Abstract
Polygonum orientale L. (Polygonaceae) fruits have various medicinal uses, but their hepatoprotective effects have not yet been studied. This study investigated the hepatoprotective activity of the ethanolic extract of P. orientale (POE) fruits against carbon tetrachloride (CCl4)-induced acute liver injury (ALI). Mice were pretreated with POE (0.1, 0.5, and 1.0 g/kg) or silymarin (0.2 g/kg) for 5 consecutive days and administered a dose of 0.175% CCl4 (ip) on the 5th day to induce ALI. Blood and liver samples were collected to measure antioxidative activity and cytokines. The bioactive components of POE were identified through high-performance liquid chromatography (HPLC). Acute toxicity testing indicated that the LD50 of POE exceeded 10 g/kg in mice. Mice pretreated with POE (0.5, 1.0 g/kg) experienced a significant reduction in their serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), and alkaline phosphatase (ALP) levels and reduction in the extent of liver lesions. POE reduced the malondialdehyde (MDA), nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) levels, and increased the activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GRd) in liver. HPLC revealed peaks at 11.28, 19.55, and 39.40 min for protocatechuic acid, taxifolin, and quercetin, respectively. In summary, the hepatoprotective effect of POE against CCl4-induced ALI was seemingly associated with its antioxidant and anti-proinflammatory activities.
Keywords: Hepatoprotective activity, Polygonum orientale, Carbon tetrachloride
1. Introduction
The liver is a crucial organ in the human body. It regulates functions including the synthesis of proteins, secretion of biochemical enzymes, and detoxification of xenobiotics. Acute liver injury (ALI) is usually caused by toxic chemicals, drugs, or pathogen infections [1]. Carbon tetrachloride (CCl4), a well-known agent used in animal models of hepatotoxicity, is metabolized by cytochrome P450 enzymes into highly reactive trichloromethyl radicals that can activate Kupffer cells and mediate the hepatic inflammation process by producing proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) [2,3]. Lipid peroxidation is also caused by trichloromethyl radicals [4]. Necrotic or apoptotic cells are regenerated and replaced by parenchymal cells after ALI. This process is associated with an inflammatory response and a limited extracellular matrix (ECM) deposition. If the hepatic injury persists, liver regeneration eventually fails and hepatocytes are replaced by a large amount of ECM that induces fibrillar collagen. Because continuous liver injury causes fibrosis, a reduction in ALI might avert this outcome [2].
Polygonum orientale L. (Polygonaceae) has been used as medicine and food in Chinese herbal pharmacopeias [5]. Numerous natural products possess medicinal properties; for example, flavonoids are among the most well-known examples of powerful pharmaceutical ingredients found in plants. Phytochemical studies have shown abundant flavonoids in this plant such as taxifolin and quercetin. Phenolics such as gallic acid and protocatechuic acid were also identified [6]. The abilities of various extracts of P. orientale (POE) to scavenge free radicals were reported in an in vitro study [5]. The whole plant has been used in China for the treatment of various conditions such as arthritis, edema, dysentery, fractures, urticarial, and muscle injuries [7]. Pharmacological studies have indicated that all parts of P. orientale possess anti-inflammatory, anti-fibrosis, antioxidative, anticancer, immune-stimulating, swelling-subsiding, antimyocardial ischemic, and vaso-dilating activities, and that these properties are concentrated in its fruit [5,8]. However, the hepatoprotective effects of this plant remain unknown.
2. Methods and materials
2.1. Chemicals and methods
Silymarin, taxifolin hydrate (≥90%), and quercetin (≥98%) were purchased from Sigma–Aldrich Chemical Co. (USA). Protocatechuic acid (≥98%) was purchased from Tokyo Chemical Industry Co. Ltd. (Japan). CCl4 was purchased from Merck Co. (Germany) and was dissolved in olive oil to form a 0.175% (v/v) solution. Silymarin was suspended in 1% carboxymethylcellulose (CMC). All other reagents used were of analytical grades. Serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GRd) assay kits were purchased from Randox Laboratory Ltd., and nitric oxide (NO) and malondialdehyde (MDA) assays were obtained from Cayman Chemical Co. (Ann Arbor, MI, USA). TNF-α, IL-1β, and IL-6 were obtained from eBioscience Inc. (San Diego, CA, USA).
2.2. Preparation of POE
The fruits of POE were collected in Toushe, Nantou County, Taiwan in July 2013. This plant was identified by Professor Chao-Lin Kuo, Chair of the Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, China Medical University (CMU-CMR). A voucher specimen (CMU-CMR-PO-103001) was deposited at CMU-CMR for further reference. The crude extract preparation procedure was modified from that described in a previous study [9]. Whole fruits were dried in a circulating air oven, crushed, and ground into a coarse powder (1.8 kg) that was extracted by triple maceration with 70% ethanol. The filtered extracts were collected and concentrated under reduced pressure to produce 91.5 g of extract with a yield of 5.08% (w/w). The remaining solution was lyophilized before the final POE was obtained. The extract was stored at −20 °C before the experiment.
2.3. Analysis of POE by high-performance liquid chromatography
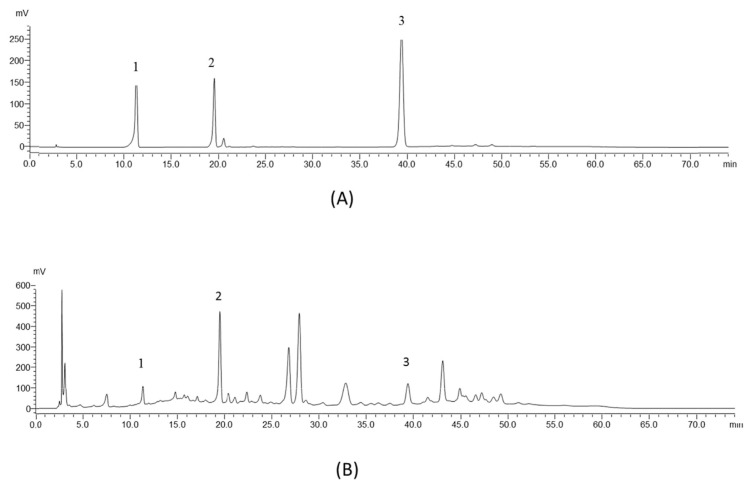
High-performance liquid chromatography (HPLC) profiles were established for the standards (protocatechuic acid, taxifolin hydrate, and quercetin) and the POE. The methodology followed that of a previous study with minor modifications [10]. The analysis was performed on an Ascentis C18 column (4.6 mm × 250 mm × 5 μm) purchased from Sigma–Aldrich by using a Shimadzu LC-20AT HPLC and SPD-20A UV–Vis detector. Optimum separation was achieved using gradient elution with 0.1% aqueous phosphoric acid (A) and methanol (B) programmed as time and percentage of B: 0 min (10%), 10 min (39%), 20 min (47%), 30 min (47%), 40 min (58%), 55 min (56%), 65 min (10%), and 75 min (10%). All standards and samples were passed through a 0.45 μm Minipore filter before injection into the column with a sample size of 20 μL. The flow rate was set at 1.0 mL/min and detected at 270 nm. The chromatograms of the standards and POE are shown in Fig. 1.
Fig. 1.
HPLC chromatograms of (A) standards and (B) POE. Peaks: (1) protocatechuic acid; (2) taxifolin; (3) quercetin.
2.4. Experimental animals
ICR male mice (20 ± 2 g) were purchased from BioLasco Charles River Technology, Taipei, Taiwan. They were housed in standard cages at a constant temperature of 22 ± 1 °C and relative humidity of 55 ± 5% with a 12-h light–dark cycle for at least 1 week before the experiment. They were fed with food and water ad libitum. The animals used in this study were housed and cared in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the Committee on Animal Research, China Medical University, under the Nos. 104–248.
2.5. Acute toxicity test
Our method was based on those of our previous study [11]. Ten male ICR mice (22–25 g) were orally administered POE (10 g/kg). The experimental mice were given food and water ad libitum and were kept under regular, recorded observation for any mortality or behavioral changes over 14 consecutive days.
2.6. Experimental design of CCl4-induced hepatotoxicity
Our methods in this segment of the study were based on those employed by Tsai et al. [12]. The experimental animals were randomly divided into 6 experimental groups (10 mice in each group). The mice in the normal control group (NC) and CCl4 group (CCl4) were orally administered distilled water. Silymarin was used as a positive control. The mice in the silymarin group were orally administered silymarin (0.2 g/kg in 1% CMC), and those in the POE groups were orally administered POE (0.1, 0.5, and 1.0 g/kg) for 5 consecutive days. The doses of POE were translated from traditional consumption levels in humans [13]. Each group of mice, except for the NC, received an intraperitoneal injection of CCl4 (10 mL/kg BW, 0.175% in olive oil) 1 h after the final daily treatments. The NC mice received an equivalent volume of olive oil (ip). The mice were sacrificed under anesthesia 24 h later. Blood was collected and liver tissues were removed. The levels of AST, ALT, and ALP were measured. In addition, liver biopsies were examined for pathological changes. The levels of MDA and NO, in addition to the activities of the antioxidative enzymes SOD, GPx, and GRd, were measured. We also estimated the levels of proinflammatory cytokines TNF-α, IL-1β, and IL-6.
2.7. Serum biochemistry
The blood was centrifuged at 3000 rpm (Beckman GS-6R, Germany) at 4 °C for 30 min to separate the serum. Moreover, ALT, AST, and ALP were measured using spectrophotometric diagnostic kits.
2.8. Histological analysis
Liver tissues were collected from the same lobes and trimmed to approximately 2 mm in thickness. The tissues were then fixed in a 10% buffered formaldehyde solution and embedded in paraffin. They were further cut into 2-μm sections, stained with hematoxylin and eosin, and examined under light microscopy.
2.9. MDA assay
The MDA levels in the liver were evaluated with the thiobarbituric acid reacting substance (TBARS) method [11]. Briefly, MDA reacted with thiobarbituric acid under an acidic condition at a high temperature and formed a red-complex TBARS. The absorbance of the TBARS was determined at 532 nm.
2.10. NO assay
NO was measured using previously reported methods [14,15]. Nitrate was converted to nitrite by using nitrate reductase. Nitrite subsequently reacted with sulfanilic acid to produce diazonium ions, which reacted with N-(1-naphthyl) ethylenediamine to produce a chromophoric azo derivative (purplish red), which was recorded at 540 nm. The sum of nitrite and nitrate obtained in this procedure are represented as μM.
2.11. Measurements of antioxidant enzymatic activity
Liver tissue homogenates were collected to estimate the levels of SOD, GPx, and GRd enzymes, and a ChemWell-T Automated Chemistry Analyzer was used to detect the antioxidant activity of POE.
2.12. Inflammatory cytokines
TNF-α, IL-1β, and IL-6 were measured by ELISA kits according to the instructions provided by the manufacturer. The captured antibodies of TNF-α, IL-1β, and IL-6 were seeded in each well of a 96-well plate overnight. The next day, a second set of biotinylated antibodies was incubated with sample tissues or standard antigens in the plate before streptavidin-HRP was added. TNF-α, IL-1β, and IL-6 were measured at 450 nm to determine their amount. The amounts of TNF-α, IL-1β, and IL-6 are represented as pg/mg protein.
2.13. Statistical analyses
All data are expressed as mean ± SEM. Statistical analyses were performed with SPSS software, using one-way ANOVA followed by Scheffe’s multiple range tests. Histological analyses were conducted using the nonparametric Kruskal–Wallis test. The criterion for statistical significance was p < 0.05.
3. Results
3.1. Analyses of POE by HPLC fingerprint
The results of the standards and POE are shown in Fig. 1. In the chromatogram of the standards, we detected peaks at the retention times of 11.28, 19.55, and 39.40 min, representing protocatechuic acid, taxifolin, and quercetin, respectively. Those peaks were also found in the POE chromatogram, with the relative amounts of each at 270 nm. Chromatograms were baseline separated according to the time program and identification was achieved through spiking each standard in the sample.
3.2. Acute toxicity tests
The acute toxicity of POE was evaluated in mice at doses of 10 g/kg. After 14 days of oral administration, no behavioral changes or mortalities were observed. Therefore, we conclude that the LD50 of POE is greater than 10 g/kg in mice, indicating that it is most likely not acutely toxic.
3.3. Effect of POE on CCl4-induced hepatotoxicity
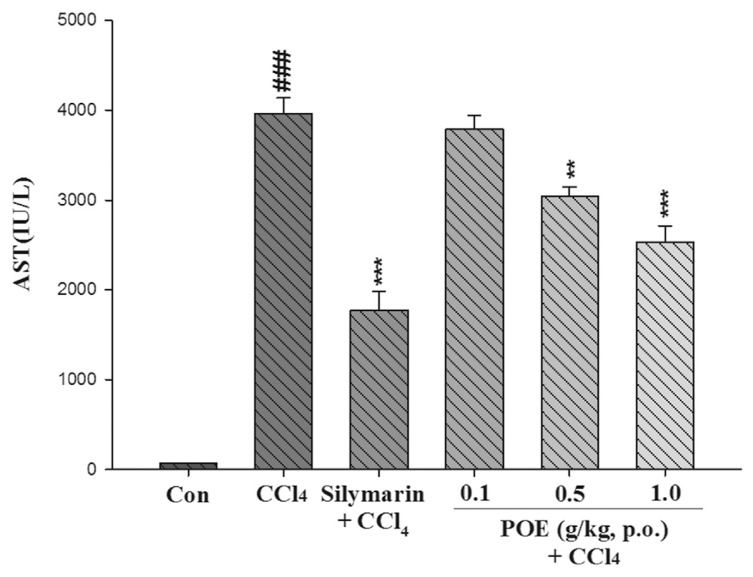
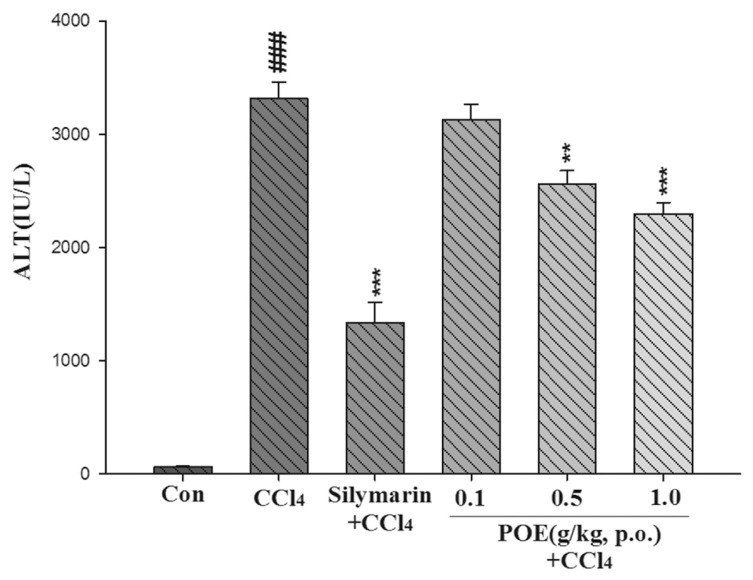
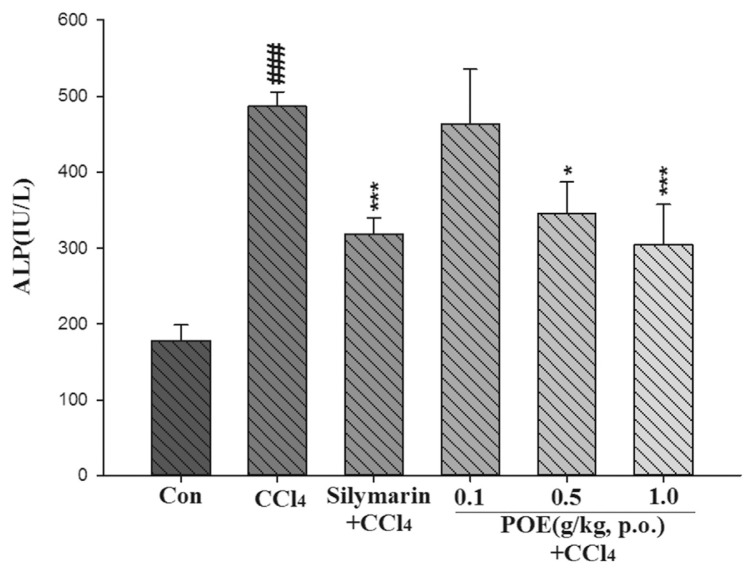
The hepatotoxicity effect of POE on CCl4-induced liver injury is shown in Figs. 2–4. The CCl4 group exhibited a significant increase in AST, ALT, and ALP. However, this increase noticeably decreased after treatment with POE (0.5 and 1.0 g/kg) and silymarin (0.2 g/kg). These results suggested that POE possessed protective properties against CCl4-induced liver injury.
Fig. 2.
Effect of silymarin (0.2 g/kg) and POE (0.1, 0.5, 1.0 g/kg) on activities of AST in the control group (Con) and CCl4 treated groups. Values are mean ± SEM (n = 10). #Indicates significant difference from the control group (###p < 0.001). *Indicates significant difference from the CCl4 group (**p < 0.01, ***p < 0.001).
Fig. 3.
Effect of silymarin (0.2 g/kg) and POE (0.1, 0.5, 1.0 g/kg) on activities of ALT in the control group (Con) and CCl4 treated groups. Values are mean ± SEM (n = 10). #Indicates significant difference from the control group (###p < 0.001). *Indicates significant difference from the CCl4 group (**p < 0.01, ***p < 0.001).
Fig. 4.
Effect of silymarin (0.2 g/kg) and POE (0.1, 0.5, 1.0 g/kg) on activities of serum ALP in the control group (Con) and CCl4 treated groups. Values are mean ± SEM (n = 10). #Indicates significant difference from the control group (###p < 0.001).
*Indicates significant difference from the CCl4 group (*p < 0.05, ***p < 0.001).
3.4. Histological analyses
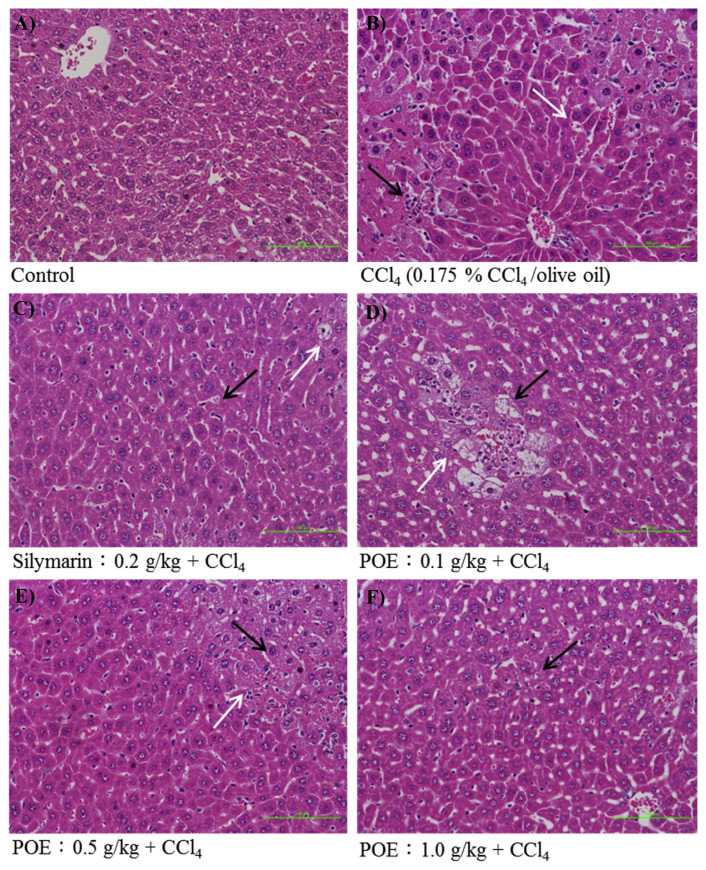
Our results showed that major changes in histology induced by CCl4 resulted in increased vacuolization, inflammation, and coagulative necrosis (Fig. 5B) when compared with the control group (Fig. 5A). Liver injuries were reduced by pre-treatment with POE (0.5 and 1.0 g/kg) (Fig. 5E and F), and the injury scores of vacuolization, inflammation, and hepatocellular necrosis significantly decreased (Table 1). Our histological findings also demonstrated that pretreatment with POE significantly prevented CCl4-induced liver injury.
Fig. 5.
The hepatic histological analyses of silymarin (0.2 g/kg) and POE (0.1, 0.5, 1.0 g/kg) on CCl4-induced acute liver injury in mice. Liver tissues were stained with H & E (200×). Cell necrosis was displayed by black arrow and vacuole formation was displayed by white arrow. (A) Control group; (B) animals treated with 0.175% CCl4 (10 mL/kg of bw); (C) animals pre-treated with silymarin (0.2 g/kg) and then treated with CCl4; (D–F) animals pre-treated with POE (0.1, 0.5 and 1.0 g/kg) and then treated with CCl4.
Table 1.
Quantitative summary of the protective effects of silymarin and POE on CCl4-induced hepatic damage based on histological observations.
| Groups | Injury of score | ||
|---|---|---|---|
|
| |||
| Vacuolization | Hepatocellular Necrosis | Inflammation | |
| Control | 0 | 0 | 0 |
| CCl4 (0.175% CCl4/olive oil) | 2.40 ± 0.20### | 3.89 ± 0.99### | 1.83 ± 0.14### |
| Silymarin: 0.2 g/kg + CCl4 | 0.63 ± 0.23** | 2.00 ± 0.41 | 0.67 ± 0.15** |
| POE: 0.1 g/kg + CCl4 | 1.13 ± 0.26 | 3.14 ± 0.26 | 1.22 ± 0.13 |
| POE: 0.5 g/kg + CCl4 | 1.29 ± 0.25 | 2.13 ± 0.40 | 0.67 ± 0.18* |
| POE: 1.0 g/kg + CCl4 | 0.75 ± 0.28** | 1.50 ± 0.36* | 0.63 ± 0.23** |
To quantify the histological index of vacuolization and hepatocellular necrosis of liver; the liver damage was graded 0–4 as following: 0 = no visible cell damage; 1 = slight (1–25%); 2 = moderate (26–50%); 3 = moderate/severe (51–75%); 4 = severe/high (76–100%) [36]. Values are mean ± SEM (n = 10).
Indicates significant difference from the control group (###p < 0.001).
Indicates significant difference with respect to the CCl4 group (*p < 0.05, **p < 0.01).
3.5. Effects of POE on NO level in liver
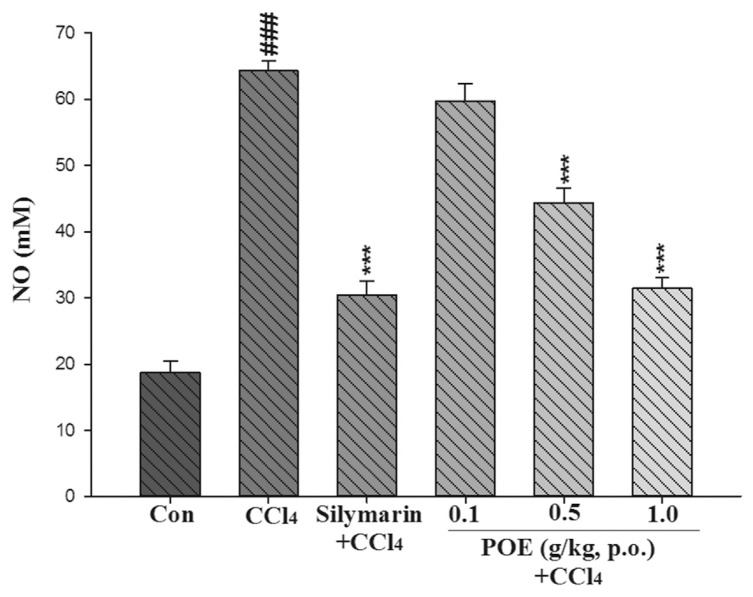
The level of NO in the liver was significantly elevated in the CCl4 group compared with the control group. Pretreatment with silymarin and 0.5 and 1.0 g/kg of POE significantly inhibited the increase in NO levels, as compared with those in the CCl4 group (Fig. 6).
Fig. 6.
Effect of silymarin (0.2 g/kg) and POE (0.1, 0.5, 1.0 g/kg) on level of NO in liver treated with CCl4. Values are mean ± SEM (n = 10). #Indicates significant difference from the control group (Con) (###p < 0.001). *Indicates significant difference from the CCl4 group (***p < 0.001).
3.6. Effect of POE on MDA level in liver
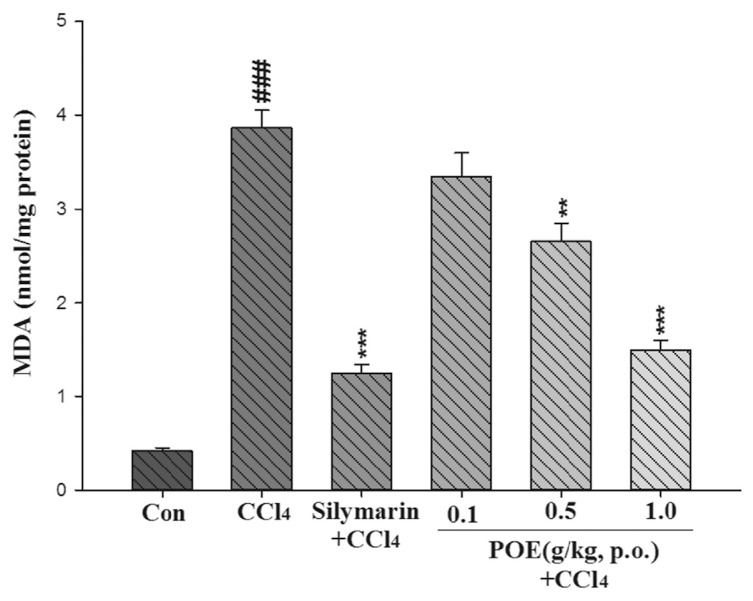
The MDA level was used to signify hepatic lipid peroxidation. The effects of POE on CCl4-induced lipid peroxidation are illustrated in Fig. 7. The levels of MDA in the CCl4 group were significantly elevated (p < 0.01). However, the MDA levels were reduced by pretreatment with POE (0.5 and 1.0 g/kg) and silymarin (0.2 g/kg).
Fig. 7.
Effect of silymarin (0.2 g/kg) and POE (0.1, 0.5, 1.0 g/kg) on level of malondialdehyde (MDA) induced with CCl4 in the mice liver. Values are mean ± SEM (n = 10). #Indicates significant difference from the control group (Con) (###p < 0.001).
*Indicates significant difference from the CCl4 group (**p < 0.01, ***p < 0.001).
3.7. Measurements of antioxidant enzymatic activities
SOD, GPx, and GRd were measured to evaluate the antioxidant effects of POE in the liver. The activity of hepatic enzymes in the CCl4 group significantly decreased compared with that in the control group (Table 2). Treatment with POE at doses of 0.5 and 1.0 g/kg and silymarin at a dose of 0.2 g/kg increased the levels of SOD, GPx, and GRd activity significantly.
Table 2.
Effect of POE on the activities of different antioxidant enzymes in CCl4-treated mice.
| Groups | Activity (U/mg protein) | ||
|---|---|---|---|
|
| |||
| SODa | GPxb | GRdc | |
| Control | 105.80 ± 4.11 | 21.31 ± 0.64 | 12.25 ± 0.22 |
| CCl4 (0.175% CCl4/olive oil) | 11.82 ± 1.44### | 5.34 ± 0.27### | 6.26 ± 0.14### |
| Silymarin: 0.2 g/kg + CCl4 | 89.11 ± 2.77*** | 15.85 ± 0.58*** | 10.37 ± 0.35*** |
| POE: 0.1 g/kg + CCl4 | 19.65 ± 4.99 | 6.8 ± 0.33 | 7.55 ± 0.37 |
| POE: 0.5 g/kg + CCl4 | 28.70 ± 1.68* | 9.04 ± 0.44** | 9.43 ± 0.50** |
| POE: 1.0 g/kg + CCl4 | 59.18 ± 5.80*** | 12.29 ± 0.51*** | 11.55 ± 0.37*** |
Indicates significant difference from the control group (###p < 0.001).
Indicates significant difference from the CCl4 group (*p < 0.05, **p < 0.01, ***p < 0.001). Values are mean ± SEM (n = 10).
SOD: superoxide dismutase.
GPx: glutathione peroxidase.
GRd: glutathione reductase.
3.8. Effect of POE on TNF-α, IL-1β, IL-6 in liver
As shown in Table 3, TNF-α, IL-1β, and IL-6 levels in CCl4-induced ALI were remarkably high. Treatment with silymarin at 0.2 g/kg or POE at 0.5 and 1.0 g/kg significantly suppressed the concentration of TNF-α. Similarly, the IL-1β and IL-6 levels were significantly lowered by silymarin or POE.
Table 3.
Effect of POE on the level of different pro-inflammatory cytokines in CCl4-treated mice.
| Groups | Pro-inflammatory cytokines (pg/mg protein) | ||
|---|---|---|---|
|
| |||
| TNF-α | IL-1β | IL-6 | |
| Control | 50.43 ± 1.97 | 170.67 ± 014.52 | 8.57 ± 0.53 |
| CCl4 (0.175% CCl4/olive oil) | 97.24 ± 2.31### | 1698 ± 48.66### | 31.35 ± 1.38### |
| Silymarin: 0.2 g/kg + CCl4 | 61.19 ± 2.18*** | 1002.17 ± 13.78*** | 16.88 ± 0.77*** |
| POE: 0.1 g/kg + CCl4 | 94.49 ± 1.36 | 1289.72 ± 69.91 | 28.66 ± 1.18 |
| POE: 0.5 g/kg + CCl4 | 83.04 ± 3.27 | 1128.10 ± 71.32** | 22.86 ± 2.01** |
| POE: 1.0 g/kg + CCl4 | 73.77 ± 11.27** | 874.05 ± 42.77*** | 18.36 ± 0.58*** |
Indicates significant difference from the control group (###p < 0.001).
Indicates significant difference from the CCl4 group (**p < 0.01, *** p < 0.001).
Values are mean ± SEM (n = 10).
4. Discussion
Phytochemical studies demonstrated that the fruits of POE are rich in flavonoids, which are the major active components of POE [16]. Flavonoids are a large class of phenolic compounds and constitute one of the largest groups of secondary metabolites in plants. Moreover, these compounds significantly protect cells against the damaging effects of reactive oxygen species (ROS) such as singlet oxygen, superoxide, peroxyl radicals, hydroxyl radicals, and peroxynitrite. Oxidative stress contributes to cancer, aging, atherosclerosis, ischemic injury, inflammation, and neurodegenerative diseases [17].
CCl4-induced ALI in mice is widely used as an experimental animal model to screen drugs for preventing liver injury [3]. The doses of human were translated based on BSA formula. In our research, 0.5 g/kg (medium dose) is conventional dose in human transfer to mice. The low dose (0.1 g/kg) is 5-fold dilution of 0.5 g/kg. The high dose (1.0 g/kg) is twice dose of 0.5 g/kg. Oxidative stress heightened by an imbalance between oxidants and antioxidants is known to be a critical factor in disrupting the health of biological systems [1]. For the treatment of hepatic diseases, numerous studies have emphasized the importance of finding antioxidant compounds that can inhibit liver injuries by scavenging free radicals [18,19].
Typically, ALT and AST are used to assess liver function; ALT is highly precise in monitoring hepatocellular status, and AST is a sensitive indicator of mitochondrial problems, particularly in centrilobular areas of the liver. Increases in the levels of cytoplasmic enzymes such as AST, ALT, and ALP are considered indicators of hepatic dysfunction and damage [1]. The leakage of cytosolic contents into the systemic circulation alters liver function. In our study, the activities of AST, ALT, and ALP in serum increased after CCl4 was introduced. This increase can be attributed to hepatic damage resulting in an increased rate of synthesis or release of functional enzymes from biomembranes [20]. However, these increases were significantly reduced by treatment with POE at 0.5 and 1.0 g/kg (Figs. 2–4). These findings suggest that POE and silymarin prevented CCl4-induced hepatotoxicity.
Hepatic cell injury was accompanied by vacuolization, inflammation, and hepatocellular necrosis of the liver around the central vein in the CCl4-treated group, developments that did not occur in the control group. This phenomenon was significantly attenuated by pretreatment with POE and silymarin (Fig. 5). The levels of vacuolization, inflammation, and necrosis of hepatocytes were considerably elevated after CCl4 treatment. However, the injury score of hepatocytes was reduced significantly by pretreatment with POE (0.5 and 1.0 g/kg) and silymarin (Table 1).
Oxidative stress plays a major role in CCl4-induced liver injury; it is mediated by the production of free radical derivatives of CCl4 and is responsible for cell membrane damage and the consequent release of marker enzymes of hepatotoxicity [21]. Oxidative injury induced by CCl4 can be monitored in experimental animals by detecting oxidative stress parameters such as NO, MDA, SOD, GPx, and GRd [22]. SOD is an effective antioxidant enzyme for catalyzing the dismutation of superoxide radicals into H2O2 [23]. GPx and GRd are glutathione-related enzymes that catalyze the reduction of H2O2 and hydroperoxides into nontoxic products and then end the chain reaction of lipid peroxidation [17]. The free radical NO is a highly active nitrogen species produced by both parenchymal and nonparenchymal liver cells from l-arginine through NO synthase [24]. NO may have a cytotoxic effect on neutrophils by forming peroxynitrite after it reacts with various ROS, especially [15,25]. Lipid peroxides or ROS can easily block these antioxidant enzymes. In the current study, a significant difference in hepatic NO levels was noted in mice pretreated with POE or silymarin compared with mice administered CCl4 alone and the control group. IL-1β also plays a crucial role in the regulation of hepatic NO synthesis [25]. Suppression of NO production is likely due to pre-treatments that increase SOD, GPx, and GRd activity and reduce the IL-1β level. Lipid peroxidation has been hypothe-sized to be a principal cause of CCl4-induced liver injury and can therefore be used as a marker of oxidative damage. MDA is the final product of lipid peroxidation, and therefore has a long history of use as a biomarker for this process [26]. The increase in MDA as a feature of liver injury is also considered a marker of lipid peroxidation [27]. In the current study, the levels of hepatic MDA were significantly attenuated by pre-treatment with silymarin and POE (0.5 and 1.0 g/kg) that increased the activity of hepatic antioxidant enzymes (SOD, GPx and GRd) and reduced the level of IL-1β. Proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, are rapidly induced from activated Kupffer cells. These mediators act on endothelial cells, hepatic stellate cells, and hepatocytes and subsequently recruit immune cells to initiate inflammatory responses [28,29]. In the present study, the levels of proinflammatory mediators including TNF-α, IL-1β, and IL-6 were considerably higher in the CCl4-treated mice. However, pre-treatment with POE led to a significant reduction in the CCl4-stimulated activity of TNF-α, IL-1β, and IL-6. These results suggest that the hepatoprotective effects of POE (0.5 and 1.0 g/kg) result from its ability to reduce the levels of proinflammatory cytokines.
Our HPLC fingerprinting shows that POE contains proto-catechuic acid, taxifolin, and quercetin. Previous studies have demonstrated that these compounds all possess significant antioxidative and anti-inflammatory activities [30–32]. Moreover, protocatechuic acid and quercetin have protected against CCl4-induced liver injury in mice [33,34]. Taxifolin is the most potent flavonoid found in silymarin, and is a strong hepatoprotective agent [35]. Therefore, it is reasonable to suggest that aforementioned phytochemicals might be parts of active constituents with the hepatoprotective profile of POE. The effects observed in this study also provide evidence for medicinal or functional food uses for the fruits of POE to prevent ALI. Further research is still imperative to elucidate the other active compounds and biochemical mechanisms involved in antifibrotic activity in the liver.
In conclusion, our results offer the first indication that POE possesses hepatoprotective potential seemingly through enhancing hepatic antioxidant enzyme activity, inhibiting lipid peroxidation, and reducing the activity of proinflammatory mediators. The hepatoprotective activity of POE might be partially associated with protocatechuic acid, taxifolin, and quercetin.
Acknowledgments
This study was supported by the Ministry of Health and Welfare program (MOHW104-CMAP-M-114-000423), Taiwan, R.O.C.
Funding Statement
This study was supported by the Ministry of Health and Welfare program (MOHW104-CMAP-M-114-000423), Taiwan, R.O.C.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Wang Y, Tang C, Zhang H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J Food Drug Anal. 2015;23(2):310–7. doi: 10.1016/j.jfda.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, Contreras R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10(4):429–34. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 3. Lin YC, Cheng KM, Huang HY, Chao PY, Hwang JM, Lee HH, et al. Hepatoprotective activity of Chhit-Chan-Than extract powder against carbon tetrachloride-induced liver injury in rats. J Food Drug Anal. 2014;22(2):220–9. [Google Scholar]
- 4. Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189(1–2):113–27. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 5. Jiang XY, Chen XQ, Wei Y. Free radical scavenging activity and flavonoids contents of Polygonum orientale leaf, stem and seed extracts. Arch Biol Sci. 2009;28(2):284–7. [Google Scholar]
- 6. Lv JH, Zhang HF, Teng K, Sun J. Research on the chemical constituents and pharmacological activities of Polygonum orientale L. Chin J Pharmacovigil. 2011;8(12):744–5. [Google Scholar]
- 7. Liao SG, Li YT, Zhang LJ, Wang Z, Chen TX, Huang Y, et al. UPLC-PDA-ESI-MS/MS analysis of compounds extracted by cardiac h9c2 cell from Polygonum orientale. Phytochem Anal. 2013;24(1):25–35. doi: 10.1002/pca.2374. [DOI] [PubMed] [Google Scholar]
- 8. Sheng HG. Advances in research of chemical constituents and pharmacological actions of Polygoni orientalis fructus. Chem Ind Times. 2013;2:44–6. [Google Scholar]
- 9. Yang GX, Song L, Li KL, Hu CQ. Studies on the chemical constituents of Polygonum orientale. Chin Pharm J. 2003;38:338–40. [Google Scholar]
- 10. Zhou XY, Su BH, Zhang YY, Zhai YJ. Effects of different processing on active components in fructus polygoni orientalis by HPLC analysis. Zhong Yao Cai. 2012;35(4):540–2. [PubMed] [Google Scholar]
- 11. Chiu YJ, Huang TH, Chiu CS, Lu TC, Chen YW, Peng WH, et al. Analgesic and antiinflammatory activities of the aqueous extract from Plectranthus amboinicus (Lour.) Spreng. Both in vitro and in vivo. Evid Based Compl Alt Med. 2012;2012:508137. doi: 10.1155/2012/508137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsai JC, Peng WH, Chiu TH, Huang SC, Huang TH, Lai SC, et al. Hepatoprotective effect of Scoparia dulcis on carbon tetrachloride induced acute liver injury in mice. Am J Chin Med. 2010;38(4):761–75. doi: 10.1142/S0192415X10008226. [DOI] [PubMed] [Google Scholar]
- 13. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 14. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 15. Ródenas J, Mitjavila MT, Carbonell T. Simultaneous generation of nitric oxide and superoxide by inflammatory cells in rats. Free Radic Bio Med. 1995;18(5):869–75. doi: 10.1016/0891-5849(94)00215-6. [DOI] [PubMed] [Google Scholar]
- 16. Xie ZT, Hu J. Studies on the chemical constituents in ethyl acetate extraction from the fruit of Polygonum orientale. Zhong Yao Cai. 2009;32(9):1397–9. [PubMed] [Google Scholar]
- 17. Chao J, Liao JW, Peng WH, Lee MS, Pao LH, Cheng HY. Antioxidant, analgesic, anti-inflammatory, and hepatoprotective effects of the ethanol extract of Mahonia oiwakensis stem. Int J Mol Sci. 2013;14(2):2928–45. doi: 10.3390/ijms14022928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casas-Grajales S, Muriel P. Antioxidants in liver health. World J Gastrointest Pharmacol Ther. 2015;6(3):59–72. doi: 10.4292/wjgpt.v6.i3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Videla LA. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J Hepatol. 2009;1(1):72–8. doi: 10.4254/wjh.v1.i1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pari L, Prasath A. Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chem Biol Interact. 2008;173(2):77–83. doi: 10.1016/j.cbi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 21. Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Szymonik-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, Slomka M, Madro A, Celinski K, et al. Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J Hepatobiliary Pancreat Surg. 2003;10(4):309–15. doi: 10.1007/s00534-002-0824-5. [DOI] [PubMed] [Google Scholar]
- 23. Bowling AC, Schulz JB, Brown RH, Jr, Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993;61(6):2322–5. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 24. Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65(2):166–76. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 25. Geller DA, de Vera ME, Russell DA, Shapiro RA, Nussler AK, Simmons RL, et al. A central role for IL-1 beta in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase. IL-1 beta induces hepatic nitric oxide synthesis. J Immuno. 1995;155(10):4890–8. [PubMed] [Google Scholar]
- 26. Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Bio Med. 1990;9(6):515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 27. Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J Chromatogr B Anal Technol Biomed Life Sci. 2005;827(1):76–82. doi: 10.1016/j.jchromb.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 28. Salazar-Montes A, Rincón AR, Panduro A, Armendáriz-Borunda J. Chemically induced liver regeneration is characterized by specific IL-6 gene expression. Hepatol Res. 1999;15(1):10–21. [Google Scholar]
- 29. Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride-induced liver injury and fibrosis in rats. Exp Mol Pathol. 2001;71(3):226–40. doi: 10.1006/exmp.2001.2399. [DOI] [PubMed] [Google Scholar]
- 30. Guo H, Zhang X, Cui Y, Zhou H, Xu D, Shan T, et al. Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicol Appl Pharml. 2015;287(2):168–77. doi: 10.1016/j.taap.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 31. Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:9. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim CS, Kwon Y, Choe SY, Hong SM, Yoo H, Goto T, et al. Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1. Nutr Metab (Lond) 2015;12:33. doi: 10.1186/s12986-015-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hung MY, Fu TY, Shih PH, Lee CP, Yen GC. Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibits CCl4-induced hepatic damage in rats. Food Chem Toxicol. 2006;44(8):1424–31. doi: 10.1016/j.fct.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 34. Ma JQ, Li Z, Xie WR, Liu CM, Liu SS. Quercetin protects mouse liver against CCl(4)-induced inflammation by the TLR2/4 and MAPK/NF-kappaB pathway. Int Immunopharmacol. 2015;28(1):531–9. doi: 10.1016/j.intimp.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 35. Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DY, Liu Y, et al. Identification of hepatoprotective flavonolignans from silymarin. P Natl Acad Sci U S A. 2010;107(13):5995–9. doi: 10.1073/pnas.0914009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shackelford C, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 2002;30(1):93–6. doi: 10.1080/01926230252824761. [DOI] [PubMed] [Google Scholar]