Abstract
QT prolongation is present in 26-52% of cases of Takotsubo cardiomyopathy (TCM). It has been postulated to result from reduced cardiac repolarization reserve and reflects the transient myocardial insult observed in TCM. Bradycardia-induced QT interval prolongation is amplified by the occurrence of TCM, a combination that potentially carries a significant risk for torsade de pointes (TdP). We present a unique case of an 80-year-old female with TCM-related cardiac arrest. The patient had acquired long QT syndrome in which TCM myocardial insult led to the precipitation of a third-degree atrioventricular (AV) block and subsequent bradycardia-induced TdP. Due to the lack of robust literature, there is no clear guideline in the management of third-degree AV block in the setting of TCM. In our case, because of recurrent ventricular tachycardia (VT) and ventricular fibrillation (VF) arrest, we opted for temporary pacing at a high ventricular rate, followed by a biventricular implantable cardioverter-defibrillator (BiV/ICD). Follow-up 3 months later revealed improvement of left ventricular (LV) dysfunction and resolution of QT prolongation. However, the noticed AV conduction defects persisted. In the available literature, we identified five reported cases that bear similarity with our patient’s presentation. The identified cases were middle-aged to elderly females with no significant cardiac history, who exhibited a similar triad of TCM associated with high-grade AV block, acquired long QT syndrome, and a rapid progression of bradycardia-induced TdP, resulting in a near cardiac arrest within the first 24 - 48 h of admission. It is crucial to monitor corrected QT (QTc), correct electrolyte abnormalities, and minimize QT-prolonging medications in patients with TCM. The recognition of AV conduction defects in patients with TCM is critical, especially if it is associated with significant QT prolongation. Such situations are underrecognized, and are potentially fatal, necessitating close monitoring and timely intervention.
Keywords: Takotsubo cardiomyopathy, Stress-induced cardiomyopathy, Torsade de pointes, Polymorphic ventricular tachycardia, Permanent pacemaker, Biventricular implantable cardiac defibrillator, Complete heart block, Cardiac arrest
Introduction
Takotsubo cardiomyopathy (TCM) is also known as broken heart syndrome, apical ballooning syndrome, or stress cardiomyopathy. It was first described in Japan in 1990 by Hikaru Sato, MD, Ph.D. [1]. The condition’s hallmark is a transient acute left ventricular (LV) dysfunction associated with apical ballooning, without evidence of acute coronary artery pathology. Increasing evidence suggests that microvascular myocardial ischemia and catecholamine excess play a central role in the disease’s pathogenesis [2]. Despite all the proposed mechanisms, the exact pathogenesis of TCM remains a mystery.
Despite the long-term favorable prognosis and transient nature of the condition, some cases of TCM exhibit more life-threatening conditions, such as intermittent asystole, sudden cardiac death, and ventricular tachycardia (VT) (2-10%) [3-5]. The association between TCM and transient QT prolongation has been well described in prior literature, with an estimated prevalence of around 26% to 51% [6]. It is postulated to result from the reduced repolarization reserve, which reflects the transient myocardial insult of TCM [6]. Bradycardia-induced QT prolongation is amplified by the occurrence of TCM, which is a combination that carries a significant risk for torsade de pointes (TdP) [7, 8]. Nevertheless, polymorphic VT in the setting of QT prolongation (TdP) was thought to be uncommon and occurs only in 1.2% of early studies [9]. The prognostic significance of QT prolongation in TCM remains uncertain, with conflicting reports in the available literature.
Atrioventricular (AV) conduction defects are rarely associated with TCM, with a prevalence of about 2.9% [3, 9]. Very few reports have described third-degree heart block requiring pacing as a complication of TCM. Despite the placement of a pacemaker, AV node dysfunction persisted even after the recovery of the LV dysfunction [10, 11]. The conduction normalized for some patients who were reassessed during a long-term follow-up period [12]. The best method for managing AV blocks associated with TCM remains unclear.
We present a unique case of an 80-year-old female with TCM-related cardiac arrest associated with third-degree AV block, sporadic long QT syndrome, and bradycardia-induced TdP. Furthermore, we conducted a thorough literature review of this rare presentation and will discuss the tremendous therapeutic challenges encountered in such patients.
Case Report
Investigations
An 80-year-old female with a past medical history of hypertension on hydrochlorothiazide was brought to the emergency department (ED) after a cardiac arrest. She lost consciousness at a live baseball game, following which the bystander’s started cardiopulmonary resuscitation (CPR), lasting for 10 - 15 min. Automated external defibrillator (AED) showed a shockable rhythm, and she received two shocks. The rhythm was noted to be VT. She regained consciousness on her way to the hospital.
Upon arrival to the ED, the patient was alert and oriented to time, place, and person. She denied any complaints of chest pain, palpitations, shortness of breath, lightheadedness, or dizziness. She reported no history of similar episodes, syncope, myocardial ischemia, angina, or stroke in the past. She denied any family history of sudden cardiac death, myocardial infarction, or stroke. On exam, she was afebrile, and her blood pressure was 147/46 mm Hg, with a heart rate of 42/min. The respiratory rate was 18/min, and oxygen saturation was 93% on room air, point of maximal impulse (PMI) nondisplaced, normal first and second heart sounds, no murmurs, rubs, or gallops. Lungs were clear on auscultation bilaterally. No Jugular venous distension or lower extremity edema.
Laboratory investigations revealed normal hemoglobin 12.2 g/dL (reference range (RR): 12.0 - 16.0 g/dL), white blood cell (WBC) count 10 × 103/µL (RR: 3.3 - 10.7 × 103/µL) and platelets count 286 × 103/µL (RR: 150 - 400 × 103/µL). The serum potassium was 3.9 mmol/L (RR: 3.5 - 5.2 mmol/L), serum calcium was 8.9 mg/dL (RR: 8.6 - 10.8 mg/dL), and serum magnesium was significantly decreased at 1.3 mg/dL (RR: 1.6 - 3.0 mg/dL). Patient had anion gap metabolic acidosis with elevated lactic acid of 3.7 (RR: 0.5 - 2 mmol/L) and elevated serum creatinine of 1.34 mmol/L (RR: 0.5 - 1.2 mmol/L). The high-sensitivity troponin I was elevated at 17 ng/mL and brain natriuretic peptide (BNP) level was 249 pg/mL (RR: < 450 pg/mL). Laboratory results obtained on admission and their reference ranges are shown in Table 1.
Table 1. Laboratory Results on Presentation and Their Reference Ranges.
| Laboratory test | Patient’s result | Reference range |
|---|---|---|
| White cells count (WCC) | 10 × 103/µL | 3.3 - 10.7 × 103/µL |
| Hemoglobin | 12.2 g/dL | 12.1 - 15.0 g/dL |
| Mean corpuscular volume (MCV) | 95 fL | 80 - 100 fL |
| Platelets | 286 × 103/µL | 150 - 400 × 103/µL |
| Serum sodium | 138 mmol/L | 133 - 144 mmol/L |
| Serum chloride | 99 mmol/L | 98 - 107 mmol/L |
| Serum potassium | 3.9 mmol/L | 3.5 - 5.2 mmol/L |
| Serum bicarbonate | 20 mmol/L | 21.0 - 28.0 mmol/L |
| Anion gap | 13 mEq/L | 3 - 11 mEq/L |
| Serum calcium | 8.9 mg/dL | 8.6 - 10.8 mg/dL |
| Serum magnesium | 1.3 mg/dL | 1.6 - 3.0 mg/dL |
| Blood urea nitrogen (BUN) | 31 mg/dL | 9.0 - 25.0 mg/dL |
| Serum creatinine | 1.34 mmol/L | 0.5 - 1.2 mmol/L |
| Serum glucose | 158 g/dL | 70 - 140 g/dL |
| Aspartate aminotransferase (AST) | 87 U/L | 7 - 52 U/L |
| Alanine aminotransferase (ALT) | 51 U/L | 13 - 39 U/L |
| International normalized ratio (INR) | 1.07 | < 1.1 |
| Lactic acid | 3.7 mmol/L | 0.5 - 2.2 mmol/L |
| High-sensitivity troponin I | 17 ng/mL | 0 - 0.4 ng/L |
| Brain natriuretic peptide (BNP) | 249 pg/mL | < 450 pg/mL |
| Thyroid-stimulating hormone (TSH) | 3.95 µIU/mL | 0.5 - 5 µIU/mL |
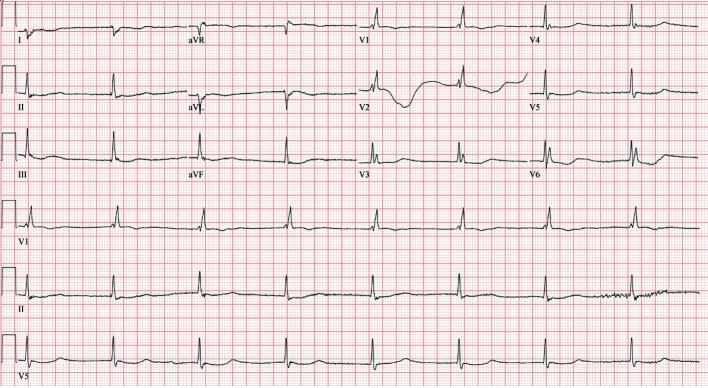
The electrocardiogram (ECG) showed sinus bradycardia with a heart rate of 47/min and corrected QT (QTc) prolongation to 509 ms (Fig. 1). Transthoracic echocardiogram (TTE) revealed a left ventricular ejection fraction (LVEF) of 60-65% with normal LV function and no regional wall motion abnormalities. The chest X-ray showed no congestion, consolidation, or effusion.
Figure 1.
ECG on admission demonstrated a junctional escape rhythm with right bundle branch block, 47 beats/min ventricular rate, and QTc of 509 ms. ECG: electrocardiogram.
The patient received 4 g of intravenous (IV) magnesium sulfate for hypomagnesemia and 1 L of normal saline. Lactic acid continued trending down to 1.8 mmol/L. The second set of high-sensitivity troponin I was 85 ng/mL and subsequently the patient received aspirin 325 mg, clopidogrel 75 mg, statin, as well as therapeutic heparin drip. The third set of troponin I trended up to 206 ng/L. Two hours later, a repeat ECG showed wide complex bradycardia with QTc of 545 ms and a heart rate of 41 beats/min. This was interpreted as a junctional escape rhythm with a right bundle branch block.
Diagnosis
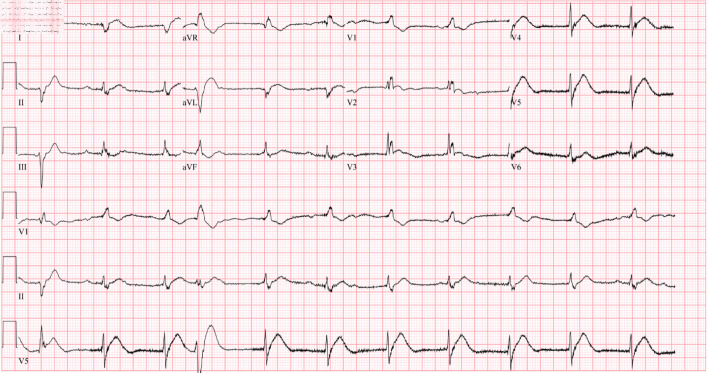
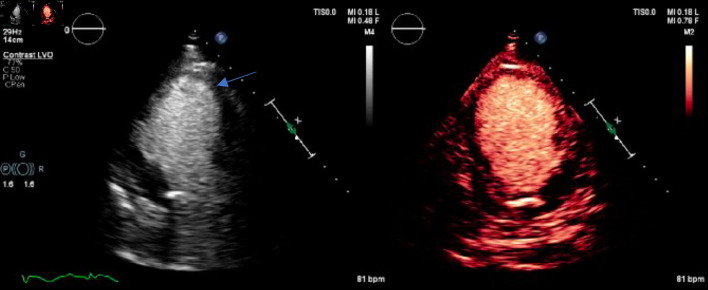
The patient was waiting transfer to the cardiac critical care unit when she developed ventricular fibrillation (VF) arrest. The episode happened coincidently when the patient requested to use the commode for micturition. The patient immediately received cardiac defibrillation with 200 J and CPR was continued for 6 min. Return of spontaneous circulation was achieved, and the patient mentation returned quickly. She denied having any chest pain, palpitations, or lightheadedness prior to the event. ECG obtained thereafter revealed a complete heart block (CHB) with an accelerated junctional rhythm of 67 beats/min, QTc of 407 ms, and no acute ischemic change was noticed (Fig. 2) (Table 2). She underwent temporary venous pacing (TVP) with a programed lower rate limit (LRL) of 70 beats/min. A repeat TTE showed a newly reduced ejection fraction (EF) of 20-25% with mid to apical segment severe hypokinesis (Fig. 3) (Supplementary Materials 1, 2, www.journalmc.org). Contractility was relatively preserved in the basal segments. The distribution of regional dysfunction raised the suspicion of TCM. Diagnosis was confirmed when no obstructive coronary lesions were seen on coronary angiography.
Figure 2.
ECG obtained immediately after ventricular fibrillation arrest showing complete heart block with an accelerated junctional rhythm with a heart rate of 67 beats/min and QTc of 407 ms. ECG: electrocardiogram.
Table 2. QTc and Heart Rate (HR) Trends in Real-Time From the Time of Admission.
| Hospital staya | Time | HR (beats/min) | QTc (ms) |
|---|---|---|---|
| Day 1 | 16:28 | 47 | 509 |
| 16:31 | 46 | 519 | |
| 17:00 | 41 | 537 | |
| 17:49 | 41 | 545 | |
| 17:50 | 41 | 536 | |
| 18:05 | 67 | 407 | |
| Day 2 | 16:20 | 85 | 466 |
| 16:21 | 67 | 405 | |
| 22:00 |
aPatient was admitted at 16:25. Notice the resolution of QTc interval prolongation following temporary venous pacing (TVP) with a programed lower rate limit (LRL) of 70 beats/min. QTC: corrected QT interval.
Figure 3.
Echocardiogram with (right image) and without (left image) contrast enhancement showing typical apical ballooning (blue arrow) with relatively spared basal segment and mid to apical segment akinesis.
Treatment
During her hospital stay, the patient started to encounter dyspnea with minimal exertion and bilateral lower limb extremity swelling. The clinical examination showed oxygen saturation of 90% on room air, regular pulse of 69 beats/min, and the jugular venous was elevated. The chest examination showed bilateral inspiratory crackles on auscultation. The lower extremity examination showed bilateral pitting edema. The chest X-ray was consistent with features of bilateral pulmonary edema. Laboratory workup revealed worsening serum BNP to 2,984 pg/mL. The patient was started on 3 L of oxygen via nasal cannula, IV diuretics, and goal-directed heart failure therapy. On day 3 of admission, she underwent an electrophysiologic (EP) study with the removal of TVP and insertion of a biventricular implantable cardioverter-defibrillator (BiV/ICD) to prevent further episodes of ventricular arrhythmia.
Postoperative course was uneventful, and the patient’s symptoms continued to improve, as well as her clinical examination. The heart rate was within the normal limit, and she was weaned from the supplemental oxygen. She was discharged home on day 10 of her hospital stay on oral torsemide, atorvastatin, aspirin, losartan, spironolactone, and oral magnesium supplements.
Follow-up and outcomes
Three months after the pacemaker placement, the patient had no new complaints. The ECG showed resolution of the QT prolongation and the persistence of high-grade AV block. The repeated TTE showed EF of 55-60% with a resolution of the regional wall motion abnormality as well as LV dysfunction.
Discussion
Various types of arrhythmias have been described in the acute phase of TCM, which constitute a significant determinant of patient outcome [11]. Arrhythmias associated with TCM include sustained VT (1.2%), non-sustained VT (1%), VF (2.2%), atrial fibrillation (4.7%) and sinus node dysfunction (1.3%) [9]. The International Takotsubo Registry has demonstrated that on admission ECG, the prevalence of QTc prolongation is higher in patients with TCM compared to acute coronary syndrome (ACS) [13]. The prevalence of QTc prolongation in TCM is variable, ranging from 26% to 51% [6]. It is postulated to result from the reduction in repolarization reserve and intra-myocyte calcium overload. Thus, it was proposed that this temporary QT prolongation might reflect the acute disturbances in cardiac autonomic function and the transient myocardial insult of TCM [14]. Abe et al described 17 patients, most of whom had a prolonged QTc interval in the acute and subacute phases of the condition. The QT interval normalized in all cases between 97 and 191 days from the onset of symptoms [15].
The prognostic significance of QT prolongation in the setting of TCM remains uncertain with conflicting data in the literature. In earlier studies, polymorphic VT is considered uncommon and occurs only in 1-1.5% of cases with acquired long QT due to TCM [9]. Hohneck et al retrospectively analyzed 105 patients with TCM and concluded that QT interval prolongation is a negative independent predictor of adverse outcome [16]. In a prospective cohort study, Imran et al have shown that TCM patients with a long QT interval, or developed acquired long QT syndrome, are more likely to be intubated, require vasopressor support, develop cardiogenic shock, ventricular arrhythmias, and death than those with a normal QT interval [7]. Santoro et al further described that TCM patients with prolonged QTc interval on admission had a higher risk of cardiovascular rehospitalization. In contrast, patients with a dynamic increase in QTc intervals after admission tend to have a better prognosis [17]. This indicates a difference in outcome between TCM-induced QT prolongation and patients with underlying long QT syndrome. This critical distinction could explain the significant heterogeneity in literature.
Severe repolarization abnormalities in TCM are expected to manifest within 24 - 48 h of presentation [6]. However, in our case, severe QTc prolongation was present on admission, thus indicating an underlying long QT syndrome that was unmasked by ventricular slowing in the setting of an AV nodal insult. This likely resulted in an R over T phenomenon, which precipitated the VF with sudden cardiac arrest. The occurrence of TCM amplifies QT interval prolongation in multiple similar literature [14, 18, 19]. Additionally, hypomagnesemia and the administration of ondansetron likely contributed to the QT prolongation. The negative genetic analysis indicated that our patient had acquired a sporadic long QT syndrome.
AV conduction defects in the TCM setting have been previously described, with a prevalence of about 2.9% [9]. The exact association between AV block and TCM is unclear, but the continuous ischemic status can explain it due to the microvascular dysfunction [2, 8]. On the other hand, previous AV block and its interventions, such as temporary or permanent pacemaker implantation, have been described as triggers of TCM [20, 21]. Myocardial abnormalities associated with AV nodal dysfunction, such as fibrosis and edema, are believed to create ventricular stimuli and contractile dysfunction leading to TCM [8, 22]. However, the definitive demonstration of a cause-and-effect relationship between myocardial edema and electrical abnormalities is still lacking [22]. Thus, it is difficult to know whether AV block is the cause or the result of TCM.
A handful of reports have described TCM patients presenting with third-degree AV blocks [12, 20, 23-25]. Most cases of complete AV block associated with TCM have been described in elderly individuals, who are more prone to have a degenerative conductive system disease. Among those reports requiring pacemaker placement, AV node dysfunction persisted long after the recovery of the LV function [26]. Nevertheless, conduction normalized for some patients over a long-term follow-up period [12, 25]. Therefore, the best method for managing high-grade AV block associated with TCM is difficult to discern at the time of diagnosis and remains uncertain due to the lack of robust literature. Stiermaier et al [10] assessed the treatment strategies for arrhythmias in a cohort of 286 patients with TCM. Patients who had complete AV block or ventricular asystole on presentation, and did not undergo pacemaker implantation, had a higher likelihood of sudden death. They recommended that the bradyarrhythmia in the acute setting of TCM may require permanent pacemaker implantation. In contrast, polymorphic ventricular arrhythmias may be managed with a temporary approach (e.g., wearable cardioverter defibrillators) until the recovery of repolarization time and LV function [10].
Because the specific degree of damage in the AV conduction system in TCM is unknown, along with recurrent VTs and cardiac arrest, we opted for temporary pacing followed by the implantation of a BiV-ICD. Due to the patient’s age and the presence of the third-degree AV block on the diagnosis of TCM, it is difficult to discern whether the TCM was the cause or result of the third-degree AV block itself. In our review of the literature, we were able to identify five reports similar to our case (Table 3) [8, 19, 24, 26, 27]. Cases occurred in middle-aged or elderly females. All patients exhibited the ominous triad of CHB, acquired long QT, and bradycardia-induced TdP, resulting in either near cardiac arrest or cardiac arrest within the first 48 h of admission. Including our case, it is unclear whether TCM is the cause or effect of the arrhythmia, considering the evolution of TCM echocardiographic findings both immediately before and after the discrete episodes of TdP. All cases demonstrated the persistence of an AV nodal conduction defect, despite improvements in the LV function, requiring permanent pacing and a single or dual-chamber implantable cardioverter-defibrillator ICD. The rapid progression to TdP and VF suggests that TCM patients with a high-grade AV block, along with a QT prolongation of > 550 ms at the time of presentation, should receive a temporary pacemaker without delay.
Table 3. Similar Case of Takotsubo Cardiomyopathy With High Degree AV Block, QT Prolongation, and TdP Resulting in Near Arrest (NA) or Cardiac Arrest (CA) Within 24 - 48 h of Hospital Admission.
| Article | Age/sex | Past medical history | ECG rhythm on admission | Duration of QT interval on admission ECG | TTE findings | Timing of NA or CA | Rhythm causing NA or CA | Temporality of TTE findings with TdP and/or CA | Timing of PPP |
|---|---|---|---|---|---|---|---|---|---|
| Ahn et al, 2011 [27] | 78/F | HTN | Third-degree HB with EJR of 35 b/min, combined with TWI precordial leads | 580 ms | Akinesia of mid and apical LV walls with the systolic ballooning of the ventricular apex; LVEF: 35% | 24 - 48 h | Third-degree HB with EJR of 20 b/min QTc 720 ms followed by TdP (treated by CV and TVP) | Before | Day 21 |
| de Santana et al, 2021 [24] | 56/F | HTN, grade 2 obesity | Second degree AVB 2:1 with EJR of 50 b/min, RBBB, LAFB, biphasic and inverted T waves in precordial and limb leads | 689 ms | Basal hypercontractility and midventricular and apical ballooning. LVEF: 40% | 24 h | VF (treated by CV and TVP) | Before | Day 14 |
| Inayat et al, 2017 [8] | 59/F | None | Third-degree AVB with EJ VR 48 b/min, multiple PVCs, prolonged, and deep TWI in limb leads | 643 ms | Basal hyperkinesia and apical hypokinesis with LVEF of 35-40% | 24 - 48 h | TdP (treated medically and TVP) | Before | Day 6 |
| Kurisu et al, 2008 [26] | 87/F | Syncope | RBBB during 2:1 AVB with a VR of 42 b/min, TWI in leads I, II, aVL, aVF and V1 to V6 | 880 ms | Akinesia of the distal portion of the LV chamber, LVEF 62% | Immediately | TdP (treated medically and TVP) | After | Day 10 |
| Kurisu et al, 2008 [26] | 78/F | Not reported | RBBB during complete AVB with a VR of 40 b/min, ST segment elevation in leads V2 to V6, T wave inversion in leads I, II, III, aVF and V1 to V6 | 920 ms | Akinesia of the mid to distal portion of the LV chamber, LVEF 38% | Immediately | TdP (treated medically and TVP) | After | Day 7 |
ECG: electrocardiogram; VF: ventricular fibrillation; CV: cardioversion; EJR: escape junctional rhythm; HB: heart block; RBBB: right bundle branch block; AVB: atrioventricular block; TWI: T wave inversion; VR: ventricular rate; PVC: premature ventricular contractions; TdP: torsade de pointes; TTE: transthoracic echocardiogram; b/min: beats/min; HTN: hypertension; TVP: temporary venous pacing; PPP: permeant pacemaker placement; LAFB: left anterior fascicular block; LVEF: left ventricular ejection fraction.
Conclusions
TCM could trigger QT prolongation in certain cases. Patients with an underlying long QT syndrome represent a particular subset of patients predisposed to TdP early during TCM and are at a higher risk of adverse outcomes. It is crucial to monitor the QTc in patients with TCM, correct electrolyte abnormalities, and minimize QT-prolonging medications. The recognition of AV conduction defects in patients with TCM is critical, especially if associated with a significant QT prolongation. Such situations are underrecognized but are potentially fatal, necessitating close monitoring and timely intervention.
Learning points
TCM could precipitate or worsen underlying AV conduction defects.
Bradycardia-induced QT interval prolongation is amplified by the occurrence of TCM, a combination that potentially fatal and carries a significant risk for TdP.
The recognition of AV conduction defects and long QT interval in patients with TCM is critical. Close monitoring and timely interventions are key in preventing catastrophic outcomes.
CHB associated with TCM may require permanent pacing.
Supplementary Material
Echocardiogram showing severely reduced left ventricular function and relatively spared basal segment and mid to apical segments akinesis (apical ballooning).
Contrast-enhanced echocardiogram showing severely reduced left ventricular function and relatively spared basal segment and mid to apical segments akinesis (apical ballooning).
Acknowledgments
We would like to thank the medical staff of the Cardiology Unit at Detroit Medical Center/Heart Hospital who were involved in the care of this patient.
Financial Disclosure
None of the authors have any source of funding to declare.
Conflict of Interest
None of the authors have any conflict of interest to declare.
Informed Consent
Verbal informed consent was obtained from the patient for the anonymized information to be published in this article.
Author Contributions
AA and AY contributed to the conceptualizing and writing the first manuscript. II, ES, and LA have performed the critical review and editing of the final draft. Rest of authors were involved in the clinical management of the reported patients. All authors agreed to the final draft submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1.Merchant EE, Johnson SW, Nguyen P, Kang C, Mallon WK. Takotsubo cardiomyopathy: a case series and review of the literature. West J Emerg Med. 2008;9(2):104–111. [PMC free article] [PubMed] [Google Scholar]
- 2.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of takotsubo syndrome. Circulation. 2017;135(24):2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 3.Moller C, Eitel C, Thiele H, Eitel I, Stiermaier T. Ventricular arrhythmias in patients with Takotsubo syndrome. J Arrhythm. 2018;34(4):369–375. doi: 10.1002/joa3.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50(5):448–452. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Gili S, Cammann VL, Schlossbauer SA, Kato K, D'Ascenzo F, Di Vece D, Jurisic S. et al. Cardiac arrest in takotsubo syndrome: results from the InterTAK Registry. Eur Heart J. 2019;40(26):2142–2151. doi: 10.1093/eurheartj/ehz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takasaki A, Nakamori S, Dohi K. Massive ST-segment elevation and QTc prolongation in the emergency department. Circulation. 2019;140(5):436–439. doi: 10.1161/CIRCULATIONAHA.119.041736. [DOI] [PubMed] [Google Scholar]
- 7.Imran TF, Rahman I, Dikdan S, Shah R, Niazi OT, Thirunahari N, Alhaj E. et al. QT prolongation and clinical outcomes in patients with takotsubo cardiomyopathy. Pacing Clin Electrophysiol. 2016;39(6):607–611. doi: 10.1111/pace.12864. [DOI] [PubMed] [Google Scholar]
- 8.Inayat F, Virk HUH, Ullah W, Riaz I. Takotsubo cardiomyopathy-related complete heart block and torsades de pointes. BMJ Case Rep. 2017;2017:bcr-2016-218017. doi: 10.1136/bcr-2016-218017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syed FF, Asirvatham SJ, Francis J. Arrhythmia occurrence with takotsubo cardiomyopathy: a literature review. Europace. 2011;13(6):780–788. doi: 10.1093/europace/euq435. [DOI] [PubMed] [Google Scholar]
- 10.Stiermaier T, Rommel KP, Eitel C, Moller C, Graf T, Desch S, Thiele H. et al. Management of arrhythmias in patients with Takotsubo cardiomyopathy: Is the implantation of permanent devices necessary? Heart Rhythm. 2016;13(10):1979–1986. doi: 10.1016/j.hrthm.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Napp LC, Bauersachs J. Takotsubo syndrome: between evidence, myths, and misunderstandings. Herz. 2020;45(3):252–266. doi: 10.1007/s00059-020-04906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanmugasundaram R, Tamilarasu K, Rajendiran G, Murali A. An uncommon presentation of a rare disease - high-degree AV block with takotsubo cardiomyopathy. Indian Heart J. 2012;64(5):511–514. doi: 10.1016/j.ihj.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL. et al. Clinical features and outcomes of takotsubo (Stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 14.Behr ER, Mahida S. Takotsubo cardiomyopathy and the long-QT syndrome: an insult to repolarization reserve. Europace. 2009;11(6):697–700. doi: 10.1093/europace/eup081. [DOI] [PubMed] [Google Scholar]
- 15.Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol. 2003;41(5):737–742. doi: 10.1016/S0735-1097(02)02925-X. [DOI] [PubMed] [Google Scholar]
- 16.Hohneck A, El-Battrawy I, Lang S, Ansari U, Schramm K, Zhou X, Borggrefe M. et al. Protective effect of acquired long QT syndrome in Takotsubo syndrome. Intern Med J. 2019;49(6):770–776. doi: 10.1111/imj.14169. [DOI] [PubMed] [Google Scholar]
- 17.Santoro F, Brunetti ND, Tarantino N, Romero J, Guastafierro F, Ferraretti A, Di Martino LFM. et al. Dynamic changes of QTc interval and prognostic significance in takotsubo (stress) cardiomyopathy. Clin Cardiol. 2017;40(11):1116–1122. doi: 10.1002/clc.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez Retamales V, Lara Garcia OE, Ranjha S, Koester C, Labedi MR. Takotsubo cardiomyopathy and QTc Prolongation with subsequent improvement of QTc interval and resolution of apical ballooning: a case report. Cureus. 2020;12(7):e9143. doi: 10.7759/cureus.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekh A, Sengupta V, Zainea M. An unusual case report of stress-induced cardiomyopathy presenting as ventricular fibrillation cardiopulmonary arrest and third-degree atrioventricular block. Eur Heart J Case Rep. 2021;5(5):ytab142. doi: 10.1093/ehjcr/ytab142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathore A, Banavalikar B, Shenthar J, Acharya D, Parvez J, Setty Srinivasa KH. An unusual case of complete atrioventricular block causing Takotsubo syndrome. Indian Pacing Electrophysiol J. 2018;18(3):123–125. doi: 10.1016/j.ipej.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunetti ND, Ieva R, Correale M, De Gennaro L, Pellegrino PL, Dioguardi E, D'Arienzo G. et al. Combined exogenous and endogenous catecholamine release associated with Tako-Tsubo like syndrome in a patient with atrio-ventricular block undergoing pace-maker implantation. Acute Card Care. 2011;13(2):112–114. doi: 10.3109/17482941.2011.553236. [DOI] [PubMed] [Google Scholar]
- 22.Migliore F, Zorzi A, Perazzolo Marra M, Iliceto S, Corrado D. Myocardial edema as a substrate of electrocardiographic abnormalities and life-threatening arrhythmias in reversible ventricular dysfunction of takotsubo cardiomyopathy: Imaging evidence, presumed mechanisms, and implications for therapy. Heart Rhythm. 2015;12(8):1867–1877. doi: 10.1016/j.hrthm.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Terui T, Iwai-Takano M, Watanabe T. Permanent pacemaker implantation in a patient with takotsubo cardiomyopathy and complete atrioventricular block. Case Rep Cardiol. 2021;2021:6637720. doi: 10.1155/2021/6637720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Santana PH, Pedreira FA, Soares PR, Scudeler TL. Takotsubo cardiomyopathy associated with high-grade atrioventricular block and ventricular fibrillation: a case report. Int Med Case Rep J. 2021;14:523–527. doi: 10.2147/IMCRJ.S317445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nef HM, Mollmann H, Sperzel J, Weber M, Bruck H, Hamm CW, Elsasser A. Temporary third-degree atrioventricular block in a case of apical ballooning syndrome. Int J Cardiol. 2006;113(2):e33–35. doi: 10.1016/j.ijcard.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakama Y, Ohkawa K. et al. Torsade de pointes associated with bradycardia and takotsubo cardiomyopathy. Can J Cardiol. 2008;24(8):640–642. doi: 10.1016/S0828-282X(08)70653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn JH, Park SH, Shin WY, Lee SW, Lee SJ, Jin DK, Lee HM. et al. Long QT syndrome and torsade de pointes associated with Takotsubo cardiomyopathy. J Korean Med Sci. 2011;26(7):959–961. doi: 10.3346/jkms.2011.26.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiogram showing severely reduced left ventricular function and relatively spared basal segment and mid to apical segments akinesis (apical ballooning).
Contrast-enhanced echocardiogram showing severely reduced left ventricular function and relatively spared basal segment and mid to apical segments akinesis (apical ballooning).
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.