Abstract
(1) Background: Ischemic stroke is one of the leading causes of death and disability. An inflammatory response is observed in multiple stages of cerebral ischemia, particularly in the acute phase. Recent publications revealed that the neutrophil–lymphocyte ratio (NLR) and lymphocyte–monocyte ratio (LMR) may be used to predict long-term prognosis in acute ischemic stroke (AIS) after thrombolysis. To test whether there is a relationship between the combination of these parameters and long-term prognosis, we analyzed the NLR–LMR combination in AIS patients treated with intravenous recombinant tissue plasminogen activator (rtPA); (2) Methods: The study included 285 adults with a diagnosis of AIS and rtPA treatment within a 4.5 h time window. Blood samples were obtained at admission and 24 h after thrombolysis to calculate pre- and post-thrombolysis NLR and LMR. Clinical data, including NIHSS was registered on admission and day 1. The long-term outcome was defined 90 days post-event by the modified Rankin Scale (mRS). Therapy-associated intracranial hemorrhage (ICH) was classified according to ECASS II. Receiver operating characteristic curve (ROC) analysis was performed to determine optimal cutoffs of NLR and LMR as predictors of therapy outcomes; (3) Results: Patients were stratified by cutoffs of 5.73 for NLR and 2.08 for LMR. The multivariate logistic regression model, including all possible confounders, displayed no significant association between NLR or LMR with 3-months functional prognosis. The combination of high NLR–low LMR vs. low NRL–high LMR as obtained 24 h after thrombolysis was found to be an independent predictor of poor 3-months functional outcome (mRS ≥ 2; OR 3.407, 95% CI 1.449 to 8.011, p = 0.005). The proportion of patients between low NLR–high LMR and high NLR–low LMR groups from admission to day 1 showed no significant change in the good outcome group. On the other hand, in the poor outcome group (mRS ≥ 2), low NLR–high LMR and high NLR–low LMR groups displayed a significant shift in patient proportions from 67% and 21% at admission (p = 0.001) to 36% and 49% at 24 h after thrombolysis (p < 0.001), respectively; (4) Conclusions: Our study demonstrated for the first time that a high NLR–low LMR combination as observed at 24 h after thrombolysis can serve as an independent predictor of 3-months poor outcome in AIS patients. This simple and readily available data may help clinicians to improve the prognostic estimation of patients and may provide guidance in selecting patients for personalized and intensified care post-thrombolysis.
Keywords: neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, ischemic stroke, thrombolysis
1. Introduction
Despite recent advances in the treatment of acute ischemic stroke (AIS), the disease puts a heavy burden on individuals, families, and the health care system. Stroke is ranked the second most common cause of death and the third leading cause of death and disability in the world. Globally, in 2019, there were 6.5 million deaths from stroke and 143 million disability-adjusted life-years due to stroke [1]. Although mechanical thrombectomy has revolutionized stroke care in the past decade [2], intravenous (i.v.) thrombolysis with recombinant tissue plasminogen activator (rt-PA) remains the most commonly used pharmacological therapy for AIS. Recent data has shown that treatment within the first hour of symptom onset produces significantly improved rates of morbidity and mortality [3]. This remarkable 60 min period from the onset of symptoms is often referred to as The Golden Hour. rt-TPA is the current gold standard for the treatment of AIS [4]. With the extended time-window of 4.5 h [5], approximately half of the patients receiving thrombolysis attain total or nearly total neurological recovery at 3 months [6]. On the other hand, a large proportion of patients will not benefit from thrombolysis, moreover, 6–8% of treated patients will develop potentially fatal intracerebral hemorrhage. The identification of patients in whom thrombolysis will not be effective or would cause potential side-effects could be a key approach to personalize acute stroke care, improve long-term quality of life, and reduce the global burden of AIS. To achieve this, a rapid, cheap, simple, easily accessible, and reliable prognostic marker of thrombolysis outcome is needed. Neutrophil–lymphocyte ratio (NLR) and lymphocyte–monocyte ratio (LMR) have been shown to be associated with various pathological conditions [7,8,9]. NLR and LMR are potential novel biomarkers of inflammation and immune response [10]. They simply drive from complete blood count, and, in this way, they are rapidly and readily available markers for clinical use before administering the thrombolytic agent. Recently, traces of evidence indicated a causal link between the prognosis of AIS, NLR, and LMR levels [11,12]. Here, we analyzed the potential relationship between NLR and LMR levels and the 3-months outcome of AIS after intravenous rtPA administration.
2. Methods
2.1. Study Population
Enrolled in this study were consecutive AIS patients admitted to the Department of Neurology, the University of Debrecen, Hungary, between September 2016 and April 2018. Inclusion and exclusion criteria of patients were identical to the standard criteria of intravenous rtPA administration according to the 2008 ESO guideline [13]. The diagnosis of ischemic stroke was confirmed by using non-contrast computerized tomography (NCCT) scan and computed tomography angiography (CTA). Clinical data, including the National Institutes of Health Stroke Scale (NIHSS), was registered on admission and day 1. Thrombolysis was performed within the 4.5 h time window from the onset of symptoms using intravenous rtPA according to standard protocols [13]. Patients receiving mechanical thrombectomy in addition to thrombolysis were not included in the study. A control NCCT was performed on day 1 and early ischemic changes based on admission NCCT and day 1 NCCT were calculated using the Alberta Stroke Program Early CT Score (ASPECTS) as assessed by four independent radiologists [14]. Stroke etiology was defined by the Trial of ORG 10,172 according to the Acute Stroke Treatment (TOAST) criteria [15]. The presence of intracerebral hemorrhage (ICH) was tested on day 1 using NCCT, and patients with hemorrhage were divided into two groups: symptomatic (SICH) or asymptomatic (aSICH), based on the European Cooperative Acute Stroke Study (ECASS) II criteria [5]. A short-term outcome evaluation was performed on day 1 after thrombolysis. The favorable short-term outcome was defined as a decrease in the NIHSS score by at least 4 points or to 0, while a poor short-term outcome was defined as an increase in NIHSS score by at least 4 points [16]. The modified Rankin Scale (mRS) was registered to define long-term outcome at 90 days. Unfavorable outcome was defined as mRS greater than 1 (mRS ≥ 2).
The study was approved by the Ethics Committee of the University of Debrecen, Hungary, and the Ethics Board of the Medical Research Council of the Hungarian Ministry of Human Capacities, Hungary. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. All patients or their relatives provided written informed consent.
2.2. Blood Sampling and Laboratory Measurements
Peripheral blood samples were drawn before the initiation of rt-PA infusion and 24 h after thrombolysis. From the blood samples taken on admission, routine laboratory examinations were performed (ions, glucose levels, renal and liver function tests, high-sensitivity C-reactive protein (hsCRP)) by standard methods (Roche Diagnostics, Mannheim, Germany).
Complete blood counts were assessed from blood samples obtained before and 24 h after thrombolysis using an automated analyzer (XE 2100, Sysmex Europe GmbH, Hamburg, Germany). Hematological parameters were determined immediately after blood collection. The absolute neutrophil-to-lymphocyte count (NLR), and the absolute lymphocyte-to-monocyte count (LMR) were calculated from blood samples obtained on both occasions.
2.3. Statistical Methods
Continuous variables were expressed either as mean ± SD or median and interquartile range (IQR) as appropriate. Categorical data were expressed as numbers and percentages. Multiple groups of continuous data were compared using one-way analysis of variance (ANOVA) using the Bonferroni post-hoc test or Kruskal–Wallis analysis with the Dunn–Bonferroni post-hoc test. Categorical data were compared using the χ2 of Fisher’s exact tests where appropriate. Receiver operating characteristic (ROC) curves were built by plotting sensitivity vs. 1-specificity and calculating the area under the curve (AUC). Optimal threshold values were calculated based on Youden’s J statistics. Multivariable logistic regression models were used to test the independent effect of NLR, LMR, their combination, or each leukocyte sub-type count on outcome measures before and after adjustment for major baseline characteristics. A p-value less than 0.05 was considered significant. Statistical analyses were performed using SPSS 18.0 (Chicago, IL, USA) and MedCalc 14.8.1 software (Mariakerke, Belgium).
3. Results
3.1. Baseline Characteristics of Enrolled Patients
During the study period, a total of 285 consecutive AIS patients undergoing i.v. thrombolysis treatment were enrolled in the study. Baseline characteristics of included patients are listed in Table 1. The mean age of the patients was 66 ± 12.9 years, with 44.2% being female. The median baseline NIHSS score was 6 (IQR (5, 9.1)) and median 90-day mRS was 1.0. Patients with poor outcome (mRS ≥ 2) at 90 days after stroke were significantly older, had higher blood pressure and atrial fibrillation more frequently, and more severe neurological deficit on admission as compared to those with a good outcome. Moreover, patients with poor outcome showed significantly higher NLR and significantly lower LMR as compared to those with good outcome (Table 1).
Table 1.
Baseline characteristics of enrolled patients according to long term outcomes (modified Rankin Scale at 90 days post event).
| All Patients n = 285 |
Good Outcome (mRS = 0–1) n = 190 |
Poor Outcome (mRS = 2–6) n = 95 |
p Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 66 ± 12.9 | 62.8 ± 12.9 | 72.0 ± 10.2 | <0.001 |
| Gender, male (%) | 159 (55.8) | 107 (56.3) | 52 (54.7) | 0.802 |
| BMI (kg/m2) | 28.5 ± 5.9 | 28.5 ± 5.6 | 28.4 ± 6.5 | 0.900 |
| Baseline laboratory results | ||||
| hsCRP (g/L) | 2.8 (1.4–6.0) | 2.5 (1.3–5.2) | 3.5 (1.7–7.7) | 0.060 |
| White blood cell count (G/L) | 8.1 (6.5–9.9) | 8.04 (6.45–9.59) | 8.15 (6.48–10.33) | 0.455 |
| Neutrophil count (G/L) | 5.2 (4.0–7.1) | 5.12 (3.99–6.86) | 5.62 (4.17–7.55) | 0.157 |
| Lymphocyte count (G/L) | 1.7 (1.2–2.3) | 1.77 (1.31–2.3) | 1.61 (1.15–2.24) | 0.053 |
| Monocyte count (G/L) | 0.56 (0.44–0.69) | 0.54 (0.43–0.69) | 0.58 (0.47–0.71) | 0.164 |
| NLR | 2.9 (1.94–4.82) | 2.72 (1.86–4.66) | 3.18 (2.17–5.94) | 0.036 |
| LMR | 3.22 (2.42–4.29) | 3.41 (2.51–4.55) | 2.97 (1.87–3.92) | 0.005 |
| Vascular risk factors, n (%) | ||||
| Smoking | ||||
| Non-smoker | 204 (71.6) | 131 (68.8) | 73 (76.8) | 0.152 |
| Previous smoker | 2 (0.7) | 2 (1.1) | 0 | |
| Current smoker | 79 (27.7) | 57 (30.2) | 22 (23.2) | |
| Previous stroke/TIA | 67 (23.5) | 38 (20) | 29 (30.5) | 0.055 |
| Atrial fibrillation | 29 (10.2) | 14 (7.4) | 15 (15.8) | 0.026 |
| PAD | 9 (3.2) | 6 (3.2) | 3 (3.2) | 1.000 |
| Hyperlipidemia | 181 (63.5) | 123 (64.7) | 58 (61.0) | 0.602 |
| DM | 71 (24.9) | 41 (21.6) | 30 (31.6) | 0.081 |
| Hypertension | 246 (86.3) | 158 (83.2) | 88 (92.6) | 0.029 |
| Therapy at stroke onset, n (%) | ||||
| ACE inhibitor | 148 (51.9) | 92 (48.4) | 56 (58.9) | 0.103 |
| Diuretic | 118 (41.4) | 71 (37.4) | 48 (50.5) | 0.056 |
| Beta blocker | 97 (34) | 62 (32.6) | 35 (36.8) | 0.509 |
| Calcium channel blocker | 69 (24.2) | 46 (24.2) | 23 (24.2) | 1.000 |
| Alfa blocker | 23 (8.1) | 14 (7.4) | 9 (9.5) | 0.645 |
| Hypertension therapy | 189 (66.3) | 121 (63.7) | 68 (71.6) | 0.231 |
| Acetylsalicylic acid | 86 (30.2) | 52 (27.4) | 34 (35.8) | 0.171 |
| Clopidogrel | 23 (8.1) | 16 (8.4) | 7 (7.4) | 0.822 |
| Anticoagulant therapy, n (%) | ||||
| Vitamin K antagonist | 9 (3.2) | 5 (2.6) | 4 (4.2) | |
| Direct thrombin inhibitor | 1 (0.4) | 1 (0.5) | 0 | |
| Direct factor Xa inhibitor | 0 | 0 | 0 | |
| Low molecular weight heparin | 3 (1.1) | 2 (1.1) | 1 (1.1) | |
| Lipid lowering therapy, n (%) | 78 (25) | 44 (23.3) | 27 (28.4) | 0.384 |
| Anti-diabetic therapy, n (%) | 52 (17) | 27 (14.2) | 21 (22.1) | 0.094 |
| Stroke severity, n (%) | ||||
| NIHSS at day 1 | ||||
| 0–5 | 110 (38.7) | 93 (48.9) | 17 (18.1) | <0.001 |
| 6–10 | 98 (34.5) | 65 (34.2) | 33 (35.1) | |
| 11–15 | 50 (17.6) | 24 (12.6) | 26 (27.7) | |
| >15 | 26 (9.2) | 8 (4.2) | 18 (19.1) | |
| NIHSS at day 7 | ||||
| 0–5 | 129 (46.9) | 109 (57.7) | 20 (23.3) | <0.001 |
| 6–10 | 113 (41.1) | 77 (40.7) | 36 (41.9) | |
| 11–15 | 24 (8.7) | 3 (1.6) | 21 (24.4) | |
| >15 | 9 (3.3) | 0 | 9 (10.5) | |
| Hemorrhagic transformation, n (%) | ||||
| aSICH | 13 (4.6) | 4 (2.1) | 9 (9.5) | 0.110 |
| SICH | 7 (2.5) | 0 | 7 (7.4) | |
| Stroke localization, n (%) | ||||
| ICA | 193 (67.7) | 112 (58.9) | 81 (85.3) | <0.001 |
| VB | 92 (32.3) | 78 (41.1) | 14 (14.7) | |
| Stroke etiology (TOAST), n (%) | ||||
| Large-artery atherosclerosis | 62 (21.8) | 55 (28.9) | 7 (7.4) | <0.001 |
| Small-vessel occlusion | 103 (36.1) | 59 (31.1) | 44 (46.3) | |
| Cardioembolic | 23 (8.1) | 17 (8.9) | 6 (6.3) | |
| Other/undetermined | 97 (34) | 59 (31.1) | 38 (40) |
3.2. White Blood Cell Counts, NLR and LMR during Thrombolysis
In the total cohort, the median neutrophil count, monocyte count, and NLR increased, whereas median LMR decreased 24 h after thrombolysis when compared with admission results (Table 2). An inverse but modest correlation was found between neutrophil count and lymphocyte count at admission (r = −0.166, p = 0.002) and at day 1 (r = −0.200, p = 0.001). Significant but modest positive correlation was found between lymphocyte count and monocyte count at admission (r = 0.261, p < 0.001) but not at day 1. Neutrophil count also correlated with monocyte count at admission (r = 0.381, p < 0.001) and at day 1 (r = 0.598, p < 0.001).
Table 2.
Leukocyte counts and ratios in acute ischemic stroke patients before and 24 h after thrombolysis.
| Before Thrombolysis | 24 h after Thrombolysis | p Value | |
|---|---|---|---|
| Neutrophil (G/L) | 5.24 (4.04–7.14) | 6.26 (4.7–8.3) | <0.001 |
| Lymphocyte (G/L) | 1.74 (1.25–2.3) | 1.69 (1.28–2.15) | 0.061 |
| Monocyte (G/L) | 0.56 (0.44–0.69) | 0.66 (0.53–0.83) | <0.001 |
| NLR | 2.9 (1.94–4.82) | 3.58 (2.48–5.6) | <0.001 |
| LMR | 3.22 (2.42–4.29) | 2.58 (1.74–3.56) | <0.001 |
NLR: neutrophil–lymphocyte ratio. LMR: lymphocyte–monocyte ratio. Statistics: Wilcoxon Signed Rank test.
Results are depicted as mean ± SD or median (interquartile range). ACE: angiotensin converting enzyme. ASPECTS: Alberta Stroke Program Early CT Score. BA: basilar artery. BMI: body mass index. DM: diabetes mellitus. hsCRP: high sensitivity C reactive protein measurement. ICA: internal carotid artery. LMR: lymphocyte–monocyte ratio. mRS: modified Rankin Scale. NIHSS: National Institutes of Health Stroke Scale. NLR: neutrophil–lymphocyte ratio. PAD: peripheral artery disease. SICH: symptomatic intracerebral hemorrhage. aSICH: asymptomatic intracerebral hemorrhage. TIA: transient ischemic attack. TOAST: Trial of ORG 10,172 in Acute Stroke Treatment. WBC: white blood cell count.
None of the leukocyte indices showed association with stroke etiology, stroke severity at admission, or with hemorrhagic transformation at admission (Table 3 and Supplementary Table S1). On the other hand, neutrophil count, monocyte count, and NLR significantly increased, while lymphocyte count and LMR significantly decreased 24 h post-rtPA in patients with more severe stroke. A similar pattern was seen in those who suffered therapy-associated intracerebral hemorrhage (Table 3).
Table 3.
Leukocyte counts and ratios at admission and 24 h after thrombolysis according to stroke severity at admission and thrombolysis safety.
| Time of Blood Sampling | Neutrophil (G/L) | Lymphocyte (G/L) | Monocyte (G/L) | NLR | LMR | |
|---|---|---|---|---|---|---|
| Hemorrhagic transformation according to ECASS II | At admission | |||||
| No hemorrhage (n = 264) | 5.2 (4.1–7.1) | 1.7 (1.2–2.3) | 0.56 (0.44–0.70) | 2.88 (1.93–4.82) | 3.22 (2.42–4.30) | |
| aSICH (n = 13) | 5.3 (3.8–7.2) | 1.7 (1.3–1.9) | 0.45 (0.39–0.58) | 3.07 (2.32–6.50) | 3.82 (2.68–5.10) | |
| SICH (n = 7) | 6.2 (3.6–8.0) | 1.8 (1.4–2.2) | 0.60 (0.53–0.68) | 3.41 (1.96–4.54) | 2.97 (2.56–3.91) | |
| p value | 0.987 | 0.688 | 0.152 | 0.805 | 0.551 | |
| 24 h after thrombolysis | ||||||
| No hemorrhage (n = 264) | 6.1 (4.6–8.2) | 1.7 (1.3–2.2) | 0.66 (0.52–0.83) | 3.44 (2.45–5.20) | 2.63 (1.75–3.59) | |
| aSICH (n = 13) | 8.2 (6.6–9.1) | 1.3 (1.1–1.9) | 0.69 (0.62–0.87) | 5.63 (3.31–8.58) | 2.07 (1.2–2.59) | |
| SICH (n = 7) | 9.7 (7.3–15.4) | 1.3 (0.8–2.2) | 0.91 (0.80–1.17) | 7.12 (4.15–19.7) | 1.51 (0.8–2.04) | |
| p value | 0.002 | 0.091 | 0.030 | 0.002 | 0.005 | |
| Stroke severity | At admission | |||||
| NIHSS 0–5 (n = 110) | 5.1 (4.0–7.0) | 1.8 (1.4–2.4) | 0.57 (0.44–0.71) | 2.75 (1.81–3.98) | 3.45 (2.51–4.51) | |
| NIHSS 6–10 (n = 97) | 5.5 (4.4–6.8) | 1.7 (1.2–2.3) | 0.57 (0.45–0.70) | 2.78 (2.00–4.95) | 3.01 (2.33–4.34) | |
| NIHSS 11–16 (n = 50) | 5.1 (4.0–7.5) | 1.6 (1.2–2.1) | 0.53 (0.42–0.66) | 2.99 (2.08–6.56) | 3.11 (2.41–4.13) | |
| NIHSS > 16 (n = 25) | 5.3 (3.6–6.9) | 1.6 (1.1–1.9) | 0.55 (0.44–0.63) | 3.27 (2.10–5.73) | 3.04 (2.36–4.06) | |
| p value | 0.782 | 0.067 | 0.581 | 0.330 | 0.441 | |
| 24 h after thrombolysis | ||||||
| NIHSS 0–5 (n = 110) | 5.4 (4.3–7.5) | 1.8 (1.4–2.4) | 0.61 (0.49–0.79) | 3.08 (2.10–4.47) | 2.95 (2.27–3.92) | |
| NIHSS 6–10 (n = 97) | 6.4 (4.7–8.0) | 1.7 (1.4–2.2) | 0.66 (0.56–0.82) | 3.30 (2.48–5.17) | 2.54 (1.85–3.59) | |
| NIHSS 11–16 (n = 50) | 7.7 (5.0–9.7) | 1.4 (1.2–2.0) | 0.67 (0.56–0.85) | 4.66 (3.04–6.85) | 2.26 (1.67–2.87) | |
| NIHSS > 16 (n = 25) | 9.7 (7.2–13.4) | 1.2 (0.9–1.7) | 0.83 (0.68–1.08) | 8.4 (4.05–12.98) | 1.34 (1.04–1.87) | |
| p value | <0.001 | <0.001 | 0.004 | <0.001 | <0.001 | |
Data depicted as median (inter-quartile range). NLR: neutrophil–lymphocyte ratio. LMR: lymphocyte–monocyte ratio. aSICH: asymptomatic intracerebral hemorrhage. SICH: symptomatic intracerebral hemorrhage. ECASS II: European Co-operative Acute Stroke Study-II. NIHSS: National Institutes of Health Stroke Scale. Statistics: Kruskal–Wallis.
Neutrophil count and NLR were significantly higher in the ASPECTS 10-8 group compared with the ASPECTS ≤7 group at day 1 (Supplementary Table S2). Univariate logistic regression proved a significant protective effect of higher LMR at admission with functional dependence at 3 months post-event (OR = 0.755, 95%CI (0.631, 0.903), p = 0.002) (Supplementary Table S3). Similar analysis showed no association between NLR and long-term functional outcome. In addition to LMR, age, hypertension, atrial fibrillation, and stroke characteristics (NIHSS, hemorrhagic transformation, and stroke localization) showed association with long-term outcome of therapy, but in the multivariate model only age, NIHSS, hemorrhagic transformation, and stroke localization remained as significant variables (Supplementary Table S3). Although in the univariate model, NLR and LMR, measured at 24 h after rtPA therapy, showed a highly significant association with functional outcomes at 3 months post-event, a multivariate logistic regression model, including all possible confounders, displayed no significant association of these parameters with 3-months functional outcome (Supplementary Table S4).
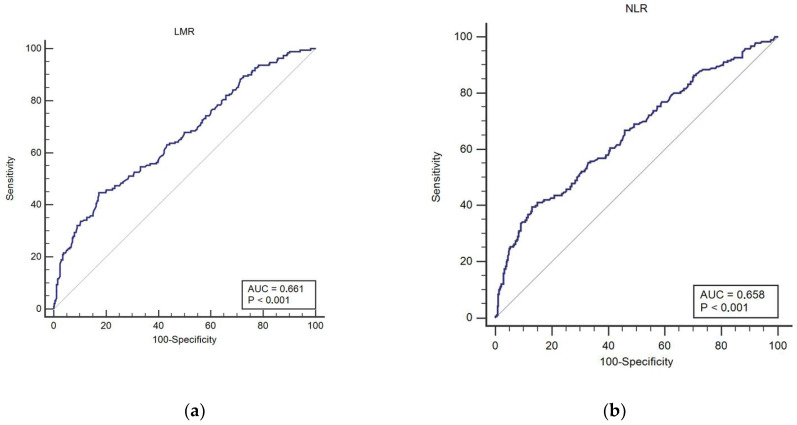
At baseline, the median values of NLR and LMR of the study population were 2.9 (inter-quartile range, IQR (1.94, 4.82) and 3.22 (IQR (2.42, 4.29)), respectively. The optimal threshold values for the prediction of poor functional outcome at 3 months post-event based on the best Youden index by ROC analysis were 5.73 for NLR and 2.08 for LMR (Figure 1). According to the optimal cutoff values of NLR and LMR at admission, patients were classified into four groups: low NLR–high LMR, high NLR–high LMR, low NLR–low LMR, and high NLR–low LMR.
Figure 1.
Receiver operating characteristics (ROC) curve analysis of admission neutrophil-to-lymphocyte ratio (NLR) (a) and lymphocyte-to-monocyte ratio (LMR) (b) values predicting functional dependence (mRS2) at 3 months post-event.
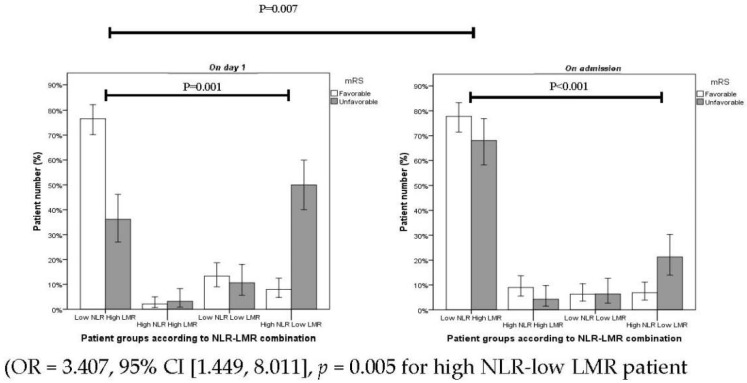
Out of 190 patients with favorable outcome, 77% of patients fell into the category of low NLR–high LMR combinations, while the high NLR–low LMR group contained only 6.8% of patients at admission. The proportion of patients with favorable outcome as stratified according to low NLR–high LMR and high NLR–low LMR were 76% and 7.8% at day 1, respectively. Out of 95 patients in the poor outcome group, 67% of patients were stratified as low NLR–High LMR, while only 21% were stratified as high NLR–low LMR before the administration of thrombolysis (p = 0.001) (Figure 2). At 24 h after thrombolysis, the proportion of patients with poor outcome displayed a significant shift in the above groups as 36% of patients could be stratified as having low NLR–high LMR while the high NLR–low LMR group included 49% (p < 0.001) of patients (Figure 2).
Figure 2.
Proportion of patients in different NLR–LMR combination groups on admission and on day 1.
The combination of NRL and LMR as determined at 24 h after thrombolysis was found to be an independent predictor of poor functional outcome at 3 months post-event (OR = 3.407, 95% CI [1.449, 8.011], p = 0.005 for high NL–low LMR patients.
Group vs. low NRL–high LMR patient group) after controlling for all potential confounders (Table 4).
Table 4.
Association of NLR–LMR combinations at admission and 24 h after thrombolysis with poor functional outcome (mRS 2) at 3 months post-event.
| Characteristics | Univariate Analysis, Crude OR (95% CI) | p Value | Multivariate Analysis, Adjusted OR (95% CI) a | p Value |
|---|---|---|---|---|
| At admission | ||||
| Low NLR–High LMR (n = 211) | Ref | - | Ref | - |
| High NLR–High LMR (n = 22) | 0.766 (0.310–1.891) | p = 0.563 | 0.338 (0.075–1.530) | p = 0.159 |
| Low NLR–Low LMR (n = 19) | 0.993 (0.516–1.914) | p = 0.507 | 1.486 (0.462–4.779) | p = 0.507 |
| High NLR–Low LMR (n = 33) | 5.496 (3.236–9.336) | p < 0.001 | 3.049 (1.205–7.714) | p = 0.019 |
| On day 1 | ||||
| Low NLR–High LMR (n = 178) | Ref | - | Ref | - |
| High NLR–High LMR (n = 10) | 1.412 (0.555–3.591) | p = 0.469 | 4.860 (0.816–28.944) | p = 0.082 |
| Low NLR–Low LMR (n = 35) | 1.831 (0.914–3.671) | p = 0.088 | 1.168 (0.439–3.107) | p = 0.755 |
| High NLR–Low LMR (n = 62) | 10.13 (5.685–18.066) | p < 0.001 | 6.353 (2.774–14.548) | p < 0.001 |
CI: confidence interval. OR: odds ratio. NLR: neutrophil–lymphocyte ratio. LMR: monocyte–lymphocyte ratio. a Controlled for: age, atrial fibrillation, hypertension, NIHSS, hemorrhagic transformation, and stroke localization.
4. Discussion
The present study, to the best of our knowledge, is the first to provide and discuss the evidence in support of a combination of measured NLR and LMR at 24 h post-thrombolysis as an independent prognostic factor that possesses clinical significance and feasibility to identify 3-months poor outcome of AIS patients treated with intravenous thrombolysis. Confirmed by a multivariate analysis, in addition to gender, NIHSS, and TOAST classification, high NLR–low LMR remained an independent prognostic factor for 3-months poor outcome in AIS patients after rtPA.
Inflammation has a very important role in ischemic brain injury. Ischemia following stroke activates microglia, and, consequently, blood-derived immune cells infiltration into the brain tissue follows within hours to a few days [17,18]. Neutrophils reach the infarct area within the first few hours of brain infarction, monocytes during the first 24 h, and lymphocytes between 24 and 48 h [19,20,21]. Immune cells release reactive oxygen species and a variety of inflammatory mediators, adhesion molecules, cytokines, chemokines, and proteases which exacerbate tissue damage [22]. Experimental stroke models have shown increased hematopoiesis and greater output of neutrophils from the bone marrow via increased stimulation of the autonomic nervous system post-stroke [23]. Post-thrombolysis NLR as a marker of increased risk for poor outcome within 3 months after stroke onset has been also found by others [24]. Another research group analyzed the ability of admission NLR for 90-day stroke outcome prediction after endovascular stroke therapy [25]. They found NLR (cutoff point at ≥5.9) a valuable prognostic marker for poor outcome. Our results are in line with others with no independent association between infarct size and NLR or LMR after 24 h post-event [26]. Pektezel et al. conducted a retrospective evaluation of acute stroke patients treated with rtPA by comparing favorable outcome (mRS ≤ 3) with excellent outcome (mRS 0 or 1) at admission and after 24 h [27]. This study showed that significant elevation of NLR from admission to 24 h post-event, and NLR ≤ 3.6 after 24 h revealed favorable prognosis. They conclude that elevated NLR during the first 24 h is an epiphenomenon of poor prognosis. On the contrary, a recent meta-analysis could not find sufficient data to prove this point [28]. Shi et al. found no significant difference in the NRL at admission of patients with good versus poor outcome. We experienced a similar cutoff point for NLR (at ≥5.73), and NRL did not show significant difference at admission between poor and good outcome groups [24]. Others found, in a small group of patients, no significant relation between NRL or LMR and long-term outcome in AIS patients treated by thrombolysis [29].
Monocytes release inflammatory mediators, such as chemokines, intercellular adhesion molecule1, interleukin-1, IL-6, IL-8, and tumor necrosis factor, and contribute to the development of inflammation by cerebral ischemia and hypoxia in the area of brain infarct. Furthermore, monocytes promote thrombosis and vascular occlusion by forming platelet monocyte aggregates, which aggravate ischemic injury [30]. In the ischemic brain, the monocyte-derived macrophages (MDMs) differentiate from monocytes [31]. MDMs are potent phagocytic cells and are involved in the long-term spontaneous functional recovery of the brain after ischemia [32]. LMR at admission was also evaluated for 3-months prognosis in patients with stroke and thrombolysis therapy [33]. They demonstrated that a higher LMR value (cutoff point at 3.48) was an independent factor to predict the clinical outcome of stroke before rtPA administration.
The exploration of temporal changes in levels of peripherally circulating leukocytes demonstrated that immediately after ischemic stroke there is an exponential decrease in the lymphocyte count [34]. Lower lymphocyte counts, as a marker of severe brain damage, were predictive of poor neurological improvement and poor functional outcome after stroke [35]. Our analysis demonstrated a similar trend in that the number of patients with poor outcome were significantly increased in the high NLR–low LMR group within one day after thrombolysis. This shows the relative association of a low lymphocyte count with poor outcome. One possible mechanism is that the number of regulatory T-cells and B-cells as disease-limiting protective cells, which maintain immune homestasis and produce anti inflammatory agents, increase in the brain tissue after ischemic injury [36]. The subset of leukocytes determines the population of adrenergic receptors and cholinergic receptors on the cell surface. A high number of cholinergic receptors and low number of adrenergic receptors express on the surface of lymphocytes, whereas granulocytes bear a high density of adrenergic receptors and a low density of cholinergic receptors [37]. Another proposed explanation is that a low lymphocyte count reflects elevated sympathetic tone and cortisol level, which can increase the production of proinflammatory cytokines, such as IL-6 and tumor necrosis factor, that aggravate ischemic injury [38]. Similarly, our results also demonstrated that a lower lymphocyte count had an obvious association with poor functional outcome after three months.
We found, as have others, that there is no correlation between aSICH, stroke severity, and leukocytes indices determined at admission [26,39,40]. The relation between leukocyte profile and stroke outcome after mechanical thrombectomy has also been investigated [26,41]. In one study, higher counts of neutrophils and NLR at admission and at day 1, as well as lower lymphocyte counts at day 1 were associated with poor prognosis (mRS > 2) [41]. In another study, higher NLR and lower LMR 24 h after mechanical thrombectomy but not at admission were significant predictors of mRS at 3-months functional outcome [26]. They found optimal cutoff values of 5.5 for NLR and 2.0 for LMR after thrombectomy.
Our study is the first to discuss the value of the combined post-thrombolysis high NLR–low LMR ratio in evaluating the prognosis of AIS patients at 3 months post-event. Our findings are corroborated by the observations of previous studies, which had revealed the lack of reliability of the pre-thrombolysis prognostic value of the NLR measurement [19]. Our results show that the NLR–LMR index as obtained 24 h post-lysis categorized patients according to 3-months outcome more precisely and with better diagnostic accuracy.
5. Conclusions
In conclusion, by combining NLR and LMR results of AIS patients as obtained 24 h after thrombolysis, the estimation of patient outcomes can be significantly improved. The combination of high NLR–low LMR was found to be an independent risk factor for poor outcome at 3 months post-event. Our study showed that, owing to higher NLR and decreased LMR, a high NLR–low LMR in patients with similar stroke severity was inclined to have poor outcomes. The predicting effect of NLR–LMR ratio on stroke prognosis still needs to be confirmed in more studies in various clinical settings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm12081221/s1, Table S1: Leukocyte counts and ratios at admission according to stroke etiology; Table S2: Leukocyte counts and ratios according to ASPECTS at admission and 24 h after thrombolysis; Table S3: Univariable and multivariable logistic regression analyses depicting the associations of admission NLR, LMR, and other baseline characteristics with functional dependence (mRS ≥ 2) at 3 months post-event; Table S4: Univariable and multivariable logistic regression analyses depicting the associations of day 1 NLR, LMR, and baseline characteristics with functional independence at 3 months post-event (mRS ≥ 2).
Author Contributions
A.H.S. conceived the study. K.S.Z., L.C., E.B. and Z.B. oversaw the statistical analysis plan. A.H.S., K.S.Z. and F.S. (Farzaneh Sadeghi) conducted statistical analysis. F.S. (Farzaneh Sadeghi), F.S. (Ferenc Sarkady), I.S., E.G.S., N.V. and R.O.-K. contributed to data acquisition. F.S. (Farzaneh Sadeghi) and I.S. contributed to data quality assurance and data quality analysis. A.H.S., K.S.Z. and Z.B. contributed to data interpretation. A.H.S., Z.B. and F.S. (Farzaneh Sadeghi) drafted the initial manuscript and all remaining authors critically revised the manuscript. All authors gave final approval for publication. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ethics Committee of the University of Debrecen, Hungary, approved the study (Approval Code: 4672/2016, Approval Date: 7 November 2016). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. All patients or their relatives provided written informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author according to “MDPI Research Data Policies” at https://www.mdpi.com/ethics. Accessed on 1 July 2022.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Supported by grants from the National Research, Development and Innovation Fund (K120042, FK128582), by GINOP-2.3.2-15-2016-00048 and the Eötvös Loránd Kutatási Hálózat (ELKH-DE Cerebrovascular and Neurodegenerative Research Group).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin V.L., Stark B.A., Johnson C.O., Roth G.A., Bisignano C., Abady G.G., Abbasifard M., Abbasi-Kangevari M., Abd-Allah F., Abedi V., et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jovin T.G., Nogueira R.G., Investigators D. Thrombectomy 6 to 24 Hours after Stroke. N. Engl. J. Med. 2018;378:1161–1162. doi: 10.1056/NEJMc1801530. [DOI] [PubMed] [Google Scholar]
- 3.Advani R., Naess H., Kurz M.W. The golden hour of acute ischemic stroke. Scand. J. Trauma Resusc. Emerg. Med. 2017;25:54. doi: 10.1186/s13049-017-0398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jivan K., Ranchod K., Modi G. Management of ischaemic stroke in the acute setting: Review of the current status. Cardiovasc. J. Afr. 2013;24:86–92. doi: 10.5830/CVJA-2013-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacke W., Kaste M., Bluhmki E., Brozman M., Davalos A., Guidetti D., Larrue V., Lees K.R., Medeghri Z., Machnig T., et al. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N. Engl. J. Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 6.Lees K.R., Emberson J., Blackwell L., Bluhmki E., Davis S.M., Donnan G.A., Grotta J.C., Kaste M., von Kummer R., Lansberg M.G., et al. Stroke Thrombolysis Trialists’ Collaborators, G. Effects of Alteplase for Acute Stroke on the Distribution of Functional Outcomes: A Pooled Analysis of 9 Trials. Stroke. 2016;47:2373–2379. doi: 10.1161/STROKEAHA.116.013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao Y., Wu B., Jia W., Zhang Z., Chen Q., Wang D. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in renal cell carcinoma: A systematic review and meta-analysis. BMC Urol. 2020;20:90. doi: 10.1186/s12894-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao X., Coull B., Lin X., Vokonas P., Sparrow D., Hou L., DeMeo D.L., Litonjua A.A., Schwartz J., Baccarelli A.A. Association of Neutrophil to Lymphocyte Ratio with Pulmonary Function in a 30-Year Longitudinal Study of US Veterans. JAMA Netw. Open. 2020;3:e2010350. doi: 10.1001/jamanetworkopen.2020.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li K.J., Xia X.F., Su M., Zhang H., Chen W.H., Zou C.L. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer. 2019;19:1004. doi: 10.1186/s12885-019-6157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues S.F., Granger D.N. Leukocyte-mediated tissue injury in ischemic stroke. Curr. Med. Chem. 2014;21:2130–2137. doi: 10.2174/0929867321666131228192119. [DOI] [PubMed] [Google Scholar]
- 11.Song S.Y., Zhao X.X., Rajah G., Hua C., Kang R.J., Han Y.P., Ding Y.C., Meng R. Clinical Significance of Baseline Neutrophil-to–Lymphocyte Ratio in Patients with Ischemic Stroke or Hemorrhagic Stroke: An Updated Meta-Analysis. Front. Neurol. 2019;10:1032. doi: 10.3389/fneur.2019.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal N., Tsivgoulis G., Chang J.J., Malhotra K., Pandhi A., Ishfaq M.F., Alsbrook D., Arthur A.S., Elijovich L., Alexandrov A.V. Admission Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker of Outcomes in Large Vessel Occlusion Strokes. Stroke. 2018;49:1985–1987. doi: 10.1161/STROKEAHA.118.021477. [DOI] [PubMed] [Google Scholar]
- 13.The European Stroke Organisation Executive Committee. ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 14.Aviv R.I., Mandelcorn J., Chakraborty S., Gladstone D., Malham S., Tomlinson G., Fox A.J., Symons S. Alberta Stroke Program Early CT Scoring of CT perfusion in early stroke visualization and assessment. Am. J. Neuroradiol. 2007;28:1975–1980. doi: 10.3174/ajnr.A0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10,172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Simonsen C.Z., Schmitz M.L., Madsen M.H., Mikkelsen I.K., Chandra R.V., Leslie-Mazwi T., Andersen G. Early neurological deterioration after thrombolysis: Clinical and imaging predictors. Int. J. Stroke. 2016;11:776–782. doi: 10.1177/1747493016650454. [DOI] [PubMed] [Google Scholar]
- 17.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiba T., Umegaki K. Pivotal roles of monocytes/macrophages in stroke. Mediat. Inflamm. 2013;2013:759103. doi: 10.1155/2013/759103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 20.Nilupul Perera M., Ma H.K., Arakawa S., Howells D.W., Markus R., Rowe C.C., Donnan G.A. Inflammation following stroke. J. Clin. Neurosci. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Simoni M.G.D. Two-way communication pathways between the brain and the immune system. Neurosci. Res. Commun. 1997;21:10. doi: 10.1002/(SICI)1520-6769(199711/12)21:3<163::AID-NRC220>3.0.CO;2-T. [DOI] [Google Scholar]
- 22.Jickling G.C., Liu D., Ander B.P., Stamova B., Zhan X., Sharp F.R. Targeting neutrophils in ischemic stroke: Translational insights from experimental studies. J. Cereb. Blood Flow Metab. 2015;35:888–901. doi: 10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruhnau J., Schulze J., Dressel A., Vogelgesang A. Thrombosis, Neuroinflammation, and Poststroke Infection: The Multifaceted Role of Neutrophils in Stroke. J. Immunol. Res. 2017;2017:5140679. doi: 10.1155/2017/5140679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi J., Peng H., You S., Liu Y., Xu J., Xu Y., Liu H., Shi R., Cao Y., Liu C.F. Increase in neutrophils after recombinant tissue plasminogen activator thrombolysis predicts poor functional outcome of ischaemic stroke: A longitudinal study. Eur. J. Neurol. 2018;25:687-e45. doi: 10.1111/ene.13575. [DOI] [PubMed] [Google Scholar]
- 25.Brooks S.D., Spears C., Cummings C., VanGilder R.L., Stinehart K.R., Gutmann L., Domico J., Culp S., Carpenter J., Rai A., et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J. Neurointerv. Surg. 2014;6:578–583. doi: 10.1136/neurintsurg-2013-010780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lux D., Alakbarzade V., Bridge L., Clark C.N., Clarke B., Zhang L., Khan U., Pereira A.C. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J. Neuroinflamm. 2020;17:60. doi: 10.1186/s12974-020-01739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pektezel M.Y., Yilmaz E., Arsava E.M., Topcuoglu M.A. Neutrophil-to-Lymphocyte Ratio and Response to Intravenous Thrombolysis in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019;28:1853–1859. doi: 10.1016/j.jstrokecerebrovasdis.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Wang C., Zhang Q., Ji M., Mang J., Xu Z. Prognostic value of the neutrophil-to-lymphocyte ratio in acute ischemic stroke patients treated with intravenous thrombolysis: A systematic review and meta-analysis. BMC Neurol. 2021;21:191. doi: 10.1186/s12883-021-02222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Switonska M., Slomka A., Korbal P., Piekus-Slomka N., Sinkiewicz W., Sokal P., Zekanowska E. Association of Neutrophil-to-Lymphocyte Ratio and Lymphocyte-to-Monocyte Ratio with Treatment Modalities of Acute Ischaemic Stroke: A Pilot Study. Medicina. 2019;55:342. doi: 10.3390/medicina55070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong X., Nao J., Gao Y. Peripheral Monocyte Count Predicts Outcomes in Patients with Acute Ischemic Stroke Treated with rtPA Thrombolysis. Neurotox. Res. 2020;37:469–477. doi: 10.1007/s12640-019-00103-0. [DOI] [PubMed] [Google Scholar]
- 31.ElAli A., Jean LeBlanc N. The Role of Monocytes in Ischemic Stroke Pathobiology: New Avenues to Explore. Front. Aging Neurosci. 2016;8:29. doi: 10.3389/fnagi.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wattananit S., Tornero D., Graubardt N., Memanishvili T., Monni E., Tatarishvili J., Miskinyte G., Ge R., Ahlenius H., Lindvall O., et al. Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J. Neurosci. 2016;36:4182–4195. doi: 10.1523/JNEUROSCI.4317-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren H., Han L., Liu H., Wang L., Liu X., Gao Y. Decreased Lymphocyte-to-Monocyte Ratio Predicts Poor Prognosis of Acute Ischemic Stroke Treated with Thrombolysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017;23:5826–5833. doi: 10.12659/MSM.907919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill D., Sivakumaran P., Aravind A., Tank A., Dosh R., Veltkamp R. Temporal Trends in the Levels of Peripherally Circulating Leukocyte Subtypes in the Hours after Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2018;27:198–202. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Kim J., Song T.J., Park J.H., Lee H.S., Nam C.M., Nam H.S., Kim Y.D., Heo J.H. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. 2012;222:464–467. doi: 10.1016/j.atherosclerosis.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 36.Liesz A., Hu X., Kleinschnitz C., Offner H. Functional role of regulatory lymphocytes in stroke: Facts and controversies. Stroke. 2015;46:1422–1430. doi: 10.1161/STROKEAHA.114.008608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abo T., Kawamura T. Immunomodulation by the Autonomic Nervous System: Therapeutic Approach for Cancer, Collagen Diseases, and Inflammatory Bowel Diseases. Ther. Apher. 2002;6:348–357. doi: 10.1046/j.1526-0968.2002.00452.x. [DOI] [PubMed] [Google Scholar]
- 38.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 39.Fang Y.N., Tong M.S., Sung P.H., Chen Y.L., Chen C.H., Tsai N.W., Huang C.J., Chang Y.T., Chen S.F., Chang W.N., et al. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biomed. J. 2017;40:154–162. doi: 10.1016/j.bj.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue J., Huang W., Chen X., Li Q., Cai Z., Yu T., Shao B. Neutrophil-to-Lymphocyte Ratio Is a Prognostic Marker in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017;26:650–657. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Semerano A., Laredo C., Zhao Y., Rudilosso S., Renu A., Llull L., Amaro S., Obach V., Planas A.M., Urra X., et al. Leukocytes, Collateral Circulation, and Reperfusion in Ischemic Stroke Patients Treated with Mechanical Thrombectomy. Stroke. 2019;50:3456–3464. doi: 10.1161/STROKEAHA.119.026743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding author according to “MDPI Research Data Policies” at https://www.mdpi.com/ethics. Accessed on 1 July 2022.