Abstract
As a typical product of microbial metabolism, the weak acid acetate is well known for its cytotoxic effects. In contrast to most other microbes, the so-called acetic acid bacteria can acquire significant resistance to high acetate concentrations when properly adapted to such hostile conditions. To characterize the molecular events that are associated with this adaptation, we analyzed global protein expression levels during adaptation of Acetobacter aceti by two-dimensional gel electrophoresis. Adaptation was achieved by using serial batch and continuous cultivations with increasing acetate supplementation. Computer-aided analysis revealed a complex proteome response with at least 50 proteins that are specifically induced by adaptation to acetate but not by other stress conditions, such as heat or oxidative or osmotic stress. Of these proteins, 19 were significantly induced in serial batch and continuous cultures and were thus noted as acetate adaptation proteins (Aaps). Here we present first microsequence information on such Aaps from A. aceti. Membrane-associated processes appear to be of major importance for adaptation, because some of the Aap bear N-terminal sequence homology to membrane proteins and 11 of about 40 resolved proteins from membrane protein-enriched fractions are significantly induced.
As one of the most prominent low-molecular-weight products of microbial metabolism, acetate is well known for its cytotoxicity that includes retardation of growth and product formation at concentrations below 5 g/liter (2, 4, 18). These toxic effects are related to the weak lipophilic nature of the undissociated acid that enables the molecule to cross the cytoplasmic membrane. This diffusion is generally thought to dissipate ion gradients, increase the internal acetate concentration, and/or disrupt membrane processes, thereby poisoning the cell (1, 4, 5, 26).
While most microorganisms are sensitive to higher concentrations of acetate, a few are known to be relatively resistant. A prominent example of such resistant organisms are the so-called acetic acid bacteria (AAB), the genera Acetobacter and Gluconobacter. Species of the former have been used for millennia in the production of acetic acid as vinegar (6, 28). In industrial settings, Acetobacter aceti can grow at acetate concentrations of up to about 60 g/liter (23) and accumulate final concentrations exceeding 140 g/liter in semicontinuous processes (6). Survival under these hostile conditions is apparently sufficient, so that such cultures can serve as inocula for subsequent batch cultures. Oxygen deficiency of Acetobacter leads within seconds to a drop in energy charge (13) and, likely as a consequence, a rapid loss of viability (6, 19). This illustrates the importance of cellular energetics for acetate resistance. The molecular mechanism of resistance in AAB, however, remains essentially unknown.
The previously reported acetate resistance genes, aarABC, of a thermophilic A. aceti strain were important for resistance on solid media (7). The identified functions of the AarA and AarC gene products in citrate synthesis (7) and acetate uptake (8), respectively, show that these proteins confer resistance by acetate assimilation via a local reduction of acetate concentrations on solid media. In liquid media, however, assimilation of acetate is not a pertinent resistance mechanism, in particular when acetate is continuously produced by the organism. Thus, it appears that different resistance mechanisms operate in acetate-containing liquid media, such as during vinegar production.
Presumably as a consequence of their genetic variability, the AAB rapidly lose acetate resistance when removed from its presence (6). For this reason, industrial AAB are maintained at high acetate concentrations, and vinegar processes are operated for years without interruption so that the industrial strain remains adapted to high acetate concentrations. This adaptation appears to be a prerequisite for high acetate tolerance, because wild-type AAB do not exhibit pronounced acetate tolerance (16). In fact, when cultivated on appropriate carbon sources, Escherichia coli exhibits a similar tolerance of acetate (17).
Previous proteome analysis of AAB revealed eight so-called acetate stress proteins (Asps) that were induced specifically by challenging unadapted A. aceti and Gluconobacter suboxydans cultures with 10 g of acetate per liter (16).
Here we investigate the changes in global protein expression levels during long-term adaptation of A. aceti to high acetate concentrations, as a first step to the characterization of the molecular mechanisms underlying acetate resistance. For this purpose, A. aceti wild-type was exposed to stepwise increased acetate concentrations in either serial batch or continuous cultures and subsequently analyzed by two-dimensional protein electrophoresis (2DE). Detection and N-terminal sequences of proteins that are induced exclusively in response to acetate adaptation, as opposed to general stress responses, are described.
MATERIALS AND METHODS
Strain and medium.
A. aceti DSMZ 2002 was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ) and routinely grown on complex YPD medium containing 30 g of glucose, 2 g of Bacto peptone, and 5 g of yeast extract (pH 6.5) per liter at 30°C. Sterile glucose was added to the separately sterilized complex components from a 30% (wt/vol) stock solution. For acetate supplementation, YPD medium containing the desired concentration of potassium acetate was titrated to pH 6.5 by addition of YPD medium containing the same concentration of acetic acid and sterilized by filtration. All bioreactor media were supplemented with 0.05% (wt/vol) polypropyleneglycol 2000 to prevent foaming.
Culture conditions.
Stress experiments were performed in 500-ml baffled flasks, filled with 100 ml of medium, at 30°C and 200 rpm. At an optical density at 600 nm (OD600) of about 1, exponentially growing cells were diluted 1:1 in fresh medium containing either 0.5 M NaCl or 10 mM H2O2. After incubation at 30°C (or 42°C for heat stress) for 3 h, cells were harvested by centrifugation and prepared for 2DE.
For acetate adaptation experiments, serial batch and continuous cultures were grown in 1.5-liter bioreactors (Bioengineering AG) at a working volume of 1 liter. The pH was maintained automatically at 6.5 by addition of 5 M NaOH or 2.5 M H3PO4. Dissolved oxygen concentrations greater than 25% were ensured by agitation at 1,000 rpm and aeration at a rate of 1 liter per min. Serial batch cultivations were performed by harvesting approximately 90% of exponentially growing cultures and replacing the depleted volume with fresh YPD medium at the appropriate acetate concentration. Continuous cultures were diluted with filter-sterilized YPD medium at a rate between 0.1 and 0.05 h−1. The reactor volume was kept constant at 1 liter by a weight-controlled pump. Aliquots were withdrawn in physiological steady state, which is defined as at least 2 days of constant optical density and carbon dioxide-oxygen concentrations in the culture effluent gas.
Analytical procedures.
Glucose and total protein concentrations were determined enzymatically (Synchron CX5CE; Beckman) with kits supplied by the manufacturer. Acetate concentrations were determined by high-pressure liquid chromatography (HPLC) (Series 200; Perkin Elmer) using an ion exclusion HPLC column (Supelcogel H; Supelco) with 0.2 N H3PO4 as the mobile phase. Oxygen and carbon dioxide concentrations in the feed and effluent gas of the bioreactor were determined with a mass spectrometer (Prima 600; Fissons Instruments).
Protein sample preparation for 2DE.
Cells were harvested by centrifugation for 15 min at 7,000 × g and 4°C. The pellets were resuspended in deionized water, aliquoted in microcentrifuge tubes, and centrifuged again for 10 min at 10,000 × g and 4°C, and the pellets were stored at −80°C. Protein samples for 2DE were prepared according to the protocol of Tonella et al. (29). First, frozen cells were resuspended in 2 ml of low-salt washing buffer (3 mM KCl, 1.5 mM KH2PO4, 68 mM NaCl, and 9 mM NaH2PO4). Typically, the equivalent of 25 to 50 ml of culture at an OD600 of 1 was used. The cells were washed four times with washing buffer and resuspended in storage buffer (10 mM Tris-HCl [pH 8], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithioerythritol, 0.5 mM Pefabloc SC, and 0.1% [wt/vol] sodium dodecyl sulfate [SDS]) at an estimated pellet-to-buffer volume ratio of 4:1. After cell disruption by vortexing for 10 min at the highest speed (Vortex Genie 2), cells were used directly for 2DE or stored at −80°C. Prior to 2DE analysis, sample quality and protein concentration were assayed by analytical one-dimensional (1D) SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% minigel (14).
Analytical 2DE.
Two-dimensional electrophoresis was adapted from the method of O‘Farrell (21). Prior to isoelectric focusing, immobilized pH gradient (IPG) strips were rehydrated along with the protein samples. For this purpose, 2 to 3 μl of sample was mixed with rehydration buffer (5 M urea, 0.333% BioLyte pH 3 to 10, 0.167% BioLyte pH 5 to 7, 4% CHAPS [3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate], 2 M thiourea, and 65 mM dithiothreitol [DTT]) and equally dispensed onto 18-cm IPG strips (pH 3 to 10 NL; Pharmacia). The exact sample volume was chosen so that equal amounts of protein were loaded on each gel. The strips were covered with cover fluid (Plusone Dry Strip cover fluid; Pharmacia) and rehydrated overnight in a dry strip reswelling tray (Pharmacia).
For isoelectric focusing, gels were run for a total of 71.75 kVh, with a voltage linearly increasing from 500 to 3,500 V for the first 5 h, followed by a constant voltage of 3,500 V for 15.5 h. The second dimension was performed with 12% acrylamide slab gels (160 by 200 by 1.5 mm), which were run for 6 h at 40 mA/gel. After electrophoresis, the gels were fixed and proteins were visualized by an ammoniacal silver stain.
2DE image analysis.
Comparisons of spot intensities were performed with digitized images of the silver-stained gels. A first detection of major differences in spot intensities was achieved visually by fast flipping of negative gel images. Detailed detection of less obvious intensity changes was done with computer-aided image analysis. For this purpose, reproducible spots were detected and quantified on different gel images with the Melanie II software (Geneva Bioinformatics S.A). Estimation of molecular weight and isoelectric point (pI) was based on 2DE analysis of protein samples that were mixed with a 2DE protein standard (Bio-Rad).
Changes in protein spot intensity that are correlated with acetate adaptation or general stress response were identified by either the above visual inspection or statistical data analysis. For statistical analysis, quantified spot intensities were first normalized to the overall intensity of the analyzed gel. To avoid experimental artifacts, gels from two independent aliquots of serial batch and stress experiments were analyzed, and the average spot intensities were used whenever possible. Continuous-culture samples were analyzed with one gel per steady state because five gels of aliquots taken at different acetate concentrations were assumed to provide statistically significant results. Further evidence for this assumption comes from the observation that a few “random” spots occurred only on gels of one or two steady states (data not shown), while the proteins that are responsive to acetate were consistently identified on all five gels.
Using linear regression analysis, we calculated slopes and R values for the intensities of 1,000 to 1,300 identified spots as a function of extracellular acetate concentration. These values were calculated independently for the five continuous-culture and three serial-batch conditions. On the bases of the calculated slopes and their statistical relevance (R values ≥70%), spots were divided into the following categories: reduced, constant, induced (two- to fourfold), and highly induced (>4-fold), compared to the control experiment without acetate. After visual verification, 17 proteins were found to be induced at high acetate concentration in both serial batch and continuous culture. About 25% of the acetate-responsive proteins identified by the statistical analysis could not be verified by visual inspection and were thus not labeled as acetate responsive. In addition to the statistical analysis, several acetate-responsive proteins were identified only by visual inspection of gel images. In the general stress experiments, the induction level was calculated from the average spot intensities in two independent gels from the reference and the stress condition.
Preparative 2DE and N-terminal sequence determination.
Preparative 2DE was performed as described for analytical 2DE but with a sample volume of 20 to 30 μl. Proteins were transferred overnight from gels to Immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore) in a Trans-Blot cell (Bio-Rad) at 100 mA with an initial current of 150 mA for 3 h using transblot buffer (192 mM glycine, 20% methanol, and 25 mM Tris base). The membrane was stained with Coomassie blue, washed for 1 to 2 days in distilled H2O, and air dried. Selected protein spots were excised and subjected to N-terminal sequencing on an automated peptide sequencer (Sequenator G1000A; Hewlett Packard).
Analytical one-dimensional gel electrophoresis of membrane proteins.
Membrane proteins of A. aceti were isolated according to a modified method of Poole (24). The equivalent of 10 to 30 ml of culture (OD600 of 1) of frozen cells from continuous cultures at different acetate concentrations was resuspended in 1 ml of buffer (30 mM Tris-HCl [pH 8.0], 20% [wt/vol] sucrose, and 50 μg/ml chloramphenicol). Potassium-EDTA (pH 7.0) and lysozyme were added to final concentrations of 10 mM and 2 mg/ml, respectively, and the cells were incubated for 1 h at 37°C under gentle agitation. Resulting spheroplasts were sedimented for 30 min at 13,000 × g and 4°C. The remaining pellet was dispersed in 1 ml of phosphate buffer (10 mM potassium phosphate [pH 6.6], 2 mM MgSO4, and 10 μg/ml each of DNase and RNase), using a syringe and a needle (1.2 by 40 mm). The suspension was incubated for 30 min at 37°C under gentle agitation, followed by centrifugation at 800 × g and 4°C for 1 h. The supernatant was centrifuged at 100,000 × g and 4°C for 1 h, and the resulting membrane pellet was resuspended in 10 to 20 μl of 50 mM potassium phosphate buffer (pH 6.6). Equal amounts of membrane proteins, preestimated on a test gel, were then resolved by analytical 1D SDS-PAGE on a 10% minigel and visualized by silver staining.
For N-terminal sequencing, membrane fractions were resolved by preparative 1D SDS-PAGE (160 by 200 by 1.5 mm gel), blotted on PVDF membrane, and isolated as described for 2DE samples.
RESULTS
Growth characteristics at increasing acetate concentrations.
To gain insight into the protective response of AAB to the toxic effects of their major metabolic product acetate, the unadapted wild-type A. aceti strain DSMZ 2002 was cultivated at increasing acetate concentrations. In serial batch cultivation, the first batch without acetate supplementation was followed by three batches at 10 g/liter and three batches at 20 g/liter total acetate concentration. The general response to acetate challenge was a slower growth rate, but adaptation to the acetate challenge manifested itself in an approximately 60% increased growth rate in the second culture at 10 g of acetate per liter, compared to the first culture at 10 g of acetate per liter (Table 1). Upon an increase to 20 g of acetate per liter supplementation, the growth rate dropped to about 0.1 h−1 and no further adaptation was observed. In a similar experiment with E. coli at acetate concentrations between 5 and 8 g/liter, we could not detect any adaptation (data not shown).
TABLE 1.
Maximum growth rates of A. aceti in serial batch cultures with increasing acetate concentrations
During continuous cultivation at low dilution rates, the acetate concentration was increased stepwise to 30 g/liter over a total of 41 generations (Table 2). Compared to the initial steady state without acetate supplementation, the biomass concentration was slightly higher at 10 g of acetate per liter, as was also reported for an industrial strain (20), but was reduced significantly at higher acetate concentrations. To prevent wash-out, the dilution rate was decreased successively with increasing acetate supplementation. Although A. aceti grew stably at the highest acetate concentration of 30 g/liter, it achieved only a very low optical density. Determination of acetate concentration in the culture broth showed no significant loss of acetate; thus, consumption or evaporation was negligible.
TABLE 2.
Physiological properties of A. aceti during continuous culture with increasing acetate concentrations
| Acetate concn (g/liter) | Cultivation time (h) | No. of generationsa | D (h−1) | OD600 |
|---|---|---|---|---|
| 0 | 123 | 0.10 | 1.6 | |
| 10 | 216 | 13 | 0.10 | 1.8 |
| 20 | 287 | 23 | 0.075 | 0.9 |
| 25 | 407 | 30 | 0.05 | 0.6 |
| 30 | 553 | 41 | 0.05 | 0.2 |
Total number of generations under increasing acetate concentrations before withdrawal of 2DE samples and further increase in acetate concentration.
The continuous culture was run in the chemostat mode. The limiting compound in the absence of acetate is not known, but it is probably one of the complex components in the feed medium. To verify that reduced biomass concentration at higher acetate levels was not caused by limiting nutrients, yeast extract and/or peptone concentrations in the medium were doubled. Additionally, the glucose concentration was reduced to 15 g/liter to avoid potential formation of other incompletely oxidized by-products such as gluconate (17). None of these manipulations, however, had any influence on the optical density at 30 g/liter (data not shown). Thus, it appears that the growth-limiting factor was the acetate concentration in the medium.
Our results clearly show that wild-type A. aceti, contrary to E. coli, can adapt to growth at higher acetate concentrations. Most prominently, this adaptation is illustrated by a more than 60% increase in specific growth rate during serial batch cultivation at 10 g of acetate per liter. Consequently, this adapted culture grew faster at 10 or 20 g of acetate per liter than previously reported for unadapted A. aceti (Table 1) (16, 17). Moreover, our adapted A. aceti grew in continuous culture at 30 g/liter, a concentration that completely inhibited growth of unadapted cultures (17).
Protein expression profile of acetate-adapted A. aceti
To identify proteins that are upregulated during adaptation of A. aceti to high acetate concentrations, we examined exponentially growing cells from serial batch (Table 1) and continuous cultures (Table 2) by 2DE analysis. Because we intended to compare proteome patterns in different physiological steady states and not short-term stress responses, we investigated expression levels rather than synthesis rates. Generally, 2DE analysis allowed us to resolve up to 1,500 distinct protein spots within a size range of 15 to 100 kDa and a pH range of 4 to 9 (Fig. 1). The 2DE patterns of independent samples harvested from the same culture were highly reproducible. Despite important differences in expression patterns under batch and continuous-culture conditions, the overall 2DE patterns were very similar and thus allowed us to quickly identify the same spots on different gels. For the following detailed analysis, all 2DE analyses were divided into three groups: serial batch, continuous culture, and general stress.
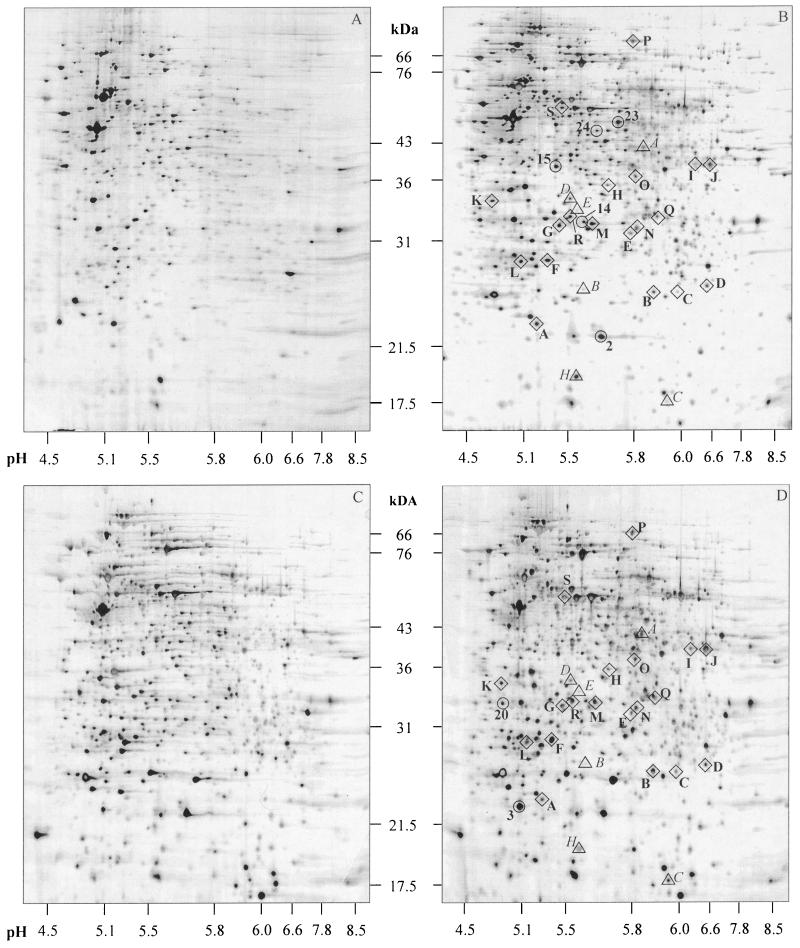
FIG. 1.
Silver-stained 2DE images of exponentially growing A. aceti in serial batch cultures at 0 (A) and 20 g of acetate (B) per liter and in continuous culture at 0 (C) and 30 g of acetate (D) per liter. Diamonds indicate acetate adaptation proteins that are induced in acetate-containing serial batch and continuous cultures. Circles indicate microsequenced proteins that are induced by acetate in either serial batch or continuous culture. Triangles highlight the previously described Asps (16).
2DE analysis of cells harvested from serial batch and continuous cultures with increasing acetate concentrations revealed 39 and 38 protein spots, respectively, with an at least twofold-increased intensity compared to cells grown in the absence of acetate (Fig. 1). Of these proteins, 21 were upregulated under both conditions and are thus prime candidates for acetate resistance proteins. While most of these acetate-responsive proteins were expressed at two- to fivefold-higher levels, six proteins exhibited even higher expression levels.
To exclude proteins that were induced under generally stressful conditions as opposed to specific induction by acetate challenge, 2DE analysis of heat, oxidatively, and osmotically stressed A. aceti cultures was performed. For this purpose, exponentially growing cultures were subjected to sublethal challenges so that they continued to grow. After 3 h, cells were harvested and the protein expression pattern was analyzed by 2DE analysis. From a total of 32 identified proteins that were significantly upregulated by other stress conditions, only two were found to be significantly induced during acetate adaptation in both cultivation systems and were thus removed from the set of acetate-induced proteins.
In conclusion, a total of 19 proteins were found to be induced exclusively during acetate adaptation in serial batch and continuous culture but not by other stress conditions (Fig. 2). Thus, these 19 proteins that met both criteria, induction by acetate in batch and continuous culture and no response to other tested stress conditions, were designated acetate adaptation proteins (Aaps A to S). In addition to these Aaps, 18 and 16 proteins were found to be specifically induced by acetate challenge in serial batch and continuous culture, respectively, but no other stress condition. These proteins are identified by a numerical system.
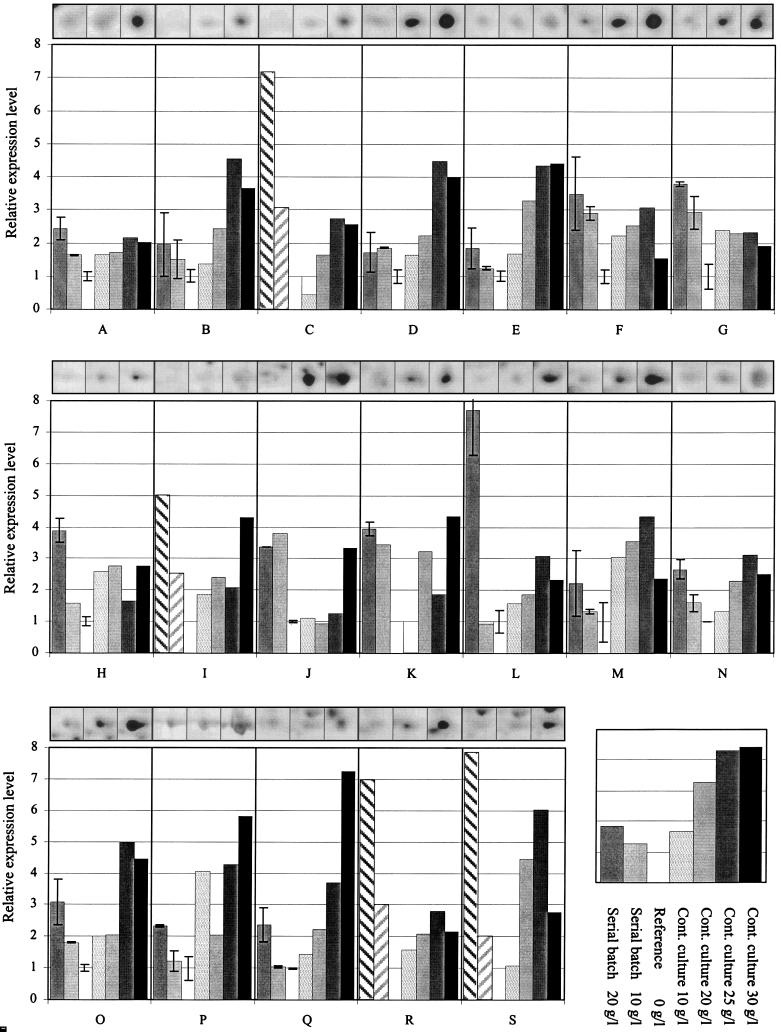
FIG. 2.
Relative expression levels of 19 identified Aaps from 2DE analysis of serial batch and continuous cultures at increasing acetate concentrations. Expression levels are normalized to the expression levels on reference gels without acetate supplementation, and seven experimental conditions are shown for each Aap in one box. These conditions are defined in the inset at the lower right. Hatched bars indicate visually estimated expression level. The three insets above each bar plot box show spot images of the corresponding Aap from 2DE of serial batches at 0, 10, and 20 g of acetate per liter, from left to right, respectively. Error bars indicate deviations of expression levels from two independent gels.
More detailed analysis of the 19 Aaps revealed that all were present at low levels under continuous culture conditions in the absence of added acetate (Fig. 2). Only AapB and AapI were almost undetectable in batch-grown cells without acetate. While many Aaps exhibited gradually increasing expression levels with increasing acetate concentrations, eight appeared to follow a single-step increase pattern (Aaps F, G, H, J, K, M, P, and S). Overall, the relative increase in expression levels was usually moderate, with only six Aaps (C, L, P, Q, R, and S) showing a more than 5-fold increase. Unexpectedly, the expression level of several Aaps was lower at 30 g/liter than at 25 g of acetate per liter in continuous culture. The vast majority of the identified Aaps belong to an intermediate molecular mass range of 25 to 40 kDa (Fig. 1).
N-terminal sequencing of Aaps.
To obtain first hints on protein function, 13 Aaps were isolated from preparative 2DE gels and subjected to N-terminal sequence analysis. Furthermore, we isolated seven protein spots that were significantly upregulated in continuous and repeated batch cultivations but not by any other stress condition. To verify the discrimination between general stress proteins and Aaps, five general stress-responsive proteins were also isolated and sequenced.
Of the 25 isolated proteins, 18 N-terminal sequences, including seven Aaps, could be obtained by microsequencing (Table 3). To obtain information on the putative functions of these proteins, the identified amino acid sequences were subjected to FASTA3 (European Bioinformatics Institute [http://www.ebi.ac.uk]) and BLASTP (National Center for Biotechnology Information [http://www.ncbi.nlm.nih.gov]) homology searches in the GenBank, Swissprot, and Trembl databases. Generally, homologies were considered significant if at least 50% identical amino acids were found within the 30 N-terminal amino acids of the homologous protein and the calculated molecular mass was within 20% of the apparent value for the A. aceti protein. Exceptions were allowed for partially sequenced homologous proteins and for N-terminal peptide sequences that were longer than 20 amino acids. To conclude heterologous protein homology from sequence homologies within short stretches is generally problematic, but has been used successfully before (10, 25). In our case, the additional criterion that sequence homology must occur within the N-terminal region of a protein proved to be relatively rigid because it allowed us in most cases to identify only one or two homologous proteins. In several cases the majority of potential homologues were rejected because the homologous region was located outside the N terminus.
TABLE 3.
A. aceti protein spots identified by microsequencing
| Proteins | Designation | Induction conditionsa | Inductionb | Size (kDa) | pI | N-terminal sequencec | Homologous proteins | Accession no. |
|---|---|---|---|---|---|---|---|---|
| Aaps | A | SC | + | 24 | 5.3 | DATPVPAPD . P | ||
| C | SC | + | 26 | 6.0 | . GQYEAIED | |||
| F | SC | + | 30 | 5.4 | AYITATDGTSLHVKD | Nonheme haloperoxidase (30 kDa) | O05691 | |
| G | SC | + | 33 | 5.5 | . . YPAPPFAT | |||
| I | SC | ++ | 39 | 6.3 | VNNQVKLQDD | Endonucleased | PC1254 | |
| J | SC | + | 39 | 6.5 | MQYRQLGRSG | Potassium channel β-subunit (31 kDa) | U46758 | |
| Oxidoreductase, aldo/keto reductase family (43 kDa) | E72284 | |||||||
| L | SC | ++ | 30 | 5.2 | ALTVLVTGAT | Putative hydroxylase (29 kDa) | AAF01810 | |
| Daunorubicin C-13 ketoreductase (31 kDa) | AAD04717 | |||||||
| Acetate-induced proteins in batch or continuous culture | 2 | S | ++ | 23 | 5.7 | . . RDRHVTQL . . NA | ||
| 3 | C | + | 23 | 5.1 | VKVAALATDGLEEIELT | Protease I (21 kDa) | AAF10772 | |
| GPQEAL . A | ||||||||
| 14 | S | + | 35 | 6.7 | PADPRTRYPR | |||
| 15 | S | + | 38 | 5.4 | MKTLLPTSTA | Pectate lyase II precursor (40 kDa) | P16530 | |
| 20 | C | ++ | 33 | 4.9 | MKTVTLRAGV | NADH:ubiquinone oxidoreductased | P25712 | |
| 23 | S | ++ | 49 | 5.7 | MKALTWQKKG | |||
| 24 | S | 47 | 5.6 | APVAFEHARL | 60-kDa chaperonin GroELd | P29134 | ||
| General stress proteins | 5 | CH | 17 | 5.9 | AEHVSGELKGTDGATHG | Superoxide dismutase (15 kDa) | AF021822 | |
| FVDVA . A | Putative aldehyde dehydrogenase | CAA19886 | ||||||
| 12 | O | 26 | 5.6 | AFELP . LAYA | Superoxide dismutase (∼22 kDa) | P81527 | ||
| 13 | SCH | 29 | 5.5 | MIGVPFPA . R | Putative thiol:disulfide interchange protein precursord | P52237 | ||
| 22 | SCH | 34 | 5.9 | MLIDIKNILL |
Induced under the following cultivation conditions: S, serial batch; C, continuous culture; O, osmotic stress; H, heat stress.
+, Two- to fourfold induction; ++, >4-fold induction.
Ambiguous residues are in italic. Dots, no amino acid identified.
Does not fulfill all significance criteria.
About half of the sequenced N termini exhibited significant homologies to known proteins in the databases. Known stress proteins, however, were not found among the sequenced Aaps, which indicates the validity of the initial Aap assignment. Of the seven Aap sequences, only Aap F, Aap J, and Aap L exhibited significant homologies to known proteins (Table 3). Homology of Aap F was most significant to a nonheme haloperoxidase from Rhodococcus erythropolis. However, less significant homologies were also found to a putative ABC transporter (Streptomyces coelicolor, CAB76078) and a putative formate transporter (E. coli, AAC73990), so that one cannot make conclusions about protein function. The latter also matched the molecular weight criterion. Aap J is homologous to the β-subunit of a potassium channel (rice) but also to other proteins of the same family (3). Finally, Aap L exhibited homology to a putative hydroxylase (Streptomyces nogalater) and a daunorubicin C-13 ketoreductase (Streptomyces peucetius).
Of the seven proteins that were induced by acetate in both batch and continuous culture, two were homologous to what may be considered general stress proteins (12), i.e., protease (Deinococcus radiodurans) and possibly GroEL (Thiobacillus ferroxidans). Additionally, protein 15 was homologous to a non-stress protein, the pectate lyase precursor of Erwinia carotovora, and, protein 20 exhibited homology to an NADH:ubiquinone oxidoreductase precursor (Bos taurus), but did not fulfill all significance criteria.
Analysis of expression of membrane proteins.
Because the 2DE analysis employed is not ideally suited for resolving membrane proteins, we investigated the membrane protein composition by 1D SDS-PAGE. Membrane protein-enriched fractions of A. aceti were analyzed at increasing acetate concentrations in continuous culture (Fig. 3). These membrane protein-enriched fractions showed a pattern on 1D SDS-PAGE that was distinct from that of the total protein fraction, but abundant cytoplasmic proteins are probably also present in this fraction (data not shown). Most of the about 40 resolved proteins were within a size range of 30 to 100 kDa. The most abundant proteins that constitute the prominent bands of the membrane fractions were found not to be affected by increased acetate cocentrations.
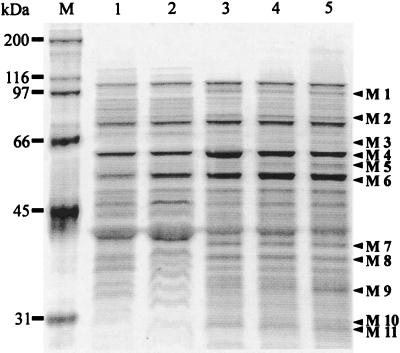
FIG. 3.
Silver-stained SDS-polyacrylamide gel electrophoresis of membrane proteins. Lanes (from left to right): M, molecular size markers; 1 to 5, membrane fractions of cells from continuous culture at 0, 10, 20, 25, and 30 g of acetate per liter, respectively. Proteins that were at least twofold induced by acetate are designated M1 to M11.
Of all the differentially expressed protein bands that were discernible on 1D SDS-PAGE, 11 proteins (designated M1 to M11) were found to be at least twofold induced during acetate adaptation. For three of these proteins, we were able to obtain N-terminal sequence information: M1, MDIQNFTERxQ; M4, AAKDVKFGADARERM; and M11, ASLHSHxEQFKAHRAFHHLLADGQRAF. The N-terminal sequence of the prominent M4 band most likely encodes the general stress protein GroEL that contaminated this protein preparation and thus is not specific to acetate adaptation. No significant homologies were found for the other two N-terminal sequences. Since the resolution on 1D SDS-PAGE did not allow us to clearly distinguish general stress proteins from acetate adaptation proteins, these M proteins cannot be identified unambiguously as Aaps.
DISCUSSION
Adaptation of A. aceti to high acetate concentrations clearly elicits a complex response at the level of protein expression, with a total of about 40 proteins induced. Some of these induced proteins presumably belong to the so-called general stress protein class because they were also induced by other stress conditions. This is a well-known phenomenon that has been described for many bacterial stress responses (12), including challenge with the weak organic acid benzoate (15). While about 35 proteins appeared to be specifically induced during acetate adaptation, only 19 exhibited the same behavior in batch and continuous culture. Only these 19 proteins that were not induced during other stress conditions were considered Aaps.
Few of the identified acetate adaptation proteins exhibited maximum expression levels at 10 g of acetate per liter, while most exhibited higher expression levels at higher acetate concentrations. Surprisingly, all Aaps were also detected in the absence of acetate, indicating that these proteins are also relevant during A. aceti’s normal life. We cannot exclude that acetate challenge leads to coexisting subpopulations in our cultures (27). This, however, does not invalidate our conclusion on Aaps, since the relative concentration of these proteins increased in the whole culture, either because it was upregulated in all cells or because the proportion of a better adapted subpopulation increased.
Unlike the more common use of 2DE to analyze rapid cellular stress responses (9, 12, 15, 16), we investigated long-term adaptation to the weak acid acetate. This constitutes a very different condition, which is also evidenced by the absence of any significant induction of eight previously identified acetate stress proteins (16). These proteins are induced by a 10 g of acetate per liter challenge of unadapted A. aceti, but only one of them, Asp C, was also induced during acetate adaptation in continuous culture (Fig 1). While five other Asps (A, B, D, E, and H) were identified on our gels, the remaining two, Asps F and G, could not be identified, either for technical reasons or because of their transient expression profile.
Generally, N-terminal sequences of organisms without a sequenced genome do not allow us to draw firm conclusions on sequence homologies and thus on protein function. However, some of the identified homologies of our sequenced proteins indicate a role of membrane transport processes in acetate resistance. This view is supported by the observed induction of several bands in 1D gels of membrane proteins. The strongest homology to a membrane transport protein was found for Aap J, which is homologous to the potassium channel β-subunit. The associated α-subunit is responsible for K+ ion conduction and voltage-dependent gating (11), and thus might be induced by the presence of potassium, the acetate counterion in our study. Preliminary DNA sequence data show that the aapJ gene of A. aceti indeed encodes a protein that is homologous to the potassium channel β-subunit (P. Steiner, U. F. Püntener, and U. Sauer, unpublished data). Less significant homology was found for Aap F to the putative formate transporter of E. coli and of protein 20 to an NADH:ubiquinone oxidoreductase. The latter homology also supports the notion that cellular energetics are important for acetate resistance, which was shown recently for Saccharomyces cerevisiae (22).
Overall, our data indicate that membrane functions, possibly including acetate transport and respiration, are involved in the acetate resistance mechanism of A. aceti. The relatively large number of identified Aaps, however, indicates that other resistance mechanisms may also be at work. Because the previously described acetate resistance proteins AarABC are involved in acetate assimilation (7, 8), it is unlikely that they contribute to resistance under the presently investigated conditions of growth in liquid culture. Thus, it is not surprising that neither of these proteins was significantly induced during acetate adaptation.
ACKNOWLEDGMENTS
We thank Cornelia Schwerdel for technical assistance, René Brunisholz for N-terminal sequencing, and James E. Bailey for providing the 2DE facility.
Funding from the ETH Forschungskommission is acknowledged.
REFERENCES
- 1.Axe D D, Bailey J E. Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol Bioeng. 1995;47:8–19. doi: 10.1002/bit.260470103. [DOI] [PubMed] [Google Scholar]
- 2.Bech Jensen E, Carlsen S. Production of recombinant human growth-hormone in Escherichia coli: expression of different precursors and physiological effects of glucose, acetate, and salts. Biotechnol Bioeng. 1990;36:1–11. doi: 10.1002/bit.260360102. [DOI] [PubMed] [Google Scholar]
- 3.Chouinard S W, Wilson G F, Schlimgen A K, Ganetzky B. A potassium channel β subunit related to the aldo-keto reductase superfamily is encoded by the Drosophilahyperkinetic locus. Proc Natl Acad Sci USA. 1995;92:6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diez-Gonzalez F, Russell J B. The ability of Escherichia coliO157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiology. 1997;143:1175–1180. doi: 10.1099/00221287-143-4-1175. [DOI] [PubMed] [Google Scholar]
- 5.Dürre P, Bahl H, Gottschalk G. Membrane processes and product formation in anaerobes. In: Erickson L E, Fung D Y-C, editors. Handbook for anaerobic fermentations. New York, N.Y: Marcel Dekker, Inc; 1988. pp. 187–206. [Google Scholar]
- 6.Ebner H, Follmann H. Acetic acid. In: Rehm H-J, Reed G, editors. Biotechnology: biomass, microorganisms for special applications, microbial products I, energy from renewable resources. Vol. 3. Weinheim, Germany: VCH; 1983. pp. 387–407. [Google Scholar]
- 7.Fukaya M, Takemura H, Okumura H, Kawamura Y, Horinouchi S, Beppu T. Cloning of genes responsible for acetic acid resistance in Acetobacter aceti. J Bacteriol. 1990;172:2096–2104. doi: 10.1128/jb.172.4.2096-2104.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukaya M, Takemura H, Tayama K, Okumura H, Kawamura Y, Horinouchi S, Beppu T. The aarC gene responsible for acetic-acid assimilation confers acetic-acid resistance on Acetobacter aceti. J Ferment Bioeng. 1993;76:270–275. [Google Scholar]
- 9.Graumann P, Marahiel M A. Some like it cold: response of microorganisms to cold shock. Arch Microbiol. 1996;166:293–300. doi: 10.1007/s002030050386. [DOI] [PubMed] [Google Scholar]
- 10.Guerreiro N, Djordjevic M A, Rolfe B G. Proteome analysis of the model microsymbiont Sinorhizobium meliloti: isolation and characterisation of novel proteins. Electrophoresis. 1999;20:818–825. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<818::AID-ELPS818>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Gulbis J M, Mann S, MacKinnon R. Structure of a voltage-dependent K+channel β subunit. Cell. 1999;97:943–952. doi: 10.1016/s0092-8674(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 12.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 13.Hitschmann A, Stockinger H. Oxygen deficiency and its effect on the adenylate system in Acetobacterin the submerse acetic fermentation. Appl Microbiol Biotechnol. 1985;22:46–49. [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lambert L A, Abshire K, Blankenhorn D, Slonczewski J L. Proteins induced in Escherichia coliby benzoic acid. J Bacteriol. 1997;179:7595–7599. doi: 10.1128/jb.179.23.7595-7599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasko D R, Schwerdel C, Bailey J E, Sauer U. Acetate-specific stress response in acetate-resistant bacteria: an analysis of protein patterns. Biotechnol Prog. 1997;13:519–523. doi: 10.1021/bp970075f. [DOI] [PubMed] [Google Scholar]
- 17.Lasko D R, Zamboni N, Sauer U. The bacterial response to acetate challenge: A comparison of tolerance among species. Appl Microbiol Biotechnol. 2000;54:243–247. doi: 10.1007/s002530000339. [DOI] [PubMed] [Google Scholar]
- 18.Luli G W, Strohl W R. Comparison of growth, acetate production, and acetate inhibition of Escherichia colistrains in batch and fed-batch fermentations. Appl Environ Microbiol. 1990;56:1004–1011. doi: 10.1128/aem.56.4.1004-1011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesa M M, Caro I, Cantero D. Viability reduction of Acetobacter acetidue to the absence of oxygen in submerged cultures. Biotechnol Prog. 1996;12:709–712. [Google Scholar]
- 20.Nanba A, Tamura A, Nagai S. Synergistic effects of acetic-acid and ethanol on the growth of Acetobactersp. J Ferment Technol. 1984;62:501–505. [Google Scholar]
- 21.O'Farrell P H. High-resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 22.Pampulha M E, Loureiro-Dias M C. Energetics of the effect of acetic acid on growth of Saccharomyces cerevisiae. FEMS Microbiol Lett. 2000;184:69–72. doi: 10.1111/j.1574-6968.2000.tb08992.x. [DOI] [PubMed] [Google Scholar]
- 23.Park Y S, Toda K, Fukaya M, Okumura H, Kawamura Y. Production of a high-concentration acetic-acid by Acetobacter acetiusing a repeated fed-batch culture with cell recycling. Appl Microbiol Biotechnol. 1991;35:149–153. [Google Scholar]
- 24.Poole R K. The isolation of membranes from bacteria. Methods Mol Biol. 1993;19:109–122. doi: 10.1385/0-89603-236-1:109. [DOI] [PubMed] [Google Scholar]
- 25.Qi S Y, Moir A, O'Connor C D. Proteome of Salmonella typhimuriumSL1344: identification of novel abundant cell envelope proteins and assignment to a two-dimensional reference map. J Bacteriol. 1996;178:5032–5038. doi: 10.1128/jb.178.16.5032-5038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell J B. Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J Appl Bacteriol. 1992;73:363–370. [Google Scholar]
- 27.Sauer U. Evolutionary engineering of industrially important microbial phenotypes. Adv Biochem Eng Biotechnol. 2001;73:129–170. doi: 10.1007/3-540-45300-8_7. [DOI] [PubMed] [Google Scholar]
- 28.Sievers M, Teuber M. The microbiology and taxonomy of Acetobacter europaeusin commercial vinegar production. J Appl Bacteriol Sym Suppl. 1995;79:84. .S-95S. [Google Scholar]
- 29.Tonella L, Walsh B J, Sanchez J C, Ou K L, Wilkins M R, Tyler M, Frutiger S, Gooley A A, Pescaru I, Appel R D, Yan J X, Bairoch A, Hoogland C, Morch F S, Hughes G J, Williams K L, Hochstrasser D F. '98 Escherichia coliSWISS-2DPAGE database update. Electrophoresis. 1998;19:1960–1971. doi: 10.1002/elps.1150191114. [DOI] [PubMed] [Google Scholar]