Abstract
Various reagents commonly used to enumerate viable helminth eggs from wastewater and sludge were evaluated for their potential to inactivate Ascaris eggs under typical laboratory conditions. Two methods were used to enumerate indigenous Ascaris eggs from sludge samples. All steps in the methods were the same except that in method I a phase extraction step with acid-alcohol (35% ethanol in 0.1 N H2SO4) and diethyl ether was used whereas in method II the extraction step was avoided by pouring the sample through a 38-μm-mesh stainless steel sieve that retained the eggs. The concentration of eggs and their viability were lower in the samples processed by method I than in the samples processed by method II by an average of 48 and 70%, respectively. A second set of experiments was performed using pure solutions of Ascaris suum eggs to elucidate the effect of the individual reagents and relevant combination of reagents on the eggs. The percentages of viable eggs in samples treated with acid-alcohol alone and in combination with diethyl ether or ethyl acetate were 52, 27, and 4%, respectively, whereas in the rest of the samples the viability was about 80%. Neither the acid nor the diethyl ether alone caused any decrease in egg viability. Thus, the observed inactivation was attributed primarily to the 35% ethanol content of the acid-alcohol solution. Inactivation of the eggs was prevented by limiting the direct exposure to the extraction reagents to 30 min and diluting the residual concentration of acid-alcohol in the sample by a factor of 100 before incubation. Also, the viability of the eggs was maintained if the acid-alcohol solution was replaced with an acetoacetic buffer. None of the reagents used for the flotation step of the sample cleaning procedure (ZnSO4, MgSO4, and NaCl) or during incubation (0.1 N H2SO4 and 0.5% formalin) inactivated the Ascaris eggs under the conditions studied.
The class of organisms known as helminths that are of direct concern to public health include the intestinal parasitic worms. It is estimated that 1.5 billion, 1.3 billion, and 1.0 billion persons are infected by human roundworm (Ascaris lumbricoides), human hookworm (Ancylostoma duodenale and Necator americanus), and human whipworm (Trichuris trichiura), respectively (10). Wastewater that is contaminated by the eggs of these species, as well as the sludge produced during wastewater treatment, may contribute to the transmission of helminth infections by dispersing the eggs in the environment. To prevent such transmission, the concentration of helminth eggs in wastewater and sludge is regulated in many countries, particularly if the treated products are to be reused beneficially in agriculture (21, 23, 25). However, there is little consensus among researchers, regulators, and practitioners about the most reliable method for measuring helminth eggs in environmental samples.
Current techniques for enumerating viable helminth eggs in wastewater and sludge typically have two parts: (i) concentrating the eggs and separating them from other particulate matter in the sample, and (ii) counting and determining the viability of the eggs in the cleaned sample by direct microscopy. Most techniques use a series of steps, such as sedimentation, filtration through one or more sieves, density flotation, and phase extraction to separate the eggs from other material in the sample so that the eggs can be more easily identified and counted under the microscope. Before the eggs are counted, the samples are incubated under aerobic conditions for about 1 month at 26 to 30°C, during which time an infective larva develops inside viable eggs (2, 7). Typically, the sample is incubated in an antimicrobial solution that prevents the growth of other organisms, especially fungi, that may interfere with egg development. Following incubation, the samples are observed under a light microscope and the number of eggs with and without larvae are counted.
Two widely used helminth methods are the standard U.S. Environmental Protection Agency (EPA) method (22) and that recommended by the World Health Organization (3); numerous other variations have also been reported in the literature. A revised version of the U.S. EPA method was recently published (23); this method has yet to undergo validation and contains several problematic steps, some of which are addressed in this paper. Several researchers have evaluated the recovery efficiency of different methods (4, 8; B. Jimenez, C. Maya, and J. Schwartzbrod, submitted for publication); however, validating the methods also requires ensuring that the viability of the eggs is not adversely affected by any of the treatment steps.
A wide range of reagents have been reported in the literature for use in isolating helminth eggs from environmental samples. Sludge and compost samples are often blended in a detergent solution of Tween 80 or Linbro 7X at the start of the procedure to increase the separation of eggs from other wastewater solids (17, 20, 23). After the sample is passed through a coarse sieve to remove large solids, density flotation is used to separate the helminth eggs, which float on top of the high-density solution, from heavier solids. The solutions used for density flotation include ZnSO4 (3, 22), MgSO4 (17, 23), and NaCl (13). The purpose of the next step, phase extraction, is to remove lipid-soluble and ether-absorbing material from the sample (6). Lipophilic and hydrophilic reagents are added to the sample and rapidly partition into two phases; a plug of waste material forms between the phases, and the Ascaris eggs are concentrated in the bottom of the tube. The reagents used for the lipophilic solution in the extraction step include diethyl ether and ethyl acetate (3, 13, 18, 23). The reagents used for the hydrophilic part of the extraction step include a mixture of H2SO4 and ethanol (22) and acetoacetic buffer (3). The most common incubation solutions include 0.1 N H2SO4 (22) and 0.5% formalin (17). In addition, a bleach treatment has been used to remove the sticky outer layer of the eggshell (7, 16), to decolorize the eggs to make determination of their developmental stage easier (9), or to induce larval mobility in already developed eggs (19).
Some of these reagents appear to have a negative impact on the viability of the helminth eggs. It has been reported that ZnSO4 is toxic to eggs (13), that soaking in MgSO4 overnight may inactivate embryonated eggs (19), that eggs incubated in 1% formalin showed retarded development compared to those incubated in water or 0.1 N H2SO4 (16), and that the viability of eggs decreased after phase extraction with acid-alcohol and diethyl ether (17). Nonetheless, quantitative data on the effects of the various reagents have not been published.
The objective of this research was to evaluate the effect of commonly used reagents, including those recommended by the U.S. EPA method (22, 23), on the viability of Ascaris eggs under typical laboratory conditions. The study focused on the eggs of Ascaris because they are usually present in the highest concentrations in wastewater and sludges and are also the most resistant to inactivation (12).
MATERIALS AND METHODS
The first set of experiments was conducted using sludge samples that contained indigenous Ascaris eggs (presumably A. lumbricoides) to determine if the reagents in the U.S. EPA procedure have the potential to inactivate eggs under typical laboratory conditions. Then, to isolate the effect of each individual reagent or relevant combination of reagents on the viability of the eggs, a second set of experiments was performed using pure solutions of Ascaris suum eggs collected from the intestines of infected pigs. In addition to the reagents used in the first set of experiments, several other reagents reported in the literature were tested.
Although genetic differences have recently been identified between the adult worms of A. suum, which infects pigs, and A. lumbricoides, which infects humans, and some degree of host specificity has been demonstrated, it is not yet clear that they represent distinct species (1, 26). To date, no morphological or physiological difference has been observed between eggs from the two hosts. Thus, A. suum eggs are commonly used as a model for A. lumbricoides eggs because they are easier to obtain in large quantities. In terms of the source of the eggs, eggs dissected from the intestines of mature female worms and eggs isolated from feces are similar in terms of their infectivity and inactivation (14, 16). Nevertheless, some doubt remains as to whether the eggshell becomes more resistant to environmental conditions after exposure to intestinal contents due to a “tanning” process (24). Therefore, the eggs used in the second set of experiments were concentrated from the intestines of infected pigs. These eggs are believed to adequately reflect the properties of eggs in feces but have the advantage that no chemicals were used to concentrate them.
Eggs from sludge samples.
Thirteen independent sludge samples were obtained from different locations in the sludge layer of a municipal wastewater stabilization pond in Mexicaltzingo, Mexico (South of Toluca in Mexico State). The samples were stored at 4°C until analysis. Two different methods were used to enumerate Ascaris eggs in each sample (Table 1). The first three steps (sedimentation, sieving, and flotation) were the same for all samples and were based on the U.S. EPA method (22). Two replicates from each sludge sample were processed through the flotation step, after which one sample was processed by method I (phase extraction) and one was processed by method II (sieving). Note that the phase extraction step is part of both the 1992 and 1999 U.S. EPA methods whereas the sieving step is included only in the 1999 method.
TABLE 1.
Summary of procedures used to clean sludge samples for analysis of Ascaris eggs
| Step | Method | Procedurea |
|---|---|---|
| Sedimentation | Both | Place equivalent of 2 g of dry solids in blender with 200 ml of 0.1% Tween 80 and blend for 1 min. Recover sample in 2-liter container using 800 ml of 0.1% Tween 80 to rinse blender. Settle overnight. Aspirate supernatant. |
| Sieving | Both | Filter sediment through 160-μm-mesh sieve. Rinse the filtrate with 1 liter of water into the original container. Settle for 3 h. Aspirate supernatant. Transfer sediment to a 200-ml centrifuge bottle. Centrifuge and aspirate supernatant. |
| Flotation | Both | Suspend sediment (∼15 ml) in 150 ml of ZnSO4 (specific gravity, 1.2). Mix carefully with glass stir rod or plastic spatula. Centrifuge. |
| Extraction | I | Recover supernatant in a 2-liter container and add 1 liter of distilled water. Settle for 3 h. Aspirate supernatant. Transfer sediment to a 200-ml centrifuge bottle. Centrifuge, and aspirate supernatant. Transfer sediment to a 50-ml centrifuge tube. Centrifuge, and aspirate to 5 ml. Add 15 ml of acid-alcohol and 10 ml of diethyl ether. Shake vigorously for 2 min. Allow gas to escape periodically. Centrifuge, and aspirate to 5 ml. Add 5 ml of 0.1 N H2SO4. |
| Sieving | II | Pour supernatant through 38-μm-mesh sieve. Rinse sieve using squirt bottle, concentrating eggs and any remaining sediment at the bottom edge. Wash material collected on sieve into a 50-ml centrifuge tube. Centrifuge, and aspirate to 5 ml. Add 40 ml of 0.1 N H2SO4. Centrifuge, and aspirate to 10 ml. |
| Incubation | Both | Incubate at 26°C for 4 wk. Mix once per wk by hand. Transfer to Doncaster Disk. Count the eggs with and without larvae. |
All centrifugations were at 1,000 × g for 5 min.
The total solids concentration in the sludge samples ranged from 60 to 250 g/liter, as determined by Standard Method 2540 G (11). For all samples, a wet weight of sample approximately equivalent to 2 g of dry sludge was weighed and blended with 100 ml of 0.1% Tween 80 for 1 min, rinsed with 1 liter of distilled water into a 2-liter container, and left to settle overnight. The supernatant was removed by aspiration, and the sediment was poured through a 160-μm sieve to remove coarse solids. The filtrate was left to settle overnight, after which the supernatant was removed by aspiration. The sediment was recovered in a 200-ml centrifuge bottle and centrifuged, and the supernatant was removed by aspiration. For the flotation step, 150 ml of ZnSO4 solution (specific gravity, 1.2) was added to the recovered sediment and the samples were thoroughly mixed and centrifuged. All centrifugations in the procedure were performed at 1,000 × g for 5 min.
After this point, the two different methods were used. For method I, the flotation supernatant was recovered in a 2-liter container and 1 liter of distilled water was added to reduce the specific gravity. After settling overnight, the supernatant was removed by aspiration and the sediment was transferred to a 50-ml centrifuge tube and centrifuged, and the supernatant was aspirated, leaving 5 ml of sample. (A 5-ml volume corresponds to approximately 2 mm of liquid volume above the cone of a 50-ml centrifuge tube. Because some eggs were observed to collect on the walls of the cone, it was decided that removing any additional supernatant could involve removal of eggs from the sample. In the U.S. EPA procedure, the volume of sample remaining after supernatant aspiration is not specified.) Then, 15 ml of acid-alcohol (0.1 N H2SO4 in 35% ethanol) and 10 ml of diethyl ether or ethyl acetate were added. The samples were shaken vigorously for 2 min and centrifuged, during which time each sample separated into two distinct phases. The diethyl ether or ethyl acetate floated on top of the acid-alcohol, and a plug of material formed at the interface. The sediment containing the eggs formed a pellet in the bottom of the tube. The supernatant, including the plug of material, was removed by aspiration, leaving 5 ml of sample that included the sediment containing the eggs. The samples were in direct contact with the reagents for approximately 1 h. Finally, 5 ml of 0.1 N H2SO4 was added to each 5-ml sample.
For method II, there was no extraction step. The supernatant from the flotation step was poured through a 38-μm-mesh stainless steel sieve to retain the eggs. The sieve was thoroughly rinsed with a water stream from a squirt bottle, concentrating the eggs and any remaining sediment at the bottom edge. The material collected on the sieve was washed into a 50-ml centrifuge tube by inverting the sieve and directing the water stream at the sample through the mesh. The sample was centrifuged, the supernatant was aspirated to a volume of 5 ml, and the tube was filled with 0.1 N H2SO4, centrifuged, and aspirated, leaving a final volume of 10 ml in the tube.
All of the cleaned sludge samples were incubated at 26°C for 4 weeks with loose caps to allow air exchange. The samples were mixed once a week by hand, and any evaporated liquid was replaced by adding distilled water. Before counting, the samples were treated with a dilute bleach solution to decolorize the eggs and facilitate observation of the larvae (the treatment also removed the outer mamillated layer of the eggshell from many of the eggs). To each sample, 10 ml of 10% household bleach was added. After 10 min, the tube was filled with distilled water and centrifuged, and the supernatant was aspirated. This rinsing step was repeated one more time to remove the residual chlorine, leaving a final sample volume of about 5 ml.
The samples were observed under ×100 magnification after transfer to a Doncaster Disk, which is a 9-cm-diameter clear plastic disk with circular wells. The entire volume of each sample was counted by dividing the sample into several aliquots. Eggs that contained fully developed larvae were assumed to be viable; all other eggs were assumed to be nonviable. The egg concentration was calculated by dividing the total number of eggs by the dry weight of the sludge, which was determined on a separate aliquot of each sludge sample by Standard Method 2540G (11). The percent viability was calculated as the number of viable eggs divided by the total number of eggs, multiplied by 100.
Pure egg solutions.
Samples of pure eggs in solution were subjected to 14 different treatments to test different reagents used in the flotation, extraction, and incubation steps of the procedure (Table 2). The purpose of the different treatments was to isolate the effect of each individual reagent and relevant combination of reagents. Each treatment is representative of a step used to separate the eggs from other material in the sample and is based on the steps in the U.S. EPA method (22, 23). Each treatment was repeated on five replicate samples.
TABLE 2.
Summary of treatments given to solutions of pure Ascaris suum eggsa
| Treatment and step | Reagent
|
Procedureb
|
||||
|---|---|---|---|---|---|---|
| All steps | Hydrophilic extraction (reagent 1) | Lipophilic extraction (reagent 2) | All steps | Minimum exposurec | Maximum exposured | |
| Flotation | ||||||
| 1 2 3 | ZnSO4 MgSO4 NaCl | Add 30 ml of reagent, and mix thoroughly. Centrifuge and pour off supernatant into 200-ml centrifuge bottle. Add 170 ml of distilled water. Settle overnight. Aspirate supernatant, and transfer sediment to 15-ml tube. Centrifuge, and aspirate to 1.5 ml. Add 13.5 ml of 0.1 N H2SO4. Centrifuge, and aspirate to 3 ml. | ||||
| Extraction | ||||||
| 4 5 6, 7 8, 9 10, 11 | None Acid-alcohol Acid-alcohol Acid-alcohol Acetoacetic buffer | Diethyl ether None Diethyl ether Diethyl ether | Add 4.5 ml of reagent 1 and 3 ml of reagent 2. Shake vigorously for 2 min. Centrifuge and aspirate to 1.5 ml. Add 13.5 ml of 0.1 N H2SO4. Centrifuge and aspirate to 1.5 ml. Add 13.5 ml of 0.1 N H2SO4. Centrifuge and aspirate to 3 ml. | Add 4.5 ml of reagent 1 and 3 ml of reagent 2. Shake vigorously for 2 min. Let sample sit for 30 min. Centrifuge and aspirate to 1.5 ml. Add 1.5 ml of 0.1 N H2SO4. | ||
| Incubation 12 13 14 | Distilled water 0.1 N H2SO4 0.5% formalin | Add 13.5 ml of reagent. Centrifuge and aspirate to 3 ml. | ||||
After treatment, all samples were incubated for 4 weeks at 26°C.
Initial samples consisted of eggs suspended in phosphate-buffered saline. Except for the samples treated with distilled water or 0.5% formalin, all samples were incubated in 0.1 N H2SO4. All centrifugations were at 1,000 × g for 5 min. The steps in these columns refer to all treatments in the flotation, the extraction, or the incubation.
30-min direct exposure to reagents. Rinsing steps decreased residual ethanol concentration to approximately 2 g/liter during incubation.
1-h direct exposure to reagents. Samples were incubated with an approximate residual ethanol concentration of 102 g/liter.
A. suum eggs were purchased from Excelsior Sentinel Inc. (Ithaca, N.Y.). The company collected the intestinal contents of infected pigs, from which the eggs were separated and concentrated via serial filtration through stainless steel sieves. The eggs were stored in 0.5% formalin at 4°C for up to 6 months before being used in the experiments. A working egg solution with approximately 6,000 eggs/ml was prepared by suspending the eggs in a buffered salt solution. Individual samples were prepared by placing 1.5 ml of the working solution in 15-ml polypropylene centrifuge tubes, except for the samples that were treated with the flotation reagents, which were prepared by adding 1.5 ml of working solution and 1.5 ml of distilled water to 50-mL polypropylene centrifuge tubes.
In treatments 1 through 3 (Table 2), different flotation solutions with specific gravity of 1.2 were tested: ZnSO4, MgSO4, and NaCl. To each 3-ml sample, 30 ml of reagent was added and the sample was thoroughly mixed and centrifuged. In all cases, the samples were centrifuged at 1,000 × g for 5 min. The supernatant containing the eggs was poured into a 200-ml centrifuge bottle, and 170 ml of distilled water was added. After settling overnight, the supernatant was removed by aspiration and the sediment was transferred to a 15-ml centrifuge tube. The sample, including rinse water, was centrifuged, and the supernatant was removed, leaving 1.5 ml. (A 1.5-ml volume corresponds to approximately 2 mm of liquid volume above the cone of a 15-ml centrifuge tube. To each sample, 13.5 ml of 0.1 N H2SO4 was added, the sample was centrifuged again, and the supernatant was removed by aspiration, leaving a final volume of 3 ml.
For the extraction step, five different treatments and two different exposures were tested (treatments 4 through 11). Two hydrophilic reagents were tested, acid-alcohol solution and acetoacetic buffer (15 g of sodium acetate trihydrate per liter, 3.6 ml of glacial acetic acid per liter [pH = 4.5]) (3), as well as two lipophilic reagents, diethyl ether and ethyl acetate. The reagents were tested alone and in combination (Table 2). To each 1.5-ml sample, 4.5 ml of the hydrophilic reagent and 3.0 ml of lipophilic reagent were added. The samples were shaken vigorously for 2 min and centrifuged, and the supernatant was aspirated, leaving 1.5 ml of sample. For the minimum exposure, the samples were processed immediately, whereas for the maximum exposure, the samples were left to sit for 30 min before being centrifuged. Thus, the length of time that the samples were in direct contact with the reagents was less than 30 min for the minimum exposure and about 1 h for the maximum exposure.
The exposure time was also affected by the residual concentration of reagents that remained in the 1.5-ml sample. For the minimum exposure, the samples were thoroughly rinsed to remove any residual traces of the reagents before incubation. To rinse the samples, 13.5 ml of 0.1 N H2SO4 was added to the centrifuge tube and the samples were mixed and centrifuged and the supernatant was aspirated to a volume of 1.5 ml. The rinsing was repeated one more time. A total of 3 ml of liquid volume was left after the final aspiration. For the maximum exposure, 1.5 ml of 0.1 N H2SO4 was added directly to the sample after treatment, which also resulted in a final volume of 3 ml. The maximum exposure used in the experiments with pure solutions of eggs was similar to the exposure that occurred in the sludge samples.
The purpose of treatments 12 through 14 was to test different solutions for incubating the eggs: distilled water, 0.1 N H2SO4, and 0.5% formalin. To each 1.5-ml sample, 13.5 ml of reagent was added, the sample was centrifuged, and the supernatant was removed by aspiration, leaving a final volume of 3 ml. (Note that except for the samples treated with distilled water and 0.5% formalin, all samples were incubated in 0.1 N H2SO4.)
The samples were incubated and treated with bleach before counting, as for the sludge samples. For enumeration, an aliquot from each well-mixed sample was placed on a glass microscope slide with coverslip and a minimum of 200 eggs were counted under the microscope and observed for the presence or absence of larvae. The counts were performed within 10 min, before pressure from the coverslip caused the eggs to rupture. The percentage of viable eggs was calculated by dividing the number of viable eggs by the total number of eggs observed and multiplying by 100.
RESULTS
Eggs from sludge samples.
The percentage of viable Ascaris eggs and the total number of eggs recovered were significantly lower in all of the sludge samples treated by method I than in those treated by method II (Table 3). The percent viability measured by method I ranged from 0 to 34.5%, with an average of only 7.5%, whereas the percent viability measured by method II ranged from 0.2 to 66.4%, with an average of 24.7%. In addition to the lower percentage of viable Ascaris eggs, fewer total (viable and nonviable) eggs were recovered using method I. The concentration of total eggs measured by method I ranged from 40 to 116 eggs/g of total solids, with an average of 66 eggs/g, whereas the concentration measured by method II ranged from 65 to 225 eggs/g, with an average of 128 eggs/g. Thus, on average, the viability of the recovered eggs was 70% lower using method I and 48% fewer eggs were recovered using method I.
TABLE 3.
Percent viability and concentration of total Ascaris eggs in sludge samples processed by two different methodsa
| Sample | Viability (%)
|
Total no. of eggs/g of total solids
|
||||
|---|---|---|---|---|---|---|
| Method Ib | Method IIc | Difference (%)d | Method Ib | Method IIc | Difference (%)d | |
| 1 | 34.5 | 66.4 | −48 | 66 | 81 | −19 |
| 2 | 13.7 | 64.7 | −79 | 57 | 71 | −20 |
| 3 | 19.4 | 36.5 | −47 | 30 | 65 | −54 |
| 4 | 0.9 | 33.1 | −97 | 116 | 135 | −14 |
| 5 | 3.9 | 33.1 | −88 | 56 | 225 | −75 |
| 6 | 18.3 | 24.3 | −24 | 48 | 103 | −54 |
| 7 | 3.3 | 23.7 | −86 | 74 | 121 | −39 |
| 8 | 0.2 | 22.0 | −99 | 102 | 170 | −40 |
| 9 | 0.0 | 7.6 | −100 | 79 | 95 | −16 |
| 10 | 2.3 | 7.2 | −68 | 71 | 175 | −59 |
| 11 | 0.4 | 2.0 | −82 | 63 | 172 | −63 |
| 12 | 0.4 | 0.8 | −42 | 56 | 131 | −57 |
| 13 | 0.0 | 0.2 | −100 | 40 | 120 | −67 |
| Mean | 7.5 | 24.7 | −70 | 66 | 128 | −48 |
In method I, samples were exposed to acid-alcohol solution and diethyl ether as per U.S. EPA (1992 and 1999), whereas in method II no exposure occured. The samples are listed in order of highest to lowest percent viability as determined by method II.
Direct exposure to acid-alcohol and diethyl ether was for about 1 h, and the samples were incubated with an approximate residual ethanol concentration of 102 g/liter.
A sieving step was substituted for the extraction step such that no exposure to acid-alcohol or diethyl ether occured.
[(method I − method II)/(method II)] × 100.
Pure egg solutions.
The percentage of viable eggs in the pure egg solutions after treatment is reported in Table 4. A one-way analysis of variance (ANOVA) model was used to determine if significant differences existed among the 14 different treatments (Minitab Statistical Software; Minitab Inc., State College, Pa.). The count data (viable or nonviable) were assumed to have a binomial distribution; thus, to achieve asymptotic normality (an assumption of ANOVA), the data were first transformed using the expression
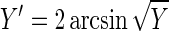 |
where Y is the percent viability of the sample divided by 100. This transformation is recommended when the response variable is a proportion (15). The ANOVA model confirmed that significant differences existed between the treatments (F = 53.3, P < 0.001).
TABLE 4.
Effect of different treatments on the viability of A. suum eggs in pure solutiona
| Treatment and step | Reagent
|
% Viability (standard deviation)
|
Tukey groupingb
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| All steps | Hydrophilic extraction (reagent 1) | Lipophilic extraction (reagent 2) | All steps | Minimum exposurec | Maximum exposured | All steps | Minimum exposurec | Maximum exposured | |
| Flotation | |||||||||
| 1 | ZnSO4 | 81.4 (1.4) | A | ||||||
| 2 | MgSO4 | 85.0 (1.0) | A | ||||||
| 3 | NaCl | 83.9 (3.3) | A | ||||||
| Extraction | |||||||||
| 4 | None | Diethyl ether | 85.3 (1.9) | A | |||||
| 5 | Acid alcohol | None | 52.2 (7.2) | B | |||||
| 6, 7 | Acid alcohol | Diethyl ether | 85.7 (3.3) | 26.5 (18.4) | A | C | |||
| 8, 9 | Acid alcohol | Ethyl acetate | 82.4 (1.6) | 4.0 (6.1) | A | D | |||
| 10, 11 | Acetoacetic buffer | Diethyl ether | 84.5 (4.3) | 87.2 (2.1) | A | A | |||
| Incubation | |||||||||
| 12 | Distilled water | 72.8 (15.5) | A | ||||||
| 13 | 0.1 N H2SO4 | 82.4 (3.2) | A | ||||||
| 14 | 0.5% formalin | 82.7 (4.1) | A | ||||||
The value reported for percent viability is the mean of five replicate samples.
Values marked with the same letter are not significantly different with α = 0.05
30-min direct exposure to reagents. Rinsing steps decreased the residual ethanol concentration to approximately 2 g/liter during incubation.
1-h direct exposure to reagents. Samples were incubated with an approximate residual ethanol concentration of 102 g/liter.
For post hoc analysis, Tukey's method was used to determine the nature of the differences between treatments (15). The results of testing all pairwise combinations are reported in Table 4. No significant difference in percent viability was found between the treatments that are marked with the same letter (α = 0.05); treatments that are marked with different letters were significantly different. A significant difference in the percentage of viable eggs was observed only among the three treatments that had a maximum exposure to acid-alcohol. In the samples treated with acid-alcohol alone, the percentage of viable Ascaris eggs decreased to 52.2%. In the samples that were treated with acid-alcohol plus diethyl ether or ethyl acetate, the viability decreased even further, to 26.5 and 4.0%, respectively. Although the diethyl ether alone did not decrease the viability of the eggs, even with a maximum exposure, a synergistic effect was observed when it was combined with the acid-alcohol. In all other treatments, the percentage of viable eggs ranged from 81.4 to 87.2%; it is concluded that none of these remaining treatments affected the viability of the Ascaris eggs.
DISCUSSION
In the experiment with sludge samples, the difference between method I and II was the extraction step; in method I acid-alcohol and diethyl ether were used to extract the lipid-soluble and ether-absorbing material from the sample, whereas in method II this step was eliminated and replaced by passing the sample through a 38-μm-mesh stainless steel sieve that retained the eggs. The lower percentage of viable eggs measured in the samples treated by method I demonstrated that exposure to the extraction reagents inactivated, on average, 70% of the Ascaris eggs (Table 3). In addition, because the concentration of eggs was 48% lower in the samples treated by method I, it appears that many of the eggs were not even recovered by method I. Possible explanations for the lower recovery are that some eggs were removed during the extraction step or that they were physically destroyed by the extraction reagents such that they were not visible by the time the samples were observed under the microscope. It should be noted that if a greater percentage of the eggs that were not recovered were nonviable (compared to the recovered eggs), then the observed percentage of viable eggs underestimates the actual number of eggs that were inactivated.
A wide range in egg concentration and percent viability was observed among the 13 independent sludge samples, whether measured by method I or method II, which reflects the heterogeneous nature of the sludge layer in wastewater stabilization ponds. (The sludge samples were taken from different locations throughout the pond and from different depths within the sludge layer; the egg concentration and viability varied according to the settling conditions in the pond and the age of the sludge.) The sample location and sludge age were not correlated, however, with the variation that was observed in the percent difference between paired sludge samples measured by method I compared to method II. One factor that could have contributed to this observed variation is that the initial 2 g of sample was collected from the original sample container and processed on different days for methods I and II. In future studies, it is recommended that (i) paired samples be processed together during the first three steps of the procedure and split at the point where the methods diverge and (ii) replicate samples be processed for each method. Nevertheless, the main finding of the experiment with sludge samples is evident—the reagents used in the extraction step significantly reduced both the number and viability of Ascaris eggs in the samples.
The results of the experiments on pure solutions of A. suum eggs provide further insight into the cause of inactivation. Consistent with the results of the sludge experiment, none of the reagents used for flotation (ZnSO4, MgSO4, and NaCl) or incubation (distilled water, 0.1 N H2SO4, and 0.5% formalin) had a significant effect on the egg viability (Table 4). Furthermore, none of the reagents used for the extraction step caused inactivation of the eggs under the conditions defined as a minimum exposure. Under the maximum-exposure conditions, however, all three treatments that employed the acid-alcohol solution caused a significant decrease in egg viability. The acid-alcohol alone caused a significant decrease in egg viability, and when it was combined with diethyl ether or ethyl acetate, a synergistic effect was observed that caused an even greater decrease. Given that there was no decrease in the viability of samples incubated only in 0.1 N H2SO4, it appears to be the ethanol content (35%) of the acid-alcohol solution that caused the inactivation. No decrease in egg viability occurred from treatment with diethyl ether alone or in combination with acetoacetic buffer, even at maximum exposure. (The ethyl acetate was not tested alone, so it is not known whether it would decrease the egg viability by itself.)
Because the number of eggs in the pure solutions was not determined, it is not known whether the recovery of eggs was affected by any of the treatments. Thus, the parameter used to quantify changes in egg viability in the pure egg solutions (percent viability) may not provide an identical measure to the parameter used with the sludge samples (number of viable eggs). Under the conditions of this research, however, any bias introduced by the difference between these two parameters is believed to be minimal compared to the magnitude of the observed changes in egg viability.
One implication of the results from the pure egg solutions is that ethyl acetate does not appear to offer any advantage over diethyl ether in terms of effect on egg viability. In fact, ethyl acetate caused an even greater inactivation of Ascaris eggs than did diethyl ether when combined with acid-alcohol at the maximum exposure. However, ethyl acetate is preferable in terms of safety and health (it is less flammable and less toxic than diethyl ether) and is as effective as diethyl ether at recovering Ascaris eggs from sludge (18). More research is needed to determine the effectiveness of diethyl ether with other types of sample matrices.
It is believed that the observed decrease in egg viability from exposure to the extraction reagents was due to an increase in the permeability of the lipid membrane of the Ascaris eggs. Although both the acid-alcohol solution and diethyl ether have lipophilic properties, apparently the acid-alcohol was more effective at penetrating the Ascaris membrane. The absence of an effect from diethyl ether alone may be because it is not soluble in water. After mixing, the acid-alcohol and diethyl ether partitioned rapidly into separate phases, limiting contact of the eggs with the ether, whereas the acid-alcohol remained in contact with the eggs.
Response to a toxic substance is commonly modeled as a function of the dose, which is the product of the toxin concentration and the exposure time. This approach is often used to develop a quantitative dose-response relationship for a specific toxin or combination of toxins, and it can be applied to evaluate the inactivation of Ascaris eggs as a result of exposure to harmful reagents. The dose concept is also useful for elucidating a response to multiple exposures. Although insufficient data were collected in this research to develop a dose-response curve, a calculation of the ethanol dose can be used to determine at which point the greatest exposure of the eggs to ethanol occurred.
In the samples treated with acid-alcohol (treatments 5 to 9), exposure to ethanol occurred both during the extraction step and during the 1-month incubation period. During the extraction step, 4.5 ml of 35% ethanol solution was mixed with 3 ml of diethyl ether (or ethyl acetate) and 1.5 ml of sample. After mixing, two phases formed, with the diethyl ether (or ethyl acetate) floating on top of the acid-alcohol mixture containing the eggs. Thus, the concentration of ethanol in contact with the eggs after mixing was approximately 350 ml/liter × 4.5 ml/6 ml × 0.78 g/ml = 205 g/liter. The resulting ethanol doses during the extraction step were 205 g/liter × 0.5/24 day = 4.3 g · days/liter and 205 g/liter × 1/25 day = 8.5 g · days/liter, in the samples with minimum and maximum exposures, respectively.
In the samples with a maximum exposure, 1.5 ml of incubation reagent (0.1 N H2SO4) was added directly to the 1.5 ml of sample remaining after the extraction step, reducing the residual ethanol concentration by one-half, to 102 g/liter. Thus, during incubation the ethanol dose was approximately 102 g/l × 28 days = 2867 g · days/liter. In the samples with a minimum exposure, the rinsing steps resulted in a 100-fold dilution of the ethanol concentration. The resulting concentration during incubation was approximately 2 g/liter, resulting in a dose of 2 g/liter × 28 days = 57 g · days/liter.
The combined ethanol dose during the extraction and incubation steps in the samples with a minimum exposure was 62 g · days/liter, whereas the combined dose in the samples with a maximum exposure was about 2875 g · days/liter. Given that the minimum dose resulted in no response in the eggs, it is unlikely that the exposure of 8.5 g · days/liter during extraction in the samples with maximum exposure caused any inactivation of the eggs. It is concluded that the observed decrease in egg viability in the samples with maximum exposure was primarily due to the ethanol dose of approximately 2,867 g · days/liter that occurred during the 28-day incubation period.
A complete dose analysis would also account for the synergistic effect of the diethyl ether or ethyl acetate during the extraction step but is not undertaken here. It is proposed that the general protocol for dose analysis outlined above can be used to assess the response of helminth eggs to any reagent of interest. In particular, additional experiments are needed to determine the dose-response relationship of Ascaris eggs to ethanol, both alone and in combination with diethyl ether or ethyl acetate. From the dose-response relationship, the maximum allowable dose before inactivation occurs could be clearly defined.
The results of this study are important, given that a recommended exposure time to the extraction reagents is not stated in the U.S. EPA procedure, nor is a rinsing step required to remove residual traces of the reagents prior to incubation. The importance of the rinsing step depends, of course, on the residual volume of acid-alcohol solution that remains in the sample after the supernatant is removed following the final centrifugation and the volume of culturing solution that is added before incubation. These volumes may vary depending on the practices of each particular laboratory.
Based on the results of this study, it is recommended that helminth procedures that utilize acid-alcohol be modified to ensure that no inactivation of Ascaris eggs occurs. Three options are proposed. (i) Clearly state the maximum allowable exposure time to acid-alcohol and diethyl ether (or ethyl acetate), and include a rinsing step before incubation. For example, under the conditions of this study, the negative effect on viability was avoided by limiting the exposure time to the extraction reagents to 30 min and reducing the residual concentration of reagents in the incubation solution to 1/100 of the original concentration by a series of rinsing steps. (ii) Replace the reagents used during the extraction step with reagents that do not affect egg viability. For example, in this study the decrease in viability was avoided by using acetoacetic buffer instead of the acid-alcohol solution. However, more research is needed to compare the effectiveness of acid-alcohol and acetoacetic buffer for cleaning the sample. The ethanol in the acid-alcohol is believed to play a role in separating the eggs from the particulate matter in the sample and has been widely used for processing sludge samples. The acetoacetic buffer was developed to optimize the recovery of helminth eggs from feces (5), but a comparative study is needed on the effectiveness of these reagents with wastewater and sludge samples. (iii) Eliminate the phase extraction step. For example, the step can be eliminated by pouring the entire sample (after flotation) through a 38-μm-mesh sieve; this option was used on the sludge samples in this study and has also been used by other researchers (D. D. Bowman and M. D. Little, Proc. Water Environ. Fed. Technol. Conf., 1998). One potential disadvantage with the 38-μm-mesh sieve is that smaller eggs such as Trichuris pass through it and are lost from the sample (data not shown); however, the most recent U.S. EPA procedure aims only to recover the eggs of Ascaris (23). In addition, the sieving step may be insufficient to clean samples that contain a high concentration of particles similar in size to the eggs (e.g., algae).
Ultimately, the most effective method for enumerating viable helminth eggs will be one that is flexible enough to account for differences in sample matrices and egg concentrations yet provides sufficient guidelines to preserve the viability of the eggs. To inform the development of such a method, more research is needed on the effectiveness of the various reagents employed.
ACKNOWLEDGMENTS
We thank the Engineering Institute at the National Autonomous University of Mexico, Mexico City, Mexico, for providing the laboratory facilities, office space, and institutional support that made this research possible.
Financial support from the Fulbright Foundation and the University of California Institute for Mexico and the United States was invaluable.
REFERENCES
- 1.Anderson T J C, Jaenike J. Host specificity, evolutionary relationships and macrogeographic differentiation among Ascaris population from humans and pigs. Parasitology. 1997;115:325–342. doi: 10.1017/s0031182097001339. [DOI] [PubMed] [Google Scholar]
- 2.Arene F O I. Ascaris suum: influence of embryonation temperature on the viability of the infective larva. J Thermal Biol. 1986;11:9–15. [Google Scholar]
- 3.Ayres R M, Mara D D. Analysis of wastewater for use in agriculture: a laboratory manual of parasitological and bacteriological techniques. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 4.Ayres R M, Stott R, Lee D L, Mara D D, Silva S A. Comparison of techniques for the enumeration of human parasitic helminth eggs in treated wastewater. Environ Technol. 1991;12:617–623. [Google Scholar]
- 5.Bailenger J. Mechanisms of parasitological concentration in coprology and their practical consequences. J Am Med Technol. 1979;41:65–71. [Google Scholar]
- 6.Beaver P C, Jung R C, Cupp E W. Clinical parasitology. 9th ed. Philadelphia, Pa: Lea & Febiger; 1984. [Google Scholar]
- 7.Boisvenue R J. Effects of aeration and temperature in in vitro and in vivo studies on developing and infective eggs of Ascaris suum. J Helminthol Soc Wash. 1990;7:51–56. [Google Scholar]
- 8.Bouhoum K, Schwartzbrod J. Quantification of helminth eggs in wastewater. Zentbl Hyg Umweltmed. 1989;188:322–330. [PubMed] [Google Scholar]
- 9.Bowman D D, Little M D, Reimers R S. Procedure for the examination of wastewater sludge or sludge product for helminth eggs. New Orleans, La: School of Tropical Health and Medicine, Tulane University; 1998. [Google Scholar]
- 10.Crompton D W T. How much human helminthiasis is there in the world? J Parasitol. 1999;85:379–403. [PubMed] [Google Scholar]
- 11.Eaton A D, Clesceri L S, Greenberg A E. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C.: American Public Health Association, American Water Works Association, and Water Environment Federation; 1995. [Google Scholar]
- 12.Feachem R G, Bradley D J, Garelick H, Mara D D. Sanitation and disease: health aspects of excreta and wastewater management. New York, N.Y: John Wiley & Sons, Inc.; 1983. [Google Scholar]
- 13.Gaspard P, Wiart J, Schwartzbrod J. A method for assessing the viability of nematode eggs in sludge. Environ Technol. 1996;17:415–420. [Google Scholar]
- 14.Ghiglietti R, Rossi P, Ramsan M, Colombi A. Viability of Ascaris suum, Ascaris lumbricoides, and Trichuris muris eggs to alkaline pH and different temperatures. Parassitologia. 1995;37:229–232. [PubMed] [Google Scholar]
- 15.Neter J, Kutner M H, Nachtsheim C J, Wasserman W. Applied linear statistical models. 4th ed. Boston, Mass: WCB/McGraw-Hill; 1996. [Google Scholar]
- 16.Oksanen A, Eriksen L, Roepstorff A, Ilsøe B, Nansen P, Lind P. Embryonation and infectivity of Ascaris suum eggs: a comparison of eggs collected from worm uteri with eggs isolated from pig faeces. Acta Vet scand. 1990;31:393–398. doi: 10.1186/BF03547520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reimers R S, Little M D, Akers T G, Henriques W D, Badeaux R C, McDonnell D B, Mbela K K. Persistence of pathogens in lagoon stored sludge. EPA 600/2-89/015, PB 89-190359. Washington, D.C.: National Technical Information Service; 1989. [Google Scholar]
- 18.Rude R A, Peeler J T, Risty N G. Comparison of diethyl ether and ethyl acetate as extracting agents for recovery of Ascaris spp. and Trichuris spp. eggs. J Assoc Offic Anal Chem. 1987;70:1000–1002. [PubMed] [Google Scholar]
- 19.Smith C. Induced larval motility: a possible viability test for embryonated Ascaris lumbricoides ova. Trans R Soc Trop Med Hyg. 1991;85:760. doi: 10.1016/0035-9203(91)90447-7. [DOI] [PubMed] [Google Scholar]
- 20.Steer A G, Nell J H, Wiechers S G. A modification of the Allen and Ridley technique for the recovery of Ascaris lumbricoides ova from municipal compost. Water Res. 1974;8:851–853. [Google Scholar]
- 21.U.S. Environmental Protection Agency. 40 CFR Part 257. Standards for the use and disposal of sewage sludge: final rules. Fed Regist. 1993;58:9248. [Google Scholar]
- 22.U.S. Environmental Protection Agency. Control of pathogens and vector attraction in sewage sludge EPA/625/R-92/013. U.S. Washington, D.C.: Environmental Protection Agency; 1992. [Google Scholar]
- 23.U.S. Environmental Protection Agency. Control of pathogens and vector attraction in sewage sludge EPA/625/R-92/013. 1999. Revised edition. U.S. Environmental Protection Agency, Washington, D.C. [Google Scholar]
- 24.Wharton D. Nematode egg-shells. Parasitology. 1980;81:447–463. doi: 10.1017/s003118200005616x. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Health guidelines for the use of wastewater in agriculture and aquaculture. Report of a WHO scientific group. W H O Tech Rep Ser. 1989;778:1–74. [PubMed] [Google Scholar]
- 26.Zhu X, Chilton N B, Jacobs D E, Boes J, Gasser R B. Characterization of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. Int J Parasitol. 1999;29:469–478. doi: 10.1016/s0020-7519(98)00226-4. [DOI] [PubMed] [Google Scholar]


