Abstract
Corneal collagen cross-linking (CXL) is an effective treatment for arresting progression in patients with keratoconus. CXL was approved by United States Food and Drug Administration for the treatment of progressive keratoconus in 2016. It is a relatively safe procedure with a low complication rate. As this approach becomes more popular, it is paramount to be familiar with the potential complications associated with the procedure and its management. This article aims to report and review the complications of CXL for the treatment of keratoconus and post-LASIK ectasia.
Keywords: Complications, corneal collagen cross-linking, CXL, epi-on CXL, keratoconus, post-LASIK ectasia, transepithelial cross-linking
Corneal collagen cross-linking (CXL) is a minimally invasive procedure for keratoconus and post-LASIK ectasia, to arrest progression of keratectasia.[1] Induction of cross-links in corneal stroma result in stiffening and strengthening of ectatic cornea. It has also been used for the management of other conditions such as bullous keratopathy, infective and ulcerative keratitis.[2] CXL involves the application of commercially manufactured ophthalmic riboflavin (vitamin B2) solution and UVA to the corneal stroma. The combination of riboflavin and UV-A induces the production of free oxygen radicals, which allows for the formation of new covalent bonds in stromal collagen improving the biomechanical and biochemical properties of the cornea, especially essential in management of keratoconus and postlasik ectasia.[3] Clinical results of CXL in keratectasia include arresting progression of the condition, vision preservation, possible improvement of topographic shape, better fit of contact lenses, and preventing the future need for keratoplasty.[4,5,6] The antimicrobial activity of riboflavin and UVA and corneal resistance to CXL have been described,[7,8] and these characteristics make CXL treatment a good option for managing infective keratitis and infectious ulcers, as CXL can improve the corneal ability to resist proteolytic enzymes secreted by infective microorganisms. This concept of treating corneal ectatic disorder with riboflavin/ultraviolet A (UVA) corneal cross-linking (CXL) was postulated by Spoerl and colleagues at the University of Dresden.[3,4] It was first done in porcine eyes which showed up to 70% increase in corneal rigidity compared to controls[3] and then was repeated in rabbit[9] and human cadaver eyes.[9,10] Based on these studies, the safety of cross-linking was ascertained and was also found to be related to corneal thickness to avoid damage to the corneal endothelium and other ocular structures.[11] The first report of the use of CXL in keratoconus was published in 2003.[4] Wollensak et al.[4] crosslinked 23 eyes with progressive keratoconus, resulting in halting progression in all eyes and corneal flattening in up to 70%. Further clinical studies showed similar promising results in patients with ectasia after refractive surgery.[12] The estimated incidence of keratoconus and post-LASIK ectasia are 0.001–0.03[13] and 0.04–0.6%,[14] respectively. Several techniques have been developed in the past decade to increase the efficacy of CXL. The conventional Dresden protocol (C-CXL), accelerated corneal crosslinking (A-CXL), transepithelial (T-CXL) techniques using chemical enhancers, and iontophoresis crosslinking techniques (I-CXL) are some of the commonly used and currently investigated procedures in clinical practice.
CXL has proved to be an effective treatment for most cases. However, besides the transient reversible side effects, some cases with complications such as corneal haze and scarring, reduced uncorrected and best spectacle-corrected visual acuity, infectious and noninfectious keratitis, stromal melting, and treatment failure with the progression of ectasia have been reported.[15] This article aims to report and review the complications of CXL, otherwise a safe procedure, for the treatment of keratoconus and post-LASIK ectasia.
Methods of Literature Search
A search of the PubMed database was conducted for “complications of CXL” and each of the following keywords: CXL, corneal cross linking, collagen cross linking, keratoconus, ectasia after LASIK, complications and cross-linking, progression, and trans-epithelial CXL. We limited our search to articles published from 1998 to present day that were written in English or the abstracts were in English. The search was conducted in August 2020. All abstracts were screened, and relevant articles were included in this review.
Complications
Removal of corneal epithelium is performed conventionally to facilitate penetration of riboflavin into the corneal stroma.[16] The classic Dresden protocol, introduced by Wollensask et al.[4] in 2003, involves the removal of the corneal epithelium followed by application of riboflavin solution (0.1% riboflavin in 20% dextran solution) to the deepithelialized cornea for 30 min, then the cornea is irradiated with UVA (wavelength, 370 nm and power, 3 mW/cm2) for another 30 min. The riboflavin solution is applied every 3–5 min during the irradiation process. This corresponds to a total energy administration of 5.4 J/cm2, which was found to be efficacious for tissue stiffening.[4,17]
Epithelial removal can lead to potential complications. Pain, epithelial defects, infectious and non-infectious keratitis, corneal opacity, reduced visual acuity, and blurred vision were the most common reported adverse events in various clinical trials.[15,18,19,20]
Pain
Removal of epithelium causes significant pain and discomfort during the procedure and in postoperative days. Pain is a common complaint with crosslinking. In a prospective study by Ghanem et al.,[19] pain after conventional CXL was worse on the day of surgery and postoperative day 1 (mean 2.8 and 2.1, respectively, on a scale of 0–5 with 5 being worst pain) and improved on subsequent days (mean 0.12 on postoperative day 5). Subjects were given standard postoperative topical drops to include 0.4% ketorolac as well as oral nonsteroidal anti-inflammatory drug (NSAID) daily and paracetamol with codeine as needed for severe pain. They reported 43% of patients had 4 or 5 pain level on the first day, which dropped to 24% on the following day.[19]
Li et al. in 2017,[21] compared the postoperative pain in conventional and subepithelial accelerated CXL in a retrospective study. The pain score (on a scale of 0–4 with 4 being worst) of patients in both the groups at 0–3 days postoperatively were recorded (questionnaire based) and the differences were statistically significant. Patients in subepithelial group experienced medium level of pains and irritation symptoms without any need for analgesics, which were significantly lighter than the pains of patients in the conventional group.
Placement of a bandage contact lens in conjunction with systemic pain medications and cold compresses are currently some of the remedies to reduce postoperative pain.
Photophobia
Transient light-sensitivity syndrome (TLSS), is characterized by moderate-to-severe photophobia bilaterally that appears 2–8 weeks after ocular procedures and is associated with inflammation of peripheral structures, such as the ciliary body, and possibly the trabecular meshwork and iris.[22] TLSS typically does not present with clinical signs of inflammation or any other abnormalities of the anterior and posterior segments on slit-lamp exam.[22,23] Moshirfar et al.,[24] in their case series, reported several cases of acute TLSS-like symptoms weeks after CXL. Among the seven patients, four had conventional CXL and the other three patients underwent transepithelial CXL (TE CXL). The symptoms of TLSS acutely emerged within a range of 5 weeks to 3 months post-CXL. There were no signs of inflammation in the anterior chamber (AC) in six cases, and one patient had endothelial inflammation. All seven patients were given fluorometholone with subsequent relief of symptoms within 1–6 weeks. These TLSS-like symptoms may be due to a secondary inflammatory response involving activated keratocytes and cytokine release during tissue remodeling and healing or may also be associated with possible phototoxicity from free radical damage from riboflavin and UVA extending into the AC. When treated with aggressive topical corticosteroid therapy, TLSS symptoms typically resolve within a few days or weeks.[23,25] Cyclosporine ophthalmic emulsion 0.05% and punctal plugs may also be helpful to relieve the photophobia symptoms.[25]
Dry eyes
After CXL, epithelial regrowth completes within several days of treatment.[26] The corneal nerve fibers start to regenerate at an early stage (1 month after CXL treatment) and within 6 months posttreatment, the corneal sensitivity is fully restored with a complete regeneration of nerve fibers within the corneal stroma.[26] However, another study described a progression in the abnormal nerve migration even after 5 years post-CXL treatment and the corneal subbasal nerves did not regenerate to reach the level of a healthy cornea following CXL treatment.[27] Therefore, given the widespread use of this treatment for management of keratoconus, abnormal nerve migration post-CXL treatment should be recognized and further investigated. Corneal denervation leads to dry eye, and it is one of the most recognized complications of corneal refractive surgeries.[28]
Epithelial healing problems: persistent epithelial defects and stromal melt
Epithelial regrowth occurs within few days of CXL. Mazzotta et al.[26] reported a complete epithelial healing after 4 days of soft contact lens bandage. Epithelial healing problems have been reported in about 3–8% of cases.[20] A study reported delayed epithelial healing in 9 of 21 cases. Four of these eyes had accelerated CXL and five underwent the standard Dresden protocol. All cases healed within 9 days.[29]
Patients with persistent epithelial defect are at increased risk for additional complications, including infection and corneal melting [Fig. 1]. Keratocyte damage and apoptosis after CXL may affect the wound healing process and collagen deposition.[30] Epithelial defect after CXL may increase MMP levels in the cornea and tears,[31] therefore increasing the risk for corneal melting. Unregulated UV exposure may also play a role. Several cases of corneal melt after CXL have been reported.[32,33,34,35] All were after the C-CXL and most had a pre op corneal thickness of <400 mm, which could be the potential risk factor.[33,35] One of them also had a history of prolonged topical NSAID use.[35] Faschinger et al.[33] reported a case of bilateral corneal melting after CXL for keratoconus with Down syndrome. A case of corneal melt post-CXL was reported in a patient with lagophthalmos and blepharitis.[32] It was suggested that corneal exposure due to lagophthalmos delayed epithelial healing and blepharitis caused an increased propensity for inflammatory activity. Careful patient selection and recognizing and optimizing potential risk factors may prevent this sight threatening complication. Another case of corneal melt had a pre-op pachymetry of 443 mm and no NSAID was used. Postoperatively, the patient had AC reaction, severe corneal haze, and a persistent epithelial defect resulting to corneal melting and perforation within 2 months without any other predisposing factor.[34] There is no definitive risk factor for PED or corneal melt; therefore, it is critical that the patient be followed carefully until epithelial healing is complete. Predisposing factor if any should be looked for and removed and lubricating eyedrops should be administered frequently. In case of corneal melt, topical antibiotic eyedrop should be started with patching of eye in between medication in case of impending perforation/perforated cornea. Temporary tarsorrhaphy can also be done in such cases. Surgical treatment like amniotic membrane grafting and tectonic penetrating keratoplasty (PK) should be considered in case of corneal perforation.
Figure 1.
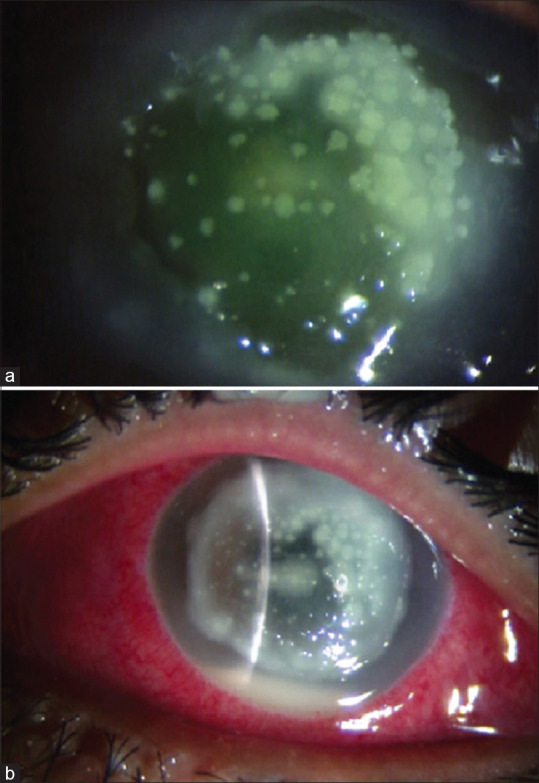
Stromal melt post-CXL in a 15-year-old boy with stage 3 keratoconus and severe VKC. (a) Third post-op day: diffuse infiltrates. (b) Sixth post-op day: stromal melt. Penetrating keratoplasty was done
Keratitis
Epithelial removal in C-CXL has the potential risk of keratitis and sterile infiltrates. Many cases of keratitis, both infectious and sterile, have reported post-CXL.
In a prospective study of 117 eyes, sterile infiltrates occurred in 7.6% of eyes after conventional epi-off CXL, which resolved within 4 weeks with treatment of dexamethasone 4 times a day.[15] Prior history of atopy could be associated with the development of sterile infiltrates in some patients, while others had no known preexisting risk factors.[36] The reported prevalence of VKC co existing with keratoconus is variable, ranging from 7 to 35%.[37] It is not uncommon in this part of subcontinent to have patients in early teens presenting with keratoconus and VKC undergoing CXL. A pediatric case reported by the authors had sterile keratitis after CXL with prior history of severe vernal conjunctivitis (VKC) [Fig. 2]. The patient was pre-treated with topical 2% cyclosporine eye drops for 6 weeks prior to CXL to control his VKC. The keratitis resolved after topical antibiotic and intensive topical steroid therapy.[38] Other cases of sterile keratitis after CXL with a history of prior VKC had variable patterns of corneal infiltrates. They resolved with residual scarring after being treated with topical steroid therapy [Fig. 3]. In another case managed by the authors, a 10-year-old child with severe VKC who developed shield ulcer with sterile infiltrates post conventional CXL received intensive steroid therapy but showed no improvement. Slit-lamp examination showed a thick plaque covering the ulcer surface hindering the healing process. Debridement of the plaque and continued steroid therapy led to resolution of the signs and symptoms in this patient [Fig. 4]. Until epithelial defects have healed, close monitoring is imperative in the immediate postoperative period especially in cases with coexisting VKC. In view of higher incidence of sterile corneal infiltrates in patients of keratoconus with co-existing vernal conjunctivitis undergoing CXL, it is imperative to control and treat VKC with topical steroids and/or cyclosporine at least 2–4 weeks prior to CXL.
Figure 2.
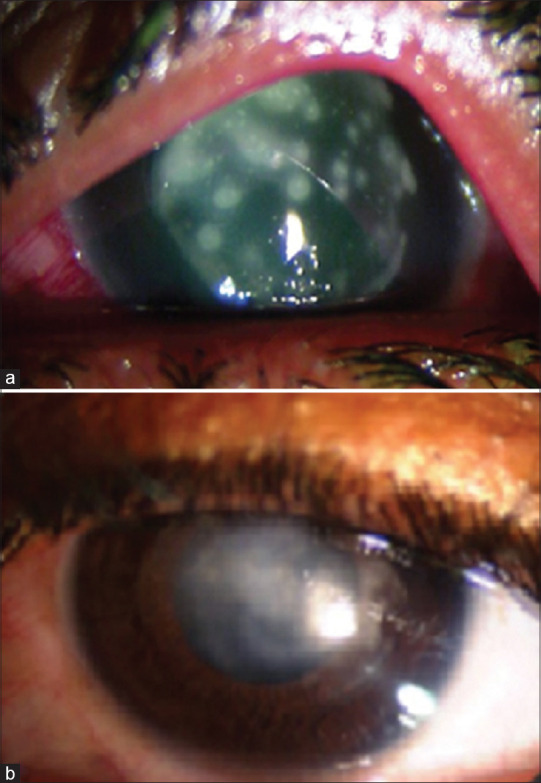
Sterile keratitis post-CXL in a patient with stage 2 keratoconus and severe VKC. (a) First post-op day: multiple diffuse infiltrates. (b) At 8 weeks: resolution with scarring. Patient awaiting corneal graft
Figure 3.
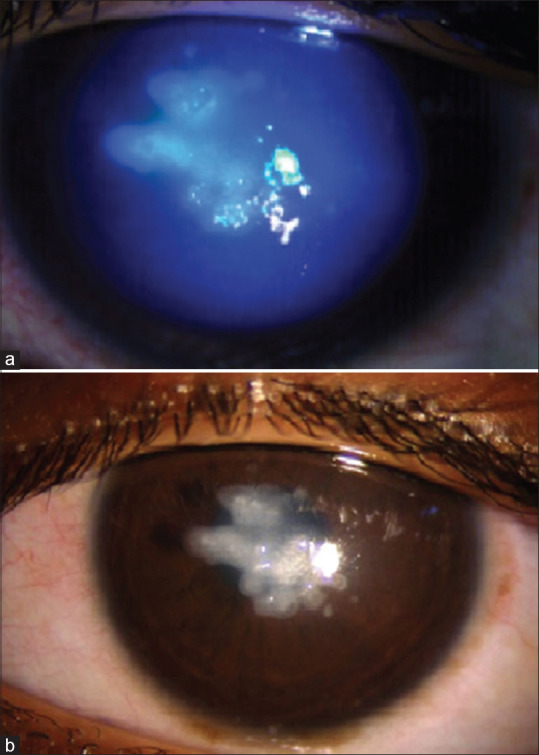
Sterile keratitis post-CXL in a patient with stage 2 keratoconus and moderate VKC. (a) First post-op day: central amoeboid shaped infiltrates. (b) At 4 weeks: resolution with scarring
Figure 4.
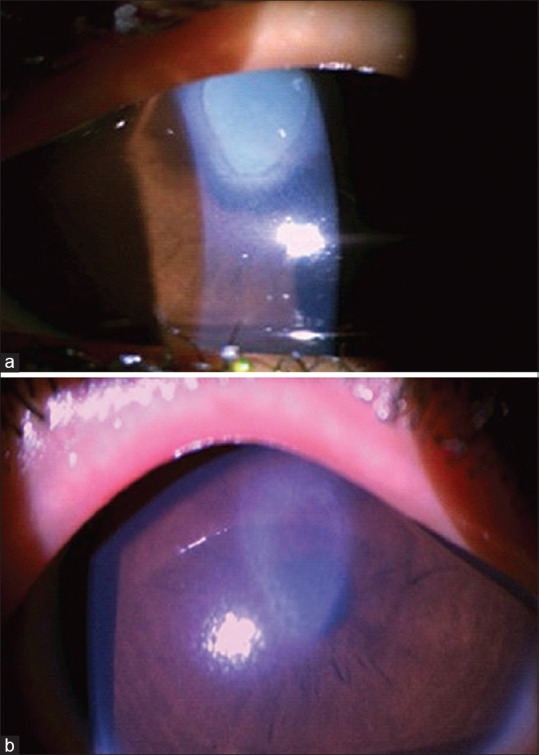
Shield ulcer post-CXL in a 10-year-old boy with stage 2 keratoconus and severe VKC. (a) At 2 weeks’ post-op: ulcer covered with plaque. (b) At 4 weeks: complete reepithelization
In a retrospective study of 771 eyes, 11 eyes (1.4%) of eight patients developed late-onset PUK with or without sterile infiltrates between 3 months and 6 years post-CXL. Four affected patients had inflammatory and autoimmune conditions which may be a risk factor inducing ulcerative keratitis post-CXL. The patients were treated with topical steroid eye drops, with resolution of symptoms within 1–2 weeks.[39] A case of diffuse lamellar keratitis after CXL in a patient with iatrogenic post-LASIK ectasia was reported, which resolved after both intensive topical and oral steroid treatment.[40]
Varying presentations of infectious keratitis post-CXL have been reported, depending on the etiologic agent. Reported causes of infectious keratitis include Gram-positive bacteria (Staphylococcus epidermidis, S. aureus, polymicrobial Streptococcus salivarius with S. oralis), Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa), fungi (microsporidia, fusarium solani), acanthamoeba, and herpes simplex virus.[18,41] According to a systematic review, the most virulent organisms reported were pseudomonas and acanthamoeba, which led to corneal melt and severe scarring, respectively.[18] All cases occurred within the first 5 days of procedure except the cases of fungal keratitis which presented on day 6 (microsporidia) and day 30 (fusarium). This implies that most infections in these cases occur early in the immediate postoperative period and require strict vigilance during this time. Broad spectrum antibiotic should be started immediately after sending the ulcer scrapings for microbiological examination for bacterial and fungal culture and antibiotic sensitivity testing. Treatment is then changed according to the result of culture and antibiotic sensitivity reports.
Epithelial defect is the fundamental cause for corneal infections in the postoperative period. In some diseases, such as atopic disease and diabetes, the epithelial healing time is longer and the cornea is more vulnerable to infection. The use of bandage contact lenses may shorten the time to healing but increase the risk of infection associated with the manipulation of the contact lens. Poor contact lens hygiene, eye rubbing, and rinsing the eye with tap water have been reported as potential mechanisms of the infections reported earlier.[18] Herpetic keratitis post-CXL can be due to reactivation of latent herpes simplex virus infection[41,42] or can occur as a primary infection.[18,42] UV exposure and epithelial debridement could be the potent stimulus to trigger the reactivation.[41,42] Treatment with topical antiviral agents should be started immediately along with topical steroid in case of stromal involvement and oral antiviral in cases of reactivation. Patients with recurrent herpetic keratitis should be given prophylactic low-dose oral antiviral in the preoperative period to prevent the reactivation.
Corneal haze and opacity
Corneal haze, a transient finding, is commonly observed after CXL and was found to be associated with corneal edema and keratocyte loss.[15,43] In 2009, Koller et al.[15] prospectively evaluated 117 eyes and found that all eyes had anterior stromal haze with a mean grade of 0.78 G (range 0–2) at 1-month post-op, which decreased to 0.06 G at 12 months. They pointed out that stromal haze after CXL is deeper and different than the sub epithelial haze seen after photorefractive keratectomy and associated it with the depth of CXL and loss of keratocytes. Kim et al. in 2016[44] did a quantitative analysis of corneal haze and reported an increase in the mean corneal densitometry postoperatively which peaked at 1 month, decreasing to baseline level after 6 months without the application of topical corticosteroids highlighting the natural history of haze development and resolution over time. Preoperatively, a lower CCT could be correlated with higher densitometry suggesting thinner cornea are more likely to develop haze.
Dense, persistent, visually significant haze or scarring may affect vision and may also respond to intensive topical steroid treatment.[43] Koller et al.[15] reported a 2.9% incidence of stromal scars with corresponding topographic flattening after CXL. Advanced keratoconus with higher keratometry values and thinner corneas have been found to be associated with greater risk of persistent haze after CXL. Other suggested risk factors for persistent haze include the presence of hypo-reflective stromal microstriae preoperatively, presence of activated keratocytes in the anterior stroma preoperatively, or presence of Langerhan cells after contact lens removal postoperatively.[43] Treatment with topical steroid reduces the occurrence of corneal haze.
Stromal degeneration
Stromal degeneration is a rare and late complication caused due to severe stromal thinning up to Descemet’s membrane. In a case series in 2020, Prabhakar et al.,[45] reported stromal degeneration in three patients who underwent CXL 3–6 years back for keratoconus. Deep anterior lamellar keratoplasty was done with good outcomes. They highlighted the possible late effects of UVA irradiation post-CXL. Zare et al.[46] conducted a study which showed that anterior and mid-stromal keratocyte density decreases greatly after CXL. Keratocyte repopulation occurs but does not complete after 6 months of the procedure. If considerable regeneration of stromal keratocytes fails to occur, it could result in stromal thinning. Lim LS et al.[47] in their study showed that, UVA exposure which causes keratocyte death may show a sublethal effect in the deeper stroma, which receives lower dose of radiation, resulting in fibroblastic transformation of the keratocytes with subsequent scar formation which may further decrease the supply of nutrition from aqueous to the superficial stroma. Hence, intraoperative parameters during CXL treatment such as exposure time and UVA dose play an important role in the outcomes.
Endothelial damage and corneal edema
UVA is toxic to corneal endothelial cells, crystalline lens, and retina. To prevent these structures from toxic effects, 400 mm is the recommended minimum corneal thickness after de-epithelization for UV-A radiant exposure of 5.4 J/cm2 used in conventional CXL.[4,48] Decreased endothelial cell density after epi-off CXL with the standard Dresden protocol was previously reported in thin corneas.[49]
Temporary corneal edema may be seen after CXL. Persistent corneal edema necessitating further treatment has also been reported. A retrospective study reported 10 cases of corneal edema in 350 patients (2.9%) on postoperative day 1. Half of those cases did not improve and were offered PK, but only two underwent PK. They suggested corneal endothelial damage as a possible explanation for persistent corneal edema in their study. The authors recommend not using a lid speculum during riboflavin instillation to minimize corneal dehydration, frequent equipment calibration, and measuring pachymetry to ensure adherence to the minimum 400 mm guideline during CXL.[50] A meta-analysis comparing epithelial-off standard (SCXL) to accelerated corneal collagen cross-linking (ACXL), indicated that SCXL created greater endothelial cell density (ECD) loss than ACXL. This may imply that ACXL may be superior to SCXL in this respect because of the possible cytotoxicity caused by prolonged ultraviolet radiation or less posterior irradiation with ACXL.[51]
Newer Modalities and Associated Problems
Moderate-to-advanced keratoconus cases are often found to have a pachymetry less than 400 mm and require protocol modifications in CXL to reduce the risk of endothelial damage. There are various modifications to the conventional method to circumvent this issue of CXL in thin corneas while avoiding the possible complications.
CXL with hypoosmolar riboflavin
Iatrogenically swelling the cornea with resultant increased temporary corneal thickening may allow for safer CXL in thin corneas. When exposed to a hypo-osmolar solution, the cornea can swell up to double its thickness.[52] Hafezi et al.[53] reported stabilization of the keratectasia at 6 months of follow-up with no endothelial cell loss in any case. Hafezi et al.[54] in another study also reported failure of CXL in a cornea that was 268 microns thick suggesting that a certain minimum stromal thickness is required for successful CXL to occur and it was proposed that the preoperative thickness should be least 330 microns. Stojanovic et al.[55] found that although the procedure using hypoosmolar riboflavin halted the progression of keratoconus, the effect was lower than that seen with CXL in normal corneas. The increase in the corneal stromal thickness after using hypoosmolar riboflavin is due to the hydrophilic properties of the stromal proteoglycans causing “collagen-free lakes” to form.[53] This could effectively dilute the number of collagen fibrils available for CXL. Also, the diffusion factor of oxygen through hydrated corneal stroma is lower than normal corneal stroma.[56] This could lead to a lower oxygen transport, reducing the efficacy of CXL.
Customised epithelial debridement technique
In 2009, Kymionis et al.[57] described a technique that involved epithelial debridement of the keratoconic cornea sparing the epithelium over the apex of the cone as determined on topography. The intact island of epithelium causes UVA attenuation and acts as a protective shield over the thinnest corneal point. It was observed that the stromal demarcation line was detectable in the deepithelialized, peripheral cornea, but not in the area where epithelium was left intact. CXL effect was seen at a depth of 150 microns in the epithelium-on area as compared to 250 microns in the epithelium-off area indicating a definite, but lower CXL effect under the intact epithelium.[58] A study by Cagil et al.[59] in 19 eyes, demonstrated that there was a halt in the progression of keratoconus at 12 months but also a significant endothelial cell loss after this procedure. Mean ECD decreased from 2550 to 2030 cells/mm2, but there was no evidence of corneal edema in any of the cases.
Lenticule-assisted crosslinking
Sachdev et al.[60] described a technique of using the stromal lenticule removed from patients undergoing small incision lenticule extraction for myopic correction. Stromal lenticule was placed over the patient’s cornea after deepithelization which augmented stromal thickness. They claimed the technique to be safe and effective in their few cases at 6 months follow-up. The potential limitations of this technique include reduced oxygen availability for CXL; however, there need to be long-term studies to establish the safety and efficacy of this technique.
Contact lens-assisted crosslinking
Jacob et al.[61] described this technique for thin corneas using a bandage contact lens placed over deepithelized cornea. They found this technique to be effective in their study of 14 eyes at 6–7 months of follow-up. This technique is not dependent on the swelling properties of the cornea, but the riboflavin-soaked contact lens reduces oxygen availability and absorbs UVA radiation to reduce the surface irradiance level by 40–50%.[62]
Epi-on CXL: Trans-epithelial CXL
CXL without epithelial debridement is another technique that allows thinner corneas to be treated. Performing CXL with intact epithelium reduces the risk of infective keratitis, improves patient comfort, reduces stromal haze, and intraoperative corneal thinning,[63] probably because of less tissue damage and reduced wound healing reaction. TE CXL has the potential advantage of faster recovery, less pain, and broadening treatment options for patients with thinner corneas. Patients may usually return to contact lens wear in 7–10 days after TE CXL compared to 1 month after epi-off CXL.[64] However, an intact epithelium poses a few challenges. Riboflavin is a hydrophilic macromolecule and has less permeability into the cornea through an intact epithelium.[16] Riboflavin-soaked epithelium might absorb the incident UVA light reducing its penetration into deeper parts of the stroma[65,66] and also oxygen diffusion is reduced because of epithelial presence causing attenuation of the CXL effect.[67]
Several studies used this technique and reported inferior effect of TE-CXL compared with the conventional method.[20,63,68] Caporossi et al.[68] reported UCVA, BCVA, or corneal topography was stable at 6 and 12 months, but a subsequent worsening was observed after 12 months. Leccisotti and Islam reported that there was a definite but limited favorable effect of TE CXL. The effect was less than conventional CXL by epithelial removal.[63] Transepithelial accelerated protocols using more intense radiation in a shorter amount of time reported acute actinic keratitis with diffuse punctate epitheliopathy that resolved in 3–4 days with soft bandage contact lens and lubricants.[69] Yuksel et al.[70] using an accelerated trans-epithelial protocol reported diffuse corneal punctate epitheliopathy and edema soon after the procedure. Healing of epithelial defects was slower in those eyes compared to eyes which underwent CXL (9 mW/cm2 for 10 min) with epithelial removal. Pain scores were also worse in TE CXL. In another study, Taneri et al.[71] compared various TE CXL protocols and reported corneal epithelial defects to be common on the first postoperative day following accelerated protocols. The presence of chemical enhancers in riboflavin solution was suggested to loosen tight junctions and thus induce epithelial disruptions and epithelial defects in the above studies. In a case series, 7 out of 11 eyes developed epithelial complications and significant pain, shortly after combined accelerated TE CXL, which healed within 5 days after placing a bandage contact lens. They suggested using bandage contact lens in these cases in the early postoperative period to reduce epithelial healing problems despite being an epithelium-on technique.[72] Given its diminished effect, TE CXL may be reserved for use in patients who present with early disease stages or in specific patient populations, such as patients with Downs syndrome and other special considerations, who may pose challenges with postoperative compliance.
CXL Failure/Progression after CXL
CXL failure is largely defined as keratoconic progression following treatment. The definition of progression after CXL varies in the literature. A common variable in the definition is an increase of more than 0.5–1.0 diopter in maximum keratometry (Kmax) after CXL.[5,15,20,73] Raiskup-Wolf et al.[5] reported less than 1% (2/241 cases) rate of progression 18 months after epi-off CXL, while Koller et al.[15] reported progressions in 7.6% of epi-off cases during the first postoperative year. They identified K Max of more than 58 diopters as a significant risk factor for progression.[15] Kuechler et al.[74] evaluated post-CXL progression in corneas with a Kmax >=58.0 D. Although a significant overall postoperative decrease of mean Kmax was found, progression rates of 23% (after 1 year) and 18% (after 2 years) were reported. Accordingly, the concept of a relative contraindication for CXL in corneas with a preoperative Kmax of 58 D or more is used.[15] A comparative analysis of topographical changes post-CXL in early and advanced keratoconus by Arora et al.[75] also showed CXL to be more effective in flattening the cornea when performed early in the course of the disease and in patients with an average central K <=53 D.
Other risk factors for CXL failure included a preoperative patient age of 35 years or older, being female, eye rubbing, and allergic conjunctivitis, cornea thickness <400 mm and best-corrected visual acuity better than 20/25.[15] Various studies also tried to identify risk factors that may predict progression. A prospective study used multivariate analysis and found that a more eccentric cone in keratoconus patients was found to result in higher keratometry values a year after CXL.[76] In pediatric patients, a retrospective study showed progression in about a third of the cases within 2 years after epi-off CXL. Paracentrally located cones and thinner corneas were more likely found to progress than central cones and thicker corneas.[77] Since risk factors for progression were found in studies using epi-off protocol, primary use of epi-on protocol in patients with thin and steep corneas was suggested.[64] Protocol modifications mentioned previously may prevent progression in thin corneas. However, progression in a patient with a very thin cornea using hypoosmolar riboflavin was reported.[54] Also, in a comparative trial, Soeters et al.[20] reported 23% progression in the TE CXL group and no progression in the epi-off group at 1-year follow-up. Some cases of progression in the studies mentioned earlier were treated with repeat CXL.[5,73] Akkaya Turhan et al.,[78] evaluated the effectiveness of repeated CXL in eyes with progressive keratoconus after primary CXL. They reported a failure rate of 1.5% at a mean interval of 19.3 months after the first CXL procedure. After repeated CXL treatments with a mean follow-up period of 36 months, Kmax values regressed in eight eyes (66.6%) and remained stable in two eyes (16.6%) but progressed in two eyes (16.6%), with continued eye rubbing. The regression experienced in eight eyes indicated the additive effect of repeat CXL on biomechanical stiffness.
Conclusion
CXL is a relatively safe procedure with a low complication rate. A majority of CXL complications occur due to epithelial debridement. Newer protocol modifications have been used to help improve safety and efficacy in thinner corneas.[53,57,60,61,63,79] Although CXL complication rates are low, there may be pre-existing medical conditions that might increase the risk for complications such as herpetic eye disease, atopy, vernal keratoconjunctivitis, prior LASIK, diabetes, and other autoimmune conditions. Clinicians should be aware of these risk factors and potential complications and may need to pre-treat certain conditions and be prepared to manage postoperative issues. As CXL is gaining popularity, being aware of different approaches of CXL may help reduce complication rates and improve outcome. Additional research in this area is paramount to maximizing results of CXL and limiting complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.O'Brart DP, Patel P, Lascaratos G, Wagh VK, Tam C, Lee J, et al. Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: Seven-year follow-up. Am J Ophthalmol. 2015;160:1154–63. doi: 10.1016/j.ajo.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Sorkin N, Varssano D. Corneal collagen crosslinking: A systematic review. Ophthalmologica. 2014;232:10–27. doi: 10.1159/000357979. [DOI] [PubMed] [Google Scholar]
- 3.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 4.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 5.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Wittig-Silva C, Chan E, Islam FMA, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: Three-year results. Ophthalmology. 2014;121:812–21. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Tsugita A, Okada Y, Uehara K. Photosensitized inactivation of ribonucleic acids in the presence of riboflavin. Biochim Biophys Acta. 1965;103:360–3. doi: 10.1016/0005-2787(65)90182-6. [DOI] [PubMed] [Google Scholar]
- 8.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 9.Spörl E, Schreiber J, Hellmund K, Seiler T, Knuschke P. Untersuchungen zur verfestigung der hornhaut am kaninchen. Der Ophthalmol. 2000;97:203–6. doi: 10.1007/s003470050515. [DOI] [PubMed] [Google Scholar]
- 10.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–5. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 11.Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg. 2003;29:1786–90. doi: 10.1016/s0886-3350(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 12.Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:2035–40. doi: 10.1016/j.jcrs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RP. Age-specific incidence and prevalence of keratoconus: A nationwide registration study. Am J Ophthalmol. 2017;175:169–72. doi: 10.1016/j.ajo.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Spadea L, Cantera E, Cortes M, Conocchia NE, Stewart CW. Corneal ectasia after myopic laser in situ keratomileusis: A long-term study. Clin Ophthalmol. 2012;6:1801–13. doi: 10.2147/OPTH.S37249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35:1358–62. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Baiocchi S, Mazzotta C, Cerretani D, Caporossi T, Caporossi A. Corneal crosslinking: Riboflavin concentration in corneal stroma exposed with and without epithelium. J Cataract Refract Surg. 2009;35:893–9. doi: 10.1016/j.jcrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Randleman JB, Khandelwal SS, Hafezi F. Corneal cross-linking. Surv Ophthalmol. 2015;60:509–23. doi: 10.1016/j.survophthal.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Abbouda A, Abicca I, Alió JL. Infectious keratitis following corneal crosslinking: A systematic review of reported cases: Management, visual outcome, and treatment proposed. Semin Ophthalmol. 2016;31:485–91. doi: 10.3109/08820538.2014.962176. [DOI] [PubMed] [Google Scholar]
- 19.Ghanem VC, Ghanem RC, De Oliveira R. Postoperative pain after corneal collagen cross-linking. Cornea. 2013;32:20–4. doi: 10.1097/ICO.0b013e31824d6fe3. [DOI] [PubMed] [Google Scholar]
- 20.Soeters N, Wisse RP, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: A randomized controlled trial. Am J Ophthalmol. 2015;159:821–8.e3. doi: 10.1016/j.ajo.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Xie H, Xu M, Li M, Liu C, He J, et al. Comparison of pain after subepithelial versus conventional accelerated corneal collagen cross-linking for keratoconus. Int Ophthalmol. 2019;39:1249–54. doi: 10.1007/s10792-018-0935-x. [DOI] [PubMed] [Google Scholar]
- 22.Desautels JD, Moshirfar M, Quist TS, Skanchy DF, Hoopes PC. Case of presumed transient light-sensitivity syndrome after small-incision lenticule extraction. Cornea. 2017;36:1139–40. doi: 10.1097/ICO.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz G, Albarrán-Diego C, Sakla HF, Javaloy J, Alió JL. Transient light-sensitivity syndrome after laser in situ keratomileusis with the femtosecond laser. Incidence and prevention. J Cataract Refract Surg. 2006;32:2075–9. doi: 10.1016/j.jcrs.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Moshirfar M, Vaidyanathan U, Hopping GC, Ronquillo YC, Hoopes PC. Delayed-onset transient light sensitivity syndrome after corneal collagen cross-linking: A case series. Med Hypothesis Discov Innov Ophthalmol. 2019;8:250–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Stonecipher KG, Dishler JG, Ignacio TS, Binder PS. Transient light sensitivity after femtosecond laser flap creation: Clinical findings and management. J Cataract Refract Surg. 2006;32:91–4. doi: 10.1016/j.jcrs.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Mazzotta C, Traversi C, Baiocchi S, Caporossi O, Bovone C, Sparano MC, et al. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: Early and late modifications. Am J Ophthalmol. 2008;146:527–33. doi: 10.1016/j.ajo.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 27.Parissi M, Randjelovic S, Poletti E, Guimarães P, Ruggeri A, Fragkiskou S, et al. Corneal nerve regeneration after collagen cross-linking treatment of keratoconus: A 5-year longitudinal study. JAMA Ophthalmol. 2016;134:70–8. doi: 10.1001/jamaophthalmol.2015.4518. [DOI] [PubMed] [Google Scholar]
- 28.Kontadakis GA, Kymionis GD, Kankariya VP, Pallikaris AI. Effect of corneal collagen cross-linking on corneal innervation, corneal sensitivity, and tear function of patients with keratoconus. Ophthalmology. 2013;120:917–22. doi: 10.1016/j.ophtha.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Kanellopoulos AJ. Long term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross linking for progressive keratoconus. Clin Ophthalmol. 2012;6:97–101. doi: 10.2147/OPTH.S27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gkika M, Labiris G, Kozobolis V. Corneal collagen cross-linking using riboflavin and ultraviolet-A irradiation: A review of clinical and experimental studies. Int Ophthalmol. 2011;31:309–19. doi: 10.1007/s10792-011-9460-x. [DOI] [PubMed] [Google Scholar]
- 31.Sakimoto T, Sawa M. Metalloproteinases in corneal diseases: Degradation and processing. Cornea. 2012;31(Suppl 1):S50–6. doi: 10.1097/ICO.0b013e318269ccd0. [DOI] [PubMed] [Google Scholar]
- 32.Chiu HH, Sade S, Chew HF. Corneal melt following collagen crosslinking and topography-guided customized ablation treatment for keratoconus. Can J Ophthalmol. 2017;52:e88–91. doi: 10.1016/j.jcjo.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Faschinger C, Kleinert R, Wedrich A. Corneal melting in both eyes after simultaneous corneal cross-linking in a patient with keratoconus and Down syndrome. Ophthalmologe. 2010;107:951–5. doi: 10.1007/s00347-009-2127-6. [DOI] [PubMed] [Google Scholar]
- 34.Labiris G, Kaloghianni E, Koukoula S, Zissimopoulos A, Kozobolis VP. Corneal melting after collagen cross-linking for keratoconus: A case report. J Med Case Rep. 2011;5:152. doi: 10.1186/1752-1947-5-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohamed-Noriega K, Butrón-Valdez K, Vazquez-Galvan J, Mohamed-Noriega J, Cavazos-Adame H, Mohamed-Hamsho J. Corneal melting after collagen cross-linking for keratoconus in a thin cornea of a diabetic patient treated with topical nepafenac: A case report with a literature review. Case Rep Ophthalmol. 2016;7:119–24. doi: 10.1159/000444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghanem RC, Netto MV, Ghanem VC, Santhiago MR, Wilson SE. Peripheral sterile corneal ring infiltrate after riboflavin-UVA collagen cross-linking in keratoconus. Cornea. 2012;31:702–5. doi: 10.1097/ICO.0b013e318226da53. [DOI] [PubMed] [Google Scholar]
- 37.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 38.Arora R, Jain P, Gupta D, Goyal JL. Sterile keratitis after corneal collagen crosslinking in a child. Cont Lens Anterior Eye. 2012;35:233–5. doi: 10.1016/j.clae.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Chanbour W, Mokdad I, Mouhajer A, Jarade E. Late-onset sterile peripheral ulcerative keratitis post-corneal collagen crosslinking. Cornea. 2019;38:338–43. doi: 10.1097/ICO.0000000000001842. [DOI] [PubMed] [Google Scholar]
- 40.Kymionis GD, Bouzoukis DI, Diakonis VF, Portaliou DM, Pallikaris AI, Yoo SH. Diffuse lamellar keratitis after corneal crosslinking in a patient with post-laser in situ keratomileusis corneal ectasia. J Cataract Refract Surg. 2007;33:2135–7. doi: 10.1016/j.jcrs.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 41.Sharma N, Maharana P, Singh G, Titiyal JS. Pseudomonas keratitis after collagen crosslinking for keratoconus: Case report and review of literature. J Cataract Refract Surg. 2010;36:517–20. doi: 10.1016/j.jcrs.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 42.Yuksel N, Bilgihan K, Hondur AM. Herpetic keratitis after corneal collagen cross-linking with riboflavin and ultraviolet-A for progressive keratoconus. Int Ophthalmol. 2011;31:513–5. doi: 10.1007/s10792-011-9489-x. [DOI] [PubMed] [Google Scholar]
- 43.Mazzotta C, Balestrazzi A, Baiocchi S, Traversi C, Caporossi A. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: In vivo confocal microscopic evaluation. Clin Exp Ophthalmol. 2007;35:580–2. doi: 10.1111/j.1442-9071.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim BZ, Jordan CA, McGhee CNJ, Patel DV. Natural history of corneal haze after corneal collagen crosslinking in keratoconus using Scheimpflug analysis. J Cataract Refract Surg. 2016;42:1053–9. doi: 10.1016/j.jcrs.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Prabhakar G, Panickar N, Reddy J, Sivasubramaniam S, Singh A. Severe focal stromal degeneration up to Descemet membrane after corneal collagen cross-linking. Indian J Ophthalmol. 2020;68:224–6. doi: 10.4103/ijo.IJO_475_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zare M, Mazloumi M, Farajipour H, Hoseini B, Fallah M, Mahrjerdi H, et al. Effects of corneal collagen crosslinking on confocal microscopic findings and tear indices in patients with progressive keratoconus. Int J Prev Med. 2016;7:132. doi: 10.4103/2008-7802.196527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim LS, Beuerman R, Lim L, Tan DT. Late-onset deep stromal scarring after riboflavin-UV-A corneal collagen cross-linking for mild keratoconus. Arch Ophthalmol. 2011;129:360–2. doi: 10.1001/archophthalmol.2011.23. [DOI] [PubMed] [Google Scholar]
- 48.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–9. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 49.Kymionis GD, Portaliou DM, Diakonis VF, Kounis GA, Panagopoulou SI, Grentzelos MA. Corneal collagen cross-linking with riboflavin and ultraviolet-A irradiation in patients with thin corneas. Am J Ophthalmol. 2012;153:24–8. doi: 10.1016/j.ajo.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 50.Sharma A, Nottage JM, Mirchia K, Sharma R, Mohan K, Nirankari VS. Persistent corneal edema after collagen cross-linking for keratoconus. Am J Ophthalmol. 2012;154:922–6.e1. doi: 10.1016/j.ajo.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Wen D, Li Q, Song B, Tu R, Wang Q, O'Brart DP, et al. Comparison of standard versus accelerated corneal collagen cross-linking for Keratoconus: A meta-analysis. Investig Ophthalmol Vis Sci. 2018;59:3920–31. doi: 10.1167/iovs.18-24656. [DOI] [PubMed] [Google Scholar]
- 52.Maurice DM, Giardini AA. Swelling of the cornea in vivo after the destruction of its limiting layers. Br J Ophthalmol. 1951;35:791–7. doi: 10.1136/bjo.35.12.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg. 2009;35:621–4. doi: 10.1016/j.jcrs.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 54.Hafezi F. Limitation of collagen cross-Linking with hypoosmolar riboflavin solution: Failure in an extremely thin cornea. Cornea. 2011;30:917–9. doi: 10.1097/ICO.0b013e31820143d1. [DOI] [PubMed] [Google Scholar]
- 55.Stojanovic A, Zhou W, Utheim TP. Corneal collagen cross-linking with and without epithelial removal: A contralateral study with 0.5% hypotonic riboflavin solution. Biomed Res Int 2014. 2014 doi: 10.1155/2014/619398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larrea X, Büchler P. A transient diffusion model of the cornea for the assessment of oxygen diffusivity and consumption. Invest Ophthalmol Vis Sci. 2009;50:1076–80. doi: 10.1167/iovs.08-2479. [DOI] [PubMed] [Google Scholar]
- 57.Kymionis GD, Diakonis VF, Coskunseven E, Jankov M, Yoo SH, Pallikaris IG. Customized pachymetric guided epithelial debridement for corneal collagen cross linking. BMC Ophthalmol. 2009;9:10. doi: 10.1186/1471-2415-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazzotta C, Ramovecchi V. Customized epithelial debridement for thin ectatic corneas undergoing corneal cross-linking: Epithelial island cross-linking technique. Clin Ophthalmol. 2014;8:1337–43. doi: 10.2147/OPTH.S66372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cagil N, Sarac O, Can GD, Akcay E, Can ME. Outcomes of corneal collagen crosslinking using a customized epithelial debridement technique in keratoconic eyes with thin corneas. Int Ophthalmol. 2017;37:103–9. doi: 10.1007/s10792-016-0234-3. [DOI] [PubMed] [Google Scholar]
- 60.Sachdev MS, Gupta D, Sachdev G, Sachdev R. Tailored stromal expansion with a refractive lenticule for crosslinking the ultrathin cornea. J Cataract Refract Surg. 2015;41:918–23. doi: 10.1016/j.jcrs.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 61.Jacob S, Kumar DA, Agarwal A, Basu S, Sinha P, Agarwal A. Contact lens-assisted collagen cross-linking (CACXL): A new technique for cross-linking thin corneas. J Refract Surg. 2014;30:366–72. doi: 10.3928/1081597X-20140523-01. [DOI] [PubMed] [Google Scholar]
- 62.Chen X, Stojanovic A, Eidet JR, Utheim TP. Corneal collagen cross-linking (CXL) in thin corneas. Eye Vis (Lond) 2015;2:15. doi: 10.1186/s40662-015-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. J Refract Surg. 2010;26:942–8. doi: 10.3928/1081597X-20100212-09. [DOI] [PubMed] [Google Scholar]
- 64.Cummings AB, Sinjab MM, Hatch KM, Talamo J, Randleman B, Kanellopoulos AJ, et al. Combined Corneal Cross Linking and Other Procedures: Indications and Application Models. In: Sinjab M.M., Cummings A., editors. Corneal Collagen Cross Linking. 1st ed. Switzerland: Springer International Publishing; 2017. pp. 87–165. [Google Scholar]
- 65.Bottós KM, Schor P, Dreyfuss JL, Nader HB, Chamon W. Effect of corneal epithelium on ultraviolet-A and riboflavin absorption. Arq Bras Oftalmol. 2011;74:348–51. doi: 10.1590/s0004-27492011000500008. [DOI] [PubMed] [Google Scholar]
- 66.Wollensak G, Aurich H, Wirbelauer C, Sel S. Significance of the riboflavin film in corneal collagen crosslinking. J Cataract Refract Surg. 2010;36:114–20. doi: 10.1016/j.jcrs.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 67.Hersh PS, Lai MJ, Gelles JD, Lesniak SP. Transepithelial corneal crosslinking for keratoconus. J Cataract Refract Surg. 2018;44:313–22. doi: 10.1016/j.jcrs.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 68.Caporossi A, Mazzotta C, Paradiso AL, Baiocchi S, Marigliani D, Caporossi T. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 2013;39:1157–63. doi: 10.1016/j.jcrs.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 69.Mazzotta C, Hafezi F, Kymionis G, Caragiuli S, Jacob S, Traversi C, et al. In vivo confocal microscopy after corneal collagen crosslinking. Ocul Surf. 2015;13:298–314. doi: 10.1016/j.jtos.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Yuksel E, Novruzlu S, Ozmen MC, Bilgihan K. A study comparing standard and transepithelial collagen cross-linking riboflavin solutions: Epithelial findings and pain scores. J Ocul Pharmacol Ther. 2015;31:296–302. doi: 10.1089/jop.2014.0090. [DOI] [PubMed] [Google Scholar]
- 71.Taneri S, Oehler S, Lytle G, Burkhard Dick H. Evaluation of epithelial integrity with various transepithelial corneal cross-linking protocols for treatment of keratoconus. J Ophthalmol. 2014;2014:614380. doi: 10.1155/2014/614380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chow SS, Chan TC, Wong IY, Fan MC, Lai JS, Ng AL. Early epithelial complications of accelerated trans-epithelial corneal crosslinking in treatment of keratoconus: A case series. Int Ophthalmol. 2018;38:2635–8. doi: 10.1007/s10792-017-0734-9. [DOI] [PubMed] [Google Scholar]
- 73.Agrawal V. Long-term results of cornea collagen cross-linking with riboflavin for keratoconus. Indian J Ophthalmol. 2013;61:433–4. doi: 10.4103/0301-4738.116072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuechler SJ, Tappeiner C, Epstein D, Frueh BE. Keratoconus progression after corneal cross-linking in eyes with preoperative maximum keratometry values of 58 diopters and steeper. Cornea. 2018;37:1444–8. doi: 10.1097/ICO.0000000000001736. [DOI] [PubMed] [Google Scholar]
- 75.Arora R, Jain P, Goyal JL, Gupta D. Comparative analysis of refractive and topographic changes in early and advanced keratoconic eyes undergoing corneal collagen crosslinking. Cornea. 2013;32:1359–64. doi: 10.1097/ICO.0b013e3182a02ddb. [DOI] [PubMed] [Google Scholar]
- 76.Wisse RP, Godefrooij DA, Soeters N, Imhof SM, Van Der Lelij A. A multivariate analysis and statistical model for predicting visual acuity and keratometry one year after cross-linking for keratoconus. Am J Ophthalmol. 2014;157:519–25.e1-2. doi: 10.1016/j.ajo.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Sarac O, Caglayan M, Cakmak HB, Cagil N. Factors influencing progression of keratoconus 2 years after corneal collagen cross-linking in pediatric patients. Cornea. 2016;35:1503–7. doi: 10.1097/ICO.0000000000001051. [DOI] [PubMed] [Google Scholar]
- 78.Turhan SA, Aydin FO, Toker E. Clinical results of repeated corneal collagen cross-linking in progressive keratoconus. Cornea. 2020;39:84–7. doi: 10.1097/ICO.0000000000002128. [DOI] [PubMed] [Google Scholar]
- 79.Bikbova G, Bikbov M. Transepithelial corneal collagen cross-linking by iontophoresis of riboflavin. Acta Ophthalmol. 2014;92:e30–4. doi: 10.1111/aos.12235. [DOI] [PubMed] [Google Scholar]


