Abstract
A 28-year-old female who underwent an uneventful femtosecond laser enabled keratoplasty (FLEK) in her left eye presented with pain, redness, and blurring of vision in the operated eye two weeks after getting immunized with COVID-19 vector vaccine (ChAdOx1 nCoV19 Vaccine Recombinant COVISHIELD, AstraZeneca). Slit-lamp examination showed donor stromal edema with Descemet’s membrane folds and Khodadoust line (KP’s on endothelium) with anterior chamber cells and flare. The patient was diagnosed with acute corneal graft rejection and advised hourly topical steroids with cycloplegics and oral steroids. The patient responded to treatment and there was progressive reversal of graft rejection with the patient achieving best spectacle-corrected visual acuity (BSCVA) of 20/30 after five weeks of treatment. Our case highlights possible immune corneal graft rejection after COVID19 vaccination and the need to step up topical steroids before vaccination.
Keywords: COVID-19, graft rejection, keratoplasty, Khodadoust line, vaccination
Corneal transplantation or keratoplasty is the most successful allogeneic transplant as cornea is devoid of vasculature, thereby minimizing risk of graft rejection.[1]
The impact of COVID-19 pandemic on the world has largely been disruptive in terms of obtaining corneal tissue for transplant as well as higher cases of transplant rejection due to inability to follow up regularly. Vaccination started in India on 16 January 2021 and presently, 64% of the population has been fully vaccinated.[2]
We report a case where a patient, who had undergone femtosecond laser enabled keratoplasty (FLEK), developed acute corneal graft rejection two weeks after receiving their first shot of ChAdOx1 nCoV-19 vaccine (COVISHIELD, AstraZeneca).[3]
Case Report
A 28-year-old female patient diagnosed with pellucid marginal degeneration underwent uneventful FLEK in her left eye in 2010. Patient was under regular follow-up and at her last follow-up in March 2021, had a best spectacle-corrected visual acuity (BSCVA) of 20/20. Slit-lamp examination showed central clear corneal graft of 9 mm with well-opposed graft-host junction with no evidence of vascularization of the peripheral recipient cornea. She continued using topical loteprednol 0.2% eye drops once a day along with topical lubricants, as advised. The patient had no known risk factors for graft rejection like loose sutures, neovascularization, peripheral anterior synechiae, trauma, or any previous episode of rejection preceding vaccination. She had not tested positive for SARS-CoV-2 at any point.
Patient reported to us in July 2021 with history of sudden onset of redness, pain, watering and blurring of vision in her left eye for the past three days. BSCVA was 20/200 in the left eye and intraocular pressure (IOP) was 19 mmHg. Slit-lamp examination showed diffuse circumcorneal congestion, localized corneal stromal edema limited to the inferotemporal area by keratic precipitates on the endothelium (Khodadoust line), Descemet membrane folds, anterior chamber (AC) inflammation (cells +1, flare +1) and a clear lens. The right eye showed no evidence of inflammation. Dilated fundoscopy was normal in both eyes. Furthermore, patient gave history of taking a single intramuscular injection of COVID-19 vaccine two weeks before the onset of symptoms [Fig. 1].
Figure 1.
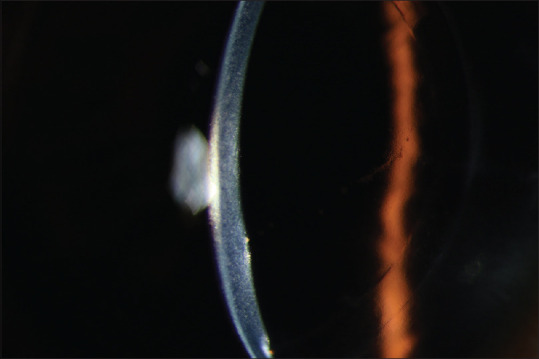
Acute endothelial corneal transplant rejection with stromal edema and KPs on endothelium
Patient was diagnosed as acute corneal graft rejection and started on topical prednisolone 1% w/v hourly, topical homatropine eye drops three times and with oral methyl prednisolone 50 mg once daily for seven days.
At follow-up three days later, symptoms and signs of inflammation were resolving. Slit-lamp examination showed resolving stromal edema and AC reaction [Fig. 2]. BSCVA improved to 20/45. Frequency of topical steroids was reduced to six times a day for seven days, afterwards tapered weekly.
Figure 2.
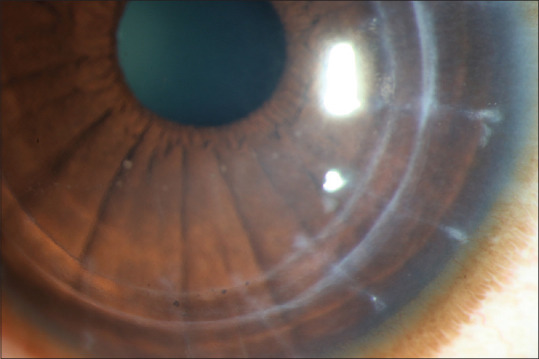
Diffuse circumcorneal congestion with localized corneal stromal edema limited to inferotemporal area by keratic precipitates on endothelium (Khodadoust line) along with Descemet membrane folds
At five weeks post-rejection onset, BSCVA improved to 20/30 with no active inflammation.
Discussion
Late onset corneal graft rejection can occur following local factors such as vascularization, peripheral anterior synechiae, large graft, intraocular surgeries, etc., and also following systemic factors such as blood transfusion, other solid organ transplants, or vaccination.[4] Corneal graft rejection following vaccination has been reported, though meta-analyses show no correlation between vaccination and graft rejection.[5]
Corneal graft rejection has before been described post influenza vaccination where, it is believed, the response was due to the compromised immune status of the recipients. But direct causal relationship couldn’t be established between the vaccine and rejection process. The influenza vaccines most commonly administered can be either the inactivated whole virus, “detergent”-split or subunit vaccines.
The ChAdOx1 nCoV-19 messenger RNA vaccine, however, uses a chimpanzee adenovirus (AZD1222 or ChAdOx1), which carries the SARS-CoV-2 spike protein. Following administration, the genetic material of the part of coronavirus is expressed which stimulates an immune response. Despite the immune privilege of the cornea, immune-mediated corneal allograft rejection can occur, especially after penetrating keratoplasty due to breakdown in the blood aqueous barrier.[6]
The main mediators for corneal allograft rejection are the CD4 Th1 cells which preferentially produce IFN-gamma and mediate delayed type hypersensitivity, leading to rejection. Vaccination incites immune response that can induce Class II MHC complex antigens in all layers of the grafted cornea and could trigger allograft rejection.[7] The effector response in endothelial rejection is characterized by AC infiltration of monocyte-derived macrophages, CD4+ and CD8+ T cells. Wasser et al.[8] reported that topical corticosteroid therapy is effective in reversing this type of graft rejection.
Conclusion
Thus, there is a possibility of immune corneal graft rejection after COVID 19 vaccination even 11 years post penetrating keratoplasty. Timing the second dose of vaccination in patients presenting with rejection as well as the need for pre-vaccination immunosuppression regimen in high-risk eyes is equally important. We suggest starting corneal graft recipients on topical corticosteroids or stepping up the topical steroids at least 1 week before taking the vaccine to suppress the immune response and bring down chances of graft rejection. We also advocate informing the patients with corneal transplant to follow-up immediately after the vaccination to look for any signs of graft rejection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh R, Gupta N, Vanathi M, Tandon R, et al. Corneal transplantation in the modern era. Indian J Med Res. 2019;150:7–22. doi: 10.4103/ijmr.IJMR_141_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shivanna Y, Nagaraja H, Kugar T, Shetty R, et al. Femtosecond laser enabled keratoplasty for advanced keratoconus. Indian J Ophthalmol. 2013;61:469–72. doi: 10.4103/0301-4738.116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panda A, Vanathi M, Kumar A, Dash, Priya S, et al. Corneal graft rejection. Surv Ophthalmol. 2007;52:375–96. doi: 10.1016/j.survophthal.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Mulley WR, Dendle C, Ling JEH, Knight SR, et al. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae?A systematic review and meta-analysis. J Heart Lung Transpl. 2018;37:844–52. doi: 10.1016/j.healun.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Wertheim MS, Keel M, Cook SD, Tole DM, et al. Corneal transplant rejection following influenza vaccination. Br J Ophthalmol. 2006;90:925–6. doi: 10.1136/bjo.2006.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravichandran S, Natarajan R, et al. Corneal graft rejection after COVID-19 vaccination. Indian J Ophthalmol. 2021;69:1953–4. doi: 10.4103/ijo.IJO_1028_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasser LM, Roditi E, Zadok D, Berkowitz L, Weill Y, et al. Keratoplasty Rejection After the BNT162b2 messenger RNA vaccine. Cornea. 2021;40:1070–2. doi: 10.1097/ICO.0000000000002761. [DOI] [PMC free article] [PubMed] [Google Scholar]


