Abstract
Pearl is one of the well-known traditional Chinese medicine (TCM) prescribed for treating various skin and bone related disorders due to its abundant proteins and mineral contents. The present investigation focused on antioxidation and life span prolonging effects from different extracts of pearl powder. During in vitro studies, various oxidative indices were evaluated, along with lifespan-prolonging effect were checked using wild-type Caenorhabditis elegans. For the clinical trial, 20 healthy middle-aged subjects were recruited and separated into 2 groups as experimental and placebo group, who received 3 g of pearl powder/d (n = 10) and 3 g of placebo/d (n = 10) for 8 weeks, respectively. During the initial, 2nd, 4th, 6th, 8th and 10th weeks the blood samples were collected for biochemical analysis. The protein extract of pearl powder recorded maximum (p < 0.05) antioxidant activity (20–68%) as well as efficiently prolonged the life span of C. elegans by 18.87%. Pearl powder supplemented subjects showed a substantial increase (p < 0.05) in total antioxidant capacity from 0.45 to 0.69 mM, total thiols from 0.23 to 0.29 mM, Glutathione content from 5.89 to 9.19 μM, enzymic antioxidant activity (SOD-1248 to 1308; Gpx-30 to 32; GR-2.4 to 2.9) as well as considerably suppressed the lipid peroxidation products from 4.95 to 3.27 μM. The outcome of both in-vitro and in-vivo antioxidant activity inferred that protein extract of pearl powder was a potent antioxidant and thereby prolonged the lifespan of C. elegans. Hence, pearl powder could be recommended for treating various age-related degenerative disorders.
Keywords: Caenorhabditis elegans, Pearl powder, Antioxidant, Clinical trial, Longevity
1. Introduction
The ocean contains an array of bioactive molecules that are actively being researched for their health benefits, one such aquatic material is a pearl. The formation of natural pearl are by the accidental lodging of sand or parasite (irritant) into the body of oyster and layered by nacre to form a pearl [1]. It is made up of various carbonates in minute crystalline form, which has been deposited in concentric layers [2]. Pearl and its grounded pure pearl powder are commonly used in traditional Chinese medicine (TCM) for beauty treatment and various ailments [3]. Moreover, in Taiwan, Pregnant women are highly recommended to intake pearl powder for improving fetal skin beauty [4]. Therefore, numerous pharmaceutical companies (Dragon Herbs, Jing Herbs, Vita Fede) are started to produce various pearl related products especially as a dietary supplement or nutraceuticals to improve health status and for beauty purpose. Pearl contains mainly calcium carbonate and magnesium carbonate, which accounted for 91%, followed by silica, calcium phosphate, aluminum oxide and ferric oxide as well as some trace elements such as sodium, magnesium, manganese, selenium, aluminum, and copper. It also contains essential amino acids such as histidine (His), lysine (Lys), arginine (Arg), valine (Val), threonine (Thr), proline (Pro), methionine (Met), leucine (Leu), phenylalanine (Phe), tryptophan (Trp) and non-essential amino acids such as aspartic acid (Asp), glycine (Gly), alanine (Ala), glutamic acid (Glu), tyrosine (Try), and serine (Ser) [5,6].
Proteins, peptides, and amino acids also contribute to the body antioxidant defense system [7]. Saiga and his coworkers [8] reported that Asp and Glu possess antioxidant properties. In addition, Val, Ile, Leu, Met, Phe, Trp, and Cys belonging to hydrophobic amino acids were also reported to exhibit better free radical quenching ability [9]. Furthermore, the presence of trace elements (calcium, magnesium, selenium) in pearl powder also favors antioxidant activity, by acting as cofactors to antioxidant enzymes [10].
Free radicals can induce lipid peroxidation by causing irreversible damage to cellular macromolecules including membrane lipids, proteins, and nucleic acids [7]. The excessive production of ROS (reactive oxygen species) is an indicator of oxidative stress (imbalance between oxidant and antioxidant) leading to cellular damage and accelerate aging process. The oxidative stress and continuous ROS production also indirectly contribute to the pathophysiology of diabetes, inflammation, neurological disorders, and obesity [11]. Natural antioxidants in the daily diet can bind to unstable free radicals and thereby render a protection from the various degenerative diseases [12,13]. Several animal experiments showed that pearl could exert numerous pharmacological properties like antioxidation, anti-aging, anti-osteoporosis as well as immunomodulatory and wound-healing activities [3,14]. However, the antioxidant effect of natural pearl powder in clinical trials as well as it anti-aging property (C elegans model) has not been evaluated. Hence, the current novel experiment was blueprinted to explore the antioxidation and life span prolonging efficacy in various extracts of pearl powder and protein extract in healthy human subjects and wild type C. elegans respectively.
2. Materials and methods
2.1. Chemicals and reagents
Folin-Ciocalteu phenol reagent, gallic acid, sodium carbonate (Na2CO3), sodium hydroxide (NaOH), sodium nitrite (NaNO2), hydrochloric acid (HCl), ascorbic acid, α-tocopherol, 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH), disodium hydrogen phosphate (Na2HPO4), ferrous chloride (FeCl3), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), hydrogen peroxide (H2O2), horseradish peroxidase (HRP), potassium ferricyanide (K3Fe(CN)6), trichloroacetic acid (TCA) were procured from Sigma (St. Louis, MO). Deionized water (dd H2O) was prepared using an UltrapureTM water purification system (Lotun Science Co., Ltd. Taipei, Taiwan).
2.2. Pearl powder capsule
Pearl powder (from naturally aqua-cultured pearl) capsule was provided by Jing Wang Nano Technology Corporation, Taiwan. Each capsule (500 mg) contain 2.27% protein, 37% calcium, 0.01% iron, 0.04% zinc and various trace elements with amino acids such as Asp, Thr, Ser, Glu, Pro, Gly, Ala, Val, Met, Leu, Tyr, Phe, His, Lys, Arg. Placebo capsules contain calcium carbonate, starch and look similar to pearl powder capsules.
2.3. Pearl powder extraction
Extraction of pearl protein was carried out by the method of Brandt and Muir [15] and estimated by Lowry [16] method. The Pearl protein profile was determined by using SDS-PAGE analysis. The protein of pearl powder was removed by the method of Hirano [17] to get a non-protein extract of pearl powder. Both protein extract and non-protein extract of pearl powder were stored at −20 °C. For in vitro studies, protein extract, non-protein extract, and whole pearl powder were dissolved in double distilled water, respectively.
2.4. In vitro studies
2.4.1. Determination of various oxidative indices
Total antioxidant ability (Trolox equivalent antioxidant capacity; TEAC) was performed by Arnao et al. method [18]. The ferrous ion chelating potential was estimated by the method of Dinis [19]. DPPH radical-scavenging activity was determined based on the method of Shimada et al. [20]. Superoxide anion scavenging effects were done by Robak and Gryglewski method [21].
2.4.2. Lifespan prolonging efficacy in wild-type nematodes
2.4.2.1. Culturing of C. elegans
The wild nematode strain was obtained from the National Taiwan University, Taipei, Taiwan and was maintained and cultured at 20 °C on Nematode Growth Medium (NGM) agar plates fed with live bacteria like Escherichia coli (food) [22].
2.4.2.2. Prolonging activity of protein extract of pearl powder
Age synchronous populations were done as described previously [23]. The life span of adult hermaphrodites was determined on NGM agar plates [24]. Prolonging activity in wild-type nematodes lifespan was measured by the method of Ishii [25].
2.5. Clinical trial (In vivo)
2.5.1. Subjects
The present randomized, placebo-controlled, and double-blind trial was conducted at Chung Shan Medical University Hospital from November 2005 to January 2006. The present intervention was performed accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Chung Shan Medical University Hospital, Taichung, Taiwan (Protocol No. CS05037). Before enrollment to study, subjects were asked to sign a consent. Twenty healthy middle-aged (38–50) subjects (equal gender) were recruited for the present experiment via advertisements. Subjects involved in the current study were requested not to take any medications or supplements during the intervention. Other exclusion criteria were a history of smoking, drinking, pregnant or lactating women, renal or hepatic dysfunction. Volunteers were asked to fill a questionnaire before and after the intervention. A physical examination and anthropometric analysis were performed to the volunteer subjects at the beginning of the intervention and were divided into two groups with 10 subjects in each (5 male and 5 female).
2.5.2. Experimental design
The experimental group (pearl) received a test capsule [500 mg × 6 capsules = 3 g pearl powder/d], placebo group received a placebo capsule [500 mg × 6 capsules = 3 g placebo/d] for 8 weeks, which were extended for two more weeks as a follow-up. During the initial, 2nd, 4th, 6th, 8th week and 10th weeks (follow-up), the anthropometric analysis was done as well as fasting blood samples were drawn for various biochemical assays to track and record the health status of each subject, for any adverse effect of the test sample (pearl powder). From the subject’s record, the average percentage of intake of the pearl powder capsule was 88.42% at the end of the experiment. During the study period, one female subject in the placebo group (due to an unwillingness to cooperate) was excluded from the study and thus ended with 19 subjects. Fig. 1 shows the schematic representation of the present study.
Fig. 1.
Schematic representation of the present study.
2.5.3. Blood collection
The fasting blood samples were collected in an EDTA (ethyl-enediamine tetra acetic acid)-coated vacuum tube and immediately stored at 4 °C. Plasma was separated by centrifuging at 1500g (Supercentrifuge. 1K15. Sigma) and used for determining the various antioxidant indexes. Separated blood samples after removing the intermediate film, settling part were washed with isometric saline and centrifuged at 1500 g to get erythrocytes and used for assaying antioxidant enzymes.
2.5.4. The antioxidation of the plasma
Total antioxidant capacity [18], total thiobarbituric acid reactive substances (TBARS) [26], total thiols [27], glutathione content [28] and ascorbic acid [29] in plasma were determined by the reported methods.
2.5.5. Assay of antioxidant enzymes in erythrocytes
Assays of superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR) were performed by using a commercial kit procured from Randox kit in according to the manufacturer’s instructions (Antrim, UK). Protein contents of erythrocytes (lysate) were determined based on the Biuret reaction of the BCA kit (Thermo, Rockford, IL, USA).
2.6. Statistical analysis
The data were expressed as a mean ± standard deviation (SD). For in vivo studies, the Paired t-test was used to compare the difference in the same group, and the Student’s t-test was conducted for comparison between the experimental and placebo group. In the case of in vitro, all the experiments were carried out in triplicate, and the data were evaluated by one-way ANOVA using statistical package for the social sciences (SPSS) version 21.0 (IBM, NY, USA). All the results with a p-value less than 0.05 were considered as statistically significant.
3. Results
3.1. Protein analysis
Table 1 revealed, the protein contents, in whole pearl powder and protein extract. The protein content of pearl powder was 22.71 mg protein/g, but the protein extract of pearl powder was 182.25 mg protein/g. Fig. 2 represents the separation of pearl protein by SDS-PAGE technique. Lane 1 showed the protein ladder-molecular weights range of 11–170 kDa. Lane 2 showed the pearl powder sample pattern, a prominent protein band was noted near the molecular weight 17 kDa and 72 kDa. However, the streaky band was also observed between 34 and 72 kDa.
Table 1.
Protein contents in pearl powder.
| Contents (mg protein/g dry wt) | |
|---|---|
| Pearl powder | 22.71 ± 2.19 |
| Protein extract of pearl powder | 182.25 ± 0.11 |
Fig. 2.
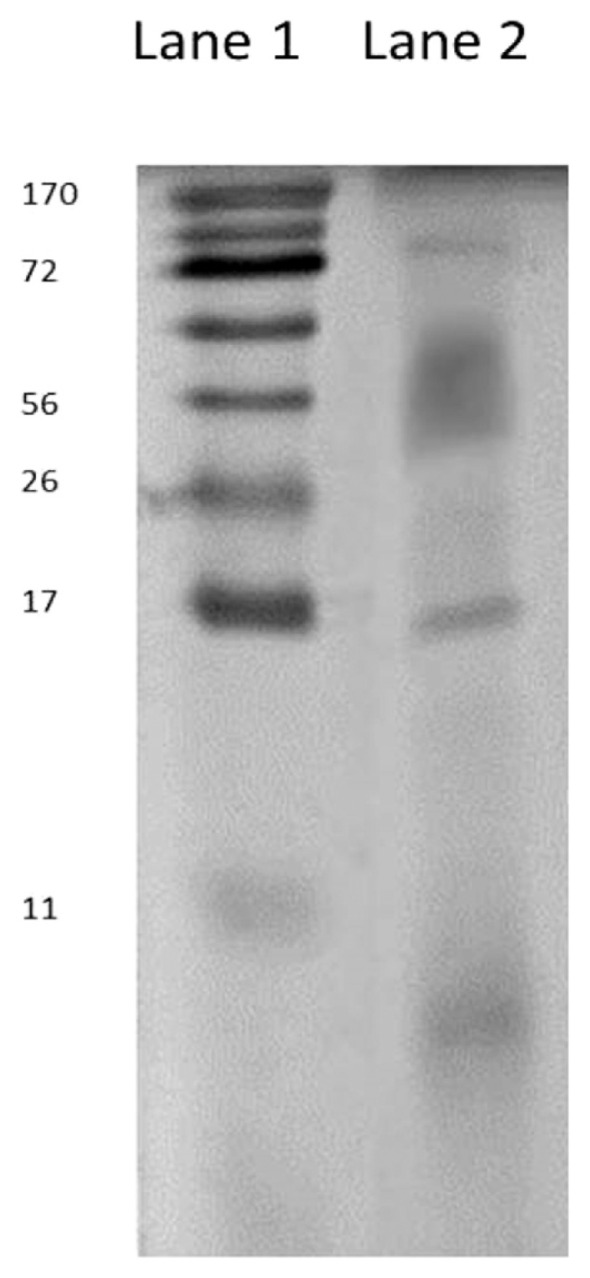
Analysis of proteins from pearl powder by SDS-PAGE.
3.2. In vitro studies
3.2.1. Total antioxidant capacity (TEAC) and chelating ability
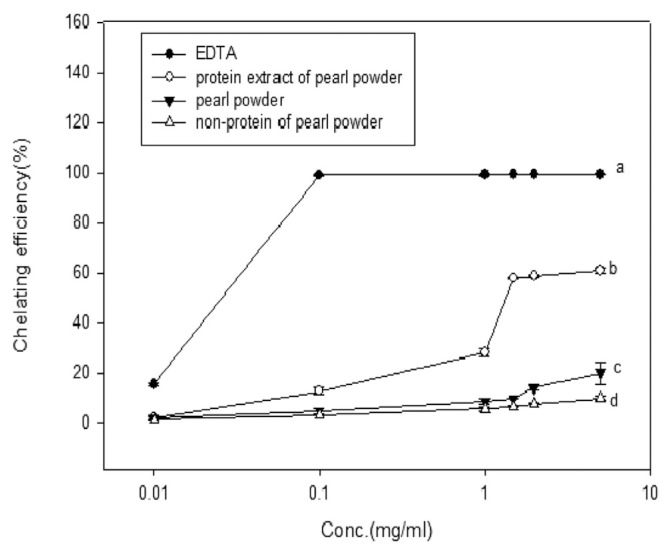
The total antioxidant capacity was shown in Table 2. The protein extract, pearl powder, and non-protein extract showed the antioxidant value of 587.12, 72.32 and 26.55 μmol/g dry wt. respectively. Thus, inferring that protein extract of pearl powder was superior (p < 0.05) to the other samples. Fig. 3 showed the chelating ability of various pearl powder extracts. EDTA (internal reference) reached its maximum chelating ability (100%) at 0.1 mg/mL concentration. Protein extract, pearl powder, and non-protein of pearl powder had the capability of the chelated ferrous ion, but the chelating efficiency of protein extract was greater than the other extracts. However, the protein extract attained its maximum chelating ability only at 5 mg/mL concentration with 61% of chelating efficiency. Whereas, pearl powder and non-protein extract recorded 20% and 10% of chelating efficiency respectively.
Table 2.
The total antioxidant capacity in pearl powder.
| TEAC (μmol/g dry wt.)* | |
|---|---|
| Pearl Powder | 72.32 ± 3.58b |
| Protein extract of pearl powder | 587.12 ± 32.55a |
| Non-protein (fraction) of pearl powder | 26.55 ± 2.79c |
TEAC (μmol/g dry wt.): μmol trolox equivalent antioxidant capacity/per g dry wt.
Values were expressed as means ± SD. Data represented with different superscript letters were significantly different (p < 0.05).
Fig. 3.
The chelating ability in various pearl powder extracts. Values were expressed as means ± SD. Data represented with different letters were significantly different (p < 0.05).
3.2.2. DPPH free radical scavenging ability
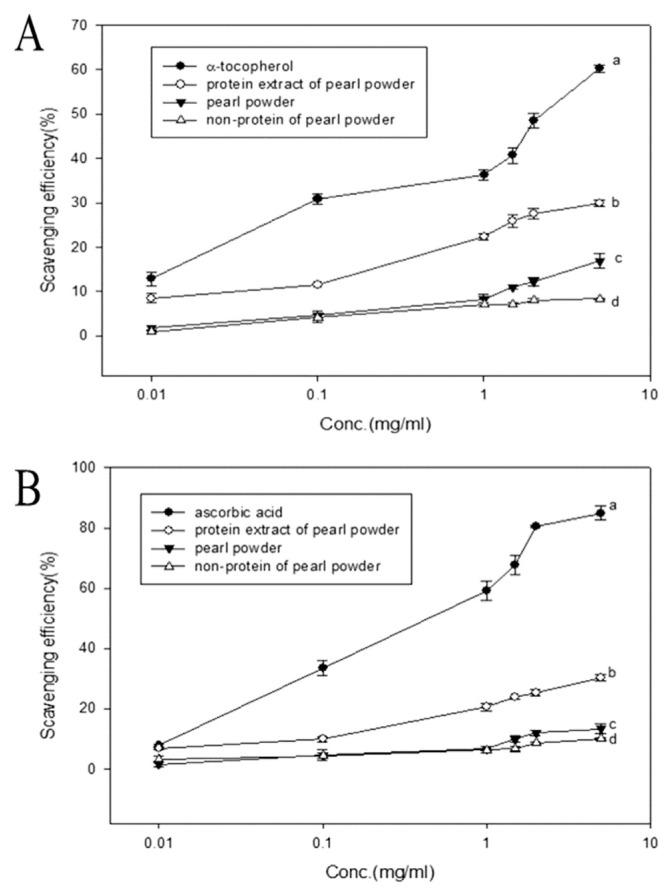
The protein pearl powder and non-protein extract showed increasing DPPH radical clearing ability in a concentration-dependent manner which is shown in Fig. 4A. At concentration 5 mg/mL protein extract, pearl powder, and non-protein extract displayed 29, 25 and 8% respectively, of DPPH scavenging ability. It appeared that protein extract of pearl powder could efficiently scavenge DPPH radicals.
Fig. 4.
The scavenging efficiency on DPPH (A) and Superoxide anion (B) in various pearl powder extracts. Values were expressed as means ± SD. Data represented with different letters were significantly different (p < 0.05).
3.2.3. Superoxide anion scavenging activity
The relative superoxide anion scavenging efficiency of various pearl powder extracts was depicted in Fig. 4B. All the three extracts showed increased superoxide anion free radical scavenging activity, as the concentration increase. When concentrations of various extract reach to 5 mg/mL, protein extract showed maximum (p < 0.05) superoxide anion clearing capacity of 30%, Pearl powder cleared 13%, and non-protein powder cleared only 9%.
3.2.4. Concentration-dependent modulation of lifespan
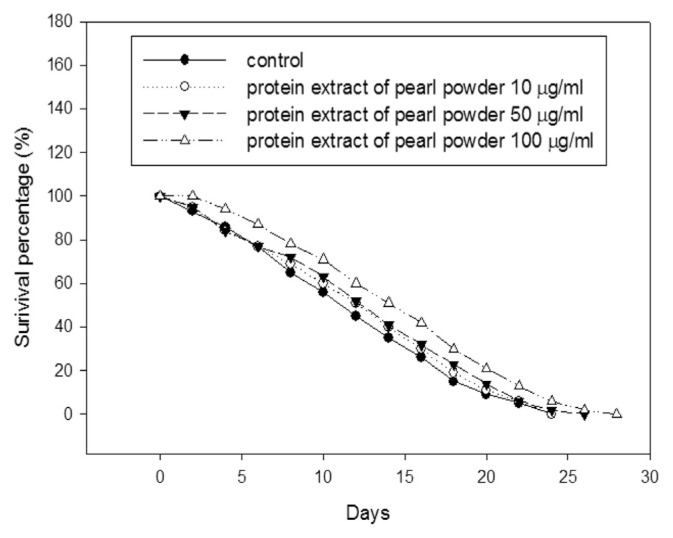
Fig. 5 showed the lifespan-prolonging efficacy of various concentrations of protein extract. The results revealed that the average life span of the control group is 23 days, but the survival percentage of C. elegans treated with 10 μg/mL and 50 μg/mL of pearl protein extract increased 4.86% (24 days) and 8.48% (26 days) of longevity when compared with the control group. When treating at 100 μg/mL concentrations, the average lifespan longevity was significantly increased 18.87% (28 days) in comparison with the control group.
Fig. 5.
The prolonging lifespan (longevity) effect of various concentrations of protein extract of pearl powder in C. elegans.
3.3. Clinical trial
The effects of pearl powder on various anthropometric parameters were epitomized in Table 3. No considerable changes were noted in body weight, height, body fat or BMI. Also, experimental subject (Exp group) who received pearl powder did not show any abnormal symptoms or phenomena and thus indicating the safety property of pearl powder.
Table 3.
The effect of pearl powder on anthropometric parameters in healthy subjects.
| Group | Height (cm) | Weight (Kg) | Body Fat (%) | *BMI(kg/m2) | |
|---|---|---|---|---|---|
| Baseline | Exp | 160.67 ± 10.66a | 65.22 ± 13.98a | 27.65 ± 7.16a | 23.43 ± 3.35a |
| Placebo | 166.10 ± 09.21a | 57.23 ± 6.83a | 24.40 ± 5.22a | 22.09 ± 1.06a | |
| 2nd week | Exp | 160.67 ± 10.66a | 65.13 ± 14.34a | 27.53 ± 6.50a | 23.38 ± 3.46a |
| Placebo | 166.10 ± 09.21a | 57.22 ± 6.65a | 24.71 ± 5.70a | 22.08 ± 1.01a | |
| 4th week | Exp | 160.67 ± 10.66a | 64.67 ± 13.76a | 28.48 ± 7.59a | 23.26 ± 3.23a |
| Placebo | 166.10 ± 09.21a | 56.79 ± 6.57a | 24.72 ± 5.20a | 21.90 ± 1.07a | |
| 6th week | Exp | 160.67 ± 10.66a | 65.23 ± 14.00a | 28.26 ± 5.72a | 23.43 ± 3.32a |
| Placebo | 166.10 ± 09.21a | 57.01 ± 6.97a | 25.06 ± 4.85a | 22.01 ± 1.21a | |
| 8th week | Exp | 160.67 ± 10.66a | 65.78 ± 14.04a | 28.42 ± 8.21a | 23.63 ± 3.35a |
| Placebo | 166.10 ± 09.21a | 57.08 ± 7.02a | 24.87 ± 5.22a | 22.02 ± 1.16a | |
| Follow Up (10th week) | Exp | 160.67 ± 10.66a | 65.29 ± 14.21a | 28.21 ± 7.87a | 23.44 ± 3.40a |
| Placebo | 166.10 ± 09.21a | 56.99 ± 6.66a | 25.36 ± 4.62a | 22.00 ± 0.90a |
Values were expressed as means ± SD. Data within the same column of each group represented by different superscript letters were significantly different (p < 0.05).
BMI: body mass index.
3.3.1. Effect of pearl powder on total antioxidant capacity in plasma
Table 4 depicts the total antioxidant capacity of healthy subjects. Two weeks of supplementation with pearl powder increased the antioxidant capacity from 0.45 to 0.52 mM, even though a slight increase was noted, but as the week passes (4th, 6th, 8th) the total antioxidant capacity also increased substantially (p < 0.05) in the experimental group by reaching 0.66 mM (8th week). During the follow-up period (10th week), experimental group subjects reached the maximum value of 0.69 mM and demonstrated that intake of pearl powder would raise the total antioxidant capacity.
Table 4.
The effect of pearl powder on various oxidative indexes in plasma of healthy subjects.
| Group | TEAC (mM)* | TBARS (μM) | Thiols (mM) | GSH (μM) | Vit-C (mg/dL) | |
|---|---|---|---|---|---|---|
| Baseline | Exp | 0.45 ± 0.04f | 4.95 ± 0.52a | 0.23 ± 0.05a | 5.89 ± 0.82e | 2.48 ± 0.17a |
| Placebo | 0.48 ± 0.08a | 4.82 ± 0.39a | 0.27 ± 0.04a | 6.16 ± 0.76a | 2.42 ± 0.26a | |
| 2nd week | Exp | 0.52 ± 0.02e | 4.55 ± 0.41b | 0.23 ± 0.04a | 6.92 ± 0.58d | 2.48 ± 0.14a |
| Placebo | 0.47 ± 0.08a | 4.83 ± 0.37a | 0.26 ± 0.04a | 6.34 ± 0.69a | 2.42 ± 0.28a | |
| 4th week | Exp | 0.59 ± 0.04d | 4.15 ± 0.36c | 0.25 ± 0.05a | 7.67 ± 0.60c | 2.47 ± 0.13a |
| Placebo | 0.44 ± 0.06a | 4.84 ± 0.34a | 0.26 ± 0.03a | 6.22 ± 0.64a | 2.41 ± 0.27a | |
| 6th week | Exp | 0.63 ± 0.04c | 3.87 ± 0.32d | 0.26 ± 0.05a | 8.27 ± 0.53b | 2.47 ± 0.14a |
| Placebo | 0.48 ± 0.06a | 4.81 ± 0.36a | 0.26 ± 0.04a | 6.40 ± 0.55a | 2.42 ± 0.25a | |
| 8th week | Exp | 0.66 ± 0.05b | 3.51 ± 0.44e | 0.29 ± 0.05b | 9.19 ± 0.42a | 2.48 ± 0.13a |
| Placebo | 0.48 ± 0.07a | 4.84 ± 0.28a | 0.26 ± 0.03a | 5.89 ± 0.82b | 2.41 ± 0.23a | |
| Follow Up (10th week) | Exp | 0.69 ± 0.05a | 3.27 ± 0.34f | 0.29 ± 0.05b | 9.21 ± 0.66a | 2.49 ± 0.11a |
| Placebo | 0.49 ± 0.08a | 4.81 ± 0.35a | 0.25 ± 0.04a | 5.93 ± 0.59b | 2.42 ± 0.21a |
Values were expressed as means ± SD. Data within the same column of each group represented by different superscript letters were significantly different (p < 0.05).
3.3.2. Effect of pearl powder on lipid peroxides in plasma
The impact of pearl powder on lipid peroxides in plasma is presented in Table 4. No substantial alterations were detected in the levels of TBARS in both experimental and placebo subjects at the initial stage (0 weeks). Maximum suppression of lipid peroxides was noted on follow-up (10th week) period, owing to prolonged ingestion of pearl powder.
3.3.3. Effect of pearl powder on total thiols, glutathione content and ascorbic acid in plasma
Table 4 showed the effect of pearl powder on total thiols, glutathione content (GSH) and ascorbic acid in plasma of healthy subjects. A substantial escalation (p < 0.05) in the levels of total thiols and glutathione content were noted within experimental groups on every subsequent two weeks (2nd, 4th, 6th and 8th weeks) due to the intake of pearl powder. However, no significant changes were observed in ascorbic acid levels.
3.3.4. Effect of pearl powder on the antioxidant enzymes in erythrocytes
The antioxidant enzymes such as SOD, GR, and GPx in erythrocyte of healthy subjects were epitomized in Table 5. At the baseline, no marked changes were observed between the experimental and placebo groups of various antioxidant enzyme activities. While treatment with pearl powder for 8 weeks posted a concomitant improvement (p < 0.05) in the activities of these antioxidant enzymes.
Table 5.
The effect of pearl powder on the antioxidant enzymes in erythrocytes of healthy subjects.
| Group | SOD (IU/g Hb) | Gpx (IU/g Hb) | GR (IU/g Hb) | |
|---|---|---|---|---|
| Baseline | Exp | 1248.95 ± 200.54a | 30.35 ± 5.61b | 2.41 ± 0.68a |
| Placebo | 1245.65 ± 159.64a | 31.77 ± 7.11a | 2.42 ± 0.67a | |
| 8th week | Exp | 1321.75 ± 134.72b | 34.22 ± 7.68a | 3.30 ± 0.81a |
| Placebo | 1267.95 ± 109.74a | 32.03 ± 6.89a | 2.37 ± 0.90a | |
| Follow Up (10th week) | Exp | 1308.16 ± 182.04b | 32.60 ± 5.99ab | 2.91 ± 0.58a |
| Placebo | 1248.92 ± 171.93a | 31.38 ± 6.24a | 2.40 ± 0.60a |
Values were expressed as means ± SD. Data within the same column of each group represented with different superscript letters were significantly different (p < 0.05).
4. Discussion
To the best of our knowledge, this is the first (novel) clinical to examine the antioxidant activity of pearl powder. The aim of the current study is to explore the antioxidation and the life span prolonging (C. elegans) effect of pearl powder. The outcome of the protein analysis by SDS-PAGE revealed two prominent protein bands near the molecular weight 17 kDa and 72 kDa. However, the streaky band was also noted between 34 and 72 kDa. Previously, proteins and peptides are reported to show several health promoting activities [14,30]. Therefore, to compare the beneficial property of protein extract from the non-protein extract of pearl powder, we designed this in vitro and in vivo study to explore the anti-oxidation and life span prolonging efficacy in healthy human subjects and wild type C. elegans respectively between protein extract and non-protein extract of pearl powder.
Oxidative stress (imbalance between oxidant and antioxidant) is the main contributor for several metabolic and degenerative disorders especially trigger aging process due to cellular functional impairment [31]. Total antioxidant capacity of protein extract of pearl powder was significantly superior in comparison with other extracts. Various investigations suggested that hydrophobic amino acids exhibit strong antioxidant activity [32], it might be the reason for increasing total antioxidant capacity found in protein extract. Protein extract, pearl powder, and non-protein of pearl powder had the capability of the chelated ferrous ion, but the chelating efficiency of protein extract was much better than the other extracts due to higher proton donating the property. Megias and his colleague reported that protein rich in histidine would have the maximum chelating ability because of the presence of an imidazole ring [33]. This study also encountered similar kind of results, it would be attributed to the presence of histidine in pearl protein extract. Free radical-scavenging is one of the known mechanisms by which antioxidants mitigate lipid peroxidation. The DPPH radical scavenging activity has been extensively used for screening antioxidants from natural compounds [34]. This study clearly indicated that all the extracts displayed greater DPPH clearing ability in a concentration-dependent fashion. Nonetheless, protein extract showed the maximum DPPH scavenging ability it might be due to its strong hydrogen-donating capacity. Similarly, Li and his coworkers inferred that chickpea protein hydrolyzate had strong DPPH radical scavenging activity [14].
Superoxide ( ) are generated by various enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, lipooxygenase, xanthine oxidase, and peroxidase. It is the major causative agent for lipid peroxidation and various degenerative diseases [35]. Superoxide anion scavenging efficiency of protein extract was greater than other extracts. Pearl powder is enriched with essential amino acids, which can act as an electron (e−) or proton (H+) donor and could terminate radical chain reactions by converting free radicals to more stable products. Thus, the protein was probably an important constituent of pearl powder to enhance its antioxidation and free radical quenching activity. Shao and others proved that pearl powders have strong capacities for restraining and removing free radicals [36].
Aging can be defined as a metabolic alteration that ends up in physiological or pathological changes with diminished ability to tackle stress as the time progresses. The current study was undertaken to determine whether protein extract can prolong lifespan in a whole organism (nematodes). For this investigation, an organism with a relatively short lifespan that could be assayed reproducibly and robustly. The experimental organism that could better suit these requirements was the nematode, C. elegans, which was a commonly used model for evaluating aging or longevity process owing to its short life span of 3–4 weeks, with higher reproducibility rate and experimental flexibility [37].
When treating protein extract at 100 μg/mL concentrations, the average lifespan longevity was significantly increased in comparison with the control group. The increase in the life span of nematodes by the protein extract was probably due to the increasing antioxidant activity in a dose-dependent manner. Few studies indicated that the compound or molecule that can improve the antioxidant status would presumably prolong the lifespan [38,39]. These findings showcase that C. elegans model probably provides a useful blueprint for mapping out how antioxidant compounds might exert longevity property in a clinical trial.
During clinical trial 20 healthy volunteer subjects were recruited and randomly separated into two groups, each group of participants in the trial period, took daily 6 capsules of pearl powder (Exp group) or a placebo. However, no marked changes were noted in anthropometric parameters. Meanwhile, the total antioxidant capacity was increased substantially in the experimental group in comparison with placebo group it might be the reason for a concomitant rise in the total antioxidant capacity. Kim and his coworker [30] suggest that essential amino acids such as histidine, proline, alanine, and leucine have been reported to contribute to the scavenging of free radicals, thereby increasing total antioxidant capacity. By correlating with the in vitro antioxidant studies, the presence of protein (essential amino acids and peptide) in pearl powder might be the reason for a concomitant increase in the total antioxidant capacity. Meanwhile, no marked changes were noted in the placebo group from the initial or baseline (0 week) to end of intervention and follow-up (10th week) period.
A free radical is the important causative factor for many degenerative disorders, especially cardiovascular disease, cancer, diabetes mellitus. As increased free radical may act the cell membrane and thereby trigger the excessive production of lipid peroxidation products like TBARS/MDA [13]. Maximum lipid peroxide suppressing activities of pearl powder were noted on follow-up (10th week) period, it is because of prolonged ingestion of pearl powder. Our results are in concordance with those of Duan and others, who demonstrated that intake of pearl powder could significantly reduce serum lipid peroxide products in a mouse model [40].
Glutathione (GSH) is one of the vital endogenous antioxidants in various organisms by serving as an electron donor to exert its free radical scavenging ability and thus preventing damage to important cellular components caused by free radicals. A significant elevation in the levels of glutathione content and total thiols were noted within experimental groups on every subsequent two weeks (2nd, 4th, 6th and 8th weeks) due to the intake of pearl powder. Balcerczyk and Bartosz demonstrated that thiols are the main determinants of the total antioxidant property of the cellular system [41]. Similarly, in the present study, the elevated levels of total thiols might be due to the increased total antioxidant capacity of pearl powder. As the pearl powder increases the total thiol, thereby protecting SH group of glutathione and hence significant increase in the levels of glutathione content were noted. Li and his colleagues showed that the supplementation with chickpea protein rich in cysteine and methionine could increase the plasma glutathione levels [14]. Ascorbic acid is the most widely cited forms of water soluble exogenous antioxidant. In the case of ascorbic acid, no significant modification was noted in pearl powder treated group. The reason might be pearl powder is not a good source of ascorbic acid, even though it increases glutathione content (GSH), but it maintains the optimal level of ascorbic acid.
SOD is the frontline enzyme involved in a defense process that protects against oxygen free radicals by catalyzing the removal of the superoxide radical, which damages the membrane and biological structures. It catalyzes the dismutation of superoxide ( ) into oxygen and hydrogen peroxide (H2O2). GPx has been shown to handle the detoxification of H2O2, which is produced by SOD. Finally, GPx with the help of reduced glutathione (GSH) converts H2O2 to oxygen. During this enzymic reaction, GR is responsible for the reduction of oxidized glutathione (GSSG) to reduced glutathione [31].
Oral supplementation with pearl powder for 8 weeks in the experimental group posted a concomitant increase in the antioxidant levels (SOD, GPx, and GR). It could be attributed to the abundant amount of protein in pearl powder (amino acids and minerals). Saiga and his coworkers reported that Asp and Glu own antioxidant properties. Moreover, hydrophobic amino acids like Val, Ile, Leu, Met, Phe, Trp, and Cys were reported to show higher free radical scavenging ability [9]. Furthermore, the presence of trace elements (calcium, magnesium, selenium) in pearl powder also favors some antioxidant capacity, by acting as cofactors to antioxidant enzymes [10]. In correlation with this present study Chou and coworkers also highlighted that amino acid and minerals (Mn and Se) in black vinegar could enhance the antioxidant activity [42]. Few limitations of the present study are not evaluated the ROS levels and unable to separate the peptide (amino acid) present in the pearl powder to display the exact mechanism behind the longevity and antioxidant properties.
5. Conclusions
From the present data, the abundant presence of protein content (amino acids and minerals) in pearl powder demonstrated an increased total antioxidant capacity, antioxidant activity, total thiols (SH group) and glutathione content with suppressing lipid peroxides products (TBARS). Moreover, the protein extract of pearl powder supplementation can significantly prolong the lifespan of C. elegans owing to antioxidant activity. The results of both in vivo and in-vitro highlight that pearl powder is a potent antioxidant and in the future can be employed for treating various age-related degenerative diseases/disorders.
Acknowledgements
We thank Dr. Tzy-Yen Chen and Dr. Chien-Ning Huang, Chung Shan Medical University Hospital, Taichung, Taiwan for their valuable support and cooperation. This study was financially supported by Chung Shan Medical University Hospital, Taichung, Taiwan (CS06003).
Funding Statement
This study was financially supported by Chung Shan Medical University Hospital, Taichung, Taiwan (CS06003).
Footnotes
Conflict of interest
No conflict of interest to disclose.
REFERENCES
- 1. Gao H, Chen H, Chen W, Tao F, Zheng Y, Jiang Y, et al. Effect of nanometer pearl powder on calcium absorption and utilization in rats. Food Chem. 2008;109:493–8. [Google Scholar]
- 2. Huang Y, Yu H, Xiao C. Effects of Ca2+ crosslinking on structure and properties of waterborne polyurethane-carboxymethylated guar gum films. Carbohydr Polym. 2006;66:500–13. [Google Scholar]
- 3. Chen H, Chang J, Wu J. Calcium bioavailability of nanonized pearl powder for adults. J Food Sci. 2008;73:H246–51. doi: 10.1111/j.1750-3841.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 4. Chuang CH, Chang PJ, Hsieh WS, Tsai YJ, Lin SJ, Chen PC. Chinese herbal medicine use in Taiwan during pregnancy and the postpartum period: a population-based cohort study. Int J Nurs Stud. 2009;46:787–95. doi: 10.1016/j.ijnurstu.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 5. Tsukamoto D, Sarashina I, Endo K. Structure and expression of an unusually acidic matrix protein of pearl oyster shells. Biochem Biophys Res Commun. 2004;320:1175–80. doi: 10.1016/j.bbrc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 6. Zhang C, Xie L, Huang J, Liu X, Zhang R. A novel matrix protein family participating in the prismatic layer framework formation of pearl oyster, Pinctada fucata. Biochem Biophys Res Commun. 2006;344:735–40. doi: 10.1016/j.bbrc.2006.03.179. [DOI] [PubMed] [Google Scholar]
- 7. Park SY, Ahn CB, Je JY. Antioxidant and anti-inflammatory activities of protein hydrolysates from Mytilus Edulis and ultrafiltration membrane fractions. J Food Biochem. 2014;38:460–8. [Google Scholar]
- 8. Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agri Food Chem. 2003;51:3661–7. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- 9. Ren J, Zhao M, Shi J, Wang J, Jiang Y, Cui C, et al. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008;108:727–36. doi: 10.1016/j.foodchem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 10. Iranzo O. Manganese complexes displaying superoxide dismutase activity: a balance between different factors. Bioorg Chem. 2011;39:73–87. doi: 10.1016/j.bioorg.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 11. Zhao GR, Xiang ZJ, Ye TX, Yuan YJ, Guo ZX. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem. 2006;99:767–74. [Google Scholar]
- 12. Hsieh CC, Liao CC, Liao YC, Hwang LS, Wu LY, Hsieh SC. Proteomic changes associated with metabolic syndrome in a fructose-fed rat model. J Food Drug Anal. 2016;24:754–61. doi: 10.1016/j.jfda.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–50. [Google Scholar]
- 15. Brandt KD, Muir H. Heterogeneity of protein–polysaccharides of porcine articular cartilage. The sequential extraction of chondroitin sulphate-proteins with iso-osmotic neutral sodium acetate. Biochem J. 1971;121:261–70. doi: 10.1042/bj1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Bio Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 17. Hirano M, Rakwal R, Shibato J, Agrawal GK, Jwa N, Iwahashi H, et al. New protein extraction/solubilization protocol for gel-based proteomics of rat (female) whole brain and brain regions. Mol Cells. 2006;22:119. [PubMed] [Google Scholar]
- 18. Arnao M, Casas J, Del Rio J, Acosta M, Garcia-Canovas F. An enzymatic colorimetric method for measuring naringin using 2, 2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid)(ABTS) in the presence of peroxidase. Anal Biochem. 1990;185:335–8. doi: 10.1016/0003-2697(90)90304-r. [DOI] [PubMed] [Google Scholar]
- 19. Dinis TCP, Maderia VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-amino salicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 20. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agri Food Chem. 1992;40:945–8. [Google Scholar]
- 21. Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37:837–41. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 22. Stiernagle T. C elegans. Maintenance of C elegans. 1999;2:51–67. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Honda S, Ishii N, Suzuki K, Matsuo M. Oxygen-dependent perturbation of life span and aging rate in the nematode. J Gerontol. 1993;48:B57–61. doi: 10.1093/geronj/48.2.b57. [DOI] [PubMed] [Google Scholar]
- 24. Adachi H, Fujiwara Y, Ishii N. Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. J Gerontol A Biol Sci Med Sci. 1998;53:B240–4. doi: 10.1093/gerona/53a.4.b240. [DOI] [PubMed] [Google Scholar]
- 25. Ishii N, Senoo-Matsuda N, Miyake K, Yasuda K, Ishii T, Hartman PS, et al. 10 can prolong C. elegans lifespan by lowering oxidative stress. Mech Ageing Dev. 2004;125:41–6. doi: 10.1016/j.mad.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 26. Draper H, Hadley M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 27. Hamvas A, Palazzo R, Kaiser L, Cooper J, Shuman T, Velazquez M, et al. Inflammation and oxygen free radical formation during pulmonary ischemia-reperfusion injury. J Appl Physiol. 1992;72:621–8. doi: 10.1152/jappl.1992.72.2.621. [DOI] [PubMed] [Google Scholar]
- 28. Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 29. Zannoni V, Lynch M, Goldstein S, Sato P. A rapid micromethod for the determination of ascorbic acid in plasma and tissues. Biochem Med. 1974;11:41–8. doi: 10.1016/0006-2944(74)90093-3. [DOI] [PubMed] [Google Scholar]
- 30. Kim SK, Kim YT, Byun HG, Nam KS, Joo DS, Shahidi F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J Agric Food Chem. 2001;49:1984–9. doi: 10.1021/jf000494j. [DOI] [PubMed] [Google Scholar]
- 31. Kamesh V, Sumathi T. Antihypercholesterolemic effect of Bacopa monniera linn. on high cholesterol diet induced hypercholesterolemia in rats. Asian Pac J Trop Med. 2012;5:949–55. doi: 10.1016/S1995-7645(12)60180-1. [DOI] [PubMed] [Google Scholar]
- 32. Zhang SB, Wang Z, Xu SY. Antioxidant and antithrombotic activities of rapeseed peptides. J Am Oil Chem Soc. 2008;85:521–7. [Google Scholar]
- 33. Megí2as C, Pedroche J, Yust MM, Girón-Calle J, Alaiz M, Millán F, et al. Production of copper-chelating peptides after hydrolysis of sunflower proteins with pepsin and pancreatin. LWT-Food Sci Technol. 2008;41:1973–7. [Google Scholar]
- 34. Sánchez-Moreno C. Review: methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int. 2002;8:121–37. [Google Scholar]
- 35. Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–94. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shao DZ, Wang CK, Hwang HJ, Hung CH, Chen YW. Comparison of hydration, tyrosinase resistance, and antioxidant activation in three kinds of pearl powders. J Cos Sci. 2010;61:133. [PubMed] [Google Scholar]
- 37. Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–62. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 38. Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81:313S–6S. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- 39. Wiegant F, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post J. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontol. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- 40. Duan L, Xiang X, Dan L, Jianguo S, Peijun W, Jing L. The antiaging effects of pearl-powder on mice aging model induced by d-glactose or ozone. Pharmacol Clin Chin Mater Medica. 1996;5:014–8. [Google Scholar]
- 41. Balcerczyk A, Bartosz G. Thiols are main determinants of total antioxidant capacity of cellular homogenates. Free Rad Res. 2003;37:537–41. doi: 10.1080/1071576031000083189. [DOI] [PubMed] [Google Scholar]
- 42. Chou CH, Liu CW, Yang DJ, Wu YHS, Chen YC. Amino acid, mineral, and polyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effects in vivo. Food Chem. 2015;168:63–9. doi: 10.1016/j.foodchem.2014.07.035. [DOI] [PubMed] [Google Scholar]