Abstract
Purpose:
Assessment of long-term effects of high altitude on choroidal thickness.
Methods:
This prospective cross-sectional study included 88 and 79 age- and sex-matched healthy individuals who were living at sea-level (SL group) and high-altitude (HA group), respectively. Participants were required to have resided in the same place for at least 10 years. Spectral-domain optical coherence tomography (SD-OCT) scans were conducted in two different and were performed within the same time (08:00 am to 10:00 am). Central macular thickness (CMT) and choroidal thickness were measured at five different points (i.e., at the central fovea and 1 mm and 2 mm temporal and nasal of the fovea). Blood hemoglobin (Hb), red blood cell (RBC), hematocrit (Htc) levels, blood oxygen saturation, heart rate, and body mass index (BMI) were compared between groups statistically.
Results:
The HA group had a mean age of 47.5 ± 13.3 years, whereas the SL group was 48.7 ± 13.4 years (P = 0.57). There was no significant difference between the groups in terms of CMT. Subfoveal choroidal thickness (SCFT) was 282.73 ± 87.82 mm in the HA group and 310.49 ± 74.73 in the SL group (P = 0.02). The choroid was found to be thinner at all the measured locations in the HA group except the 2 mm nasal point of the fovea. However, only the difference at an SFCT was statistically significant. Furthermore compared with the SL group statistically significant higher Hb, RBC, Htc levels were determined in the HA group. In the multiple linear regression model analysis, age was found an only effective confounder factor for SCFT (P = 0.001, 95% CI 4.132–2.476).
Conclusion:
The systemic adaptive changes due to chronic high altitude exposure may cause structural changes in the choroidal vascular network. The current study results revealed significant thinning only at SFCT. Large-scale longitudinal studies are needed to obtain more definitive data on this subject.
Keywords: Altitude, choroid, hypercapnia, hypoxia, optical coherence tomography
The choroid and retina consist of dense complex microvascular networks, and they tend to be the potential target tissues in many systemic diseases. Because the spectral-domain optical coherence tomography (SD-OCT) has become commercially available in 2006, the continuous developments in the device technology and scanning methods (enhanced depth imaging-OCT, [EDI-OCT]) have allowed for the easy and reproducible measurement of choroid thickness.[1] As a result, the evaluation of the choroidal vascular network in many systemic diseases as well as in posterior segment diseases has increasingly attracted interest.
An elevation of 1,500–3,500 meters (m) is considered to be a characteristic of high-altitude places.[2] High-altitude habitats can induce some physiological changes in the human body for two reasons: hypobaric hypoxia and low ambient temperature. Ambient temperatures decrease by approximately 1°C for every 150 m increase in elevation. Survival in high-altitude habitats is made possible only by specific adaptive mechanisms that result in the partial restoration of the oxygen uptake capacity of the blood.
Numerous studies have investigated the effects of chronic hypoxia on retinal and choroidal circulation in the course of chronic diseases, such as obstructive sleep apnea syndrome (OSAS) and chronic obstructive pulmonary disease (COPD).[3,4,5,6,7,8,9,10] In addition, we have encountered case series evaluating the effects of acute high-altitude exposure on ocular microcirculation. Currently, no study has evaluated choroidal thickness in healthy individuals living at high altitudes. Thus, this study aims to evaluate the effects of high-altitude exposure on choroidal circulation in the chronic process. Perhaps the changes in choroidal thickness caused by altitude may alter the occurrence of pachychoroid spectrum diseases for persons living at various elevations.
Methods
This prospective cross-sectional study comprised 88 healthy sea-level residents (Ordu City, Turkey; average altitude: 5 m) and 79 high-altitude residents (Ağrı City, Turkey; average altitude: 1,630 m) who were age- and sex-matched over 18 years of age. The research protocol was carried out in accordance with the tenets of the Helsinki declaration, and this study was approved by the local ethics committee. All participants were informed about the examinations and procedures to be performed and signed informed consent forms were obtained from each of them.
All subjects underwent detailed ocular examinations, including best-corrected visual acuity (BCVA) assessment, intraocular pressure (IOP) measurements (using the Goldmann applanation tonometer), slit-lamp biomicroscopy, and fundus viewing. Patients with BCVA values of ≥20/25, with refraction defect values ranging from +3.0 D to −3.0 D, and with IOP values of <21 mm Hg were included. Only individuals who have been residing in their locality for at least 10 years were included in this study.
Patients with systemic and/or ocular diseases that may lead to structural changes in the choroidal vascular bed, such as glaucoma, uveitis, previous vein occlusions, macroaneurysm, age-related macular degeneration, pachychoroid spectrum diseases, and previous eye surgery, were excluded. Meanwhile, blood oxygen saturation levels and heart rate were measured using a classical finger tip-type pulse oximetry (Model 8500A, Nonin Medical, Inc., Minneapolis, MN, USA).
All blood samples taken for the assessment of hemogram parameters were stored in tubes containing ethylenediaminetetraacetic acid, and measurements were performed on the same day on a Sysmex hematology analyzer (Sysmex XT-2000i, Kobe, Japan). Finally, the body mass indexes (BMIs) of the patients were calculated.
OCT scanning
OCT scans were conducted in two different cities using the same device (3D OCT-2000® Topcon, Topcon Inc., Tokyo, Japan) and were performed within the same time (08:00 am to 10:00 am) to avoid diurnal changes in choroidal thickness. All measurements were taken from the myoticpupilla and were performed in a semi-illuminated operating room.
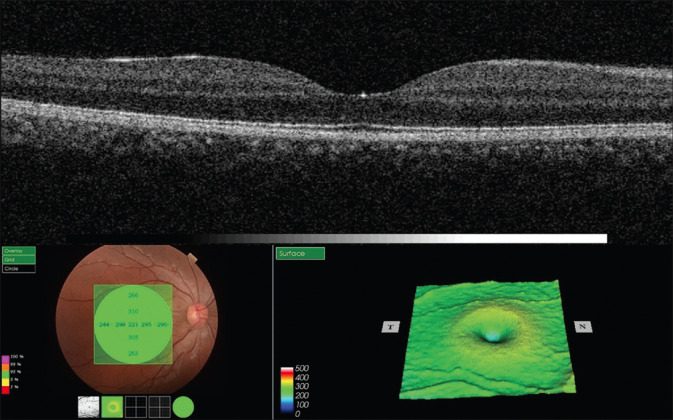
During the same visit, a high-resolution (l = 840 nm, 27,000 A-scans/5 mm axial resolution) 6-mm single-line horizontal scanning (centering on the fovea) was conducted using an SD-OCT platform (3D OCT-2000® Topcon, Topcon Inc., Tokyo, Japan) operating in the enhanced depth imaging (EDI) mode. This device also provided automated central macular thickness (CMT) measurements.[Fig. 1].
Figure 1.
Representative macular thickness map of a 46-year-old male patient including central macular thickness
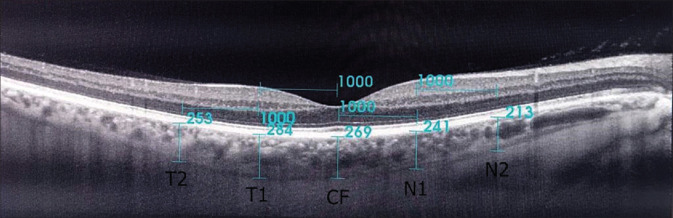
All OCT measurements were performed by two experienced technicians during a single patient visit. For each eye, CTs were measured three times at a 20-min interval and then the mean values were recorded. The eyes in which there is a difference of >5% among the three measurements were excluded. The choroidal thickness, the extent of which starts from the outer edge of the retinal pigment epithelium (RPE) to the inner scleral border, was manually measured [Fig. 2]. The measurements were performed by two independent observers (Dr. M.G. and Dr. S.K). Interobserver differences were within 10% of the mean values.
Figure 2.
Measurement of the choroidal thickness on an OCT image. CF-central fovea, N1-1 mm nasal of the fovea, N2-2 mm nasal of the fovea, T1-1 mm temporal of the fovea, T2-2 mm temporal of the fovea
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) program (v 22.0, IBM-SPSS, Chicago, Illinois, USA). The normality of data distribution was first evaluated using the Kolmogorov–Smirnov test. Then, the Chi-square test was used to compare categorical variables, and a between-group comparison of the normally distributed variables was performed using an independent student’s t-test. Data were presented as mean ± standard deviation. Statistical significance was set at P ≤ 0.05. The subfoveal choroidal thickness (SCFT) measurements were introduced into a multiple linear regression model (as dependent variables).
Results
A total of 79 participants (41 males and 38 females; mean age: 47.55 ± 13.35 years) were included in the high altitude (HA) group, whereas 88 participants (42 males and 46 females; mean age: 48.73 ± 13.44 years) were included in the low altitude (sea level, SL) group. The two groups did not significantly differ in terms of gender and age. The participants’ demographic data, namely, hemogram parameters, blood oxygen saturation, body mass index (BMI), and heart rate, are summarized in Table 1.
Table 1.
Demographic data of the groups
| HA group | SL group | P | |
|---|---|---|---|
| Age (years) | 47.55±13.35 | 48.73±13.44 | 0.57** |
| Sex (F,%/M,%) | 41,%51.9/38, %48.1 | 42, %47.7/46,%52.3 | 0.59* |
| Hgb (g/dL) | 13.71±1.21 | 12.97±1.13 | 0.001** |
| Rbc (×1012/L) | 4.70±0.61 | 4.26±0.48 | 0.001** |
| Hct (%) | 44.20±3.19 | 41.38±3.26 | 0.001** |
| Heart rate | 71.98±7.54 | 70.44±7.15 | 0.17** |
| O2 Saturation (%) | 98.01±0.62 | 97.92±0.72 | 0.37** |
| Smoking status (+,%/–,%) | 32,40.5%/47, 59.5% | 39,54.9%/49,55.7% | 0.61** |
| Body mass index (kg/m2) | 21.93±2.10 | 22.46±2.22 | 0.11** |
HA; high altitude, SL; sea-level, Hgb; hemoglobin, Rbc; red blood cells *Chi-Square test, **Independent Student’s t-test
Blood hemoglobin (Hgb), hematocrit (Hct), and red blood cells (RBC) levels were significantly higher in the HA group than in the SL group. Choroidal thickness measurements were performed at five different points (i.e., at the central fovea and 1 mm and 2 mm nasal and temporal of the fovea).
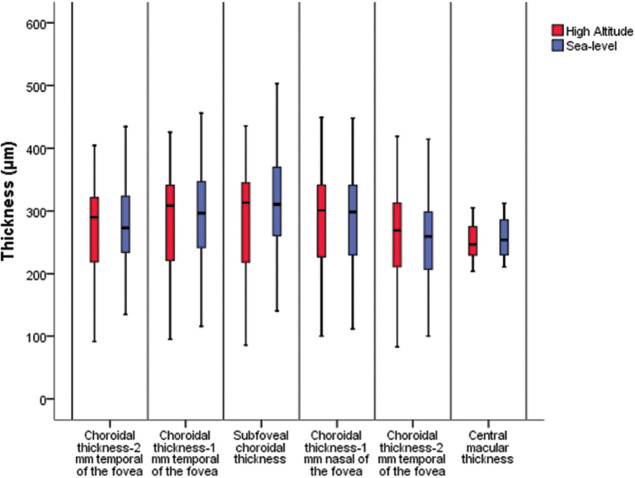
Choroidal thicknesses were lesser in the HA group than in the SL group in all points, except at 2 mm nasal of the fovea. However, only the SCFT was significantly thinner in the HA group than in the SL group (P = 0.02) [Table 2]. The CMT measurements did not significantly differ between the groups [Fig. 3].
Table 2.
Central macular thickness and choroidal thickness measurements of the groups
| HA group | SL group | P* | |
|---|---|---|---|
| CMT (μm) | 252.39±20.44 | 256.55±20.19 | 0.49 |
| SFCT (μm) | 282.73±87.82 | 310.49±74.73 | 0.02 |
| N1 (μm) | 279.75±82.16 | 283.80±73.20 | 0.73 |
| N2 (μm) | 254.87±80.92 | 253.43±66.51 | 0.90 |
| T1 (μm) | 286.27±80.01 | 292.85±68.44 | 0.56 |
| T2(μm) | 270.39±75.09 | 274.30±64.43 | 0.71 |
HA-high altitude, SL-sea-level CMT-central macular thickness SFCT-subfoveal choroidal thickness, N1-1 mm nasal of the fovea, N2-2 mm nasal of the fovea, T1-1 mm temporal of the fovea, T2-2 mm temporal of the fovea. *Independent Student’s t-test
Figure 3.
Comparative Boxplot analysis graphic of data in both groups
In the multiple linear regression model analysis, age was found the only effective confounder factor for SCFT (P = 0.001, 95% confidence interval [CI]: 4.132–2.476) [Table 3].
Table 3.
Multiple linear regression analysis of confounder factors for the subfoveal choroidal thickness
| B | Std. Error | t | Significance | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Lower Bound | Upper Bound | |||||
| (Constant) | 1187.5 | 756.7 | 1.569 | 0.119 | -307.1 | 2682.1 |
| Age | -3.304 | 0.419 | -7.879 | 0.001* | -4.132 | -2.476 |
| BMI | 1.329 | 2.459 | 0.540 | 0.590 | -3.528 | 6.185 |
| O2 Saturation | -11.133 | 7.813 | -1.425 | 0.156 | -26.56 | 4.297 |
| Hgb | 9.536 | 12.286 | 0.776 | 0.439 | -14.73 | 33.80 |
| Rbc | -15.76 | 19.20 | -0.821 | 0.413 | -53.70 | 22.17 |
| Hct | 1.336 | 3.555 | 0.376 | 0.708 | -5.685 | 8.357 |
Adj. R2=0.362 F (12.799) P≤0.001 BMI; body-mass index, Hgb; hemoglobin, Rbc; red blood cells, Hct; hematocrit dependent variable: subfoveal choroidal thickness
Discussion
The retina is one of the most metabolically active tissues in the human body. Outer retinal layers, which contain photoreceptors, are nourished by the choroidal circulation through diffusion. By contrast, the inner retinal layers are fed by superficial and deep retinal vascular plexuses.[11] The retina and choroid could autoregulate the circulation to maintain their adequate oxygen supply.[12,13] Unlike the absence of autonomic innervation, the retinal vascular network has active autoregulation ability.[14] By contrast, choroidal circulation is mainly regulated by the autonomic nervous system and by hormones.[15] Photoreceptors, which are concentrated in the foveal avascular zone, require a continuous supply of high levels of oxygen. This particular need is satisfied only by the choroidal circulation via diffusion.[16]
A limited number of case series and studies have investigated the acute effects of high altitude on choroidal circulation. However, no study has evaluated the effects of high altitude on choroidal thickness in the chronic process.
In our study, choroidal thicknesses were lesser in the HA group than in the SL group, except at 2 mm nasal of the fovea. However, these two groups significantly differed only in terms of the SFCT. Meanwhile, studies have revealed that the choroidal topography shows a marked nasal thinning.[17] Accordingly, no significant difference was observed between the groups at the 2 mm nasal CT. The two groups also did not significantly differ in terms of CMT measurements. In the individuals living at high altitudes, some physiological adaptive changes, such as increased blood Hgb, Hct, and RBC levels, were observed due to hypobaric hypoxic conditions.
Choroidal circulation is characterized by a high blood flow rate and low oxygen extraction and is predominantly sensitive to blood PaCO2 changes.[18] Whereas Bosch et al.[19] found no significant changes in choroidal blood flow in climbers with acute exposure to a high altitude of up to 5,500 m; however, they found an increase in choroidal blood flow at higher altitudes. Moreover, Bosch et al.[19] claimed that the tissue oxygen demand was compensated by the increase in the oxygen-carrying capacity and the oxygen release of the choroid without an increase in choroidal blood flow up to a certain altitude (5,500 m). At higher altitudes, choroidal blood flow increased as a result of hypoxia and the accompanying hypercapnia.
Fischer et al.[20] evaluated the changes in choroidal thickness and the correlation between choroidal thickness alteration and acute mountain sickness parameters following the acute exposure of 14 healthy climbers to high altitudes of up to 4,559 m. However, they obtained only eight robust choroidal thickness measurements due to poor delineation between the choroid and the underlying sclera. Their results revealed a significant increase in central choroidal thickness during altitude exposure. This finding could be explained by the compensatory choroidal hyperperfusion as a response to acute hypoxia. Also, the authors argued that this increase in choroidal blood flow was not correlated with acute mountain sickness parameters (AMS cerebral score, heart rate, and peripheral capillary oxygen saturation [SpO2]) and that such an increase was completely reversible after the climbers returned to low altitude.[20]
Hirukawa-Nakayama et al.[21] presented the case of a 36-year-old individual exposed to an altitude of 4,600 m for 1 week and consequently developed high-altitude retinopathy. Also, they detected a significant choroidal thickening (530 mm OD and 490 mm OS).
Animal studies have shown that chronic hypoxia stimulates the sympathoadrenal system and causes an increase in plasma catecholamine levels. Calbet[22] reported that the plasma noradrenaline and adrenaline concentrations in individuals living at sea level increased significantly in the ninth week of high altitude exposure. Another report has demonstrated the tendency for high blood pressure to develop and for plasma erythropoietin, catecholamine, and endothelin-1 (ET-1) levels to markedly increase in individuals with continued exposure to high-altitude habitats in the Tibetian highlanders and Andean highlanders.[23] Similarly, in diseases with chronic hypoxia (e.g., COPD and OSAS), vascular dysfunction, increased sympathoadrenal activity, and increased blood levels of vasoconstrictor agents (e.g., ET-1 and catecholamines) were observed.[24]
We have encountered many studies evaluating the effects of COPD and OSAS on choroidal circulation.[4,5,6] The choroidal thickness, especially in severe OSAS patients, significantly decreased compared with that in the control group and patients with mild-to-moderate disease stages.[4,5,6] In a meta-analysis, He et al.[7] found that OSAS causes a decrease in CT, which is negatively correlated with disease severity.
Meanwhile, our findings and those presented in another report showed that the SCFT and in other points was lesser in COPD patients than in healthy individuals; however, these differences were not significant.[8,9] In another study, the SFCT values were also significantly lesser in COPD patients with acute exacerbation and/or stable period than in the controls.[10] Similarly, Alim et al.[3] reported that the severe COPD patients demonstrated significantly reduced SCFT compared with the healthy subjects.
Nevertheless, no previous studies have evaluated the effects of chronic high altitude exposure on retinal thickness. Our study and that of Alim et al. have found no significant difference between the COPD patients and the control group in terms of CMT.[3,9] The CMT values obtained in the current work are attributed to the active autoregulatory mechanisms in the retinal circulation.[14] In addition, compared with choroidal circulation the retinal circulation is more sensitive to blood PaO2 levels and responds to changes in blood oxygen levels more rapidly and actively to protect the central retinal functions.[25] Meanwhile, as mentioned above, the increased sympathoadrenal activity as well as the high levels of blood noradrenaline, adrenaline, and ET-1 may cause long-term atherosclerotic changes in the choroidal vascular bed and may cause a decrease in choroidal thickness by causing an increase in vascular resistance in individuals exposed to chronic high altitudes.
The cross-sectional nature and the lack of automated choroidal thickness measurement are the shortcomings of this study. In addition, a wider choroidal topographic analysis could have been done, including other quadrants. This work is a pilot study; longitudinal studies involving larger sample sizes, especially those involving individuals living at higher altitudes, such as the Andes and the Tibetan Plateau, can provide more descriptive information on the effects of chronic hypobaric hypoxia on choroidal thickness. Furthermore, although the linear regression model found age to be the only confounding factor on SCFT, R2 values show that other factors not considered in this study may also have an impact on SCFT values. Accordingly, more research is needed in which the exclusion criteria are strictly adhered to and standardization is better achieved with a larger sample structure.
Conclusion
Studies evaluating choroidal topography have shown that the SCFT is thicker than in the other choroidal regions. The concentration of photoreceptors in this region, along with the presence of cone cells and the high oxygen requirements, may render this region more sensitive to hypoxic conditions. Considering the results of our study and other studies with chronic hypoxia in the literature, we predict that chronic hypobaric hypoxia may cause a possible decrease in choroidal thickness as a result of some systemic hormonal changes (increased levels of vasoconstrictor hormones, and by the activation of the sympathoadrenal system).[22,23,24] According to our research, high-altitude residents had statistically thinner SCFT than sea-level residents. As mentioned above, the decrease in SCFT may be because this region is more sensitive to hypoxic conditions due to its special physiological structure. As a result, this study is a pilot study and should be supported by large-scale longitudinal studies to reach more definite conclusions.
Author contributions
Dr. Mustafa Gok (Associate Professor): study planning, collecting data, writing the article, Dr. Suleyman Karaman MD: study planning, collecting data, Dr. Burak Erdem MD: writing the article, statistical analysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Non-Physician Altitude Tutorial, 2005. International Society for Mountain Medicine Archived from the original on 23 December 2005 [Google Scholar]
- 3.Alim S, Demir HD, Yilmaz A, Demir S, Güneş A. To evaluate the effect of chronic obstructive pulmonary disease on retinal and choroidal thicknesses measured by optical coherence tomography. J Ophthalmol. 2019;8:7463815. doi: 10.1155/2019/7463815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayhan HA, Aslan Bayhan S, İntepe YS, Muhafiz E, Gürdal C. Evaluation of the macular choroidal thickness using spectral optical coherence tomography in patients with obstructive sleep apnoea syndrome. Clin Exp Ophthalmol. 2015;43:139–44. doi: 10.1111/ceo.12384. [DOI] [PubMed] [Google Scholar]
- 5.Karaca EE, Ekici F, Yalcin NG, Ciftci TU, Ozdek S. Macular choroidal thickness measurements in patients with obstructivesleep apnea syndrome. Sleep Breath. 2015;19:335–41. doi: 10.1007/s11325-014-1025-6. [DOI] [PubMed] [Google Scholar]
- 6.Xin C, Wang J, Zhang W, Wang L, Peng X. Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS) Eye. 2014;28:415–21. doi: 10.1038/eye.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He M, Han X, Wu H, Huang W. Choroidal thickness changes in obstructive sleep apnea syndrome: A systematic review and meta-analysis. Sleep Breath. 2016;20:369–78. doi: 10.1007/s11325-015-1306-8. [DOI] [PubMed] [Google Scholar]
- 8.Ugurlu E, Pekel G, Altinisik G, Bozkurt K, Can I, Evyapan F. New aspect for systemic effects of COPD: Eye findings. Clin Respir J. 2018;12:247–52. doi: 10.1111/crj.12523. [DOI] [PubMed] [Google Scholar]
- 9.Gok M, Ozer MA, Ozen S, Yildirim BB. The evaluation of retinal and choroidal structural changes by optical coherence tomography in patients with chronic obstructive pulmonary disease. Curr Eye Res. 2018;43:116–21. doi: 10.1080/02713683.2017.1373824. [DOI] [PubMed] [Google Scholar]
- 10.KocamışÖ, Zorlu D. Choroid and retinal nerve fiber layer thickness in patients with chronic obstructive pulmonary disease exacerbation. J Ophthalmol. 2018;8:1201976. doi: 10.1155/2018/1201976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975;55:383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- 12.Lovasik JV, Kergoat H, Riva CE, Petrig BL, Geiser M. Choroidal blood flow during exercise-induced changes in the ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2003;44:2126–32. doi: 10.1167/iovs.02-0825. [DOI] [PubMed] [Google Scholar]
- 13.Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986;27:722–6. [PubMed] [Google Scholar]
- 14.Lange CA, Bainbridge JW. Oxygen sensing in retinal health and disease. Ophthalmologica. 2012;227:115–31. doi: 10.1159/000331418. [DOI] [PubMed] [Google Scholar]
- 15.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Querques G, Lattanzio R, Querques L, Del Turco C, Forte R, Pierro L, et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:6017–24. doi: 10.1167/iovs.12-9692. [DOI] [PubMed] [Google Scholar]
- 17.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Alm A, Bill A. The oxygen supply to the retina II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Acta Physiol Scand. 1972;84:306–19. doi: 10.1111/j.1748-1716.1972.tb05182.x. [DOI] [PubMed] [Google Scholar]
- 19.Bosch MM, Merz TM, Barthelmes D, Petrig BL, Truffer F, Bloch KE, et al. New insights into ocular blood flow at very high altitudes. J Appl Physiol (1985) 2009;106:454–60. doi: 10.1152/japplphysiol.90904.2008. [DOI] [PubMed] [Google Scholar]
- 20.Fischer MD, Schatz A, Seitz IP, Schommer K, Bartz-Schmidt KU, Gekeler F, et al. Reversible ıncrease of central choroidal thickness during high-altitude exposure. Invest Ophthalmol Vis Sci. 2015;56:4499–503. doi: 10.1167/iovs.15-16770. [DOI] [PubMed] [Google Scholar]
- 21.Hirukawa-Nakayama K, Hirakarta A, Tomita K, Hiraoka T, Inoue M. Increased choroidal thickness in patient with high-altitude retinopathy. Indian J Ophthalmol. 2014;62:506–7. doi: 10.4103/0301-4738.116483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calbet JA. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J Physiol. 2003;551:379–86. doi: 10.1113/jphysiol.2003.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narvaez-Guerra O, Herrera-Enriquez K, Medina-Lezama J, Julio AC. Systemic hypertension at high altitude. Hypertension. 2018;72:567–78. doi: 10.1161/HYPERTENSIONAHA.118.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taranto-Montemurro L, Messineo L, Perger E, Salameh M, Pini L, Corda L, et al. Cardiac sympathetic hyperactivity in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. COPD. 2016;13:706–11. doi: 10.1080/15412555.2016.1199668. [DOI] [PubMed] [Google Scholar]
- 25.Kergoat H, Marinier JA, Lovasik JV. Effects of transient mild systemic hypoxia on the pulsatile choroidal blood flow in healthy young human adults. Curr Eye Res. 2005;30:465–70. doi: 10.1080/02713680590956739. [DOI] [PubMed] [Google Scholar]