Abstract
Objectives
To identify key epidemiological factors relevant to fetal development that are associated with biliary atresia
Study design
This population-based registry study examined infants born in Texas between 1999 and 2014. Epidemiological data relevant to fetal development was compared between cases of biliary atresia identified in the Texas Birth Defects Registry (n=305) versus all live births (n=4,689,920), and Poisson regression was used to calculate prevalence ratios (PRs) and 95% confidence intervals (CIs).
Results
The prevalence of BA was 0.65 per 10,000 live births over the study period. BA was positively associated with female sex (adjusted PR 1.68, 95% CI: 1.33–2.12), delivery before 32–37 weeks gestation (adjusted PR 1.64, 95%CI 1.18–2.29), delivery before 32 weeks gestation (adjusted PR 3.85, 95%CI 2.38–6.22), and non-Hispanic Black versus non-Hispanic White maternal race/ethnicity (adjusted PR 1.54, 95%CI 1.06–2.24), and BA was inversely associated with season of conception in the fall relative to spring (adjusted PR 0.62, 95%CI 0.45–0.86). In addition, BA was associated with maternal diabetes (adjusted PR 2.34, 95%CI 1.57–3.48), with a stronger association with pre-gestational compared with gestational diabetes. In sub-group analyses, these associations were present in isolated BA cases which do not have any additional birth defects.
Conclusions
BA is associated with multiple factors related to fetal development, including pre-gestational maternal diabetes, female sex, and preterm birth. These associations were also observed in isolated cases of biliary atresia without other malformations or laterality defects. Our results are consistent with early life events influencing the pathogenesis of biliary atresia, and support further studies investigating in utero events to better understand the etiology and time of onset.
Keywords: prematurity, maternal diabetes
Biliary atresia, a serious liver disease of infancy, which has an estimated prevalence of 5 to 10 per 100,000 births, is the leading indication for pediatric liver transplantation worldwide (1). BA is characterized by obstruction of the biliary duct system. As a result, bile cannot exit the liver to the intestines to help digest fats. Instead, bile is retained in the liver, leading to liver injury, progressive liver fibrosis, and, if untreated, end-stage liver disease by the first year of life (2).
Despite significant morbidity of biliary atresia, key aspects of the etiology—including when the disease starts—remain unknown. One possibility is that BA is acquired after birth, which is consistent with infants typically appearing healthy as newborns, discordance in identical twins, and no recurrence in families (3). However, other important lines of evidence argue that BA starts in utero. For example, infants with BA have elevated laboratory markers of bile retention at birth, indicating that disease may already be present (4). In addition, abnormalities on fetal ultrasound and amniotic fluid analysis have been reported, suggesting defects in biliary development occur as early as gestational week 15 (5). Approximately 10–30% of patients with BA also have malformations of other organs, indicating broader problems during embryonic development (6,7). In this study, we propose the novel hypothesis that if BA starts in utero, the disease would be associated with key early epidemiological factors relevant to fetal development. To explore this hypothesis, we leveraged data from a large birth defects registry.
Methods
Study Population
This study was approved by the Texas Department of State Health Services (DSHS) and Baylor College of Medicine institutional review boards. All patients were part of the Texas Birth Defects Registry (TBDR), one of the largest active birth defects surveillance systems operated by the Birth Defects Epidemiology and Surveillance Branch of the Texas DSHS. TBDR staff members routinely visit all maternity hospitals, pediatric hospitals, birthing centers, and midwife facilities in Texas, and examine discharge logs diagnostic codes for birth defects in all infants under one year of age. The diagnostic codes used are six-digit entries based on the British Pediatric Association (BPA) Classification of Diseases and the World Health Organization’s International Classification of Diseases, 9th revision, (ICD-9) system, with modifications by the Centers for Disease Control and Prevention (the codes are referred to as “BPA codes”).
The study population included infants delivered in Texas between January 1, 1999 and December 31, 2014. Infants considered to have BA met three criteria. First, they had at least one medical record under one year of age with the BPA code for BA (751.60). Second, their records were reviewed manually by TDBR staff to ensure the clinical course was consistent with BA. Third, the TDBR records were re-reviewed by a pediatric hepatologist, to exclude patients who were likely to have another condition. Similar to other studies using TDBR data, patients from Texas Public Health Region 5/6 were excluded from this analysis because of differences in case surveillance and ascertainment strategies.(8–10)
Data Collection and Variable Definitions
Early epidemiological factors relevant to fetal development were obtained from vital records. Infant variables included sex, gestational age, season of conception, and plurality. Gestational age was divided into >37 weeks (term), between 32–37 weeks (moderate to late preterm), and <32 weeks (very to extremely preterm), and season of conception was categorized into Spring (March-May), Summer (June-August), Fall (September-November), and Winter (December-February) based on the estimated date of the mother’s last menstrual period. Maternal variables included race/ethnicity (as categorized by TDBR), age, previous pregnancies resulting in a live birth, previous pregnancies not resulting in a live birth, diabetes, body mass index (BMI), education, and residence near the Texas/Mexico border during pregnancy. BMI was divided into categories of underweight (<18.5 kg/m2), normal weight (18.5 to <25.0 kg/m2), overweight (25.0 to <30.0 kg/m2), and obese (≥30 kg/m2). Paternal factors included race/ethnicity, age, and education.
We also evaluated the presence of co-occurring malformations among children with BA (7). Cases of isolated BA (sub-group 1) had no additional major birth defects. Cases of BA with non-laterality birth defects (sub-group 2) had at least one other major malformation not related to malpositioning of organs. Cases of BA with laterality birth defects (sub-group 3) had at least one major malformation related to malpositioning of organs. Similar to the previous report, this included incorrect locations of vessels (anomalies of the aorta, anomalies of the pulmonary artery, partial anomalous pulmonary veins, persistent left superior vena cava, bilateral superior vena cava, or anomalous portal vein termination), thoracic organs (dextrocardia), and/or abdominal organs (displacement or transposition of the stomach, malrotation of the cecum/colon, malrotation of the small intestine, annular pancreas, asplenia, polysplenia, right-sided spleen, or situs inversus).
Statistical Analyses
To identify associations between BA and factors which could influence fetal development, unadjusted prevalence ratios (PRs) and 95% confidence intervals (CIs) were first calculated using Poisson regression analysis with data from all live births as the denominator. Variables were considered significantly associated with BA when the 95%CI excluded 1.00. Adjusted PRs and 95%CIs were then calculated by testing all significant variables in a multivariable adjusted Poisson regression model. For the adjusted models, paternal race/ethnicity was excluded because of a high percentage of missing responses (14.5%). In addition, analyses were repeated for sub-groups of cases based on the presence of co-occurring malformations as described above. All calculations were performed using Stats 14.0 (StataCorp LP, College Station, TX).
Results
There were 4,689,920 births during the study period, including 341 infants with a diagnostic code for BA in the TDBR (Figure 1). Of these, 21 patients were excluded because they were deemed to have a liver disease other than BA after manual review of their TDBR case descriptors, and 15 patients were excluded because they had chromosomal abnormalities or other Mendelian disorders (Table I; available at www.jpeds.com). The remaining 305 patients were considered to have BA without other known genetic syndromes, resulting in a birth prevalence of 6.5 per 100,000 births. Year-by-year, the prevalence varied from 0.49 to 0.78 per 10,000 births without any recognizable temporal trends (Figure 2; available at www.jpeds.com).
Figure 1.
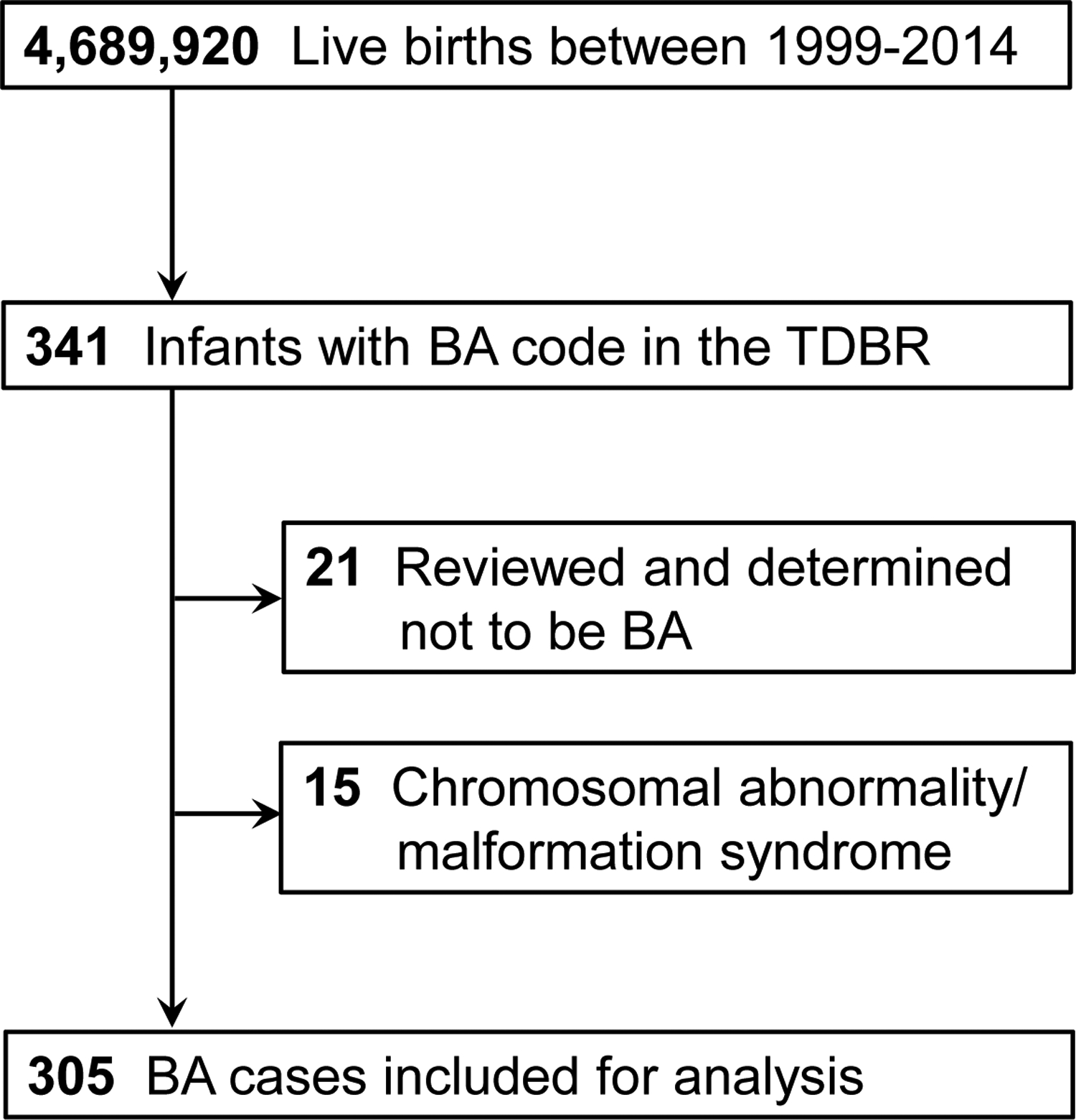
Cases included in this study. Of the births captured by the TDBR between 1999–2014, there were 305 cases of BA not associated with chromosomal abnormalities or Mendelian disorders.
Table I.
Cases coded as BA but excluded because of chromosome abnormalities/malformation syndromes
| BPA Codes | Chromosome abnormality, Mendelian syndrome, malformation complex | N |
|---|---|---|
| 753.xxx | Congenital Anomalies of Urinary System
|
<5 |
| 758.xxx | Chromosomal Anomalies
|
5 |
| 759.xxx | Other and Unspecified Congenital Anomalies
|
9 |
Figure 2.
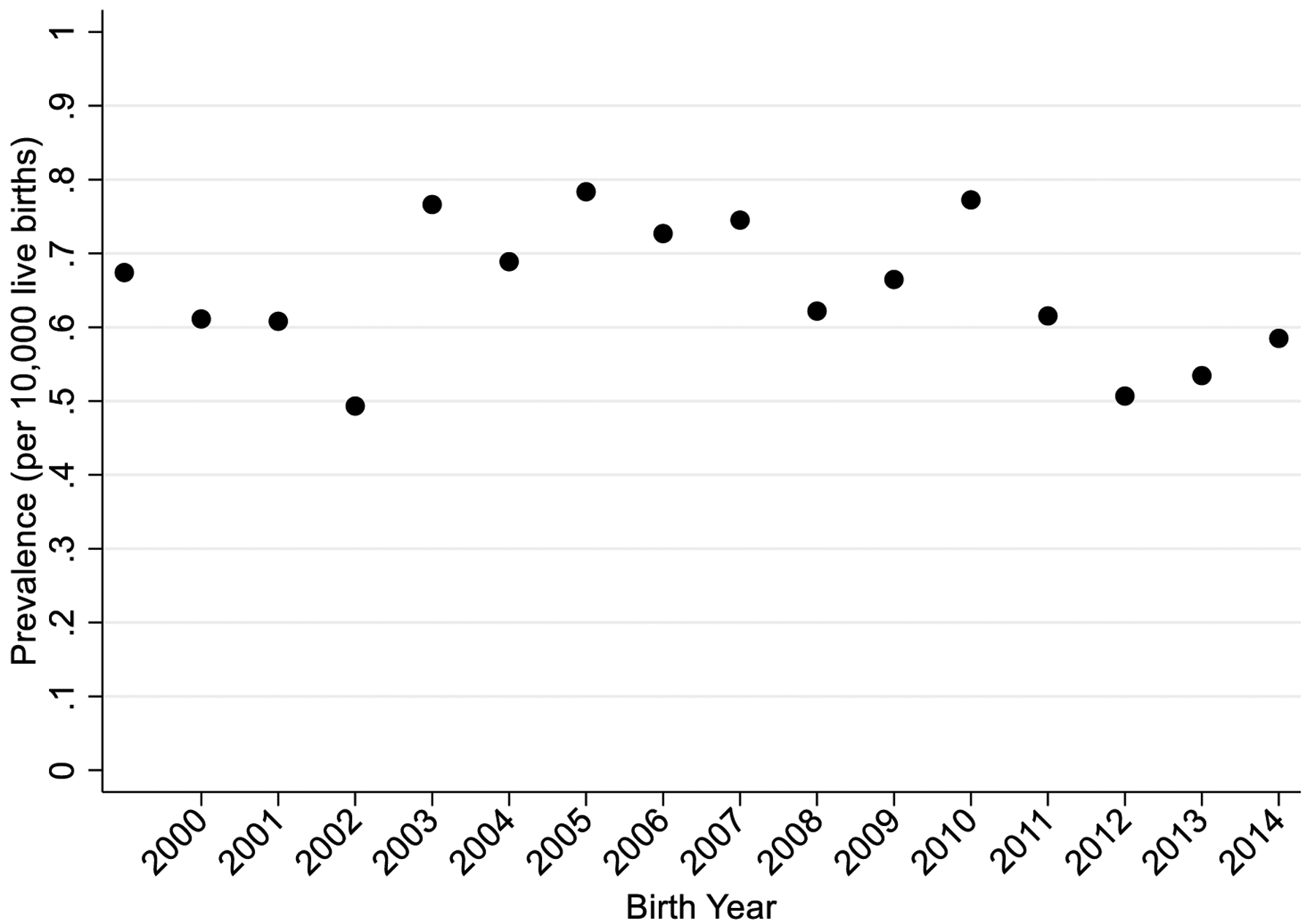
BA birth prevalence in Texas by birth year. There were no significant differences in BA prevalence across years during the study period (p=0.98).
We next evaluated the impact of early epidemiological factors relevant to fetal development on the birth prevalence of BA (Table II). Infant factors that were positively associated with BA included female sex (adjusted PR 1.68, 95%CI 1.33–2.12), gestational age 32–37 weeks (adjusted PR 1.64, 95%CI 1.18–2.29), and gestational age <32 weeks (adjusted PR 3.85, 95%CI 2.38–6.22). An infant factor inversely associated with BA was season of conception in the fall relative to spring (adjusted PR 0.62, 95%CI 0.45–0.86). Maternal factors that were significantly more common in BA cases included non-Hispanic Black relative to non-Hispanic White race/ethnicity (adjusted PR 1.54, 95%CI 1.06–2.24) and maternal diabetes (adjusted PR 2.34, 95%CI 1.57–3.48). The association with maternal diabetes was significant for pre-gestational (adjusted PR 4.94, 95%CI 2.32–10.54) but not gestational diabetes. Paternal factors were excluded from the adjusted model because of a high percentage of missing responses.
Table II.
Unadjusted and adjusted associations of BA with epidemiological factors relevant to fetal development
| Characteristic | Live Births (N=4,689,920) | Cases (N=305) | PR (95%CI) | |
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Infant factors | ||||
| Sex | ||||
| Male | 2,398,625 | 117 | 1.00 (Ref) | 1.00 (Ref) |
| Female | 2,291,295 | 188 | 1.68 (1.34–2.12) | 1.68 (1.33–2.12) |
| Gestational Age | ||||
| 37 weeks | 4,166,862 | 244 | 1.00 (Ref) | 1.00 (Ref) |
| 32 to 37 weeks | 413,469 | 41 | 1.69 (1.22–2.36) | 1.64 (1.18–2.29) |
| 32 weeks | 77,644 | 18 | 3.96 (2.45–6.39) | 3.85 (2.38–6.22) |
| Season of Conception | ||||
| Spring | 1,168,576 | 93 | 1.00 (Ref) | 1.00 (Ref) |
| Summer | 1,123,261 | 70 | 0.78 (0.57–1.07) | 0.79 (0.58–1.08) |
| Fall | 1,177,803 | 58 | 0.62 (0.45–0.86) | 0.62 (0.45–0.86) |
| Winter | 1,207,419 | 82 | 0.85 (0.63–1.15) | 0.86 (0.64–1.15) |
| Plurality | ||||
| Singleton | 4,550,904 | 293 | 1.00 (Ref) | – |
| Multiple | 138,805 | 12 | 1.34 (0.75–2.39) | |
| Maternal Factors | ||||
| Race/ethnicity | ||||
| NH White | 1,722,437 | 66 | 1.00 (Ref) | 1.00 (Ref) |
| NH Black | 449,082 | 25 | 1.45 (0.92–2.30) | 1.54 (1.06–2.24) |
| Hispanic | 2,334,327 | 108 | 1.21 (0.89–1.64) | 1.22 (0.94–1.59) |
| Other | 178,551 | 13 | 1.90 (1.05–3.44) | 1.94 (1.19–3.19) |
| Age | ||||
| <20 years | 634,163 | 36 | 1.00 (Ref) | – |
| 20–24 years | 1,312,061 | 90 | 1.21 (0.82–1.78) | |
| 25–29 years | 1,271,536 | 83 | 1.15 (0.78–1.70) | |
| 30–34 years | 952,013 | 58 | 1.07 (0.71–1.63) | |
| 35–39 years | 428,106 | 31 | 1.28 (0.79–2.06) | |
| ≥ 40 years | 91,626 | 7 | 1.35 (0.60–3.02) | |
| Previous pregnancies that resulted in live births | ||||
| 0 | 1,784,835 | 102 | 1.00 (Ref) | – |
| 1 | 1,432,004 | 94 | 1.15 (0.87–1.52) | |
| 2+ | 1,392,835 | 103 | 1.29 (0.98–1.70) | |
| Previous pregnancies that did not result in live births | ||||
| 0 | 3,678,199 | 246 | 1.00 (Ref) | – |
| 1 | 674,849 | 32 | 0.71 (0.49–1.02) | |
| 2+ | 282,472 | 23 | 1.22 (0.79–1.87) | |
| Diabetes | ||||
| No | 4,513,478 | 278 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 176,441 | 27 | 2.48 (1.67–3.69) | 2.34 (1.57–3.48) |
| Pre-gestational diabetes* | ||||
| No | 2,983,924 | 190 | 1.00 (Ref) | |
| Yes | 19,839 | 7 | 5.54 (2.61–11.78) | 4.94 (2.32–10.54) |
| Gestational diabetes* | ||||
| No | 2,891,726 | 187 | 1.00 (Ref) | |
| Yes | 112,392 | 10 | 1.38 (0.73–2.60) | 1.30 (0.69–2.46) |
| BMI* | ||||
| Underweight | 126,306 | 8 | 1.00 (Ref) | – |
| Normal | 1,450,521 | 90 | 0.98 (0.48–2.02) | |
| Overweight | 735,353 | 42 | 0.90 (0.42–1.92) | |
| Obese | 670,509 | 53 | 1.25 (0.59–2.62) | |
| Education | ||||
| <12 years | 1,290,001 | 90 | 1.00 (Ref) | — |
| 12 years | 1,378,473 | 99 | 1.03 (0.77–1.37) | |
| 12 years | 1,378,473 | 99 | 1.03 (0.77–1.37) | |
| >12 years | 1,992,755 | 113 | 0.81 (0.62–1.07) | |
| Residence near Texas/Mexico border | ||||
| Yes | 771,758 | 50 | 1.00 (Ref) | — |
| No | 3,918,161 | 255 | 1.00 (0.74–1.36) | |
| Paternal Factors | ||||
| Race/Ethnicity | ||||
| NH White | 1,509,533 | 82 | 1.00 (Ref) | – |
| NH Black | 365,479 | 33 | 1.66 (1.11–2.49) | |
| Hispanic | 1,976,540 | 124 | 1.15 (0.87–1.53) | |
| Other | 157,719 | 20 | 2.33 (1.43–3.81) | |
| Age | ||||
| <20 years | 226,886 | 17 | 1.00 (Ref) | – |
| 20–24 years | 840,910 | 48 | 0.76 (0.44–1.32) | |
| 25–29 years | 1,079,621 | 72 | 0.89 (0.52–1.51) | |
| 30–34 years | 973,270 | 60 | 0.82 (0.48–1.41) | |
| 35–39 years | 563,836 | 34 | 0.80 (0.45–1.44) | |
| ≥ 40 years | 329,460 | 28 | 1.13 (0.62–2.07) | |
| Education | ||||
| <12 years | 1,011,173 | 74 | 1.00 (Ref) | – |
| 12 years | 1,253,607 | 81 | 0.88 (0.64–1.21) | |
| >12 years | 1,710,752 | 100 | 0.80 (0.59–1.08) | |
Analysis limited to subset of patients born between 2005–2014.
Abbreviations: NH = non-Hispanic; PR = prevalence ratio; CI = confidence interval; Ref = reference
When BA cases were divided into sub-groups according to the presence of co-occurring malformations, there were 212 cases in sub-group 1 (69.5%) with isolated BA, 66 cases in sub-group 2 (21.6%) with non-laterality birth defects most commonly affecting the cardiovascular and genitourinary systems, and 27 cases in sub-group 3 (8.9%) with laterality birth defects most commonly affecting the cardiovascular and digestive systems (Table III). Overall, the impact of early epidemiological factors relevant to fetal development was consistent across BA sub-groups, especially for those with isolated BA (Table IV). Examples of exceptions included no associations with female sex in sub-group 3 or with maternal diabetes in sub-group 2, but estimates in these groups were limited by sample size.
Table III.
Organ systems affected in sub-groups of BA with major malformations
| BPA Codes | Organ System | Malformation category* | |
|---|---|---|---|
| Sub-group 2: Not related to laterality (n=66) | Sub-group 3: Related to laterality (n=27) | ||
| 742,743 | Nervous System and Eye, no. of occurrences | 11 | 0 |
| 744 | Ear, Face, and Neck, no. of occurrences | <5 | 0 |
| 745,746, 747 | Heart and Circulatory System, no. of occurrences | 50 | 14 |
| 750,751 | Digestive System, no. of occurrences | 10 | 11 |
| 752,753 | Genitourinary System, no. of occurrences | 27 | 0 |
| 754,755,756 | Musculoskeletal, no. of occurrences | 11 | 0 |
| 757 | Skin, no. of occurrences | <5 | 0 |
| 759 | Other/Unspecified, no. of occurrences | <5 | 14 |
A patient may be represented more than once within these counts as multiple patients may have ≥2 diagnoses of major malformations
Table IV.
Associations of factors with sub-groups of BA
| Characteristic | Adjusted PR (95%CI)* | ||
|---|---|---|---|
| Sub-group 1: Isolated BA (n=212) | Sub-group 2: BA with non-laterality birth defects (n=66) | Sub-group 3: BA with laterality defects (n=27) | |
| Infant sex | |||
| Male | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Female | 1.76 (1.33–2.32) | 1.71 (1.04–2.83) | 1.14 (0.54–2.43) |
| Gestational Age | |||
| >37 weeks | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 32–37 weeks | 1.09 (0.68–1.73) | 3.80 (2.13–6.77) | 2.10 (0.79–5.60) |
| <32 weeks | 2.91 (1.54–5.52) | 8.63 (3.86–19.31) | 2.32 (0.31–17.33) |
| Season of conception | |||
| Spring | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Summer | 0.70 (0.48–1.02) | 1.12 (0.57–2.22) | 0.86 (0.36–2.07) |
| Fall | 0.66 (0.45–0.97) | 0.81 (0.39–1.69) | 0.09 (0.01–0.70) |
| Winter | 0.84 (0.59–1.19) | 1.16 (0.60–2.25) | 0.53 (0.20–1.43) |
| Maternal race/ethnicity | |||
| NH White | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| NH Black | 1.39 (0.88–2.21) | 2.07 (0.94–4.58) | 1.62 (0.50–5.27) |
| Hispanic | 1.17 (0.86–1.60) | 1.60 (0.89–2.89) | 0.93 (0.39–2.21) |
| Other | 1.84 (1.01–3.33) | 2.43 (0.81–7.27) | 1.86 (0.40–8.67) |
| Maternal diabetes | |||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 2.16 (1.32–3.56) | 1.42 (0.51–3.92) | 6.83 (2.73–17.08) |
| Pre-gestational diabetes** | |||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 3.21 (1.02–10.10) | 2.71 (0.37–19.84) | 26.58 (7.51–94.04) |
| Gestational diabetes** | |||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 1.14 (0.50–2.58) | 1.17 (0.28–4.86) | 2.87 (0.66–12.56) |
In order to compare a full complement across the three sub-groups, this model adjusts for the same variables of interest regardless of significance within each individual sub-group.
Analysis limited to subset of patients born between 2005–2014 (n=137 in sub-group 1, n=42 in sub-group 2, and n=18 in sub-group 3).
Abbreviations: NH = Non-Hispanic; PR = Prevalence ratio; CI = Confidence interval; Ref = Reference
Discussion
Our findings suggest that factors related to fetal development appear to predispose infants to BA. Specifically, we found that BA was significantly more common among infants who were female, premature, conceived in seasons other than the fall, born to non-Hispanic Black mothers, or born to mothers with pre-gestational diabetes. Importantly, the associations were also present in infants with isolated BA, who have no other major malformations or signs of disrupted fetal development. The results raise the possibility that early life events influence BA occurrence, and they support the hypothesis that BA begins in utero.
Some associations identified in this study agree with previous reports, though it is still unclear how they could contribute to the pathogenesis of biliary atresia. For example, many but not all studies have reported a higher occurrence of BA in females (11–16). However, to date no female-specific factors that may contribute to problems in bile duct development have been found. Similarly, multiple studies have reported higher occurrence in preterm infants (14–17). One possibility is that an early biliary event may cause fetal distress and a premature delivery. Alternatively, infants delivered prematurely may be prone to bile duct damage when out of the in utero environment. Possible associations with season of conception/birth and maternal race/ethnicity similar have also been reported (12–14,18–23). One study in French Polynesia identified higher occurrence with birth in the dry season, and different studies on race/ethnicity suggest that BA occurs evenly across the population or more often in non-Hispanic Black mothers (18–20,22).
We found two notable associations between diabetes and BA. First, maternal diabetes was strongly associated with isoalted BA. Previous studies have reported associations with BA with laterality defects, including one study examining gestational diabetes specifically (7,24). The additional observation in isolated BA raises the possibility that fetal hyperglycemia could influence development of bile ducts in isolation, without impairing development of other organs. Second, in our sensitivity analysis of cases between 2005–2014 when diabetes onset was also recorded, pre-gestational rather than gestational diabetes had a significant association with BA. a similar observation was made with data from the National Birth Defects Prevention Study (25). The findings point to BA starting early in development, before 24 weeks of gestation which precedes the hyperglycemia from gestational diabetes (26). This potential early time period of onset agrees with other BA studies, which showed abnormal gallbladder morphology as early as 15 weeks gestation or low amniotic fluid GGT levels (indicating biliary obstruction) at 18–19 weeks gestation (5).
In addition to factors associated with BA, the study identified factors not associated with BA that provide potential clues to BA pathogenesis. For example, maternal age was not significantly associated with BA, suggesting that chromosomal aberrations which arise when gametes age do not promote BA development. In addition, birth order was not associated with the prevalence of BA. If BA were an alloimmune disease, first pregnancies would be less likely affected whereas subsequent pregnancies would the most susceptible (27). Finally, infants born to mothers living near the Texas-Mexico border were not more likely to have BA. Living near the Texas-Mexico border is an independent risk factor for development of other birth defects (28).
An important study limitation is the way cases of biliary atresia were ascertained. Although the TDBR has unprecedented coverage across Texas and has been validated in previous birth defects studies, there are scenarios in which cases could be misclassified. For example, a patient would have been missed if born in Texas but then moved to another state before BA was diagnosed. In addition, a patient without BA could have been included if the data in the TDBR data was not complete enough to exclude infants with an incorrect diagnosis of biliary atresia. However, we assume TDBR accurately captured BA, because the birth prevalence matches the birth prevalence in other studies (1). In addition, the percent in each sub-group approximates the 67–84% of cases previously reported for isolated BA, 6% of cases with BA and non-laterality defects, and 10% of cases with BA and laterality defects (7).
In conclusion, we have identified factors relevant to fetal development which are more common in infants with biliary atresia than the general population. These include female sex, preterm birth, and pre-gestational maternal diabetes. The associations are also present in isolated BA cases which do not have any other malformations. Our results are consistent with early life events influencing the pathogenesis of biliary atresia, and support further studies investigating in utero events.
Supplementary Material
Funding:
SH is supported by NIH NIDDK K23 DK109207 and R03 DK128535
Abbreviations:
- PR
Prevalence ratio
- CI
Confidence interval
- TDBR
Texas Birth Defects Registry
- BPA
British Pediatric Association
- BMI
Body Mass Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: SH serves on the DSMB for a therapeutic trial for biliary atresia (DSMB coordinated by Syneos Health). The other authors declare no conflicts of interest.
References
- 1.Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI. International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr. 2013. Apr;56:344–54. [DOI] [PubMed] [Google Scholar]
- 2.Feldman AG, Mack CL. Biliary Atresia. J Pediatr Gastroenterol Nutr. 2015. Aug;61:167–75. [DOI] [PubMed] [Google Scholar]
- 3.Fallon SC, Chang S, Finegold MJ, Karpen SJ, Brandt ML. Discordant presentation of biliary atresia in premature monozygotic twins. J Pediatr Gastroenterol Nutr. 2013. Oct;57:e22–3. [DOI] [PubMed] [Google Scholar]
- 4.Harpavat S, Finegold MJ, Karpen SJ. Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics. 2011. Dec;128:e1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mysore KR, Shneider BL, Harpavat S. Biliary Atresia as a Disease Starting In Utero: Implications for Treatment, Diagnosis, and Pathogenesis. J Pediatr Gastroenterol Nutr. 2019. Oct;69:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport M, Savage M, Mowat AP, Howard ER. Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery. 1993. Jun;113:662–8. [PubMed] [Google Scholar]
- 7.Schwarz KB, Haber BH, Rosenthal P, Mack CL, Moore J, Bove K, et al. Extrahepatic anomalies in infants with biliary atresia: results of a large prospective North American multicenter study. Hepatology. 2013. Dec;58:1724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schraw JM, Benjamin RH, Scott DA, Brooks BP, Hufnagel RB, McLean SD, et al. A Comprehensive Assessment of Co-occurring Birth Defects among Infants with Non-Syndromic Anophthalmia or Microphthalmia. Ophthalmic Epidemiol. 2021;28:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schraw J, Woodhouse J, Langlois P, Canfield M, Scheuerle A, Agopian A, et al. Risk factors and time trends for isolated craniosynostosis. Birth defects Res. 2021. Jan 1;113:43–54. [DOI] [PubMed] [Google Scholar]
- 10.Langlois P, Schraw J, Hoyt A, Lupo P. Leveraging a phenome-wide approach to identify novel exposure-birth defect associations: A proof of concept using maternal smoking and a spectrum of birth defects. Birth defects Res. 2021. Mar 15;113:439–45. [DOI] [PubMed] [Google Scholar]
- 11.Karrer FM, Lilly JR, Stewart BA, Hall RJ. Biliary atresia registry, 1976 to 1989. J Pediatr Surg. 1990. Oct;25:1076–80; discussion 1081. [DOI] [PubMed] [Google Scholar]
- 12.Lee K, Kim J, Moon J, Ko J. Epidemiology of Biliary Atresia in Korea. J Korean Med Sci. 2017;32:656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada H, Muraji T, Yokoi A, Okamoto T, Sato S, Takamizawa S, et al. Insignificant seasonal and geographical variation in incidence of biliary atresia in Japan: a regional survey of over 20 years. J Pediatr Surg. 2007. Dec;42:2090–2. [DOI] [PubMed] [Google Scholar]
- 14.Caton A, Druschel C, McNutt L. The epidemiology of extrahepatic biliary atresia in New York State, 1983–98. Paediatr Perinat Epidemiol. 2004. Mar;18:97–105. [DOI] [PubMed] [Google Scholar]
- 15.Chiu C-Y, Chen P-H, Chan C-F, Chang M-H, Wu T-C, Group TISCCS. Biliary Atresia in Preterm Infants in Taiwan: A Nationwide Survey. J Pediatr. 2013. Jul;163:100–103.e1. [DOI] [PubMed] [Google Scholar]
- 16.Fischler B, Haglund B, Hjern A. A population-based study on the incidence and possible pre- and perinatal etiologic risk factors of biliary atresia. J Pediatr. 2002. Aug 1;141:217–22. [DOI] [PubMed] [Google Scholar]
- 17.van Wessel D, Boere T, Hulzebos C, de Kleine R, Verkade H, Hulscher J. Preterm Infants With Biliary Atresia: A Nationwide Cohort Analysis From The Netherlands. J Pediatr Gastroenterol Nutr. 2017. Oct 1;65:370–4. [DOI] [PubMed] [Google Scholar]
- 18.Girard M, Jannot A, Besnard M, Leutenegger A, Jacquemin E, Lyonnet S, et al. Polynesian ecology determines seasonality of biliary atresia. Hepatology. 2011. Nov;54:1893–4. [DOI] [PubMed] [Google Scholar]
- 19.The N, Honein M, Caton A, Moore C, Siega-Riz A, Druschel C. Risk factors for isolated biliary atresia, National Birth Defects Prevention Study, 1997–2002. Am J Med Genet A. 2007. Oct 1;143A:2274–84. [DOI] [PubMed] [Google Scholar]
- 20.Yoon P, Bresee J, Olney R, James L, Khoury M. Epidemiology of biliary atresia: a population-based study. Pediatrics. 1997. Mar;99:376–82. [DOI] [PubMed] [Google Scholar]
- 21.Strickland A, Shannon K. Studies in the etiology of extrahepatic biliary atresia: time-space clustering. J Pediatr. 1982;100:749–53. [DOI] [PubMed] [Google Scholar]
- 22.Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, Golmard J, Auvert B. Epidemiology of biliary atresia in France: a national study 1986–96. J Hepatol. 1999;31:1006–13. [DOI] [PubMed] [Google Scholar]
- 23.Houwen R, Kerremans I, van Steensel-Moll H, van Romunde L, Bijleveld C, Schweizer P. Time-space distribution of extrahepatic biliary atresia in The Netherlands and West Germany. Z Kinderchir. 1988;43:68–71. [DOI] [PubMed] [Google Scholar]
- 24.Davenport M, Tizzard S, Underhill J, Mieli-Vergani G, Portmann B, Hadzić N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr. 2006. Sep;149:393–400. [DOI] [PubMed] [Google Scholar]
- 25.Tinker S, Gilboa S, Moore C, Waller D, Simeone R, Kim S, et al. Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997–2011. Am J Obstet Gynecol. 2020. Feb 1;222:176.e1–176.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyer V Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014. Mar 18;160:414–20. [DOI] [PubMed] [Google Scholar]
- 27.Muraji T, Hosaka N, Irie N, Yoshida M, Imai Y, Tanaka K, et al. Maternal microchimerism in underlying pathogenesis of biliary atresia: quantification and phenotypes of maternal cells in the liver. Pediatrics. 2008. Mar;121:517–21. [DOI] [PubMed] [Google Scholar]
- 28.Suarez L, Felkner M, Brender J, Canfield M, Zhu H, Hendricks K. Neural tube defects on the Texas-Mexico border: what we’ve learned in the 20 years since the Brownsville cluster. Birth Defects Res A Clin Mol Teratol. 2012. Nov;94:882–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


