Abstract
Essential oils (EOs) extracted from six medicinal herbs and food plants [Cinnamomum zeylanicum (CZ), Psiadia arguta (PA), Psiadia terebinthina (PT), Citrus grandis (CGp), Citrus hystrix (CH), and Citrus reticulata (CR)] were studied for any inhibitory potential against key physiological enzymes involved in diabetes (α-glucosidase), skin aging (collagenase and elastase), and neurodegenerative disorders (acetylcholinesterase). Kinetic studies of the active EOs on the aforementioned enzymes were determined using Lineweaver–Burk plots. The intracellular and extracellular antimelanogenic potential of the EOs were evaluated on B16F10 mouse melanocytes. CH and CR were found to significantly inhibit (2.476 ± 0.13 μg/mL and 3.636 ± 0.10 μg/mL, respectively) acetylcholinesterase, compared with galantamine (3.989 ± 0.16 μg/mL). CH inhibited collagenase (50% inhibitory concentration 28.71 ± 0.16 μg/mL) compared with the control (24.45 ± 0.19 μg/mL). The percentage inhibition in the elastase assay of CH was 63.21% compared to the positive control (75.09%). In addition, CH, CR, CGp, CZ, and PT were found to significantly inhibit α-glucosidase (276.70 ± 0.73 μg/mL, 169.90 ± 0.58 μg/mL, 240.60 ± 6.50 μg/mL, 64.52 ± 0.69 μg/mL, and 313.0 ± 5.0 μg/mL, respectively), compared to acarbose (448.80 ± 0.81 μg/mL). Active EOs showed both uncompetitive and competitive types of inhibition. The EOs also inhibited intracellular (50% inhibitory concentration 15.92 ± 1.06 μg/mL, 23.75 ± 4.47 μg/mL, and 28.99 ± 5.70 μg/mL for CH, CR, and CGp, respectively) and extracellular (< 15.625 μg/mL for CH, CR, CGp, and PT) melanin production when tested against B16F10 mouse melanocytes. Results from the present study tend to show that EOs extracted from these medicinal plants can inhibit key enzymes and may be potential candidates for cosmetic and pharmaceutical industries.
Keywords: acetylcholinesterase, citrus, diabetes mellitus, hyperpigmentation, oils
1. Introduction
Essential oils (EOs), also known as ethereal oils [1,2], are obtained mostly from vegetable organs (flowers, leaves, barks, woods, roots, rhizomes, fruits, and seeds) through different processes such as expression, fermentation, enfleurage, or extraction [3,4]. Although their composition consist mainly of terpenes, terpenoids, and phenylpropanoids, they represent a great diversity of chemical structures, chemical compositions, and for that matter, an assortment of properties.
EOs available on the market are mostly known for their odoriferous potential, while they can also be used in the management of many diseases [5–7]. EOs being rich in polyphenolic and terpenes components can be exploited for their potential antimicrobial, antioxidant, and anti-inflammatory properties. For instance, lavender oils, which are rich in linalool and linalyl acetate, can be found in approved drugs, for the treatment of anxiety disorder. Also, it has been purported that the use of EOs extracted from citrus fruits, rosemary, lavender, and others can be valued as effective antimicrobials, which has confirmed their use as natural preservatives in cosmetic products as well as food additives in food [8–10]. However, some components of EOs such as safrole and estragole, can also be deleterious for humans. The use of EOs thus needs to be documented and tested accordingly prior to use.
Noncommunicable diseases (NCDs) such as diabetes, cardiovascular diseases, neurodegenerative diseases, and other metabolic disorders have been reported by the World Health Organization [11] to be responsible for the death of > 38 million people every year, and 28 million of these fatalities occur in middle-income countries. Recent studies have been dedicated to the inhibition of key enzymes involved in pathologies as an effective strategy for the treatment and management of NCDs [12–15]. For instance, acetylcholinesterase (AChE) inhibition has been investigated in the management of neurodegenerative disorders such as Alzheimer’s disease (AD), due to its potential to impede the cholinergic deficit that is associated with the cognitive dysfunction occurring in AD [14]. AD is a disease that concerns ~5% of the population in developed countries. Research into its symptoms, causes, risk factors, and treatment has gained an impact in recent decades. The progression of AD has also been linked to oxidative stress. Studies have shown a relationship of free radicals with the pathology of neuronal death similar to that in AD [16]. It has also been purported by Madeo and Elsayed [14] that there is a relationship between oxidative stress and AD, as the level of peripheral markers of oxidative stress is reported to increase with the severity of the cognitive disorder.
The management of type 2 diabetes has also been documented in the literature through the actions of natural plant products, involving two major techniques. First, the scavenging of free radicals, since diabetes have been correlated to a defective antioxidant defense system and an increase in level of free radicals [17]. Second, the inhibition of key enzymes involved in the breakdown of starch, such as α-amylase and α-glucosidase, which help reduce glucose level in the blood. These two key enzymes are therapeutic targets in the management of diabetes. Their involvement in the breakdown of starch to glucose leads to an increase in blood glucose level. In contrast, inhibition of these enzymes leads to a delay in glucose absorption and thus regulating postprandial glucose level in the bloodstream.
Oxidative stress has been purported as one of the mechanisms associated with oxidative damage triggered by free radicals and leading to chronic pathology like neurodegener-ative diseases, diabetes, loss of skin elasticity, cancer, and coronary heart diseases. Aging as a skin degradation process has been categorized as the intrinsic and extrinsic aging process. Intrinsic aging refers to the changes in elasticity of the skin, which is a natural process occurring with time, while extrinsic aging is a result of overexposure of the skin to harmful UV radiation, leading to alteration of the connective tissue due to the formation of peroxides, reactive oxygen species, and other components [18].
Tropical islands like Mauritius are endowed with rich floral biodiversity having an interesting microcosm with diverse species including aromatic medicinal plants, offering interesting biological activities. Aromatic and medicinal plants have been scrutinized in the quest for alternative natural bioactive products and drugs. Drugs used for the management of diseases have shown positive results, especially for patients with AD, for which no cure exists to date. However, these drugs are also associated with several major adverse effects. Compounds such as galantamine and tacrine for AD, along with acarbose and voglibose used against diabetes mellitus have shown adverse effects leading to gastrointestinal disease and hepatotoxicity. Thus, the search for new phytomedicines that can inhibit key physiological enzymes is warranted. The present study was designed (as illustrated in Figure 10) to evaluate the efficacy and the potential of EOs extracted from medicinal plants used in traditional medicine to inhibit key enzymes involved in several NCDs.
Figure. 10.
Methodology highlights.
2. Methods
2.1. Collection of plant materials
Exotic and endemic plants included in the present study are those used traditionally by the local people as medicinal herbs and food plants [19,20]. Plants were collected as described previously [21,22]. The leaves of Cinnamomum zeylanicum Nees (CZ), Psiadia arguta Pers. (PA), Psiadia terebinthina A.J. Scott (PT), and fully ripened fruits of three Citrus species, namely Citrus grandis L. (CGp), Citrus hystrix D.C. (CH), and Citrus reticulata Blanco (CR) were collected at the University Farm. Each plant was identified by a local botanist. Voucher specimens have been deposited at the National Herbarium, Reduit, Mauritius and assigned the following codes; CZ-MAU 0022465, P-MAU 0018969, PT-MAU 0020201, CGp-MAU 0020200, CH-MAU 0020508, and CR-MAU 0022485. A local database was constructed whereby plant samples were assigned a collection number for future reference. A personal local repository database has been constructed to store primary data for future data mining and sharing.
2.2. Extraction of EOs
The leaves of the plants were processed as previously described [21,22]. Peels of the fruits were also used for the extraction of EOs. In this respect, the fruits collected were peeled off carefully using a sharp knife to avoid any damage of the oil glands and reduced to uniform size. The plant materials were then subjected to the hydrodistillation process for 3 hours using a Clevenger apparatus. The distillates of the EOs thus yielded were then dried over anhydrous sodium sulfate, filtered, and stored at −4°C until further analysis [21–23].
2.3. Enzymatic assays
2.3.1. Anti-acetylcholinesterase assay
The assay to measure AChE activity was modified from a previously published protocol [24]. The reaction mixture was prepared in 96-well microplates and consisted of 5-μL 3 mM 5,5′-dithiobis (2-nitrobenzoic acid), 5 μL of 75 mM acetylth-iocholine iodide, 110-μL 0.1M sodium phosphate buffer (pH 8.0,) and 120 μL of test item, that is, EO at different concentrations (0.39–50 μL) or positive control (galantamine). Ten microliters of AChE at a concentration of 0.25 U/mL was then added to the reaction mixture. The microplate was immediately read at 415 nm every 2.5 minutes for 12.5 minutes in a Labsystem Multiskan MS: 35200 7847 microplate reader (Japan). Percentage enzymatic inhibition was calculated with respect to the control, without inhibitor as follows: I %= [(Ab − AEO)/Ab] × 100, where Ab is the absorbance of the blank at T12.5 minutes, and AEO is the absorbance of EOs T12.5 minutes. Each EO was assessed in triplicate. The extent of inhibition was expressed as mean ± standard error of the 50% inhibition concentration (IC50) of the enzymatic activity.
In order to examine the inhibition mode of the active EOs, AChE activity was monitored as previously; except this time a concentration gradient of acetylthiocholine iodide (0.023–3 mM) was used in the absence or presence of EOs. In the wells to which EO was added to the reaction mixture (for the assessment of AChE activity in presence of EO), the concentration of EO used was 300 μg/mL. Inhibition type for the active EOs was determined by plotting the data obtained as 1/activity (1/V) against 1/substrate concentration (1/S), based on the Lineweaver–Burk method. The Michaelis–Menten constant (Km) and maximum velocity (Vmax) were determined with variable substrate concentrations in the standard reaction mixture and the inhibition type determined.
2.3.2. Anti-collagenase assay
The assay used was based on spectrophotometry as described previously [25], with slight modifications. The assay was performed in 1× reaction buffer provided in the kit at a concentration 10× (0.5M Tris–HCl, 1.5M NaCl, 50 mM NaCl2, and 2 mM sodium azide, pH 7.6). The enzyme collagenase from Clostridium histolyticum (Type IV) obtained in the kit was dissolved in the 1× reaction buffer for use at a concentration of 0.2 U/mL. DQ gelatin from pig skin, fluorescein conjugate, provided in the kit was used as substrate, diluted in 1× reaction buffer to a final concentration of 150 μg/mL. To constitute the reaction mixture, 80 μL of test item, that is, EOs dissolved in 1× reaction buffer to a final concentration gradient of 400–3.125 μg/mL was distributed in Nunc 96-well microtiter plates. Collagenase enzyme (100 μL) was added to the wells and allowed to incubate for 15 minutes at 37°C. Following incubation, 20 μL DQ gelatin was added to the reaction mixture. Fluorescence was read with parameters set for an excitation wavelength at 485 nm and emission at 515 nm. 1,10-Phenanthroline provided in the kit was used as positive control. Positive control wells thus consisted of the reaction mixture as describe but with EOs replaced by 1,10-phenanthroline. Blank wells were prepared in the microplate constituting of 100 μL 1× reaction buffer and 100 μL collagenase enzyme. Inhibition percentage of the collagenase enzyme was calculated using the area under curve (AUC) as follows: % I = [(AUCb − AUCs)/AUCb] × 100, where AUCb is AUC of the blank sample after Time, T2h; and AUCs is AUC of the sample at T2h. Concentration of EOs at which 50% of the enzyme was inhibited (IC50) was calculated using GraphPad Prism 6.0 (La Jolla, CA, USA).
2.3.3. Anti-elastase assay
The method used for the elastase assay was also based on a spectrophotometric method as described previously [26], with slight modifications. The assay was performed in 1× reaction buffer provided in the kit at a concentration 10× (1M Tris–HCl, pH 8.0, and containing 2 mM sodium azide). The enzyme elastase from pig pancreas obtained in the kit was dissolved in 1× reaction buffer for use at a concentration of 0.2 U/mL. DQ elastin from bovine neck ligament, BODIPY FL conjugate, provided in the kit was used as substrate, diluted in 1× reaction buffer to a final concentration of 100 μg/mL. To constitute the reaction mixture, 50 μL of the test item, that is, EOs dissolved in 1× reaction buffer to a final concentration gradient of 800–6.25 μg/mL was distributed in Nunc 96-well microtiter plates. One hundred microliters of elastase was added to the wells and allowed to incubate for 15 minutes at 37°C. Following incubation, 50 μL DQ elastin was added to the reaction mixture. Fluorescence was read with parameters set for an excitation wavelength at 485 nm and emission at 530 nm. N-Methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone provided in the kit was used as a positive control. Positive control wells thus consisted of the reaction mixture as described previously, but replacing EO test sample by N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone to a final concentration of 0.2 mM. Blank wells were prepared in the microplate consisting of 100 μL 1× reaction buffer and 100 μL elastase enzyme. Inhibition percentage of the elastase enzyme was calculated using the AUC as follows: % I = [(AUCb − AUCs)/AUCb] × 100, where AUCb is AUC of the blank sample after Time, T2h; and AUCs is AUC of the sample at T2h. Concentration of EOs at which 50% of the enzyme was inhibited (IC50) was calculated using GraphPad Prism 6.0.
2.3.4. Antidiabetic assay
The antidiabetic assay was evaluated using the α-glucosidase enzymatic inhibitory method, carried out as per Bachhawat et al. [27] and Mayur et al. [28] with slight modification. The reaction mixture consisted of the following, EOs (50 μL, 500–3.91 μg/mL) or positive control, acarbose (50 μL, 250–1.95 μg/mL), α-glucosidase type I from Saccharomyces cerevisiae (10 μL, 1 U/mL) and sodium phosphate buffer (50 μL, 50 mM, pH 6.9), mixed in the 96-well microplate and pre-incubated at 37°C for 10 minutes p-Nitrophenol-α-D-glucopyranoside (pNPG) used as substrate (20 μL, 1 mM), was added to start the reaction. The microplate was allowed to incubate at 37°C for 30 minutes, after which 50 μL sodium carbonate (0.1M) was added to the reaction mixture to stop the reaction and absorbance was measured at 405 nm using a UV–visible microplate reader. The percentage inhibition of α-glucosidase activity was calculated from the absorbance read out, as follows: I % = [(Ab − AEO)/Ab] × 100, where Ab is the absorbance of the blank, and AEO is the absorbance of EOs. The extent of inhibition was expressed as mean ± SEM of the IC50 of the enzymatic activity.
In order to examine the inhibition mode of the active EOs, α-glucosidase activity was measured with increasing concentrations of pNPG (0.125–2 mM), in the absence or presence of EOs. The concentration of the EOs was kept constant at 400 μg/mL. The reaction mixture consisted of 20 μL of EO (400 μg/mL), 10 μL α-glucosidase solution (1 U/mL), 50 μL sodium phosphate buffer (0.1M, pH 6.9), and 20 μL graded concentrations of pNPG. The mixture was incubated at 37°C for 10 minutes. Fifty microliters of sodium carbonate (0.1M) was added to the reaction mixture following incubation, to stop the reaction. Absorbance was measured at 405 nm. A calibration curve using graded concentrations of pNPG (0.6–0.019 mM) was used. Inhibition type for the active EOs was determined by plotting the data obtained as 1/activity (1/V) against 1/substrate concentration (1/S), according to the method of Lineweaver–Burk. The Michaelis–Menten constant (Km) and maximum velocity (Vmax) were determined with variable substrate concentrations in the standard reaction mixture and the inhibition type determined.
2.3.5. Antimelanogenic assay
The amount of intracellular and extracellular melanin produced in B16F10 mouse melanocytes after treatment with the different EOs was determined as describe previously [29]. Mouse melanocytes (B16F10) were cultured in complete Minimum Essential Eagle’s Medium (MEM), containing 10% fetal bovine serum, 1.5 g/L NaHCO3, 2 mM L-glutamate, 10 μg/mL streptomycin, and 0.25 μg/mL fungizone. The cultured B16F10 mouse melanocytes were trypsinized (0.25% trypsin and 0.1% EDTA at 37°C for 5–10 minutes), inoculated into 24-well plates (50,000 cells/well in 1.5 mL MEM) and incubated for 24 hours at 37°C in a CO2 incubator. After 24-hours incubation, 500 μL each sample solution was added to each well, containing 1.5 mL MEM and cells, in duplicate, and the 24-well plates were incubated for 3 days at 37°C in a CO2 incubator. The EOs and the positive control—theophylline (concentrations ranging between 15.63 μg/mL and 500 μg/mL)—were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO was 1.25%. In the control group, DMSO was used instead of the sample solution. After incubation, the cultured medium was assayed for extracellular melanin as follows: cultured medium was centrifuged (900 g, 20 minutes at 4°C) to yield a supernatant. One milliliter of a mixture of 0.4M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (pH 6.8) and ethanol (9:1, volume/volume) was added to 1 mL supernatant. The mixture of supernatant and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer was measured at a wavelength of 475 nm. The amount of extracellular melanin present was determined by comparing the sample optical density (OD) with the OD of the control cells. The mouse melanocytes, remaining in the 24-well plates, were digested by the addition of 400 mL 1N NaOH, washed with 100 mL of CMF-D-PBS (Calcium and Magnesium Free-Dulbecco’s-Phosphate Buffered Saline) and trypsinized (0.25% trypsin and 0.1% EDTA at 37°C for 5–10 minutes), and were left for 16 hours at room temperature. The OD was determined at 475 nm for the aforementioned mixture and the amount of intracellular melanin was determined.
2.4. Statistical analysis
Experiments were performed in triplicate and results are presented as mean ± SEM. The endpoint values, percentage inhibition, and IC50 of the EOs were calculated using GraphPad Prism version 6.0. The difference between the EOs and their relevant control was evaluated in terms of IC50. The significance of the difference between the IC50 of the EOs versus the relevant control were assessed using a one-sided independent sample t test in GraphPad Prism version 6.0. Differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Anti-acetylcholinesterase assay
A plethora of natural products are used for therapeutic purposes against several NCDs. Several of these products are purported to have beneficial effects on memory disorders, depression, and cerebral ischemia. In the same way, secondary metabolites extracted from plants materials in the form of EOs have also found their role in the management of cognitive disorders on an empirical basis but also through homeopathy [30–32].
Mortality and morbidity related to neurological diseases have seen a rise in recent years in both developed and developing countries [33]. A recent report from the World Health Organization aimed at addressing the impact of dementia as an increasing threat to global health mentioned that there are ~35.6 million people worldwide suffering from dementia and that this number will double in 2030 and triple in 2050 [34]. To this effect, research devoted to the study of neurodegenerative disorders such as AD has seen an increase. Drugs used in the treatment of AD are based on the inhibition of AChE and also aimed at hindering the cholinergic deficit that is associated with cognitive dysfunction. These drugs have shown significant results in slowing down the progress of AD but they are associated with several major negative adverse effects. For this purpose, the search for phytomedicines that can inhibit AChE while at the same time act as a good antioxidant is warranted.
The present study was designed towards investigating the potential effects of EOs to inhibit key physiological enzymes involved in some key pathologies. IC50 values obtained in the AChE inhibition assay for the EOs are shown in Table 1. The strongest inhibition was observed in the EO of CH, followed by CR and CGp. There were no significant difference between the IC50 demonstrated by these three EOs compared to galantamine, a known drug, prescribed in the management of mild to moderate AD as well as in other memory impairments. EOs with IC50 values < 1 mg/mL can be considered among potential anti-AChE candidates, as supported in the literature. These EOs being comparable to the activity of galantamine were further investigated for their inhibitory type [12,35].
Table 1.
Inhibition of acetylcholinesterase by the EOs.
| EOs | IC50 (μg/mL) | I% at 50 μg/mL | p |
|---|---|---|---|
| CH | 2.476 ± 0.13 | 92.46 ± 0.32 | 0.094a |
| CR | 3.636 ± 0.10 | 92.49 ± 0.41 | 0.091a |
| CGp | 6.321 ± 0.45 | 88.82 ± 0.60 | 0.066a |
| CZ | 16.04 ± 0.11 | 61.36 ± 0.34 | 0.038b |
| PT | 78.83 ± 0.20 | 41.50 ± 0.77 | 0.006b |
| PA | 13.83 ± 0.42 | 59.42 ± 0.51 | 0.020b |
| Gal | 3.989 ± 0.16 | 90.40 ± 0.47 | – |
Values are mean ± standard error of the mean of three assays.
CGp = Citrus grandis; CH = Citrus hystrix; CR = Citrus reticulata; CZ = Cinnamomum zeylanicum; EO = essential oil; Gal = galantamine; I% = percentage inhibition; IC50 = 50% inhibitory concentration; PA = Psiadia arguta; PT = Psiadia terebinthina.
p > 0.05, no significant difference when compared to positive control ascorbic acid.
p < 0.05, statistically significant difference when compared to positive control galantamine.
The inhibition types of the active EOs were evaluated using Lineweaver–Burk plots. The Km (the affinity of the enzyme for the substrate) and Vmax (the rate of the reaction) in the presence and absence of EOs are summarized in Table 2. In the presence of the inhibitors, there is a significant reduction in both Km and Vmax, categorizing them as uncompetitive type of inhibitors. This result may help future investigations to clarify the interaction of the EO components with AChE.
Table 2.
Kinetic study of active EOs.
| EO | Km | Vmax | Inhibitor type |
|---|---|---|---|
| CR | 169.43 | 0.0128 | UC |
| CH | 81.93 | 0.0079 | UC |
| CGp | 329.02 | 0.0111 | UC |
| Controla | 568.22 | 0.0147 | – |
CGp = Citrus grandis; CH = Citrus hystrix; CR = Citrus reticulata; EO = essential oil; UC = uncompetitive inhibitor: both Km and Vm decrease.
Control: reaction mixture without EOs.
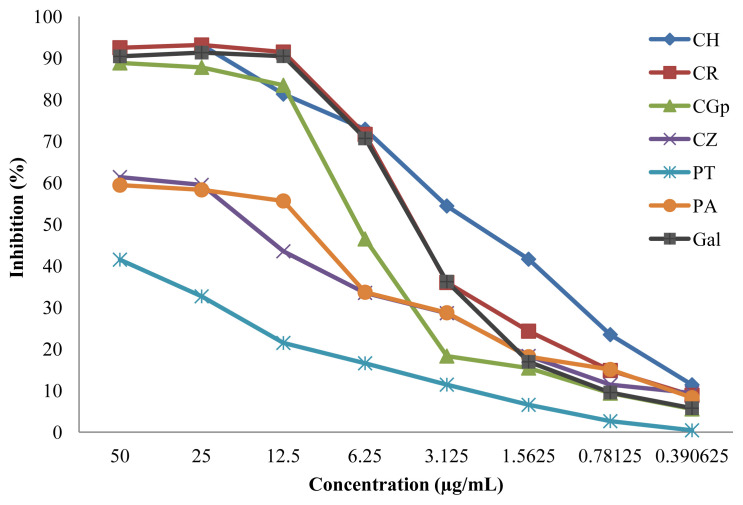
Chaiyana and Okonogi [36] reported the potential of the EO of citrus species, with the presence of limonene, citronellol, o-cymene, and 1,8-cineole as major components, in the inhibition of AChE (85.8 ± 3.9%). The citrus species in this study also exhibited strong AChE inhibition activities, with inhibition percentage greater than that reported by Chaiyana and Okonogi [36] (Figure 1). The EOs of the citrus evaluated in this study presented a gas chromatography–mass spectrometry (GC-MS) profile consisting of α-pinene, β-pinene, limonene, cymene, and linalool as major components, as reported previously [6]. Moreover, Dalai et al. [22] reported the activity of CZ as moderate AChE inhibitor (45.88 ± 1.94 μg/mL) compared to galantamine (22.34 ± 0.56 μg/mL), characterized by the presence of eugenol in the EO. CZ used in this experiment also showed moderate anti-AChE activity with an IC50 of 16.04 ± 0.11 μg/mL (Table 1), with a GC-MS profile showing eugenol at an abundance of 58.09%, as previously reported [22].
Figure. 1.
Inhibition percentage of the different essential oils against acetylcholinesterase. CGp = Citrus grandis; CH = Citrus hystrix; CR = Citrus reticulata; CZ = Cinnamomum zeylanicum; EO = essential oil; Gal = galantamine, PA = Psiadia arguta; PT = Psiadia terebinthina.
EOs from PA and PT have never been assessed for their potential as AChE inhibitors. PA and PT both belonging to the same family and genus are however different in physicochemical constituents [22]. Caryophyllene oxide, methyl eugenol, isoeugenol, limonene, and vanillin were the major components in the Eos of PA. β-Myrcene, α-curcumene, α-cubebene, and acetyl eugenol were major components in the Eos of PT. This difference in composition may account for the difference in bioactivity observed (IC50, 78.83 ± 0.20 μg/mL and 13.83 ± 0.42 μg/mL, respectively).
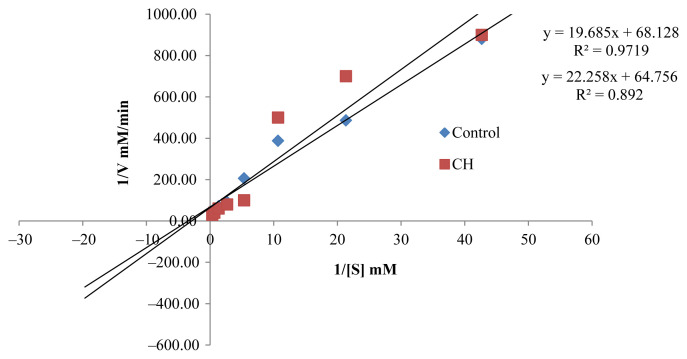
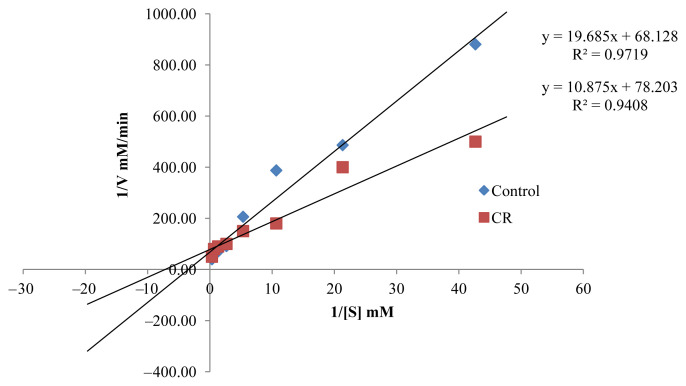
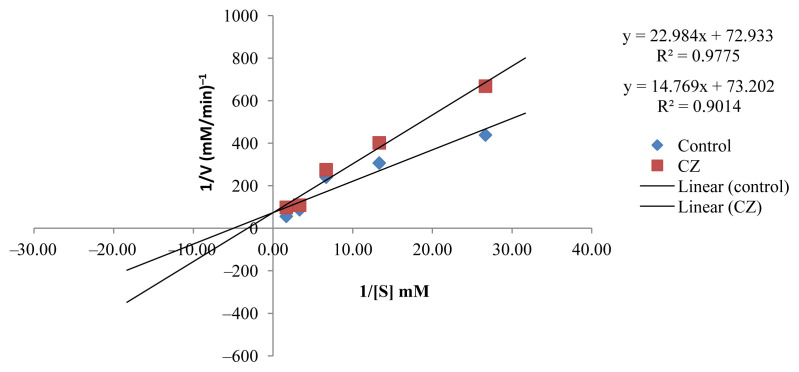
Pharmacological activities of AChE inhibitors are important in the inactivation of the enzymatic activity, resulting in the accumulation of acetylcholine in the synapse regions, which leads to improved stimulation of the cholinergic receptors. AChE inhibitors described in the literature, such as galantamine and donepezil, can be categorized as reversible and irreversible competitive and noncompetitive inhibitors. The active EOs (CR, CH, and CGp), as uncompetitive inhibitors will bind to AChE, following which, there will be the binding of the substrate, justifying the reduction in Km (Table 2, Figures 2–4). The resultant complex enzyme–EO–substrate therefore yielded a slower reaction rate to the formation of the product, thus Vmax was also reduced. The AChE inhibition potential of CR, CH, and CGp was expressed by an inhibition percentage of 92.49 ± 0.41%, 92.46 ± 0.32%, and 88.82 ± 0.60%, respectively, along with a slow rate of reaction of AChE. It can be argued these EOs have the potential to enhance cholinergic neurotransmission in the forebrain regions, which has been reported to compensate for the loss of functioning brain cells. However, further studies are needed to confirm the AChE inhibitory activities in vivo.
Figure. 2.
Lineweaver–Burk plot in the presence and absence of Citrus grandis (CGp).
Figure. 3.
Lineweaver–Burk plot in the presence and absence of Citrus hystrix (CH).
Figure. 4.
Lineweaver–Burk plot in the presence and absence of Citrus reticulata (CR).
3.2. Anti-collagenase and anti-elastase assays
Collagen, the major component of the skin, is degraded by the enzyme collagenase [37]. When the skin is overexposed to sunlight, UV light stimulates the excessive production of collagenase, leading to degradation of the protein matrix, and the photoaging process is triggered. Inhibition of collagenase activity delays the process of forming precollagen fibers and subsequently the wrinkling process [37]. Another type of skin degradation and condition is caused by proteolytic enzymes present in the dermis such as hyaluronidase and elastase. Previous studies have reported the potential of secondary metabolites such as EOs and plant derivatives for their potential anti-elastase and anti-collagenase activities due to the enrichment of these natural products with many bioactive molecules such as phenolic compounds [38].
Endpoint validation of the enzymatic activity in the anti-collagenase assay was compared to that of the 1,10-phenanthroline. 1,10-Phenanthroline is an inhibitor of metallopeptidases. Collagenase is an example of a metallopeptidase, causing degradation of the matrix. Inhibition of collagenase enzyme occurs by removal and chelation of the metal ion required for catalytic activity to occur [39,40].
The EOs tested displayed active to moderately active collagenase inhibitory effect, when compared to 1,10-phenanthroline (inhibition ranging from 47.0 ± 0.40% to 72.10 ± 0.24% for CZ and CH respectively; Table 3). The most active EO was found to be CH with 50% inhibition of the enzyme observed at a concentration of 28.71 ± 0.16 μg/mL.
Table 3.
Inhibition of collagenase and elastase.
| EOs | Anti-collagenase activity | Anti-elastase activity | ||
|---|---|---|---|---|
|
|
|
|||
| IC50 (μg/mL) | Inhibition % at 400 μg/mL | IC50 (μg/mL) | Inhibition % at 800 μg/mL | |
| CH | 28.71 ± 0.16 | 72.10 ± 0.24 | 412.76 ± 2.77 | 63.21 ± 0.16 |
| CR | 153.73 ± 0.24 | 64.09 ± 2.02 | 302.75 ± 1.56 | 61.23 ± 0.33 |
| CGp | 132.67 ± 0.15 | 61.55 ± 0.29 | 13052 ± 1.59 | 42.43 ± 0.43 |
| PA | 440.88 ± 0.72 | 47.00 ± 0.40 | 724.15 ± 0.97 | 49.08 ± 0.03 |
| PT | 329.22 ± 0.11 | 49.88 ± 0.86 | 1530.20 ± 22.54 | 32.23 ± 0.19 |
| CZ | 337.75 ± 0.15 | 52.82 ± 0.37 | NA | 34.63 ± 0.19 |
| Control | 24.45 ± 0.19a | 91.24 ± 0.94a | 33.7 ± 0.15b | 75.10 ± 0.20b |
Values are mean ± standard error of the mean of three assays.
CGp = Citrus grandis; CH = Citrus hystrix; CR = Citrus reticulata; CZ = Cinnamomum zeylanicum; EO = essential oil; Gal = galantamine, PA = Psiadia arguta; PT = Psiadia terebinthina.
Collagenase inhibitor.
Elastase inhibitor.
The moderate activity in the collagenase assay demonstrated by CZ in the present study may be partly due to it reported capacity in promoting the biosynthesis of collagen type I, through the activation of insulin-like growth factor-1 signaling, as reported by Takasao et al. [41].
Elastin is a key protein found in connective tissue that is responsible for elasticity of the skin. Elastin is catalyzed by the enzyme elastase, which is a serine protease with broad specificity. It cleaves protein at the carboxyl side of small hydrophobic amino acids and possesses a unique proficiency of digesting elastin. It has been suggested that degradation of elastin by elastase rises with age and/or repeated UV radiation, leading to skin aging [38]. Endpoint validation of the enzymatic activity in the anti-elastase assay was compared to that of N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone, a known inhibitor of the elastase enzyme. Phenolic compounds have been highlighted for their anti-elastase capacity. Reduction in elastase activity up to 70% has been observed by the EOs having eugenol, cinnamaldehyde, and geraniol as major components [38,42]. Eugenol and geraniol were not present in the EOs (except for CZ) screened previously by GC-MS [6,22]. However, β-myrcene, which is an important intermediate in the synthesis of geraniol, was found among the major components of CH, CR, CGp, and PT. From the data obtained in this study, in comparison with those in the literature, one could argue that the activities observed for the EOs are not only due to the major components present but it may also be due to the synergistic effect of all the components of the EO blends. Therefore, it can be argued that these EOs are potential inhibitors of proteolytic enzymes present in the dermis responsible for the degradation of the extracellular matrix.
3.3. Antidiabetic assay
The incidence of diabetes is on a firm increase worldwide and has been identified as one of the main threats to human health in the 21st century [43]. In Mauritius as in many developing countries, the use of herbal medicine alone or alongside prescription drugs is common. The present study highlighted the positive effect of several EOs on the inhibition of α-glucosidase enzyme.
As shown in Table 4, EOs of CH, CGp, CR, CZ, and PA demonstrated higher activity than that of the positive control, acarbose. However, the activity obtained from the leaves of CZ depicted that CZ was by far the most potent, with inhibition of 93.72 ± 0.03% and IC50 of 64.52 ± 0.69 μg/mL, compared to acarbose (inhibition = 51.39 ± 0.19%, IC50 = 448.80 ± 0.81 μg/mL). In a previous study, Salehi et al. [44] reported the potential activity of CZ in exhibiting a high hypoglycemic effect, as demonstrated by the inhibition of α-glucosidase. Ranasinghe et al. [45] also reported the various benefits of CZ, including its potential to lower blood glucose level. In addition, CZ has been used as part of traditional medicine, worldwide, for decades, as treatment against gastritis, in-flammatory diseases, and blood circulation disorders. Recently, the prophylactic effect of CZ against diabetes has been reported [46–48], particularly for their potential to mimic insulin.
Table 4.
Inhibition of α-glucosidase by EOs.
| EO | Inhibition % at 500 μg/mL | IC50 (μg/mL) |
|---|---|---|
| CH | 85.49 ± 0.26 | 276.70 ± 0.73 |
| CR | 81.15 ± 0.01 | 169.90 ± 0.58 |
| CGp | 83.19 ± 0.30 | 240.60 ± 6.50 |
| CZ | 93.72 ± 0.03 | 64.52 ± 0.69 |
| PT | 40.12 ± 0.05 | 14584.0 ± 90.34 |
| PA | 76.45 ± 0.18 | 313.0 ± 5.0 |
| Acarbose | 51.39 ± 0.19 | 448.80 ± 0.81 |
Values are mean ± standard error of the mean of three assays.
CGp = Citrus grandis; CH = Citrus hystrix; CR = Citrus reticulata; CZ = Cinnamomum zeylanicum; EO = essential oil; Gal = galantamine, PA = Psiadia arguta; PT = Psiadia terebinthina.
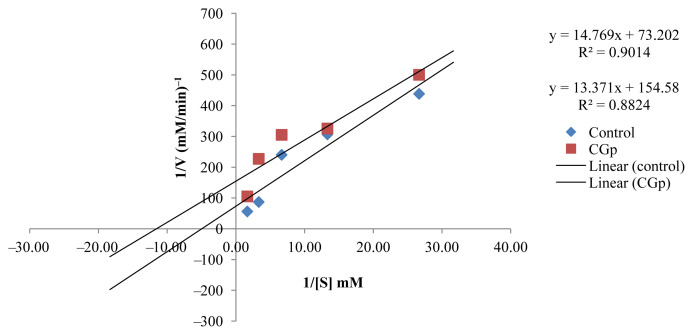
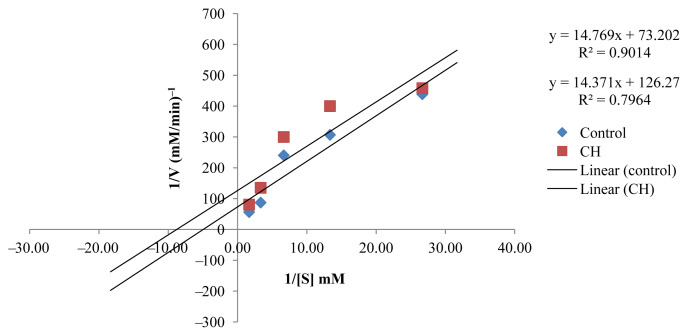
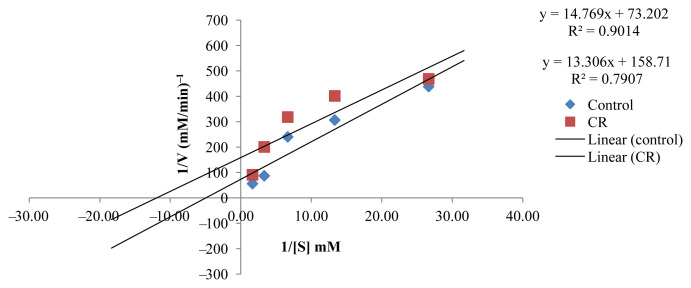
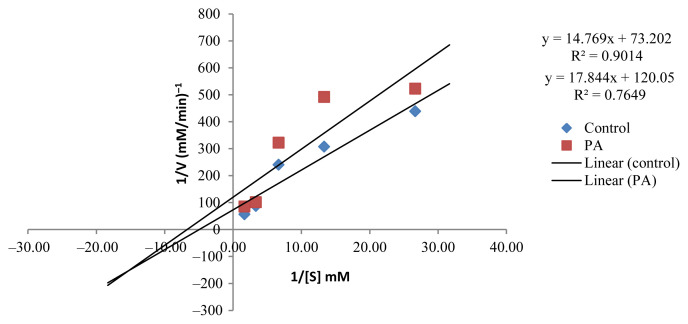
The kinetic study to investigate the inhibition type of the active EOs showed that the active EOs exhibited an uncompetitive type of inhibition except for CZ, which presented a competitive inhibition type (Figures 5–9). EOs of CH, CR, CGp, and PA once they bind to the inhibitor binding site of the enzyme lead to a reduction in Vmax and Km (Table 5). These findings can be considered an interesting as it has provided baseline data for further investigation in the design of drugs targeting postprandial peaks.
Figure. 5.
Lineweaver–Burk plot in the presence and absence of Cinnamomum zeylanicum (CZ).
Figure. 6.
Lineweaver–Burk plot in the presence and absence of Citrus grandis (CGp)
Figure. 7.
Lineweaver–Burk plot in the presence and absence of Citrus hystrix (CH).
Figure. 8.
Lineweaver–Burk plot in the presence and absence of Citrus reticulata (CR).
Figure. 9.
Lineweaver–Burk plot in the presence and absence of Psiadia arguta (PA).
Table 5.
Kinetic study of active EOs.
| EO | Km | Vmax | Inhibitor type |
|---|---|---|---|
| CR | 160.98 | 0.0063 | UC |
| CH | 249.33 | 0.0079 | UC |
| CGp | 195.76 | 0.0065 | UC |
| CZ | 1550.08 | 0.0137 | C |
| PA | 231.24 | 0.0090 | UC |
| Controla | 282.10 | 0.0137 | – |
CGp = Citrus grandis; CH = Citrus hystrix; CR = Citrus reticulata; CZ = Cinnamomum zeylanicum; EO = essential oil; Gal = galantamine, PA = Psiadia arguta; PT = Psiadia terebinthina; UC = uncompetitive inhibitor (both Km and Vm decreased).
Reaction mixture without EOs.
3.4. Antimelanogenic assay
To determine the antimelanogenic activity of the EOs, their effect on the reduction of melanin content in B16F10 melanoma cells was investigated. The B16F10 melanoma cells were treated with varying concentrations of EOs. From the results obtained (Table 6), we conclude that the EOs had a positive effect on melanin inhibition. The concentrations of EOs exhibiting 50% inhibition were substantial in both intra- and extracellular assessments, with CH exhibiting a better intracellular melanin inhibitory activity at an IC50 of 15.92 ± 1.06 μg/mL. One could highlight that the melanin inhibition assessed in the extracellular medium was more pronounced for the EOs (IC50 < 15.625 μg/mL for the citrus species and PT) compared with the positive control theophyline (IC50 23.31 ± 5.99 μg/mL). A one-sided independent samples Student t test was performed to investigate whether the mean values obtained for the EOs were significantly lower than that of the positive control theophyline. In the intracellular assessment, one can observe that even though the EOs showed promising results, they were not significantly lower than that of control theophyline. In the extracellular assessment, it can be observed that for CH, CR, CGp, and PT, the IC50 values were significantly lower to that of theophyline. The IC50 value of PA on the contrary, was not statistically significant, even though the IC50 of the EO was less than that of theophyline.
Table 6.
Melanin inhibitory activity.
| EO | Melanin inhibition IC50 (μg/mL) | |||
|---|---|---|---|---|
|
| ||||
| Intracellular | p | Extracellular | p | |
| CH | 15.92 ± 1.06 | 0.979 | 14.44 ± 0.16 | 0.023 |
| CR | 23.75 ± 4.47 | 0.986 | 14.43 ± 0.90 | 0.023 |
| CGp | 28.99 ± 5.70 | 0.989 | 7.41 ± 1.85 | 0.023 |
| CZ | NA | NA | NA | NA |
| PT | 69.51 ± 0.05 | 1.000 | 6.88 ± 3.25 | 0.023 |
| PA | 256.35 ± 16.90 | 1.000 | 17.79 ± 3.03 | 0.061 |
| PC | 2.25 ± 4.53 | – | 23.31 ± 5.99 | – |
Values are mean ± standard error of the mean of three assays.
CGp = Citrus grandis; CH = Citrus hystrix; CR = Citrus reticulata; CZ = Cinnamomum zeylanicum; EO = essential oil; Gal = galantamine; NA = no inhibition at tested concentrations; PA = Psiadia arguta; PC = positive control (theophyline); PT = Psiadia terebinthina.
Melanogenesis activity in the cultured melanoma cells has been reported by Matsuda et al. [29] to correlate with the amount of melanin expressed in the cells and assessed from the sum of the amount of melanin retained in the cells (intra-cellular melanin) and that excreted into the cultured medium (extracellular melanin). Inhibition of melanin occurred in both intra- and extracellular environments, which is a good indication of the antimelanogenic potential of the EOs.
These results tend to corroborate the potential of EOs to inhibit hyperpigmentation using the anti-tyrosinase assay [21]. The EOs were reported to be good inhibitors of tyrosinase. A significant reduction in Vmax for CGp and CH was observed. The combination of these results thus allow us to suggest the potential of these EOs as suitable candidates for the management of hyperpigmentation disorders and as potent source of whitening agent for the cosmetic industry.
4. Conclusion
EOs of CH, CR, CGp, CZ, PT, and PA studied in the present study showed interesting properties. In this context, enzyme inhibitory properties of plant-derived products such as EOs can add value to drug development, with their possible uses as natural enzyme inhibitors to replace synthetic inhibitors. However, attempts to valorize EOs have been established previously on the treatment of multiple disorders related to NCD but always on an empirical basis. Further studies are warranted to establish therapeutic safety and efficacy of active EOs such as CZ as a pharmaceutical agent.
Footnotes
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
REFERENCES
- 1.Guenther E. The essential oils. 1st ed. New York: Van Nostrand; 1948. [Google Scholar]
- 2.Hunter VM. Essential oils: art, agriculture, science, industry and entrepreneurship a focus on the Asia–Pacific region. Malaysia: University of Malaysia Perli; 2009. [Google Scholar]
- 3. Celiktas OY, Hames Kocabas EE, Bedir E, Vardar Sukan F, Ozek T, Baser KHC. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007;100:553–9. [Google Scholar]
- 4. Aleksic V, Knezevic P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol Res. 2014;169:240–54. doi: 10.1016/j.micres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Zhu L, Zeng D, Long W, Zhu S. Chemical composition and anti-inflammatory activities of essential oil from Trachydium roylei. J Food Drug Anal. 2016;24:602–9. doi: 10.1016/j.jfda.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aumeeruddy-Elalfi Z, Gurib-Fakim A, Mahomoodally MF. Chemical composition, antimicrobial and antibiotic potentiating activity of essential oils from 10 tropical medicinal plants from Mauritius. J Herb Med. 2016;6:88–95. [Google Scholar]
- 7. Mendez-Tovar I, Herrero B, Perez-Magarino S, Pereira JA, Asensio-S-Manzanera MC. By-product of Lavandula latifolia essential oil distillation as source of antioxidants. J Food Drug Anal. 2015;23:225–33. doi: 10.1016/j.jfda.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dibazar SP, Fateh S, Daneshmandi S. Clove (Syzygium aromaticum) ingredients affect lymphocyte subtypes expansion and cytokine profile responses: an in vitro evaluation. J Food Drug Anal. 2014;22:448–54. doi: 10.1016/j.jfda.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thring TSA, Hili P, Naughton DP. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med. 2009;9:27. doi: 10.1186/1472-6882-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin PC, Lee JJ, Chang IJ. Essential oils from Taiwan: chemical composition and antibacterial activity against Escherichia coli. J Food Drug Anal. 2016;24:464–70. doi: 10.1016/j.jfda.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization (WHO) Noncommunicable diseases. Fact sheet N°. 2015:355. [Google Scholar]
- 12. Adsersen A, Gauguin B, Gudiksen L, Jager AK. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J Ethnopharmacol. 2006;104:418–22. doi: 10.1016/j.jep.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 13. Barla F, Higashijima H, Funai S, Sugimoto K, Harada N, Yamaji R, et al. Inhibitive effects of alkyl gallates on hyaluronidase and collagenase. Biosci Biotechnol Biochem. 2009;73:2335–7. doi: 10.1271/bbb.90365. [DOI] [PubMed] [Google Scholar]
- 14. Madeo J, Elsayad C. The role of oxidative stress in Alzheimer’s disease. J Alzheimers Dis Park. 2013;3:116. [Google Scholar]
- 15. Dalai MK, Bhadra S, Chaudhary SK, Chanda J, Bandyopadhyay A, Mukherjee P. Anticholinesterase activity of Cinnamomum zeylanicum L. leaf extract. TANG Humanit Med. 2014;2014:4:e11. [Google Scholar]
- 16. Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23:134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 17. Maritim AC, Sanders RA, Watkins JB., III Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 18. Kim HE, Ishihara A, Lee SG. The effects of caffeoylserotonin on inhibition of melanogenesis through the downregulation of MITF via the reduction of intracellular cAMP and acceleration of ERK activation in B16 murine melanoma cells. BMB Rep. 2012;45:724–9. doi: 10.5483/BMBRep.2012.45.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurib-Fakim A, Gueho J, Sewraj MD. Plantes médicinales de Maurice Tome 1 Editions de l’ocean Indien. Stanley. Mauritius: Rose Hill; 1996. [Google Scholar]
- 20. Nunkoo DH, Mahomoodally MF. Ethnopharmacological survey of native remedies commonly used against infectious diseases in the tropical island of Mauritius. J Ethnopharmacol. 2012;143:548–64. doi: 10.1016/j.jep.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 21. Aumeeruddy-Elalfi Z, Gurib-Fakim A, Mahomoodally MF. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. S Afr J Bot. 2016;103:89–94. [Google Scholar]
- 22. Aumeeruddy-Elalfi Z, Gurib-Fakim A, Mahomoodally F. Antimicrobial, antibiotic potentiating activity and phytochemical profile of essential oils from exotic and endemic medicinal plants of Mauritius. Ind Crops Prod. 2015;71:197–204. [Google Scholar]
- 23. Hussain AI, Anwar F, Sherazi STH, Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depend on seasonal variations. Food Chem. 2008;108:986–95. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 24. Arruda M, Viana H, Rainha N, Neng NR, Rosa JS, Nogueira JMF, Do Carmo Barreto M. Anti-acetylcholinesterase and antioxidant activity of essential Oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules. 2012;17:3082–92. doi: 10.3390/molecules17033082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EnzCheck Gelatinase/Collagenase Assay Kit, manuals. Netherlands: Molecular probes, Inc; 2001. [Google Scholar]
- 26.EnzCheck Elastase Assay Kit (E-12056) manuals. Netherlands: Molecular probes, Inc; 2001. [Google Scholar]
- 27. Bachhawat JA, Shihabudeen MS, Thirumurugan K. Screening of fifteen Indian ayurvedic plants for alpha-glucosidase inhibitory activity and enzyme kinetics. Int J Pharm Pharm Sci. 2011;3:267–74. [Google Scholar]
- 28. Mayur B, Sandesh S, Shruti S, Sung-Yum S. Antioxidant and α-glucosidase inhibitory properties of Carpesium abrotanoides L. J Med Plants Res. 2010;4:1547–53. [Google Scholar]
- 29. Matsuda H, Hirata N, Kawaguchi Y, Yamazaki M, Naruto S, Shibano M, et al. Melanogenesis stimulation in murine b16 melanoma cells by Umberiferae plant extracts and their coumarin constituents. Bio Pharm Bull. 2005;28:1229–33. doi: 10.1248/bpb.28.1229. [DOI] [PubMed] [Google Scholar]
- 30. Howes MR, Perry NSL, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother Res. 2003;17:1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- 31. Perry NSL, Bollen C, Perry EK, Ballard C. Salvia for dementia therapy: review of pharmacological activity and pilot tolerability clinical trial. Pharmacol Biochem Behav. 2003;2003(75):651–9. doi: 10.1016/s0091-3057(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 32. Savelev S, Okello E, Perry NSL, Wilkins RM, Perry EK. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol Biochem Behav. 2003;75:661–8. doi: 10.1016/s0091-3057(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization (WHO) International classification of diseases. Geneva: World Health Organization; 2016. [Google Scholar]
- 34.World Health Organization (WHO) and Alzheimer’s Disease International. Dementia: a public health priority. Geneva: WHO; 2012. [Google Scholar]
- 35. Ingkaninan KP, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol. 2003;89:261–4. doi: 10.1016/j.jep.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 36. Chaiyana W, Okonogi S. Inhibition of cholinesterase by essential oil from food plant. Phytomedicine. 2012;19:836–9. doi: 10.1016/j.phymed.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 37. Mukherjee PK, Maity N, Nema NK, Sarkarm BK. Bioactive compounds from natural resources against skin ageing. Phytomedicine. 2011;19:64–73. doi: 10.1016/j.phymed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 38. Kacem R. Phenolic compounds from medicinal plants as natural anti-elastase products for the therapy of pulmonary emphysema. J Med Plants Res. 2013;7:3499–507. [Google Scholar]
- 39. Bencini A, Lippolis V. 1,10-Phenanthroline: a versatile building block for the construction of ligands for various purposes. Coord Chem Rev. 2010;254:2096–180. [Google Scholar]
- 40. Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: a functional balance in tissue degradation. Oral Dis. 1996;2:70–6. doi: 10.1111/j.1601-0825.1996.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 41. Takasao N, Tsuji-Naito K, Ishikura S, Tamura A, Akagawa M. Cinnamon extract promotes Type I collagen biosynthesis via activation of IGF-I signaling in human dermal fibroblasts. J Agric Food Chem. 2012;60:1193–200. doi: 10.1021/jf2043357. [DOI] [PubMed] [Google Scholar]
- 42. Khan MS, Ahmad I. In vitro antifungal, anti-elastase and antikeratinase activity of essential oils of Cinnamomum, Syzygium and Cymbopogon species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine. 2011;19:48–55. doi: 10.1016/j.phymed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 43. Hurt RT, Kulisek C, Buchanan LA, McClave SA. The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. J Gastroenterol Hepatol. 2010;6:780–92. [PMC free article] [PubMed] [Google Scholar]
- 44. Salehi P, Asghari B, Esmaeili MA, Dehghan H, Ghaz I. α-Glucosidase and α-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J Med Plants Res. 2013;6:257–66. [Google Scholar]
- 45. Ranasinghe P, Pigera S, Premakumara GS, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of “true” cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement Altern Med. 2013;13:275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ranasinghe P, Perera S, Gunatilake M, Abeywardene E, Gunapala N, Premakumara S, et al. Effects of Cinnamomum zeylanicum (Ceylon cinnamon) on blood glucose and lipids in a diabetic and healthy rat model. Pharmacogn Res. 2012;4:73–9. doi: 10.4103/0974-8490.94719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen Y, Fukushima M, Ito Y, Muraki E, Hosono T, Seki T. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci Biotechnol Biochem. 2010;74:2418–25. doi: 10.1271/bbb.100453. [DOI] [PubMed] [Google Scholar]
- 48. Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids in people with type 2 diabetes. Diabetes Care. 2003;26:3215–8. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]