student’s t-test, no significant difference between placebo and treatment group at week 0,
p < 0.05 vs. week 0 for each group, data are the mean ± SD.
T4, thyroxine; TSH, thyroid-stimulating hormone; CPK, creatinine phosphokinase; AC, ante cibum (fasting blood glucose); LDL, low-density lipoprotein.
4. Discussion
M. purpureus NTU 568 fermented products (Ankascin 568 plus) contain monascin (MS) and ankaflavin (AK) not monacolin K, which possess anti-inflammatory ability. Our previous animal experiment studies were the first to discover the hypolipidemic and anti-atherosclerosis functions of MS and AK; these studies also discovered that MS and AK significantly enhanced HDL-C levels, while imposing no damage on the liver or kidneys [16]. Previous study have revealed that MS and AK can reduce the concentrations of serum triglyceride and hepatic cholesterol. In addition, MS and AK can inhibit the activity and expression of inflammatory factor tumor necrosis factor alpha (TNF-α) and endothelial nitric oxide synthase (eNOS), thereby reducing the formation of nitric oxide [14]. Analysis of the mechanisms of action of MS and AK has shown that these compounds are peroxisome proliferator-activated receptor (PPAR)-γ agonists that subsequently initiate the transcription of downstream genes [12,15]. Therefore, based on the reported health benefits of MS and AK as the major constituents of Ankascin 568 plus, we performed the current clinical trial to evaluate the effects of Ankascin 568 plus on hyperlipidemia.
In this study, 57 qualified subjects were included at the beginning of the study and 17 were eventually withdrawn owing to contraindicated diseases or sensory problems. The 40 subjects finally enrolled completed this study. The dietary behavior and lifestyle of the subjects were not changed during the study and no clinical syndromes or discomfort were recorded. In addition, no difference was found in the anthropometric measurements after 8 weeks of intervention between the test and placebo groups. The results showed that TC and LDL-C the treatment group (500 mg Ankascin 568 plus/day), a significant decreased by 11.9% and 19.0%, respectively. Whereas in the placebo group, TC and LDL-C changed only 0.1% and 1.9%. Compared with the placebo group, Ankascin 568 plus can indeed reduce TC and LDL-C. The results of serum triglyceride (TG) levels in the treatment and placebo groups at week 0, 4, 8, and 12 revealed no differences between the treatment and placebo groups (Table 2). However, the TG values did show considerable differences among patients in each group. This intragroup variability might have resulted in the lack of statistically significant differences between the treatment and placebo groups. Table 2 also shows LDL-C can significantly regulate blood lipids, and LDL-C/HDL-C also decreased significantly. Furthermore, significantly lower LDL-C/HDL-C ratios were observed in the Ankascin 568 plus-treated group than in the placebo group. LDL-C is a key indicator of coronary heart disease. It is an important human lipoprotein cholesterol that can be transported to the body cells for use. However, high blood levels of LDL-C result in its accumulation in the vessel walls leading to atherosclerosis. Therefore, high LDL-C is considered a risk factor for vascular obstruction. In contrast, HDL-C is an essential substance in the in vivo prevention of atherosclerosis and is widely used to assess the incidence of coronary artery disease; low levels are an important predictor of coronary atherosclerosis. From the foregoing, Ankascin 568 plus risks regulate blood lipids and reduce cardiovascular disease. This result clearly indicates that the risk of cardiovascular diseases could be greatly reduced by the administration of Ankascin 568 plus.
The in vivo bioavailability of complex foods, herbs, and fermented products has been the focus of many studies [17]. M. purpureus NTU 568 fermented product consists of multiple materials including Monascus metabolites, Dioscorea starch, and other ingredients at such high levels that minimal amounts of Ankascin 568 plus can be completely assimilated into the digestive system. Furthermore, the regulation of cholesterol biosynthesis may alter the effect of Ankascin 568 plus without biological or toxicological significance.
Rhabdomyolysis is a clinical syndrome characterized by the necrosis of skeletal muscle and the subsequent release of toxic intracellular components into the systemic circulation [18]. Causes of rhabdomyolysis include genetic metabolic myopathies, trauma, electrical injuries, exercise, infections, envenomation, hyperthermia, and adverse drug–drug interactions. Different possible mechanisms have been proposed to account for the myotoxic effects of statins. These include statin-induced interruption of glycoprotein synthesis in the muscle membrane, deficiency in chloride channel activation in the muscle membrane, and increased intracellular calcium concentrations leading to impaired membrane function [19]. In agreement with this notion, study statistics have revealed that in approximately 26% of patients, rhabdomyolysis cannot be diagnosed based on urine myoglobin concentrations owing to the low sensitivity of the test [20,21]. However, muscle injury induces release of the enzyme CPK that generates adenosine triphosphate (ATP). In addition, CPK catalyzes the conversion of creatine to creatine phosphate, thereby generating energy for muscle movement. CPK is abundant in the muscles, brain, thyroid, and red blood cells, and CPK levels significantly increase in muscular disease conditions such as dystrophy, polymyositis, skin myositis, trauma, surgery, and conditions involving the excessive use of muscles [22]. Therefore, the CPK level is often used in the diagnosis and monitoring of clinical myocardial infarction and muscular diseases. Indeed, rhabdomyolysis-associated elevated CPK levels are a useful diagnostic indicator of the disease. In a previous study, CPK levels were shown to increase as early as 2–12 h following muscle injury. Furthermore, blood CPK levels remain elevated longer than blood myoglobin levels [23]. Studies of rhabdomyolysis have reported abnormally high CPK values of up to 5000 U/L [24].
The minor changes in liver, kidney, and blood parameters (Tables 3 and 4) revealed no evidence of Ankascin 568 plus-induced toxic effects on liver and renal function as well as electrolyte balance, and rhabdomyolysis was not induced after 8 weeks of treatment in this clinical trial. In our previous study, a wide range of HMG-CoA reductase inhibitors exerted a hypolipidemic effect in adult hamsters at daily doses of 15–75 mg. High-cholesterol diets have a negative impact on the liver and kidneys and cause metabolic burden, thereby affecting the physiological function of these organs [13]. Deterioration of renal function leads to an increase in BUN concentrations. Therefore, urea nitrogen concentration is an important indicator of kidney function. The creatinine level also is an important indicator of renal function, with increased levels being indicative of abnormalities. The in vivo extracellular cation sodium plays an important role in maintaining osmotic pressure by regulating the body fluid balance while potassium prevents muscle contraction and nerve conduction. The kidneys excrete excess potassium in the plasma. The administration of Ankascin 568 plus showed no significant effect on the metabolic or physiological function of the kidneys.
5. Conclusions
This study demonstrated that subjects who were administered one 500 mg capsule of Ankascin 568 plus per day for more than 4 weeks exhibited a significant reduction in serum TC and LDL-C. Therefore, Ankascin 568 plus produced by M. purpureus NTU 568 fermentation may be a potentially useful agent for the regulation of blood lipids and treatment of coronary artery diseases.
REFERENCES
- 1. Steinberg HO, Bayazeed B, Hook G, Johnson A, Cronin J, Baron AD. Endothelial dysfunction is associated with cholesterol levels in the high normal range in humans. Circulation. 1997;96:3287–93. doi: 10.1161/01.cir.96.10.3287. [DOI] [PubMed] [Google Scholar]
- 2. Tandra S, Vuppalanchi R. Use of statins in patients with liver disease. Curr Treat Options Cardiovasc Med. 2009;11:272–8. doi: 10.1007/s11936-009-0028-2. [DOI] [PubMed] [Google Scholar]
- 3. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, Macfarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 4. Delbosc S, Cristol JP, Descomps B, Mimran A, Jover B. Simvastatin prevents angiotensin II-induced cardiac alteration and oxidative stress. Hypertension. 2002;40:142–7. doi: 10.1161/01.hyp.0000024348.87637.6f. [DOI] [PubMed] [Google Scholar]
- 5. Manoukian AA, Bhagavan NV, Hayashi T, Nestor TA, Rios C, Scottolini AG. Rhabdomyolysis secondary to lovastatin therapy. Clin Chem. 1990;36:2145–7. [PubMed] [Google Scholar]
- 6. Nakahara K, Kuriyama M, Sonoda Y, Yoshidome H, Nakagawa H, Fujiyama J, et al. Myopathy induced by HMG-CoA reductase inhibitors in rabbits: a pathological, electrophysiological, and biochemical study. Toxicol Appl Pharmacol. 1998;152:99–106. doi: 10.1006/taap.1998.8491. [DOI] [PubMed] [Google Scholar]
- 7. Corpier CL, Jones PH, Suki WN, Lederer ED, Quinones MA, Schmidt SW, et al. Rhabdomyolysis and renal injury with lovastatin use. Report of two cases in cardiac transplant recipients. JAMA. 1998;260:239–41. [PubMed] [Google Scholar]
- 8. Tokinaga K, Oeda T, Suzuki Y, Matsushima Y. HMG-CoA reductase inhibitors (statins) might cause high elevations of creatine phosphokinase (CK) in patients with unnoticed hypothyroidism. Endocr J. 2006;53:401–5. doi: 10.1507/endocrj.k04-144. [DOI] [PubMed] [Google Scholar]
- 9. Chen CL, Pan TM. Red mold dioscorea: a potentially safe traditional function food for the treatment of hyperlipidemia. Food Chem. 2012;134:1074–80. doi: 10.1016/j.foodchem.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 10. Shi YC, Pan TM. Characterization of a multifunctional Monascus isolate NTU 568 with high azaphilone pigments production. Food Biotechnol. 2010;24:349–63. [Google Scholar]
- 11. Hsu WH, Lu SS, Lee BH, Hsu YW, Pan TM. Monacolin K and monascin attenuated pancreas impairment and hyperglycemia in BALB/c mice induced by advanced glycation endproducts. Food Funct. 2013a;4:1742–50. doi: 10.1039/c3fo60268k. [DOI] [PubMed] [Google Scholar]
- 12. Hsu WH, Chen TH, Lee BH, Hsu WY, Pan TM. Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem Toxic. 2014;64:94–103. doi: 10.1016/j.fct.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 13. Hsu WH, Lee BH, Liao TH, Hsu YW, Pan TM. Monascus-fermented metabolite monascin suppresses inflammation via PPAR-γ regulation and JNK inactivation in THP-1 monocytes. Food Chem Toxicol. 2012;50:1178–86. doi: 10.1016/j.fct.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 14. Hsu WH, Liao TH, Lee BH, Hsu YW, Pan TM. Ankaflavin regulates adipocyte function and attenuates hyperglycemia caused by high-fat diet via PPAR-γ activation. J Funct Foods. 2013b;5:124–32. [Google Scholar]
- 15. Lee BH, Hsu WH, Hsu YW, Pan TM. Dimerumic acid attenuates receptor for advanced glycation endproducts signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into dlactic acid. Free Radic Biol Med. 2013;60:7–16. doi: 10.1016/j.freeradbiomed.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 16. Lee CL, Hung YP, Hsu YW, Pan TM. Monascin and ankaflavin have more anti-atherosclerosis effect and less side effect involving increasing creatinine phosphokinase activity than monacolin K under the same dosages. J Agric Food Chem. 2013;61:143–50. doi: 10.1021/jf304346r. [DOI] [PubMed] [Google Scholar]
- 17. Yan R, Lin G, Ko NL, Tam YK. Low oral bioavailability and pharmacokinetics of senkyunolide a, a major bioactive component in Rhizoma Chuanxiong, in the rat. Ther Drug Monit. 2007;29:49–56. doi: 10.1097/FTD.0b013e31802c5862. [DOI] [PubMed] [Google Scholar]
- 18. Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002;65:907–12. [PubMed] [Google Scholar]
- 19. Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18:90–100. doi: 10.1016/j.ejim.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Bonventre J, Shah S, Walker P, Humphreys M. Rhabdomyolysis-induced acute renal failure. In: Jacobson, Striker, Klahr, editors. The principles and practice of nephrology. 2nd ed. St. Louis: Mosby; 1995. pp. 564–76. [Google Scholar]
- 21. Moore KP, Holt SG, Patel RP, Svistunenko DA, Zackert W, Goodier D, et al. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem. 1998;273:31731–7. doi: 10.1074/jbc.273.48.31731. [DOI] [PubMed] [Google Scholar]
- 22. Homsi E, Barreiro MF, Orlando JM, Higa E. Prophylaxis of acute renal failure in patients with rhabdomyolysis. Ren Fail. 1997;19:283–8. doi: 10.3109/08860229709026290. [DOI] [PubMed] [Google Scholar]
- 23. Wortmann RL, Tipping RW, Levine JG, Melin JM. Frequency of myopathy in patients receiving lovastatin. Am J Cardiol. 2005;95:983–5. doi: 10.1016/j.amjcard.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 24. Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56:1191–6. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]