Abstract
Purpose:
To assess the role of noninvasive ocular surface analyzer (OSA) in workup of meibomian gland dysfunction (MGD) and to estimate hospital-based prevalence of MGD using this objective device.
Methods:
The study recruited 113 consecutive participants attending the ophthalmology outpatient department of a tertiary care hospital. All participants were administered a symptom questionnaire. Participants underwent a comprehensive ocular examination, including slit-lamp biomicroscopy and meibomian gland expression. Lipid layer thickness (LLT), noninvasive tear breakup time (NIBUT), tear meniscus height (TMH), and meibomian gland loss (MGL) were assessed using OSA. The presence of either or both reduced/absent meibum secretion and cloudy to toothpaste-like secretion was diagnosed as MGD.
Results:
Prevalence of total MGD was 57.52% (95% confidence interval [95% CI]: 48.3%–66.8%) and that of symptomatic MGD was 42.5% (95% CI: 33.2%–51.7%). Prevalence of total and symptomatic MGD was highest in those aged ≥50 years (P < 0.001 and P = 0.004, respectively). Computer vision syndrome increased the odds of symptomatic MGD (odds ratio [OR]: 4.3). NIBUT and MGL significantly differed in MGD and non-MGD groups (P = 0.023 and P < 0.001, respectively). LLT significantly differed between asymptomatic and symptomatic cases (P = 0.033). MGL >25% increased the odds of having MGD (OR: 19.1). Significant negative correlations were observed between MGL and NIBUT (P = 0.04) and between MGL and LLT (P = 0.02). MGL demonstrated the highest diagnostic accuracy for MGD (AUC = 0.827, sensitivity = 75.4%, specificity = 85.4%, cut-off value: ≥26%).
Conclusion:
MGD is a common disorder in adults attending the ophthalmology outpatient services of a tertiary eye care hospital. Incorporating noninvasive OSA in clinical practice can aid in rapid and reliable measurements of MGD-related parameters.
Keywords: Dry Eye, meibomian gland dysfunction, meibomian gland loss, meibomian glands, ocular surface analyzer
Meibomian gland dysfunction (MGD) is a commonly encountered disorder in clinical practice and an important cause of evaporative dry eye disease (DED).[1] Digital display usage is an important contributor to this emerging public health problem, particularly in younger age groups.[2] The reported prevalence of MGD in the literature varies from 3.5% to 70.3% across studies, with relatively higher prevalence in Asians than in Caucasians.[3,4,5,6,7,8,9,10] Inconsistent diagnostic criteria and varied study groups have been partly held responsible for this disparity.[5,6,7,10] Until recently, there was no standardized definition of MGD.[11] Differing study definitions and symptom questionnaires, along with disruptive effects of clinical tests on subsequent test values, make the diagnosis and workup of MGD cumbersome in routine clinical practice. Lack of objectivity in clinical assessment compounds the problem.
Despite a high prevalence reported in Asians, the available literature on MGD in the Indian population is limited, with very few studies keeping MGD their primary focus.[5,6,12,13,14,15] This lack of focused research in MGD could be attributed to the reasons mentioned earlier. The current coronavirus disease of 2019 (COVID-19) pandemic has also impacted the ocular surface in multiple ways.[16] Besides the increased occurrence of DED reported following COVID-19 infection, attempts to curb the pandemic have led to an increased screen time in all age groups, whether in the domain of work, school, or leisure. Mask-associated dry eye is a further concern. The situation thus calls for heightened surveillance to look for MGD and associated dry eye. Developing a comprehensive diagnostic tool that provides noninvasive and objective evaluation of meibomian glands, tear film, and ocular surface, along with quantifiable, repeatable, and reliable measurements that can easily be adopted in clinical practice is imperative. The aim of this study was to estimate the prevalence and study-associated risk factors for MGD in a hospital-based population and to evaluate the role of a noninvasive ocular surface analyzer (OSA) in the diagnosis of MGD.
Methods
Study design and ethical approval
This observational, cross-sectional study was conducted at a tertiary eye care hospital from January to December 2019. The study was performed in accordance with the Declaration of Helsinki and was commenced after obtaining approval from our institute’s ethics committee.
Subject recruitment and screening
Consecutive participants, aged 18 years and above, attending the ophthalmology outpatient department of our tertiary eye care hospital and consenting to participate in the study were included. Participants with acute ocular infection or inflammation, history of ocular surgery within the past 3 months, ocular trauma, or globe abnormality; those using ocular medication; contact lens users; and those with uncontrolled systemic disease were excluded from the study.
The study was designed and powered to estimate the prevalence of MGD in a hospital-based population. The sample size of 113 adult subjects was considered assuming prevalence of 47.7%,[13] 95% confidence level, absolute error of 10%, and design effect of 1.2. The sample size calculation was conducted using the Open Epi Statistical Calculator version 3.01.
The study participants were administered a standardized sypmtom questionnaire specific to dry eye symptoms related to MGD.[17] The questionnaire has been previously used and validated by studies to effectively differentiate between subgroups of patients with MGD and controls.[4,5,14] Demographic details, presence of computer vision syndrome (CVS; prolonged use of visual display terminals with ocular symptoms), presence of systemic illness, and use of systemic medications were noted. Clinical evaluations and automated measurements using OSA (ICP OSA; SBM Sistemi, Turin, Italy) were performed in the following order to minimize the disruptive effect of preceding tests on subsequent measurements: lipid layer thickness (LLT), noninvasive tear breakup time (NIBUT), tear meniscus height (TMH), slit-lamp examination, corneal staining using 1% sodium fluorescein strips, meibomian gland expression, and noncontact infrared meibography.
Ocular surface analyzer
LLT and NIBUT were measured by interferometry using ICP OSA (SBM Sistemi) [Fig. 1]. LLT was recorded after asking the participants to blink thrice, which ensured an even distribution of the lipid layer over the cornea. For measurement of NIBUT, the median of three readings on interferometry was taken. TMH was estimated along the lower lid margin using magnification tools. Infrared meibography of the everted upper lid was performed using BG-4 M noncontact meibography system (SBM Sistemi).[18] Meibomian gland loss (MGL) was represented as the percentage of the area of the missing glands in the region of the upper tarsal plate.
Figure 1.
Examination of the ocular surface through slit-lamp–mounted ocular surface analyzer, an integrated platform for diagnosis of dry eye disease
Clinical assessment
On slit-lamp examination, the presence of meibomian orifice obstruction (MOO), lid margin telangiectasia, eyelash contamination, and tear film signs was looked for. After documenting corneal staining scores meibomian gland expression was done. Details of the clinical test procedures and grading are provided in Supplementary File 1. Meibum quality was graded as 0 = clear, 1 = cloudy, 2 = granular, and 4 = toothpaste like, and meibum expressibility was graded as 0 = all glands expressible, 1 = three or four glands expressible, 2 = one or two glands expressible, and 3 = no glands expressible. Diagnosis of MGD was based on the International Workshop on Meibomian Gland Dysfunction 2010 criteria for gland expression.[11] MGD was diagnosed in the presence of a score of 1 or more for both meibum quality and expressibility or a score of more than 1 for either meibum quality or expressibility in at least one eye. This was labeled as “total MGD” in the study. A diagnosis of “symptomatic MGD” was made if a subject with MGD reported one or more symptoms to be present often or all the time. The remainder subsets of asymptomatic participants with clinical features of MGD with no symptoms were labeled as “asymptomatic MGD.”
Statistical analysis
Pearson Chi-square/Fischer’s exact test were used for qualitative data. Mann–Whitney U test was applied for nonparametric data and a two-sample t-test for normal distribution data. The correlation between OSA parameters was performed using the Spearman correlation test. Receiver operating characteristic (ROC) curves with calculations of the area under the curve (AUC) were used to describe the accuracy of each parameter for differentiating subjects with MGD and without MGD. Results were considered statistically significant for P < 0.05.
Results
Demographic and clinical characteristics
A total of 113 participants were recruited in the study, of which 62 (54.9%) were males and 51 (45.1%) were females. The mean age of the participants was 41.56 ± 13.23 years (range: 17–65 years). Total MGD prevalence was 57.52% (95% confidence interval [95% CI]: 48.3%–66.8%; n = 65/113) [Table 1]. The MGD group had significantly older subjects than the non-MGD group (46.5 ± 13 vs. 34.8 ± 10.3 years; P < 0.001). MGD was identified in 58.1% (95% CI: 45.4%–70.7%) of males and 56.9% (95% CI: 42.8%–70.9%) of females (P = 0.898). Symptomatic MGD was identified in 42.5% of the participants (95% CI: 33.2%–51.7%; n = 48/113), that is, in 38.7% of males and 47.1% of females (P = 0.371). The prevalence of both total MGD and symptomatic MGD was highest in those aged 50 years and above (P < 0.001 and P = 0.004, respectively). A similar trend was also noted in both males (P = 0.001 and P = 0.037, respectively) and females (P = 0.047 and P = 0.057, respectively) [Table 1]. On multivariate analysis, the adjusted odds ratio (OR) showed that total MGD was associated with age [Table 2]. Those aged 50 years and above had about 25 times higher risk of developing MGD than those aged 29 years and below. Gender and prior cataract surgery were not independent risk factors for MGD. Systemic comorbidities (diabetes mellitus, hypertension, rheumatoid arthritis, and coronary artery disease) were identified in 46 participants, but none were significantly associated with total MGD. In participants diagnosed with CVS, the odds of symptomatic MGD increased by a factor of 4.3 (95% CI: 0.8–21.5; P = 0.02) [Table 2].
Table 1.
Prevalence of MGD by age and gender
| Total study subjects | Total MGDa | Symptomatic MGD | |||
|---|---|---|---|---|---|
|
|
|
||||
| n (%) | 95% CI | n (%) | 95% CI | ||
| Overall | 113 | 65 (57.5) | (48.3-66.8) | 48 (42.5) | (33.2-51.7) |
| Age groups (years) | |||||
| ≤29 | 25 | 10 (40) | (19.4-60.6) | 9 (36) | (15.8-56.2) |
| 30-39 | 20 | 6 (30) | (8-52) | 5 (25) | (4.2-45.8) |
| 40-49 | 40 | 23 (57.5) | (41.5-73.5) | 15 (37.5) | (19.6-50.4) |
| ≥50 | 28 | 26 (92.9) | (82.7-103) P<0.001 |
19 (67.8) | (53.6-89.3) P=0.004 |
| Male | 62 | 36 (58.1) | (45.4, 70.7) | 24 (38.7) | (26.2-51.1) |
| Age groups (years) | |||||
| ≤29 | 11 | 4 (36.4) | (2.5, 70.3) | 3 (27.3) | (4.1-58.7) |
| 30-39 | 13 | 4 (30.8) | (1.7, 59.8) | 4 (30.8) | (1.7-59.8) |
| 40-49 | 20 | 11 (55) | (31.1, 78.9) | 6 (30) | (4.2-45.8) |
| ≥50 | 18 | 17 (94.4) | (82.8, 106.2) P=0.001 |
11 (61.1) | (42.5-90.8) P=0.037 |
| Female | 51 | 37 (56.9) | (42.8, 70.9) | 24 (47.1) | (32.9-61.2) |
| Age groups (years) | |||||
| ≤29 | 14 | 8 (57.1) | (43.2, 73.5) | 6 (42.9) | (13.2-72.5) |
| 30-39 | 7 | 2 (28.6) | (16.5, 73.7) | 1 (14.3) | (20.6-49.2) |
| 40-49 | 20 | 12 (60) | (36.5, 83.5) | 9 (45) | (21.1-68.9) |
| ≥50 | 10 | 9 (90) | (67.4, 112.6) P=0.047 |
8 (80) | (49.8-110.1) P=0.057 |
CI=confidence interval, MGD=meibomian gland dysfunction aTotal MGD included both asymptomatic and symptomatic MGD cases P<0.05 are in bold
Table 2.
Association of various risk factors with MGD
| Total MGD | Symptomatic MGD | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | |
| Age group | ||||||||
| ≤29 | 1 | 1 | 1 | |||||
| 30-39 | 0.6 (0.2-2.2) | 0.487 | 0.6 (0.2-2.3) | 0.493 | 0.5 (0.07, 3.3) | 0.464 | 0.4 (0.06, 3.1) | 0.408 |
| 40-49 | 2.1 (0.7-5.6) | 0.172 | 1.9 (0.6-6) | 0.254 | 0.2 (0.04, 0.9) | 0.035 | 0.3 (0.05, 1.6) | 0.159 |
| ≥50 | 19.5 (3.8-101.1) | <0.001 | 24.9 (3.7-167.9) | 0.001 | 0.3 (0.05, 1.4) | 0.117 | 0.4 (0.06, 2.3) | 0.288 |
| Gender, male versus female | 0.9 (0.5-2) | 0.898 | 1 (0.4-2.5) | 0.987 | 1.3 (0.5, 3.2) | 0.56 | 1.2 (0.4, 3.4) | 0.693 |
| Diabetes, no versus yes | 1.6 (0.4-4.4) | 0.586 | 1.2 (0.1-2.9) | 0.265 | 0.2 (0.05, 0.5) | 0.002 | 0.18 (0.04, 0.8) | 0.02 |
| Hypertension, no versus yes | 2.3 (0.8-6.4) | 0.114 | 1.6 (0.4-6.6) | 0.529 | 0.7 (0.2, 2.04) | 0.518 | 1.3 (0.3, 5.6) | 0.684 |
| Ischemic heart disease, no versus yes | 0.9 (0.2-4.6) | 0.983 | 0.6 (0.1-4.2) | 0.592 | 0.7 (0.1, 3.8) | 0.673 | 1.4 (0.2, 12.2) | 0.776 |
| Rheumatoid arthritis, no versus yes | 1.5 (0.1-16.9) | 0.747 | 2.5 (0.2-33.4) | 0.5 | 0.6 (0.05, 6.4) | 0.64 | 1.3 (0.08, 19.5) | 0.858 |
| Computer vision syndrome, no versus yes | 0.4 (0.2-0.9) | 0.026 | 0.6 (0.2-1.5) | 0.224 | 5.4 (1.2, 24.4) | 0.003 | 4.3 (0.8, 21.5) | 0.02 |
| Cataract surgery, no versus yes | 1.8 (0.4-7.4) | 0.409 | 0.4 (0.1-2.8) | 0.379 | 2.7 (0.3, 22.6) | 0.351 | 5.5 (0.5, 56.1) | 0.147 |
CI=confidence interval, MGD=meibomian gland dysfunction, OR=odds ratio P<0.05 are in bold
Lid margin telangiectasia (P < 0.001), eyelash contamination (P = 0.001), MOO (P < 0.001), and tear film signs (P = 0.002) were significantly higher in the MGD group than in the non-MGD group [Table 3]. No difference was observed in best corrected visual acuity (P = 0.291) and corneal staining scores (P = 0.835) between the two groups. The distribution of clinical signs did not differ between symptomatic and asymptomatic MGD.
Table 3.
Clinical and OSA parameters in study subjects
| Overall | Based on symptoms | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Total MGD (n=65) | No MGD (n=48) | P | Symptomatic MGD (n=48) | Asymptomatic MGD (n=17) | P | |
| BCVA, LogMAR Mean (SD)a | 0.2 (0.1) | 0.1 (0.1) | 0.291 | 0.2 (0.2) | 0.2 (0.2) | 0.998 |
| Clinical signs | ||||||
| Lid telangiectasiab | 1.62±0.84 | 0.23±0.42 | <0.001 | 1.54±0.79 | 1.82±0.95 | 0.247 |
| Eyelash contaminationb | 1.14±0.97 | 0.50±0.71 | 0.001 | 1.19±1 | 1.01±0.86 | 0.627 |
| Meibomian orifice obstructionb | 1.22±0.96 | 0.56±0.5 | <0.001 | 1.23±0.9 | 1.18±1.13 | 0.605 |
| Tear film signsb | 1.22±0.8 | 0.73±0.81 | 0.002 | 1.23±0.75 | 1.18±0.95 | 0.182 |
| Corneal staining scoreb | 0.65±0.87 | 0.5±0.7 | 0.835 | 0.73±0.87 | 0.41±0.87 | 0.081 |
| OSA parameters | ||||||
| NIBUT, mma Mean (SD) | 5.9 (3.3) | 7.5 (4) | 0.023 | 5.6 (2.9) | 6.6 (4) | 0.267 |
| MGL, %a Mean (SD) | 31.2 (16.7) | 15.9 (9.8) | <0.001 | 33.4 (17.6) | 25.1 (12.2) | 0.075 |
| LLT, nma Mean (SD) | 19.3 (10.9) | 22.6 (10.4) | 0.107 | 17.6 (7.8) | 24.1 (16.2) | 0.033 |
| TMH, mma Mean (SD) | 0.2 (0.1) | 0.2 (0.1) | 0.982 | 0.2 (0.1) | 0.2 (0.1) | 0.805 |
BCVA=best corrected visual acuity, LogMAR=Logarithm of the Minimum Angle of Resolution, LLT=lipid layer thickness, MGD=meibomian gland dysfunction, MGL=meibomian gland loss, NIBUT=noninvasive tear breakup time, OSA=ocular surface analyzer, TMH=tear meniscus height aStudent’s t-test for continuous data, bMann–Whitney U test for ordinal data represented as average±SD P<0.05 are written in bold
OSA parameters
Compared to the non-MGD group, NIBUT was significantly lower (7.5 ± 4 vs. 5.9 ± 3.3; P = 0.023) and MGL was significantly higher (15.9 ± 9.8 vs. 31.2 ± 16.7; P < 0.001) in the MGD group [Table 3]. There was no difference in the values of LLT (22.6 ± 10.4 vs. 19.3 ± 10.9; P = 0.107) and TMH (0.2 ± 0.1 vs. 0.2 ± 0.1; P = 0.982) between both groups. On subgroup analyses, participants with symptomatic MGD had lower values of LLT compared to those with asymptomatic MGD (17.6 ± 7.8 vs. 24.1 ± 16.2; P = 0.033). No difference was noted in the values of NIBUT (P = 0.267), MGL (P = 0.075), and TMH (P = 0.805) between symptomatic and asymptomatic MGD [Table 3].
Regression analysis comparing the association of MGD with OSA variables is shown in Table 4. A significant positive association was seen between MGL values of >25% (OR: 19.1; 95% CI: 6.7–54.8) and the occurrence of MGD. No association was found with NIBUT, LLT, and TMH. Also, no association was noted between OSA parameters and symptomatic MGD. On correlation analysis, negative correlation was found between NIBUT and MGL (r = –0.25, P = 0.04) and MGL and LLT (r = –0.28, P = 0.02). No significant correlation was found between MGL and TMH (r = –0.12, P = 0.78), NIBUT and LLT (r = 0.03, P = 0.08), NIBUT and TMH (r = 0.15, P = 0.21), and TMH and LLT (r = 0.03, P = 0.43). ROC curves of NIBUT, MGL, LLT, and TMH are shown in Fig. 2. Highest AUC was seen with MGL (AUC = 0.827; 95% CI: 0.774–0.881) followed by NIBUT (AUC = 0.633; 95% CI: 0.576–0.672), TMH (AUC = 0.487; 95% CI: 0.441–0.528), and LLT (AUC = 0.483; 95% CI: 0.429–0.511). The cut-off value of MGL was determined as ≥26%. MGL showed a sensitivity of 75.4% and specificity of 85.4%.
Table 4.
Multi-logistic regression analyses showing association of MGD with ocular surface analyzer variables
| Total MGD OR (95% CI) | P | Symptomatic MGD OR (95% CI) | P | |
|---|---|---|---|---|
| NIBUT (s) | ||||
| ≤10 | 1 | 1 | ||
| >10 | 0.94 (0.29, 2.95) | 0.915 | 0.67 (0.22, 2.05) | 0.484 |
| MGL (%) | ||||
| ≤25 | 1 | 1 | ||
| >25 | 19.12 (6.67, 54.81) | <0.001 | 0.58 (0.22, 1.56) | 0.284 |
| LLT (nm) | ||||
| <30 | 1 | 1 | ||
| ≥30 | 0.87 (0.31, 2.42) | 0.791 | 0.72 (2.69, 1.92) | 0.513 |
| TMH (μm) | ||||
| ≤0.25 | 1 | 1 | ||
| >0.25 | 2.03 (0.19, 22.48) | 0.561 | 0.7 (0.06, 7.73) | 0.632 |
CI=confidence interval, LLT=lipid layer thickness, MGD=meibomian gland dysfunction, MGL=meibomian gland loss, NIBUT=noninvasive tear breakup time, OR=odds ratio, TMH=tear meniscus height P<0.05 are in bold
Figure 2.
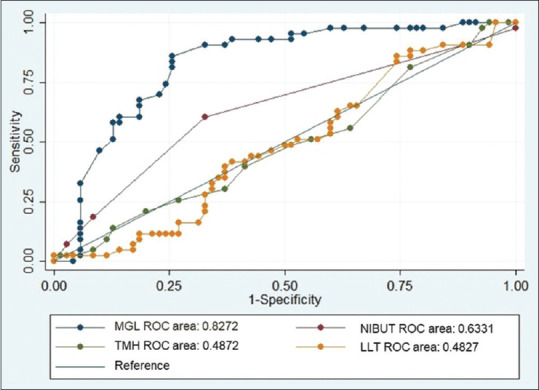
ROC curves for NIBUT, MGL, LLT, and TMH. LLT = lipid layer thickness, MGL = meibomian gland loss, NIBUT = noninvasive tear breakup time, ROC = receiver operating characteristic, TMH = tear meniscus height
Discussion
In this study, the prevalence of total and symptomatic MGD was 57.5% and 42.5%, respectively. Furthermore, the role of OSA in investigating MGD was evaluated, and we found that MGL on OSA was a significant association of total MGD, while symptomatic MGD was associated with CVS.
The prevalence of MGD reported in the current study is consistent with the high prevalence reported by other studies from Asia and is higher than that reported in studies on the western population.[4,5,6,8,13,14,17] However, it must be noted that the lack of a universally accepted definitive diagnostic criteria for MGD has led to the use of nonuniform definitions. Also, some of the reported studies have targeted specific study groups, leading to variation in reported prevalence.[9,12] These factors render a comparative analysis between studies difficult. Studies primarily investigating MGD prevalence in the Indian population are sparse, and we found only two such reports in the published literature.[12,13] A high prevalence of MGD in this region, as reported by previous publications and the current study, especially in the younger population with increased usage of digital devices, highlights the need to generate relevant and up-to-date data on MGD and its risk factors.
An increasing prevalence of total and symptomatic MGD with increasing age was observed in our study, consistent with previous studies.[4,13,14] Those aged 50 years and above had the highest prevalence of both total and symptomatic MGD. A similar trend was also noted in both males and females, although it was marginally insignificant in females (P = 0.057) for symptomatic cases. In symptomatic MGD, in contrast to our results, Chatterjee et al.[13] observed a declining trend with age and Amano et al.[14] observed no change. This discrepancy can be explained by the lack of a questionnaire specific to MGD and the use of nonuniform diagnostic criteria. Development and adoption of standardized study definitions and disease-specific questionnaire will help in achieving reproducible results. Interestingly, we noted a relatively higher prevalence of total and symptomatic MGD in the ≤29 years age group than in the 30–39 years age group. Adolescents and young adults have particularly high digital display usage, which puts them at increased risk of associated dry eye and meibomian gland changes.[19] Kim et al.[2] reported that lifetime exposure to smartphones increased the risk of dry eye symptoms in adolescents and advised caution to this age group.
In the current study, the MGD rates between males and females were similar. While Amano et al.[14] reported a similar finding, other studies have reported higher prevalence in males.[4,5,13] The role of sex hormones in regulating meibomian gland function and a protective role of estrogen in females have been identified, but the mechanism by which these hormones affect MGD causation still needs to be defined.[5,20]
Digital device usage is associated with an increased risk of MGD.[19] Blink abnormalities and alterations in the ocular surface milieu and tear film composition seen in digital display users predispose them to experience significant dry eye symptoms.[19,21] We found an increased likelihood of symptomatic MGD associated with CVS in the current study. Increasing digitization in our daily life and a shift to online mode for day-to-day workings due to the pandemic are expected to increase the health-related problems of CVS. Studies with a larger sample size are required to estimate the actual burden of this disorder in the Indian population.
In an attempt to overcome the inherent biases and limitations associated with clinical tests, automated and noninvasive diagnostic modalities have been developed.[15,22,23,24,25] These tests give repeatable and reproducible quantitative values of ocular surface parameters and provide in vivo details about the meibomian gland morphology. A comprehensive tool that incorporates these modalities in a single instrument and allows simultaneous measurements is ideal. OSA is one such modality. We observed that NIBUT was significantly reduced and MGL was significantly higher in subjects with MGD as compared to those without MGD. Our findings were consistent with those of a previous study.[26] However, unlike Giannaccare et al.,[26] we found MGL to be the parameter with the highest AUC on ROC analysis, indicating its better diagnostic accuracy for MGD in this subset of the Indian population. On regression analysis, we also found higher odds of MGD with MGL values >25%, while no increased risk was seen with NIBUT of <10 s. High diagnostic accuracy of MGL has been previously established.[27,28] As MGD is primarily a disease of the meibomian glands and reduced tear breakup time is secondary to the dysfunction, we feel that MGL, represented as the percentage ratio of area of gland loss to the total area, could be a better representative of the disease.
In the present study, tear film LLT was observed to be similar in participants with and without MGD. This finding is counterintuitive as MGD is associated with decreased secretion of lipids into the tear film.[29,30] However, on subgroup analysis, we noted that LLT showed a significant difference between symptomatic and asymptomatic MGD. Blackie et al.[24] observed that LLT correlated better with symptoms than other objective clinical tests of dry eye and concluded that thinner LLT is associated with a higher likelihood of dry eye symptoms. Nevertheless, on literature review, we found inconsistent associations of LLT with symptoms and clinical signs of MGD and dry eye across studies.[23,24,26,31,32,33] LLT values are known to be affected by several factors, which could explain the inconsistencies observed.[11,34] However, a comprehensive noninvasive approach may yield more accurate results about the relationship between different ocular surface parameters in MGD. MGL is directly linked to a reduction of LLT in tear film, which leads to an unstable tear film and shorter tear breakup time. In our study, MGL demonstrated negative correlation with both NIBUT and LLT, consistent with the results reported previously.[27]
TMH did not differ between subjects with and without MGD and showed no correlation with any other measured parameter. These findings are in accordance with previous studies.[33,35] Tear secretion increases as a compensatory response to unstable tear film seen in MGD.[36] This compensatory phenomenon can explain our finding of comparable TMH values in MGD and non-MGD groups, despite a shorter NIBUT observed in MGD cases.
Strengths of our study include the use of widely accepted standardized definition for diagnosis of MGD and correlation of the clinical diagnosis with objective, quantitative measurements of OSA. Limitations include hospital-based settings and a small sample size for subgroup analysis. A population-based study with a larger sample size would help determine community-based prevalence and assess the effect of environmental and socioeconomic factors. It would also help evaluate the feasibility of OSA in large population-based studies.
Conclusion
In conclusion, we report a high hospital-based prevalence of MGD in Indian subjects. We found notably higher prevalence in younger age groups. Use of digital devices seems to be an important contributor to symptomatic MGD. To the best of our knowledge, this study also provides the first objective assessment of MGD in the Indian population. MGL was the OSA parameter with the highest predictive ability for MGD in this study. Although the present study was conducted before the COVID-19 emerged, we believe its usefulness is more so now than before. We recommend that OSA can serve as a tool to perform simultaneous and objective measurements of ocular surface parameters and gland morphology in clinic-based settings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Mr. Vikrant is acknowledged for his contribution to data acquisition. Mr. Deepak Kumar and Ms. Subhi Jain are acknowledged for their contribution to management and analysis of the data.
Supplementary File 1.
Clinical tests and grading scores
| Clinical parameter | Grading scale | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 1 | 2 | 3 | 4 | |
| Lid margin telangiectasia | 0 | 1 | 2 | 3-5 | >5 |
| Eyelash contamination | Clear | Slight contamination | Mild | Moderate | Severe |
| Meibum orifice obstruction | None | <25% of gland orifices | 25%-<50% of gland orifices | 50%-<75% of gland orifices | 75% or more of gland orifices |
| Tear film signs | None | Mild debris | Mild tear debris, foaming at the corners, and decreased meniscus | Filamentary keratitis, mucus clumping, and increased tear debris | Filamentary keratitis, mucus clumping, increased tear debris, and ulceration |
Corneal surface staining was performed using 1% sodium fluorescein strips. Following instillation of the fluorescein dye in the inferior conjunctival sac, the subject was asked to blink several times to ensure even distribution over the corneal surface. Corneal staining scores: 0 = no staining, 0.5 = slight punctate staining, 1 = diffuse punctate staining, 2 = diffuse staining covering less than one-third of the cornea, 3 = diffuse staining covering more than one-third of the cornea, and 4 = staining covering more than two-thirds of the cornea.
Meibomian gland expression: The assessment of meibomian gland dysfunction was done based on the definition by the International Workshop on Meibomian Gland Dysfunction.[11] Moderate digital pressure was applied over the central third of the lower lids to assess the expressibility of the meibomian glands and the meibum quality.
The expressibility of the meibomian glands was graded on a scale of 0–3 based on the assessment of the central five glands of the lower lid: 0- all glands expressible, 1- three or four glands expressible, 2- one or two glands expressible, and 3- no glands expressible.
The meibum quality was assessed in the central eight glands of the lower lid, and the secretions of individual glands were scored on a scale of 0–3 based on the appearance of meibum: 0- clear secretions, 1- cloudy secretions without debris, 2- cloudy secretions with debris, and 3- thick/toothpaste-like secretions.
References
- 1.Tong L, Chaurasia SS, Mehta JS, Beuerman RW. Screening for meibomian gland disease: Its relation to dry eye subtypes and symptoms in a tertiary referral clinic in Singapore. Invest Ophthalmol Vis Sci. 2010;51:3449–54. doi: 10.1167/iovs.09-4445. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Hwang Y, Kang S, Kim M, Kim TS, Kim J, et al. Association between exposure to smartphones and ocular health in adolescents. Ophthalmic Epidemiol. 2016;23:269–76. doi: 10.3109/09286586.2015.1136652. [DOI] [PubMed] [Google Scholar]
- 3.Schein OD, Tielsch JM, Munõz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly A population-based perspective. Ophthalmology. 1997;104:1395–401. doi: 10.1016/s0161-6420(97)30125-0. [DOI] [PubMed] [Google Scholar]
- 4.Viso E, Rodríguez-Ares MT, Abelenda D, Oubiña B, Gude F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci. 2012;53:2601–6. doi: 10.1167/iovs.11-9228. [DOI] [PubMed] [Google Scholar]
- 5.Siak JJK, Tong L, Wong WL, Cajucom-Uy H, Rosman M, Saw SM, et al. Prevalence and risk factors of meibomian gland dysfunction: The Singapore Malay eye study. Cornea. 2012;31:1223–8. doi: 10.1097/ICO.0b013e31823f0977. [DOI] [PubMed] [Google Scholar]
- 6.Jie Y, Xu L, Wu YY, Jonas JB. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond) 2009;23:688–93. doi: 10.1038/sj.eye.6703101. [DOI] [PubMed] [Google Scholar]
- 7.Lekhanont K, Rojanaporn D, Chuck RS, Vongthongsri A. Prevalence of dry eye in Bangkok, Thailand. Cornea. 2006;25:1162–7. doi: 10.1097/01.ico.0000244875.92879.1a. [DOI] [PubMed] [Google Scholar]
- 8.Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: The Shihpai eye study. Ophthalmology. 2003;110:1096–101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 9.Rabensteiner DF, Aminfar H, Boldin I, Schwantzer G, Horwath-Winter J. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol. 2018;96:e707–11. doi: 10.1111/aos.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: Executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–9. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, et al. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–49. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamsheer RP, Arunachalam C. A clinical study of meibomian gland dysfunction in patients with diabetes. Middle East Afr J Ophthalmol. 2015;22:462–6. doi: 10.4103/0974-9233.167827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee S, Agrawal D, Sharma A. Meibomian gland dysfunction in a hospital-based population in central India. Cornea. 2020;39:634–9. doi: 10.1097/ICO.0000000000002217. [DOI] [PubMed] [Google Scholar]
- 14.Amano S, Inoue K. Clinic-Based study on meibomian gland dysfunction in Japan. Invest Ophthalmol Vis Sci. 2017;58:1283–7. doi: 10.1167/iovs.16-21374. [DOI] [PubMed] [Google Scholar]
- 15.Qi Y, Zhang C, Zhao S, Huang Y, Yang R. A novel noninvasive ocular surface analyzer for the assessment of dry eye with Meibomian gland dysfunction. Exp Ther Med. 2017;13:2983–8. doi: 10.3892/etm.2017.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh S, Rhee MK. COVID-19 and dry eye. Eye Contact Lens. 2021;47:317–22. doi: 10.1097/ICL.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 17.Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–8. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 18.Dogan AS, Kosker M, Arslan N, Gurdal C. Interexaminer reliability of meibography: Upper or lower eyelid? Eye Contact Lens. 2018;44:113–7. doi: 10.1097/ICL.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 19.Blehm C, Vishnu S, Khattak A, Mitra S, Yee RW. Computer vision syndrome: A review. Surv Ophthalmol. 2005;50:253–62. doi: 10.1016/j.survophthal.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009;15:1553–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez-Valerio MDR, Mohamed-Noriega K, Zamora-Ginez I, Baez Duarte BG, Vallejo-Ruiz V. Dry eye disease association with computer exposure time among subjects with computer vision syndrome. Clin Ophthalmol. 2020;14:4311–7. doi: 10.2147/OPTH.S252889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–5. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Finis D, Pischel N, Schrader S, Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea. 2013;32:1549–53. doi: 10.1097/ICO.0b013e3182a7f3e1. [DOI] [PubMed] [Google Scholar]
- 24.Blackie CA, Solomon JD, Scaffidi RC, Greiner JV, Lemp MA, Korb DR. The relationship between dry eye symptoms and lipid layer thickness. Cornea. 2009;28:789–94. doi: 10.1097/ICO.0b013e318191b870. [DOI] [PubMed] [Google Scholar]
- 25.Pult H, Riede-Pult B. Comparison of subjective grading and objective assessment in meibography. Cont Lens Anterior Eye. 2013;36:22–7. doi: 10.1016/j.clae.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 26.Giannaccare G, Vigo L, Pellegrini M, Sebastiani S, Carones F. Ocular surface workup with automated noninvasive measurements for the diagnosis of meibomian gland dysfunction. Cornea. 2018;37:740–5. doi: 10.1097/ICO.0000000000001500. [DOI] [PubMed] [Google Scholar]
- 27.Pult H, Riede-Pult BH. Non-contact meibography: Keep it simple but effective. Cont Lens Anterior Eye. 2012;35:77–80. doi: 10.1016/j.clae.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Fukuoka S, et al. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009;116:2058–63.e1. doi: 10.1016/j.ophtha.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–93. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCulley JP, Shine WE. Meibomian secretions in chronic blepharitis. Adv Exp Med Biol. 1998;438:319–26. doi: 10.1007/978-1-4615-5359-5_45. [DOI] [PubMed] [Google Scholar]
- 31.Isreb MA, Greiner JV, Korb DR, Glonek T, Mody SS, Finnemore VM, et al. Correlation of lipid layer thickness measurements with fluorescein tear film break-up time and Schirmer's test. Eye (Lond) 2003;17:79–83. doi: 10.1038/sj.eye.6700224. [DOI] [PubMed] [Google Scholar]
- 32.Eom Y, Lee JS, Kang SY, Kim HM, Song JS. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am J Ophthalmol. 2013;155:1104–10.e2. doi: 10.1016/j.ajo.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Ji YW, Lee J, Lee H, Seo KY, Kim EK, Kim T-I. Automated measurement of tear film dynamics and lipid layer thickness for assessment of non-Sjögren dry eye syndrome with meibomian gland dysfunction. Cornea. 2017;36:176–82. doi: 10.1097/ICO.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 34.Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–60. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Rico-Del-Viejo L, Benítez-Del-Castillo JM, Gómez-Sanz FJ, García-Montero M, Llorens-Quintana C, Madrid-Costa D. The influence of meibomian gland loss on ocular surface clinical parameters. Cont Lens Anterior Eye. 2019;42:562–8. doi: 10.1016/j.clae.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Arita R, Morishige N, Koh S, Shirakawa R, Kawashima M, Sakimoto T, et al. Increased tear fluid production as a compensatory response to meibomian gland loss: A multicenter cross-sectional study. Ophthalmology. 2015;122:925–33. doi: 10.1016/j.ophtha.2014.12.018. [DOI] [PubMed] [Google Scholar]


