Abstract
Multiple sclerosis and neuromyelitis optica spectrum disorder may be seen in the acute setting of coronavirus disease 2019 (COVID-19) infection or even post-recovery. Such patients may present with optic neuropathy along with weakness in the back and lower limbs. Ascending paralysis can present with respiratory distress in acute COVID-19 infection and may even prove to be fatal. We report a unique case of a 16-year-old female with past history of COVID-19 infection having optic neuropathy, and radioimaging showing demyelinating plaques in the central nervous system with spinal cord edema. Serology showed positivity for rheumatoid arthritis, and the patient was managed with steroids and rituximab.
Keywords: COVID-19, demyelinating, neuromyelitis optica, rheumatoid arthritis
Neuromyelitis optica (NMO) is an extension of central nervous system (CNS) demyelinating degeneration into the spinal cord, causing varied manifestations.[1,2,3,4,5,6,7,8,9,10] Loss of vision in one or both the eyes with optic neuropathy occurs rapidly within days to weeks, with subsequent conversion into transverse myelitis.[1,2,3,4] This disease is seen as a spectrum disorder (NMOSD) in young to middle-aged patients, and either gender may be affected.[1,2,3,4] We report a case of a coronavirus disease 2019 (COVID-19)–recovered patient with rheumatoid arthritis (RA) and NMOSD, treated with steroids and biologics.
Case Report
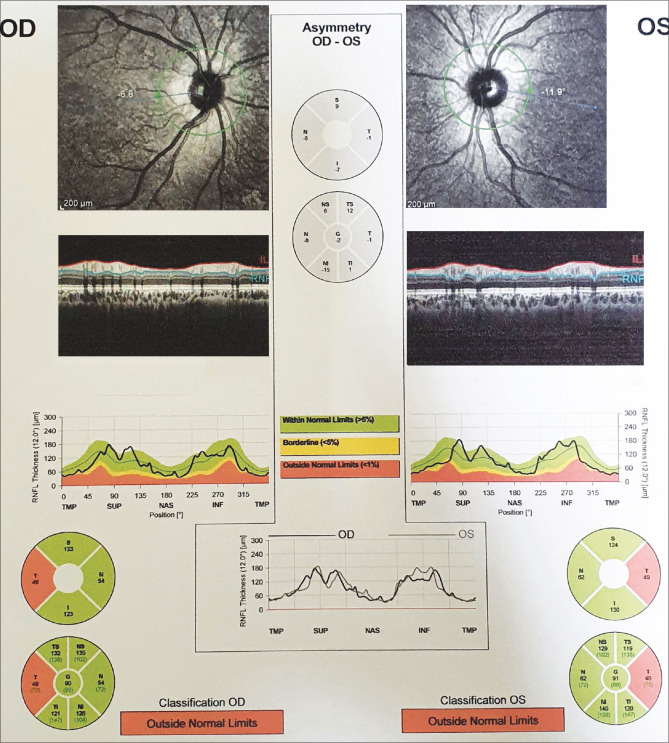
A 16-year-old female student from upper Assam presented to a tertiary care institute of Northeast India with the complaint of diminution of vision in the right eye (OD) for 1 week. The patient had history of COVID-19 infection 4 months back. She also complained of headache and eye ache in OD. There was no history of seizures or vertigo. She had also developed stiffness in her lower limbs and lower back few days ago. She was a nonvegetarian having good appetite. Bowel and bladder habits were normal, and she had no known addiction history. There was no history of drug allergy, long-term medication use, or transfusion history. Vision in OD was counting finger at 3 m and N36 improving to 20/80 (0.25) with pinhole vision (PHV). Left eye (OS) vision was 20/20, N6. There was no relative afferent pupillary defect in either eye. On examination, ocular movements in all cardinal directions of gaze were normal There was no diplopia noted. However, there was increase in pain in the OD in outward and upward gaze. Cranial nerve examination was normal. Face examination was unremarkable. Slit-lamp examination of both eyes was normal. Fundus examination revealed temporal optic disk pallor in both eyes, OD more than OS [Fig. 1a and b]. No other abnormal retinal finding was detected. Ishihara color vision test was subnormal in OD (3/16). Humphrey visual field (HVF) 30-2 in OD showed gross central field defect, and flash visual-evoked potential (VEP) in OD was subnormal with prolonged latency. Magnetic resonance imaging (MRI) of brain and orbit in fat-suppressed Fluid attenuated inversion recovery (FLAIR) mode with contrast showed mildly swollen right optic nerve in T2-weighted image compared to the left side. There was a patch of subcortical white matter T2 hyperintensity lesion in the right frontal subcortical area, suggestive of atypical demyelinating pathology [Fig. 2a]. MRI spine with contrast showed T2-weighted lesion between C2 and C7 vertebral level with swollen cervical cord and edema [Fig. 2b]. Retinal nerve fiber layer (RNFL) in optical coherence tomography (OCT) showed few abnormal points in the curves and temporal defect in both eyes in circular scan [Fig. 3]. The patient was diagnosed as NMOSD and was referred to a local neurological institute. She was thoroughly examined by a neurologist, with CNS examination showing normal Glasgow Coma Scale (GCS) of E4V5M6; function of cranial nerves was intact; motor examination showed normal muscle bulk, tone, and power; sensory examination revealed normal findings and plantar flexor response was observed bilaterally. Respiratory, cardiovascular, and abdominal systems were healthy; all vitals were within normal limits (oxygen saturation 99%). Systemic investigations revealed normal complete blood count (CBC), renal function test (RFT), liver function test (LFT), normal blood sugar, normal serum vitamin B12 estimation, normal thyroid hormone assay, and normal chest X-ray. Patient was advised serum anti-myelin-oligodendrocyte glycoprotein (MOG) antibody and anti-aquoporin-4 (AQP4) antibody testing, and both showed a negative result. She was given five doses of intravenous methyl prednisolone (IVMP) in reconstituted intravenous (IV) fluid. Post IVMP, her vision improved in OD to 20/200 (unaided), further improving to 20/30 (PHV, 0.20), while the OS vision was maintained at 20/20, N6. The patient was prescribed an extended course of oral steroids. Color vision by Ishihara was still subnormal in OD, and Retinal nerve fiber layer optical coherence tomography (RNFLOCT) showed almost similar pattern with the pre-IVMP RNFL record. Further review by another neurologist showed raised IgG immunoglobulin and glucose levels in cerebrospinal fluid (CSF) without other significant findings. Antinuclear antibody (ANA) was positive and Sjogren’s syndrome related antigen A (SSA) (anti-Ro) antibody was strongly positive, indicating systemic autoimmune rheumatic disease. Patient was started on rituximab, in addition to oral steroids. Incidentally, it was found that her father had previous history of uveitis OS and seronegative arthritis, with human leukocyte antigen (HLA) B-27 positivity. She underwent antibody titer test for severe acute respiratory syndrome-coronavirus-2 (SARS –CoV- 2) which was raised. Patient is currently under close follow-up with neurologist, rheumatologist, and neuro-ophthalmologist for her condition.
Figure 1.
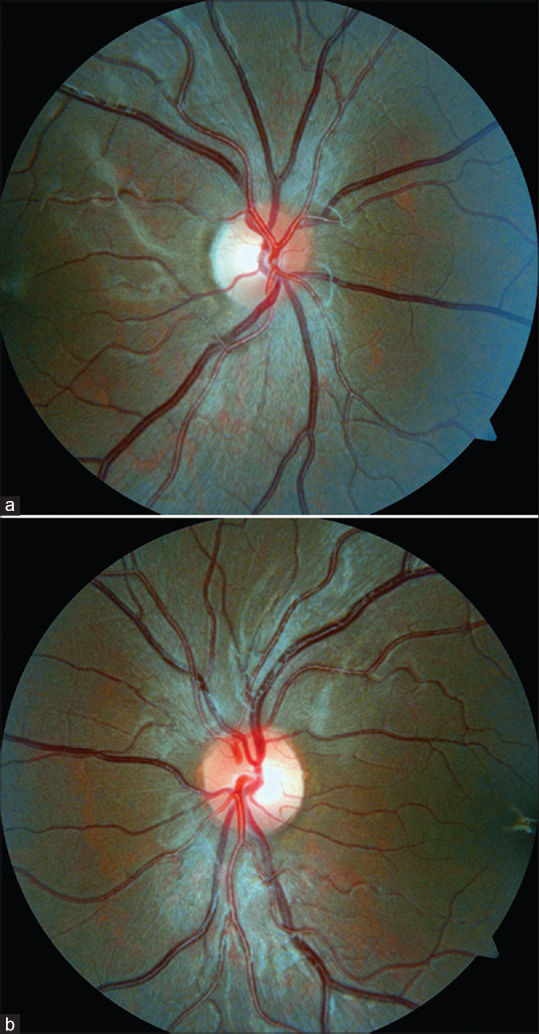
(a and b) Fundus photos showing optic disk of right and left eyes with temporal disk pallor
Figure 2.
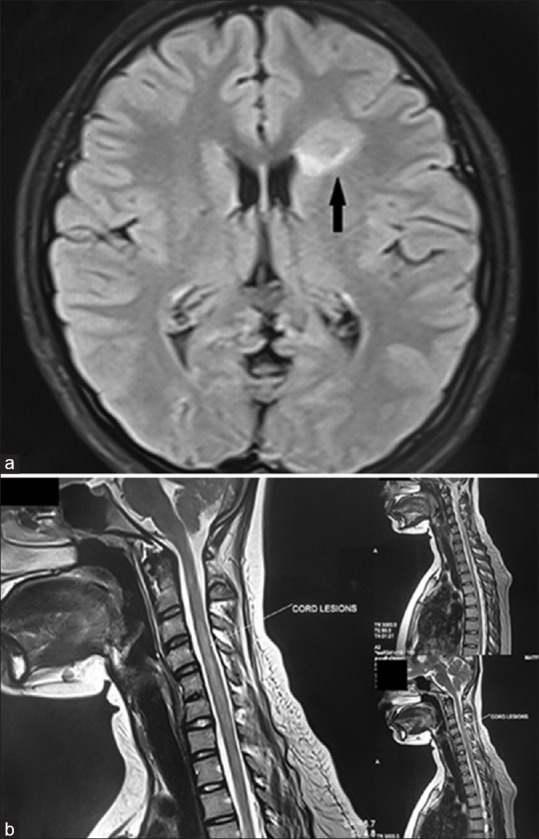
(a) MRI brain and orbit in fat-suppressed FLAIR mode showing a patch of subcortical white matter T2 hyperintensity lesion (marked with black arrow) in the right frontal subcortical area, suggestive of atypical demyelinating pathology. (b) MRI spine with contrast showed T2-weighted lesion between C2 and C7 vertebral levels with swollen cervical cord and edema (marked with white arrow).MRI = magnetic resonance imaging
Figure 3.
Retinal nerve fiber layer in optical coherence tomography showing few abnormal points in the curves and temporal defect in both eyes in circular scan
Discussion
Multiple sclerosis (MS) and NMOSD can behave in different ways.[1,2,3,4,5,6,7,8,9,10] Atypical NMOSD has been reported during the COVID-19 pandemic.[1,2,3,4,5,6,7,8,9,10] Our case was a young girl with past history of COVID-19 infection and sudden loss of vision in the OD with radicular pain radiating to her thighs. In MRI, demyelinating changes were seen in periventricular, subcortical region of brain with focal edema of vertebral column of the spinal cord. The patient was negative for AQP4 antibody, an aqua channel protein, and MOG antibody. Her ANA and antibody to SSA (anti-Ro) were strongly positive, indicating RA–related NMOSD. NMOSD can have destructive lesions in both white and gray matter.[1,2,3,4] COVID-19 virus has been described in association with these disorders in a few recent reports.[5] It has been hypothesized that the culprit RNA virus enters into the targeted cells by angiotensin converting enzyme (ACE)-2 receptors.[1,2,3,4,5] The virus then reaches the CNS by infecting the epithelial and endothelial cells of blood–brain barrier through axonal route.[1,2,3,4,5] Autoimmunity is also known to play a significant role in the pathogenesis initiating NMOSD and RA.[6] Our patient was given IV steroids with a course of rituximab. Treatment with rituximab has been found to be beneficial and should not be stopped before at least 2 years of therapy.[7,8]
Conclusion
Atypical NMOSD in COVID-19 infection is a challenging clinical entity where accurate diagnosis is utmost important and prompt treatment with corticosteroid and biologics is a must.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient’s guardian consent form. In the form, the patient’s father had given his consent for her images and other clinical information to be reported in the journal. The patient’s father understand that his daughter’s name and initial will not be published and due efforts will be made to conceal her identity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Sri Kanchi Sankara Health and Educational Foundation;
Dr. Rupjyoti Das DNB, Neurologist, GNRC-Guwahati, India;
Dr. Rajashekar Reddi MD, DM, DNB, Neurologist, Max Super Specialty Hospital, Saket, New Delhi; and
Dr. Himadri Shekar Das, MD, Radiologist; Dr. Pradip Hatimota, MD, Radiologist, Matrix Imaging, Guwahati, India are acknowledged.
References
- 1.Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65:727–32. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahmohammadi S, Doosti R, Shahmohammadi A, Mohammadianinejad SE, Sahraian MA, Azimi AR, et al. Autoimmune diseases associated with neuromyelitis optica spectrum disorders: Literature review. Mult Scler Relat Disord. 2019;27:350–63. doi: 10.1016/j.msard.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G, et al. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35:1123–38. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W, Wang L, Zhang B, Zhou L, Zhang T, Quan C, et al. Effectiveness and tolerability of immunosuppressants and monoclonal antibodies in preventive treatment of neuromyelitis optica spectrum disorders: A systematic review and network meta-analysis. Mult Scler Relat Disord. 2019;35:246–52. doi: 10.1016/j.msard.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Seah I, Agrawal R, et al. Can the coronavirus disease 2019 (COVID-19) affect the eyes?A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–5. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, et al. Diagnosis and treatment of neuromyelitis optica. Neurologist. 2007;13:2–11. doi: 10.1097/01.nrl.0000250927.21903.f8. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Zhong Y, Wang Y, Dai Y, Qiu W, Zhang L, et al. Neuromyelitis optica spectrum disorders without and with autoimmune diseases. BMC Neurol. 2014;14:162. doi: 10.1186/s12883-014-0162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damato V, Evoli A, Iorio R, et al. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: A systematic review and meta-analysis. JAMA Neurol. 2016;73:1342–8. doi: 10.1001/jamaneurol.2016.1637. [DOI] [PubMed] [Google Scholar]
- 9.Saxena R, Phuljhele S, Menon V, Gadaginamath S, Sinha A, Sharma P, et al. Clinical profile and short term outcome of optic neuritis in India. Indian J Ophthalmol. 2014;62:265–7. doi: 10.4103/0301-4738.121131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batum M, Kisabay Ak A, Mavioğlu H, et al. Covid-19 infection-induced neuromyelitis optica: A case report. Int J Neurosci. 2020;30:1–7. doi: 10.1080/00207454.2020.1860036. [DOI] [PubMed] [Google Scholar]