Abstract
Purpose:
Real-life comparison of three intravitreal drug regimens used in cases of endophthalmitis at a tertiary care center in India.
Methods:
In this prospective, comparative study, patients of bacterial endophthalmitis were grouped according to intravitreal antibiotic drug regimens into Group 1 (ceftazidime and vancomycin), Group 2 (piperacillin + tazobactam and vancomycin), and Group 3 (imipenem and vancomycin). Forty-eight hours after injection nonresponding/worsening patients underwent vitrectomy. Vitreous samples were subjected to microbiological and pharmacokinetic tests.
Results:
A total of 64 patients were included and divided into Group 1: 29, Group 2: 20, and Group 3: 15 cases. Also, 75% of patients were post-surgical endophthalmitis, whereas 25% were post-traumatic. Improvement in vision (V90-0) and vision at 3 months (V90) were comparable between the three groups. Visual recovery was poorer in post-traumatic cases. In post-surgical cases, visual recovery was poorer in those presenting beyond 72 h of onset of symptoms (P = 0.0002). Polymerase chain reaction (PCR) positivity (66%) was higher than BACTEC™ (33%) and culture (14%). Antibiotic resistance was comparable amongst the three groups. Most patients (62/64) further underwent vitrectomy. Ceftazidime and vancomycin achieved vitreous concentrations more than the minimum inhibitory concentration (MIC) at 48 h after the first injection.
Conclusion:
The choice of antibiotics did not affect the rate of vitrectomy and final vision in a real-life scenario. Ceftazidime and vancomycin can still be used as first-line intravitreal antibiotics owing to their comparable microbial sensitivity profile and adequate ocular bioavailability.
Keywords: Ceftazidime, endophthalmitis, imipenem, piperacillin/tazobactam, vancomycin
Endophthalmitis is defined as an inflammation of the inner layers of the eye with exudation in the vitreous cavity due to the colonization of microorganisms.[1,2] It is a medical emergency, which requires prompt treatment, else can lead to severe or permanent visual loss.[3] The prognosis of endophthalmitis depends on its etiology, virulence of organisms, and adequate intervention. Intravitreal antibiotics remain the mainstay of initial treatment in many cases. Pars plana vitrectomy may also be performed as the primary procedure or following intravitreal antibiotic injection. In most cases of bacterial endophthalmitis, a combination of vancomycin and ceftazidime is used for the intravitreal injections to cover both gram-positive and gram-negative bacteria. There has been a recent rise in cases of endophthalmitis that are resistant to ceftazidime,[4] which has led to the introduction of alternate drugs covering these gram-negative bacteria. These drugs include tazobactam-piperacillin or imipenem. In this study, we have compared the clinical outcomes, microbiological sensitivity, and pharmacokinetics of three different intravitreal antibiotic regimens in a real-life setting.
Methods
This was a prospective, comparative study conducted at a tertiary care hospital. Ethical clearance was taken from the Institute Ethical Committee and consecutive patients presenting to our center with endophthalmitis and those who met the inclusion criteria were included in the study from January 2018 to June 2019. Post-traumatic and post-surgical endophthalmitis cases were included. Fungal endophthalmitis, metastatic endophthalmitis, and cases with retained intraocular foreign body or retinal detachment were excluded.
A detailed history was taken from the patient with respect to the onset, duration, and severity of the symptoms. The vision was taken using Snellen’s chart and converted to corresponding logMAR units.[5,6] Patients were allocated to either one of the three antibiotic regimens. Patients with corneal involvement in the form of keratitis, corneal ulcers, and severe corneal edema were not included in the study. Initially, the first intravitreal antibiotic regimen was injected and the patient was followed up to see the response. If there was improvement in terms of reduction in vitreous haze (standard NEI grading),[7] resolution of hypopyon (height of hypopyon on slit-lamp using calibrated marking scale), or improvement in vision, the patient was observed and followed up closely. If there was worsening or no change in the further management in the form of repeat intravitreal injection or vitrectomy, it was left to the surgeon’s discretion. The same injections were repeated according to the group the patients were allocated to if there was no improvement in visual acuity or vitreous haze. Vitrectomy along with the same intravitreal injection was advocated in those who were worsening. A maximum of three intravitreal injections were given 48 h apart and when improvement could not be documented, the patients were taken up for surgery. Oral ciprofloxacin (500 mg twice a day) was given to all patients, whereas intravenous antibiotics were given to patients with fulminant endophthalmitis according to the treating ophthalmologist.
The vitreous sample obtained either during primary injection or vitrectomy was sent for microbiological analysis, which included Gram stain, KOH stain, bacterial culture and sensitivity, polymerase chain reaction (PCR), and BACTEC™. However, the results of the antibiotic sensitivity pattern did not change our intravitreal regimen as most cases underwent vitrectomy before the results were available. PCR assay was performed in a thermal cycler (Applied Biosystems, USA) with the following temperature profile: initial denaturation for 5 min at 95°C, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 45 s, extension at 72°C for 45 s, with the final extension at 72°C for 7 min. Amplified DNA bands were cut from the agarose gel using sterile scalpel blades and processed for nucleotide sequencing. Nucleotide sequences of the purified DNA were determined by Sanger’s dideoxy chain termination method using an automated capillary DNA sequencer (ABI Prism 310; PE Applied Biosystems, Foster City, California). The obtained nucleotide sequences were aligned using DNASTAR laser gene molecular biology suite software. The sequences were analyzed for homology against available sequences in the GenBank database using the NCBI BLAST computer program, and the identity of the organisms was determined as per the CLSI MM18A document guidelines with a minimum of 99.5% similarity at least ≥0.8% sequence discrepancy to the second scoring species.
Vitrectomy was performed in patients who worsened with intravitreal injections or showed no improvement after the third dose. At the time of vitrectomy, an undiluted vitreous sample was taken before starting the infusion and sent for microbiological as well as pharmacokinetics assessment of the drugs. Liquid chromatography-mass spectrometry (LC-MS) was used to analyze the amount of drug that remained in the vitreous cavity after a fixed duration. These were compared to the minimum inhibitory concentration (MIC) values for common bacteria as given by EUCAST (version 9.0).[8] Silicon oil endotamponade was used according to the surgeons’ discretion in cases of extensive vitreous or retinal exudates and retinal necrosis.
The patients were followed up for 3 months, and visual acuity at 3 months (V90) was used for analysis. Statistical analysis was performed using the statistical package SPSS (Statistical Package for the Social Sciences) version 25.
Results
Clinical results
A total of 64 patients diagnosed with endophthalmitis were included in the study. Group 1 (ceftazidime and vancomycin), Group 2 (tazobactam–piperacillin and vancomycin), and Group 3 (imipenem and vancomycin) had 29, 20, and 15 patients, respectively. The mean age of the patients was 50.78 years with no statistical difference between the groups (P = 0.12).
The mean presenting visual acuity was logMAR 2.24 (which is nearly hand movements close to the face), and this was comparable between the three groups (P = 0.5). In our study, post-surgical endophthalmitis constituted 75% of cases (48/64), whereas the remaining 25% were post-traumatic endophthalmitis cases. Among the post-surgical group, the most common cases were those secondary to small incision cataract surgery (SICS). The proportion of cases of post-surgical and post-traumatic endophthalmitis in the three groups was comparable [Table 1]. Thirty-five out of 64 patients presented with a history of more than 3 days from the onset of symptoms. Also, 20% of the patients received intravenous antibiotics, which were solely advised by the treating ophthalmologist. However, the intergroup comparison revealed that the number of cases receiving intravenous antibiotics was comparable between the groups.
Table 1.
Baseline parameters of the three study groups
| Baseline details | Group 1 n=29 | Group 2 n=20 | Group 3 n=15 | P |
|---|---|---|---|---|
| Age (years) | 46.17±18.92 | 52.85±15.21 | 56.93±15.16 | 0.12 |
| Duration from the onset of symptoms to first intravitreal injection (post-operative endophthalmitis cases) (days) | Mean: 5.45 Median: 3 SEV: 6.07 |
Mean: 6.87 Median: 3 SEV: 5.82 |
Mean: 4.55 Median: 4 SEV: 3.87 |
0.77 |
| Duration from intravitreal injection to vitrectomy (days) (mean±SD) | 3.34±2.12 | 3.72±1.77 | 3.76±1.23 | 0.48 |
| Vision at presentation (V0) | 2.28±0.3 | 2.15±0.51 | 2.27±0.39 | 0.5 |
| Post-traumatic: post-surgical | 8:21 | 4:16 | 4:11 | 0.07 |
| Eyes undergoing vitrectomy | 29 | 18 | 15 | 0.78 |
| Eyes undergoing silicon oil injection during vitrectomy | 10/29 | 6/18 | 6/15 | 0.11 |
SEV: standard error of variation; SD: standard deviation
The patients were followed up for 3 months and the improvement in vision was noted as compared to that at presentation. Of the 64 patients, 21 received a single intravitreal injection, 36 received 2 intravitreal injections, and 7 received 3 intravitreal injections 48 h apart. The mean improvement in the three groups was up to logMAR 1.22 ± 0.88, accounting for nearly 4/60 vision. On comparing the three groups, the P value was 0.272 using the Kruskal–Wallis test, which was statistically insignificant, making the groups comparable. The groups were also compared on the basis of the number of patients whose final vision (at 3 months) was less than 3/60 (blindness), between 3/60 and 6/18 (low vision), and better than 6/18 [Fig. 1]. This sub-group visual acuity analysis was also found to be comparable in the three groups (P = 0.703).
Figure 1.
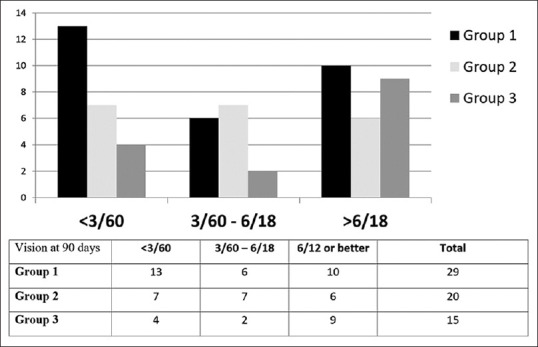
Final BCVA between different groups
The majority of the patients (62/64 = 97%) underwent vitrectomy. The mean duration from the first intervention to vitrectomy was 3.5 days (P = 0.48) with no statistical difference among the groups. Thus, the choice of antibiotics did not affect the rate of vitrectomy. Silicone oil tamponade was used in 35% of cases in our study.
Out of the post-surgical endophthalmitis cases, the patients who presented within 3 days after onset of symptoms had a lesser number of patients with vision <6/18 at 90 days as compared to those who presented later than 72 h (Wilcoxon-signed rank test, P = 0.0002). Amongst the post-traumatic and post-surgical groups, visual recovery was worse in the traumatic group (P = 0.001)[Table 2].
Table 2.
Comparison of visual outcomes according to etiology, time of presentation, and gram positivity
| Vision at presentation (V0) logMAR | Vision at 90 days (V90) logMAR | |
|---|---|---|
| Post-traumatic cases (n=16) | 2.4 | 2.17 |
| Post-surgical cases (n=48) | 1.78 | 0.78 |
| P | 0.45 | 0.001 |
| First intervention within 3 days of symptoms in postoperative group (n=23) | 2.15 | 0.78 |
| First intervention more than three days of symptoms in the postoperative group (n=23) | 2.28 | 1.62 |
| P | 0.67 | 0.002 |
| Gram-positive (n=26) | 2.18 | 0.48 |
| Gram-negative (n=14) | 2.28 | 1.78 |
| P | 0.63 | 0.07 |
Depending upon the causative agent, the final vision (V90) of cases due to gram-positive bacteria had a better final vision (logMAR 0.7 that is equivalent to 6/36) as compared to gram-negative bacteria (logMAR 1.78 that is 1/60). However, statistical significance could not be achieved in our cohorts (P = 0.07).
Nearly 20% of cases (13/64) in our study were diabetic and their final visual acuity was comparable to the non-diabetic patients using the Mann–Whitney test (51/64) (P = 0.63).
Microbiological results
On performing Gram’s staining of vitreous samples, 11% (7/64) of cases stained positive. The conventional culture positivity rate was 14% (9/64). Among these nine cases, seven were gram-positive and two were gram-negative micro-organisms. Using the BACTEC™ (automated culture system) method, the yield increased to 33% cases (21 out of 64 cases). Of these, 15 were gram-positive and six were gram-negative. The BACTEC™ positive cases were tested for antibiotics susceptibility. Among 15 positives for gram-positive bacteria, 2 of them were resistant to vancomycin. Although among the six gram-negative cases, two were resistant to ceftazidime, one for tazobactam-piperacillin, and one for imipenem.
PCR was also performed for the vitreous samples and it was positive in 66% (42 out of 64 cases) of samples. Among these, 57% of cases (26 out of 42 cases) were gram-positive, 33% of cases (14 out of 42 cases) were gram-negative, and two sequences could not be identified.
Among the gram-positive bacteria, coagulase-negative Staphylococcus (CoNS) was the most common micro-organism, whereas the most common gram-negative bacteria was Pseudomonas [Table 3].
Table 3.
Microbiological results of polymerase chain reaction
| Microorganism detected | Number |
|---|---|
| GRAM-positive bacteria (n=26) | |
| Coagulase-negative Staphylococcus | 10 |
| Staphylococcus aureus | 5 |
| Streptococcus | 5 |
| Staphylococcus hemolyticus | 2 |
| Staphylococcus warneri | 1 |
| Clostridium | 1 |
| Nocardia | 1 |
| Propionibacterium acnes | 1 |
| Gram-negative bacteria (n=14) | |
| Pseudomonas aeruginosa | 9 |
| Pseudomonas stutzeri | 2 |
| Klebsiella | 2 |
| Hemophilus influenzae | 1 |
Pharmacokinetic results
Drug concentration in the vitreous at 48 h of injection was analyzed using the LC-MS in a few samples. Eighteen samples for vancomycin, 11 for ceftazidime, and 2 each for piperacillin and imipenem were found adequate for LC-MS analysis. We found that the mean vitreous concentration of vancomycin and ceftazidime at 48 h was more than MIC values. Results for imipenem and piperacillin were considered inconclusive as the number of samples was low [Table 4].
Table 4.
Pharmacokinetics of various drugs using LC-MS
| Drug | Sample studied | Mean concentration at 48 h | MIC (minimum inhibitory concentration) |
|---|---|---|---|
| Vancomycin | 18 | 25.1 mg/L | 2 mg/L |
| Ceftazidime | 11 | 20.2 mg/L | 8 mg/L |
| Piperacillin | 2 | 1.7 mg/L | 16 mg/L |
| Imipenem | 2 | Insignificant amount | 4 mg/L |
Discussion
The duration from the onset of symptoms to the first intervention is critical to the final visual outcome. When patients present early, the bacterial load is low and the severity of tissue infection is low. Hence, early intervention in cases leads to better anatomical and visual outcomes. As the disease becomes chronic, tissue necrosis (including retinal necrosis) sets in and the microbiological load becomes manifold, decreasing the chances of tissue salvage. In the post-surgical endophthalmitis cohort of our study, we found that visual recovery was significantly better in patients in whom the first intervention was performed within 72 h of onset of symptoms. Thus, we strongly recommend timely referral of such cases so that appropriate management can be instituted early.
According to the endophthalmitis vitrectomy study (EVS), early vitrectomy had better outcomes when presenting vision was less than hand movements close to the face.[9] EVS was a landmark study conducted in 1990 and has its implications even today. However, over the last 30 years, the scenario has changed considerably. The incidence of endophthalmitis has decreased with better OT sterilization techniques and novel instruments.[10] Instrumentation for vitreo-retinal surgeries has markedly improved making vitrectomy easier and reducing chances of post vitrectomy complications. The rate of vitrectomy in our study was much higher than in other studies. This could be due to the surgeon’s preference for early surgical intervention at a tertiary care center.
On comparing different parameters such as etiology, time of presentation, and the microorganisms (which were comparable in the groups) in between the three groups we found that the final visual outcome and rates of vitrectomy were comparable within the groups and superiority could not be established with any particular drug.
Oral ciprofloxacin was prescribed to all patients and intravenous antibiotics were given to 20% of the patients. Intravenous antibiotics did not confer any added advantage in terms of visual outcome or vitrectomy rates.
Silicon oil has been used as an adjunct to early vitrectomy in the surgical management of endophthalmitis. Its role has been described in post-traumatic cases where complete vitrectomy followed by silicon oil instillation led to a better visual outcome as described by Azad et al.[11] Silicone oil tamponade was used in 35% of cases in our study and the intergroup distribution of cases was comparable. Silicon oil would have been used in cases with more fulminant infection. However, our study was not designed to assess the effect of silicon oil on visual or anatomical recovery, and a difference in the number of cases between the groups may be a limiting factor of our study.
In our study, with the use of BACTEC™ method, the culture positivity increased to 33%. Thus, we propose this to be routinely used rather than conventional culture. PCR has an even higher positivity rate; however, it does not provide information on antibiotic sensitivity. However, it can differentiate between a fungal, gram-positive, or gram-negative infection. This information is also quite valuable in the real-life scenario for the clinician.
Vancomycin resistance was seen in 2 cases out of 15 samples, which shows an increase in the trend of vancomycin resistance from previous studies.[12,13] These results were based on the disc diffusion method. This is a sensitive, quick, and reliable method of assessment of bacterial sensitivity. However, combining it with other tests such as the broth-dilution method might have increased the positive predictability value.[6,14] Intravitreal linezolid[15] or daptomycin[16] can be considered as an important treatment alternative in such cases. In our study, two out of six culture-positive gram-negative cases showed resistance to ceftazidime and one each to imipenem and piperacillin-tazobactam. In a study by Dave et al.,[17] the outcomes of ceftazidime-resistant gram-negative endophthalmitis were poor and all those cases (n = 56) were sensitive to imipenem. Eleven of them were reinjected with imipenem and eight of those showed improvement compared to 18 of 45 eyes who underwent reinjection with another drug (amikacin/gentamicin/ciprofloxacin). Univariate analysis in the same study also showed that eyes that underwent repeat imipenem had chances of better visual outcomes. Our study shows similar outcomes with all three regimens. Both the studies do not have a sufficient sample size to justify one drug superior to the other. Carbapenems are highly resistant to the b-lactamase enzymes produced by many multiple drug-resistant gram-negative bacteria, thus playing a key role in the treatment of infections not readily treated with other antibiotics.[18,19,20] Intrinsic resistance to carbapenems is not common among clinically important bacteria and in most cases carbapenem resistance is acquired by mutational events or gene acquisition via horizontal gene transfer.[21] Thus, these drugs may be a good alternative for resistant cases.
The antibiotic sensitivity could be evaluated only in BACTEC™-positive cases (33%), which were 21 in number. There was no statistical difference found in the resistance rate of the three antibiotics for gram-negative bacteria. Despite evaluating 64 cases, the number of gram-negative organisms detected in our study was small (six in number) and is thus a limitation of our study.
In our study, a pharmacokinetic study of various antibiotics was also done. Vancomycin and ceftazidime were found to have a higher concentration than MIC in the vitreous after 48 h of intravitreal injection. This corresponds to the nature of the drugs as both of these cations are eliminated via the anterior chamber. In inflamed eyes as in endophthalmitis, they have decreased elimination, and thus their concentrations in the vitreous go beyond MIC. In contrast, piperacillin and imipenem form anions and are known to have higher posterior elimination via pumps in the RPE. The function of these pumps is enhanced in an inflamed eye.[22] This may be a reason why these drugs have a lower concentration than MIC at the end of 48 h. However, because we could only adequately study two vitreous samples of piperacillin and imipenem, we recommend that the concentration of these drugs in the human vitreous should be studied further in detail in a larger study.
Small sample size is a limitation of our study; however, it is difficult to collect a large number of cases from a single tertiary center in a prospective study. Another limitation is the randomization of patients to different groups. Ours being a government tertiary care center offering ophthalmology services at a very subsidized cost. Piperacillin and imipenem were given to patients who could afford to purchase these drugs. Thus strict randomization was not employed; however, the economic criterion was the method of allocation. Because the severity of the case or clinical findings were not the criterion, we feel that there was no bias regarding the use of a particular drug in a particular case.
Conclusion
We conclude that endophthalmitis is an ocular emergency and early intervention (within 3 days) can lead to a better visual outcome. Presently, the three antibiotic drug regimens are comparable based on final visual acuity, improvement in vision from baseline, and antibiotic sensitivity. Ceftazidime and vancomycin have good ocular bioavailability even at 48 h after injection. Thus, this combination can still be used as the standard first-line empirical drug regimen for intravitreal injection in cases of endophthalmitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pijl BJ, Theelen T, Tilanus MA, Rentenaar R, Crama N, et al. Acute endophthalmitis after cataract surgery: 250 consecutive cases treated at a tertiary referral centre in the Netherlands. Am J Ophthalmol. 2010;149:482–7. doi: 10.1016/j.ajo.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 2.What is Endophthalmitis? American Academy of Ophthalmology. 2019. [Last accessed on 2019 Nov 29]. https://www.aao.org/eye-health/diseases/what-is-endophthalmitis.
- 3.Bohigian GM, Olk RJ, et al. Factors associated with a poor visual result in endophthalmitis. Am J Ophthalmol. 1986;101:332–41. doi: 10.1016/0002-9394(86)90829-9. [DOI] [PubMed] [Google Scholar]
- 4.Pan U, Jain A, Gubert J, Kumari B, Sindal MD, et al. Antibiotic sensitivity trends of pseudomonas endophthalmitis in a tertiary eye care center in South India: A 12-year retrospective study. Indian J Ophthalmol. 2020;68:627–31. doi: 10.4103/ijo.IJO_1145_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida T, Ishida K, Niwa Y, Kawakami H, Mochizuki K, Ohkusu K, et al. An eleven-year retrospective study of endogenous bacterial endophthalmitis. J Ophthalmol. 2015;2015:2610–3. doi: 10.1155/2015/261310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer AW, Kirby WM, Sherris JC, Turck M, et al. Antibiotic susceptibility testing by astandardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 7.Nussenblatt RB, Palestine AG, Chan CC, Roberge F, et al. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–71. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 8.EUCAST: MIC and zone distributions and ECOFFs [Internet] [Last accessed on 2020 Jul 11]. https://www.eucast.org/mic_distributions_and_ecoffs.
- 9.Johnson MW, Doft BH, Kelsey SF, Barza M, Wilson LA, Barr CC, et al. The endophthalmitis vitrectomy study: Relationship between clinical presentation and microbioloaic spectrum. Ophthalmology. 1997;104:261–72. doi: 10.1016/s0161-6420(97)30326-1. [DOI] [PubMed] [Google Scholar]
- 10.Safneck JR, et al. Endophthalmitis: A review of recent trends. Saudi J Ophthalmol. 2012;26:181–9. doi: 10.1016/j.sjopt.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azad R, Ravi K, Talwar D, Rajpal, Kumar N, et al. Pars plana vitrectomy with or without silicone oil endotamponade in post-traumatic endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2003;241:478–83. doi: 10.1007/s00417-003-0670-4. [DOI] [PubMed] [Google Scholar]
- 12.Sunaric-Mégevand G, Pournaras CJ, et al. Current approach to postoperative endophthalmitis. Br J Ophthalmol. 1997;81:1006–15. doi: 10.1136/bjo.81.11.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chantratita N, Rholl DA, Sim B, Wuthiekanun V, Limmathurotsakul D, Amornchai P, et al. Antimicrobial resistance to ceftazidime involving loss of penicillin-binding protein 3 in Burkholderiapseudomallei. Proc Natl Acad Sci USA. 2011;108:17165–70. doi: 10.1073/pnas.1111020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Wayne, PA: USA: CLSI; 2010. Performance Standards for AntimicrobialSusceptibility Testing; Twentieth Informational Supplement M100-S20. [Google Scholar]
- 15.Shivaramaiah HS, Relhan N, Pathengay A, Mohan N, Flynn HW., Jr Endophthalmitis caused by gram-positive bacteria resistant to vancomycin: Clinical settings, causative organisms, antimicrobial susceptibilities, and treatment outcomes. Am J Ophthalmol Case Rep. 2018;10:211–4. doi: 10.1016/j.ajoc.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sim JM, Kapoor KG, Wagner AL, et al. Intravitreal daptomycin for recalcitrant postoperative endophthalmitis. Case Rep Ophthalmol. 2016;7:103–7. doi: 10.1159/000444046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dave VP, Pathengay A, Nishant K, Pappuru RR, Sharma S, Sharma P, et al. Clinical presentations, risk factors and outcomes of ceftazidime-resistant Gram-negative endophthalmitis. Clin Exp Ophthalmol. 2017;45:254–60. doi: 10.1111/ceo.12833. [DOI] [PubMed] [Google Scholar]
- 18.Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME, et al. Prolonged versus short-term intravenous infusion of antipseudomonal b-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18:108–20. doi: 10.1016/S1473-3099(17)30615-1. [DOI] [PubMed] [Google Scholar]
- 19.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME, et al. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum b-lactamases: A systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:2793–803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 20.Clissold SP, Todd PA, Campoli-Richards DM, et al. Imipenem/cilastatin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1987;33:183–241. doi: 10.2165/00003495-198733030-00001. [DOI] [PubMed] [Google Scholar]
- 21.Carbapenem resistance: Overview of the problem and future perspectives-Georgios Meletis, 2016. [Last accessed on 2019 Nov 30]. https://journals.sagepub.com/doi/full/10.1177/2049936115621709. [DOI] [PMC free article] [PubMed]
- 22.Halder N, Velpandian T, et al. Research in ocular pharmacology: An Indian perspective in the last five years. Proc Indian Natl Sci Acad. 2018;84:185–93. [Google Scholar]


