Abstract
Endometriosis is a hormone-associated disease which has been considered as the precursor for certain types of ovarian cancer. In recent years, emerging evidence demonstrated potent roles of lncRNA in regulating cancer development. Since endometriosis shares several features with cancer, we investigated the possible involvement of cancer-related lncRNAs in endometriosis, including UCA1, GAS5 and PTENP1. By using massARRAY system, we investigated certain genetic variations in cancer-related lncRNAs that can change the thermo-stability, leading to up-regulation or down-regulation of those lncRNAs. Our data indicated three risk genetic haplotypes in UCA1 which can stabilize the RNA structure and increase the susceptibility of endometriosis. Of note, such alterations were found to be associated with long-term pain and infertility in patients. It has been known that UCA1 can function as a ceRNA to sponge and inhibit miRNAs, resulting in loss-of-control on downstream target genes. Gene network analyses revealed fatty acid metabolism and mitochondria beta-oxidation as the major pathways associated with altered UCA1 expression in endometriosis patients. Our study thus provides evidence to highlight functional/epigenetic roles of UCA1 in endometriosis development via regulating fatty acid metabolism in women.
Introduction
Endometriosis is a condition that commonly affects 5–10% women of reproductive age globally [1]. It happens when the inner tissue layer of the uterus grows at the outside, whereas the causes are still controversial. Half of the patients suffer from severe pain during menstruation because when a woman has her period, healthy people bleed only from inside of the uterus, but endometriosis patients bleed from outside of the uterus as well. This complication causes blood contact with other organs inside the abdomen, leading to long-term inflammation, band formation and pelvic pain. For some patients (around 30–50%), they become infertile either due to distorting the fallopian tubes thus fail to pick up the egg after ovulation or due to constant inflammation that affect normal functions of the ovary, egg, fallopian tubes or uterus [2, 3]. With many biochemical changes in endometriotic lesions, the genetic/epigenetic theory was purposed in recent years to explain the hereditary aspects, the predisposition, and the endometriosis-associated changes in the endometrium, immunology, and placentation during disease development [4, 5].
Notably, endometriosis shares several phenotypes with cancer such as uncontrolled proliferation, cell migration/invasion to other surrounding organs [2]. Hormone imbalance, a proposed cause for endometriosis, can be also a risk for several types of gynecological cancer [2, 6, 7]. In fact, epidemiological and molecular investigations provided evidence to support that endometriotic lesions serve as benign precursors for certain types of gynecological cancers [8, 9]. Symptoms like recurring pelvic pain and long-term inflammation are also frequently found in patients with endometrial and uterine cancers.
In recent years, the next-generation sequencing technology has discovered thousands of long noncoding RNAs (lncRNA) in mammals. LncRNAs are non-coding RNAs with length larger than 200nt, which can undergo post-transcriptional editing just like protein-coding mRNAs, such as 5’-capping, 3’-end polyadenylation and RNA splicing [10]. Although no protein encoded, lncRNAs show dynamic impacts on gene expression through at least three well-known regulatory mechanisms: guides, dynamic scaffolds and molecular decoys [11, 12], therefore lncRNAs profiles can control the content and abundance of microRNAs (miRNAs), mRNAs, and proteins in a cell. Many studies revealed potent roles of lncRNAs in regulating carcinogenesis or tumor suppression [13]. Dysregulated expression of certain lncRNAs have been linked to the development of gynecologic cancers [14]. Some other studies also demonstrated the association of certain lncRNAs with endometriosis [15]. More interestingly, genetic alterations in those lncRNAs have been suggested as the cause to drive the pathogenesis of endometriosis [16].
UCA1 and GAS5 are lncRNAs predominantly expressed in endometrial tissues based on the HPA RNA-seq results from NCBI databank (www.ncbi.nlm.nih.gov). Previous studies found that both UCA1 and GAS5 lncRNAs play roles in the development of ovarian cancer [17]. GAS5 has been reported to function as a tumor suppressor-like gene, that can block ovary cancer progression [18–20], whereas UCA1 serves as a potential novel biomarker and therapeutic target for ovarian cancer [21, 22]. Of note, certain lncRNAs including UCA1 can interact with a variety of miRNAs which subsequently alter gene expression profiles and control cancer progression [23–25]. For examples, UCA1 can function as a competing endogenous RNA (ceRNA) to sponge tumor suppressor gene-binding miRNAs, thus promote cancer development [26–31], such as ovarian cancer [32]. On the other hand, PTENP1 is another emerging cancer-related lncRNA, which can also function as ceRNA to abolish the expression of PTEN, a well-characterized tumor suppressor, thus has been considered as a cancer-promoting gene for a variety of cancers, including endometrial cancer [9]. Although the biological consequences associated with the altered lncRNAs and miRNAs have been intensively investigated in cancer, little is known about their possible roles in the development and progression of endometriosis. Since endometriosis has been known as the precursor of certain types of ovarian cancer, similar bio-functions of those potent lncRNAs detected in cancer may also determine the development of endometriosis.
Several lines of evidence have proven that genetic variations in miRNAs can promote endometriosis development [33]. In this study, we investigated the possible impacts of genetic variations in these ovary-related lncRNAs, PTENP1, GAS5 and UCA1, on endometriosis. Several SNPs (single nucleotide polymorphisms) that can alter the RNA structure and thermodynamic energy were selected and genome-typed in patients and normal controls. Clinical consequences associated with the disease-related alleles were also analyzed. Furthermore, we studied the associated regulatory effects on the lncRNA-miRNA axes and discussed their involvements in the pathogenesis of endometriosis.
Results
Functional SNPs change thermo-energy and local structures in lncRNAs
LncRNAs have been frequently linked to hormone-sensitive cancers (S1 Table in S1 File) as well as endometriosis (S2 Table in S1 File), suggesting the involvement of those oncogenic lncRNAs in regulating cell proliferation and invasiveness. In addition, several studies also indicate that alterations in RNA structures of gene products can determine the outcomes of human diseases [34], leading to our hypothesis that alterations in RNA structure by genetic variations like SNPs may affect the progression and development of endometriosis. To prove this hypothesis, MassARRAY system was performed to detect the SNPs in oncogenic lncRNAs, including PTENP1, GAS5, and UCA1. SNPs with minor allele frequencies (MAF) larger than 10% in Chinese Han Beijing population were filtered out from NCBI databank (www.ncbi.nih.gov/snp). Potentially functional SNPs were further selected in which the substitutions can alter overall free energy of the lncRNAs (ΔΔG > 0.5 or < -0.5 kcal/mol) and change the folding of the local RNA structures (S3 Table in S1 File). For this task, partial RNA sequences containing the SNP sites (total 121-bp, the SNP site with 60-bp upstream and 60-bp downstream sequences) were submitted to RNA stability calculation and structure prediction by using MaxExpect (rna.urmc.rochester.edu/RNAstructureWeb/Manual/MaxExpect.html). Based on those criteria and probe availability, three SNPs in GAS5, five SNPs in UCA1 and four SNPs in PTENP1 were finally picked up to further genotyping (S3 Table in S1 File). Our results show that the energy changes were valid (ΔΔG > 0.5 or < -0.5 kcal/mol) in all selected SNPs, and the RNA folding got altered as compared to the wildtype sequences, including GAS5 (S1 Fig in S1 File), UCA1 (S2A Fig in S1 File) and PTENP1 (S3 Fig in S1 File). Those results confirmed the genetic variations in lncRNAs can induce changes in local RNA folding and their thermo-dynamics.
Impacts of functional SNPs in lncRNAs on the susceptibility to endometriosis
Allelic and genotypic distributions of selected SNPs in this case-control study were analyzed, and our data indicated that genetic variation of A to G at rs113579010 in UCA1 showed a tendency to increase the risk to develop endometriosis (OR = 1.718; 95% CI: 1.101–2.674) (Table 1). Dominant/recessive genotype analyses further confirmed the dominant effects of this functional SNP that associated with higher susceptibility to endometriosis (OR = 1.835; 95% CI: 1.079–3.125) (Table 2). Of note, the p-values were not statistically significant after Bonferroni corrections. No notable associations, allelic/genotypic distributions and dominant/recessive effects, were found for the selected functional SNPs in GAS5 and PTENP1 (S4 and S5 Tables in S1 File).
Table 1. Genotypic and allelic distributions of five functional SNPs in UCA1 between Taiwanese endometriosis patients and controls1.
| SNPs | Genotype /allele | No. (%) of patients | HWE | No. (%) of controls | HWE | p-value | Corrected p-value2 | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| rs113579010 | GG | 8 | 0.854 | 3 | 0.663 | 0.520 | 1.000 | 3.322 (0.845–12.987) |
| AG | 48 | 35 | 1.709 (0.985–2.967) | |||||
| AA | 61 | 76 | 1.000 Reference | |||||
| G | 64 (27.4%) | 41 (13.2%) | 0.016* | 0.082 | 1.718 (1.101–2.674) | |||
| A | 170 (72.6%) | 187 (86.8%) | 1.000 Reference | |||||
| rs12462414 | TT | 30 | 0.854 | 29 | 0.132 | 0.599 | 1.000 | 1.212 (0.596–2.469) |
| GT | 61 | 52 | 1.375 (0.741–2.551) | |||||
| GG | 29 | 34 | 1.000 Reference | |||||
| T | 121 (50.4%) | 110 (47.8%) | 0.572 | 1.000 | 1.108 (0.772–1.592) | |||
| G | 119 (49.6%) | 120 (52.2%) | 1.000 Reference | |||||
| rs12610921 | GG | 3 | 0.000 | 3 | 0.000 | 0.787 | 1.000 | 1.333 (0.196–9.091) |
| GA | 100 | 91 | 1.464 (0.490–4.386) | |||||
| AA | 6 | 8 | 1.000 Reference | |||||
| G | 106 (48.6%) | 97 (47.5%) | 0.823 | 1.000 | 1.044 (0.712–1.529) | |||
| A | 112 (51.4%) | 107 (52.5%) | 1.000Reference | |||||
| rs73003273 | AA | 8 | 0.643 | 3 | 0.616 | 0.209 | 1.000 | 2.976 (0.759–11.628) |
| AG | 42 | 36 | 1.302 (0.750–2.257) | |||||
| GG | 69 | 77 | 1.000 Reference | |||||
| A | 58 (24.4%) | 42 (18.1%) | 0.097 | 0.583 | 1.458 (0.933–2.278) | |||
| G | 180 (75.6%) | 190 (81.9%) | 1.000 Reference | |||||
| rs73005445 | GG | 8 | 0.665 | 3 | 0.616 | 0.194 | 1.000 | 3.021 (0.770–11.765) |
| AG | 42 | 36 | 1.321 (0.760–2.294) | |||||
| AA | 68 | 77 | 1.000 Reference | |||||
| G | 58 (24.6%) | 42 (18.1%) | 0.088 | 0.525 | 1.475 (0.943–2.304) | |||
| A | 178 (75.4%) | 190 (81.9%) | 1.000 Reference |
1 Abbreviations: SNP, single nucleotide polymorphism; HWE, Hardy–Weinberg equilibrium; OR, odds ratio; CI, confidence interval
2 Bonferroni correction was applied to get the corrected p-value, which equals to p-value x5 in UCA1. Statistical significance: p-value < 0.05, *; p-value < 0.01, **; p-value < 0.001, ***.
Table 2. Dominant and recessive effects of five functional SNPs in UCA1 between Taiwanese endometriosis patients and controls1.
| SNPs | Genotype | No. (%) of patients | No. (%) of controls | p-value | Corrected p-value2 | OR (95%CI) |
|---|---|---|---|---|---|---|
| rs113579010 | AG+GG | 56 (47.9%) | 38 (33.3%) | 0.025* | 0.123 | 1.835 (1.079–3.125) |
| AA | 61 (52.1%) | 76 (66.7%) | 1.000 Reference | |||
| GG | 8 (6.8%) | 3 (2.6%) | 0.134 | 0.668 | 2.717 (0.702–10.526) | |
| AA+AG | 109 (93.2%) | 111 (97.4%) | 1.000 Reference | |||
| rs12462414 | GT+TT | 91 (75.8%) | 81 (70.4%) | 0.351 | 1.000 | 1.318 (0.738–2.347) |
| GG | 29 (24.2%) | 34 (29.6%) | 1.000 Reference | |||
| TT | 30 (25.0%) | 29 (25.2%) | 1.000 | 1.000 | 0.988 (0.545–1.783) | |
| GG+GT | 90 (75.0%) | 86 (74.8%) | 1.000 Reference | |||
| rs12610921 | GA+GG | 103 (94.5%) | 94 (92.2%) | 0.493 | 1.000 | 1.460 (0.489–4.367) |
| AA | 6 (5.5%) | 8 (7.8%) | 1.000 Reference | |||
| GG | 3 (2.8%) | 3 (2.9%) | 0.627 | 1.000 | 0.934 (0.184–4.739) | |
| AA+GA | 106 (97.2%) | 99 (97.1%) | 1.000 Reference | |||
| rs73003273 | AG+AA | 50 (42.0%) | 39 (31.0%) | 0.185 | 1.000 | 1.431 (0.842–2.433) |
| GG | 69 (58.0%) | 77 (69.0%) | 1.000 Reference | |||
| AA | 8 (6.7%) | 3 (2.6%) | 0.134 | 0.668 | 2.717 (0.702–10.526) | |
| GG+AG | 111 (93.3%) | 113 (97.4%) | 1.000 Reference | |||
| rs73005445 | AG+GG | 50 (42.4%) | 39 (33.6%) | 0.168 | 1.000 | 1.451 (0.854–2.469) |
| AA | 68 (57.6%) | 77 (66.4%) | 1.000 Reference | |||
| GG | 8 (6.8%) | 3 (2.6%) | 0.129 | 0.647 | 2.740 (0.708–10.638) | |
| AA+AG | 110 (93.2%) | 113 (97.4%) | 1.000 Reference |
1 Abbreviations: SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval
2 Bonferroni correction was applied to get the corrected p-value, which equals to p-value x5 in UCA1. Statistical significance: p-value < 0.05, *; p-value < 0.01, **; p-value < 0.001, ***.
Among the SNPs tested in UCA1, allelic types of SNPs at rs113579010, rs73003273 and rs73005445 show lesser risks of endometriosis development (p < 0.100, Table 1), we therefore analyzed two-locus haplotypes of genetic combinations among these three SNPs. Significantly, patients with haplotypes of two allelic variants in UCA1 showed stronger correlations with endometriosis development as compared to the controls (Table 3), suggesting more functional impacts generated by two-locus combinations than one single SNP on endometriosis development. Based on those results, we confirmed the possible involvements of three functional SNPs in UCA1, especially the two-locus hyplotypes among these three, in the development of endometriosis. Such genetic associations may be due to the changes in local RNA structures in UCA1 generated by genetic variations in those functional SNPs.
Table 3. Haplotype frequencies of selected SNPs in UCA1 gene between endometriosis patient and controls1.
| SNPs | Genotype / allele | No. (%) in patients | No. (%) in controls | p-value | OR (95%CI) |
|---|---|---|---|---|---|
| rs113579010 | GA | 74 (15.7%) | 48 (10.4%) | 0.0177* | 1.5959 (1.0821–2.3535) |
| rs73003273 | GG | 56 (11.9%) | 36 (7.8%) | 0.0388* | 1.5855 (1.0211–2.4618) |
| AA | 44 (9.3%) | 36 (7.8%) | 0.4166 | 1.2108 (0.7640–1.9189) | |
| AG | 298 (63.1%) | 340 (74.0%) | 0.0004*** | 0.6045 (0.4569–0.7996) | |
| rs113579010 | GG | 74 (15.8%) | 48 (10.4%) | 0.0154* | 1.6121 (1.0930–2.3777) |
| rs73005445 | GA | 52 (11.1%) | 36 (7.8%) | 0.0875 | 1.4722 (0.9425–2.2996) |
| AG | 44 (9.4%) | 36 (7.8%) | 0.3929 | 1.2222 (0.7711–1.9372) | |
| AA | 298 (63.7%) | 340 (74.0%) | 0.0008*** | 0.6187 (0.4673–0.8192) | |
| rs73003273 | AG | 75 (15.8%) | 48 (10.3%) | 0.0139* | 1.6209 (1.1003–2.3879) |
| rs73005445 | AA | 43 (9.0%) | 36 (7.8%) | 0.4795 | 1.1807 (0.7435–1.8749) |
| GG | 43 (9.0%) | 36 (7.8%) | 0.4795 | 1.1807 (0.7435–1.8749) | |
| GA | 315 (66.2%) | 344 (74.1%) | 0.0077** | 0.6825 (0.5151–0.9044) |
1 Abbreviations: SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval
2 Statistical significance: p-value <0.05,*; p-value <0.01,*; p-value <0.001.
Functional SNPs in UCA1 and their associations with clinical features
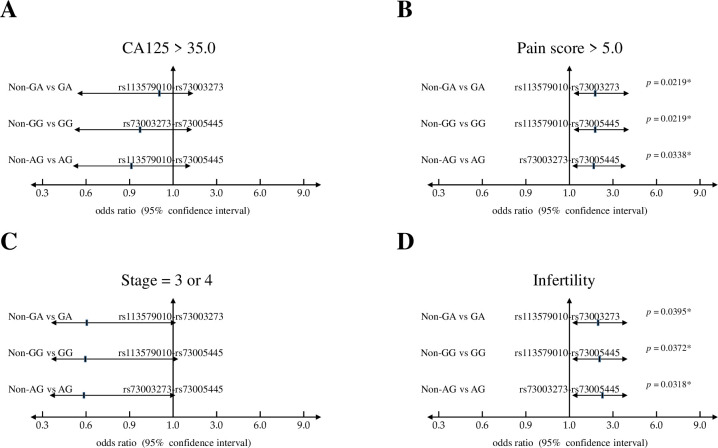
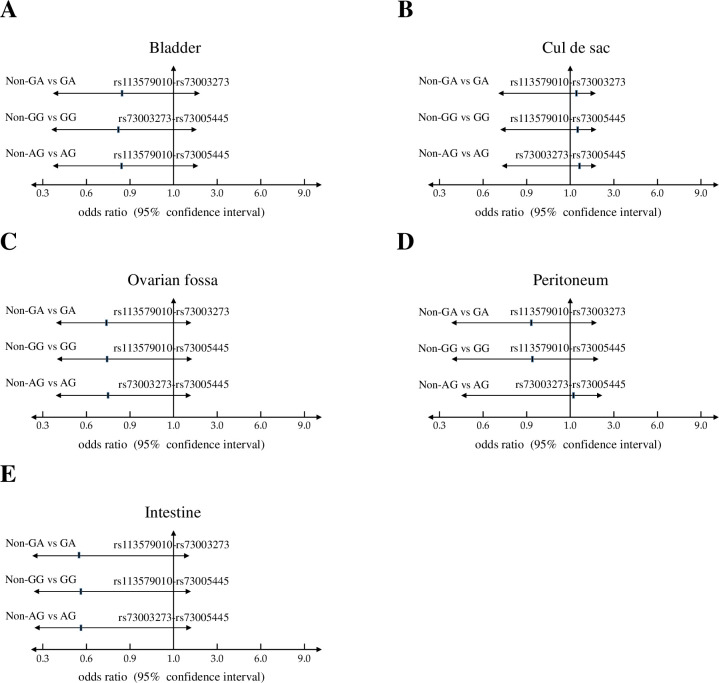
Based on the results by haplotype analyses, we further asked the possible relevance of those risk haplotypes with the clinical outcomes, including plasma CA125 level (≤ 35.0 vs. > 35.0 U/ml), pain score (1–5 vs. 6–10), clinical stage (1 & 2 vs. 3 & 4), and reproductive activity (fertile vs. infertile). Interestingly, these three potent haplotypes showed significant associations with patients who showed higher pain scores and infertility (Fig 1). Furthermore, we found that patients with those risk haplotypes show higher frequencies to harbor the lesions at cul-de-sac (Fig 2), an adhesion site that frequently associated with deeply infiltrating endometriosis and the associated pain or infertility [35, 36]. Howerver, such associations did not reach statistically significance.
Fig 1. Haplotype analyses of selected functional SNPs in UCA1 and their associations with clinical features of endometriosis patients.
Odds ratios and 95% confidence intervals of three risk haplotypes based on clinical features, including (A) CA125 levels; (B) disease stage; (C) pain score; and (D) endometriosis-associated infertility.
Fig 2. Haplotype analyses of selected functional SNPs in UCA1 and their associations with adhesion sites of endometriotic lesions.
Odds ratios and 95% confidence intervals of three risk haplotypes based on adhesion sites, including (A) bladder; (B) cul de sac; (C) ovarian fossa; (D) peritoneum; and (D) intestine.
Functional SNPs in UCA1 change the thermo-stability and structure of the RNA product
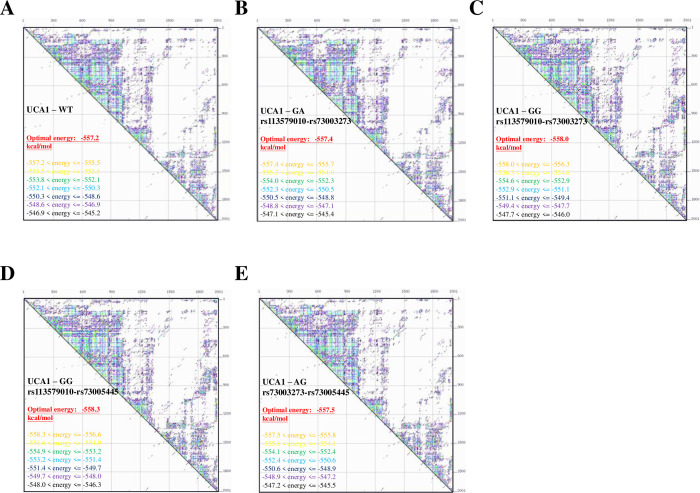
To learn how those novel haplotypes may affect the functions of UCA1, the full-length UCA1 variants with different haplotypes were analyzed by UNAfold web server (www.unafold.org/mfold/applications/rna-folding-form-v2.php) to estimate their thermo-stability. As shown in (Fig 3A), the optimal net energy for wild-type UCA1 was -557.2 kcal/mol; the optimal energy for the GA haplotype and the GG haplotype of rs113579010-rs73003273 were -557.4 kcal/mol (Fig 3B) and -558.0 kcal/mol (Fig 3C), respectively. For these two haplotypes of rs113579010-rs73003273, they showed similar but lower optimal net energy as compared to the wild-type. The optimal energy for the GG haplotype of rs113579010-rs73005445 was -558.3 kcal/mol (Fig 3D), which showed much lower thermo-stability than wild-type UCA1. The optimal energy for the AG haplotype of rs73003273-rs73005445 was -557.5 kcal/mol, also lower than the wild-type (Fig 3E).
Fig 3. Thermo-stability changes in UCA1 variants with different haplotypes.
Thermo-stability of (A) wild-type UCA1, (B) UCA1 variant with the GA haplotype of rs113579010-rs73003273, (C) UCA1 variant with the GG haplotype of rs113579010-rs73003273, (D) UCA1 variant with the GG haplotype of rs113579010-rs73005445, and (E) UCA1 variant with the AG haplotype of rs73003273-rs73005445. Thermo-stability of those UCA1 variants were predicted by UNAfold web server (www.unafold.org/mfold/applications/rna-folding-form-v2.php).
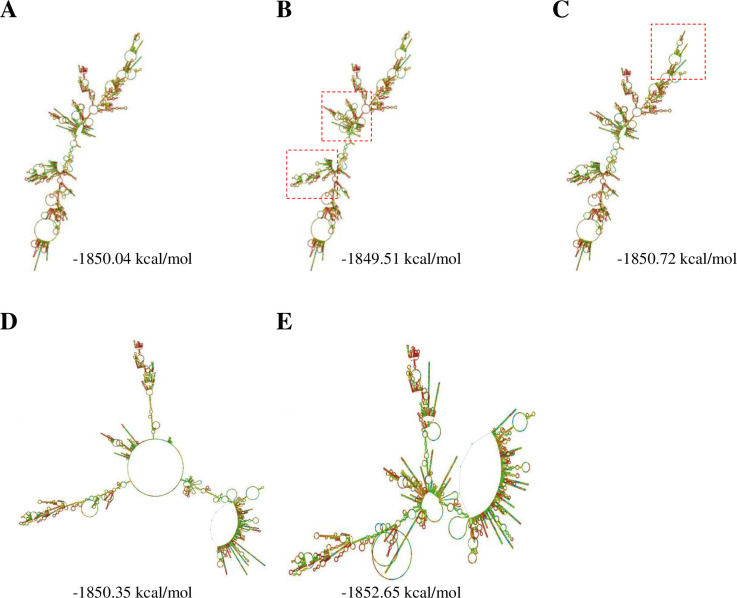
In parallel with thermo-stability calculations, RNA structures of UCA1 variants with different haplotypes were also predicted. Thermo-stability analyses revealed that the GA and GG haplotypes of rs113579010-rs73003273 produce UCA1 variants with similar structures as the wild-type (Fig 4A to 4C). Notably, haplotypes associated with rs73005445, the GG haplotype of rs113579010-rs73005445 and the AG haplotype of rs73003273-73005445, can strikingly change their full-length RNA structures (Fig 4D and 4E) as compared to the wild-type. In general, our data suggest that all the risk-associated haplotypes in UCA1 can generate more stable RNA products, some may even change their RNA structures, leading to higher expression UCA1 levels in endometrial tissues during the development of endometriosis.
Fig 4. Changes in RNA structures of UCA1 variants with different haplotypes.
The predicted RNA structures of (A) wild-type UCA1, (B) UCA1 variant with the GA haplotype of rs113579010-rs73003273, (C) UCA1 variant with the GG haplotype of rs113579010-rs73003273, (D) UCA1 variant with the GG haplotype of rs113579010-rs73005445, and (E) UCA1 variant with the AG haplotype of rs73003273-rs73005445. Full-length RNA strucures of those UCA1 variants were predicted by RNAfold web server (rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi). Dash line boxes indicate the altered local structures in the RNA transcripts.
UCA1 functions as a miRNA sponge to disrupt lipogenesis in endometrial tissues
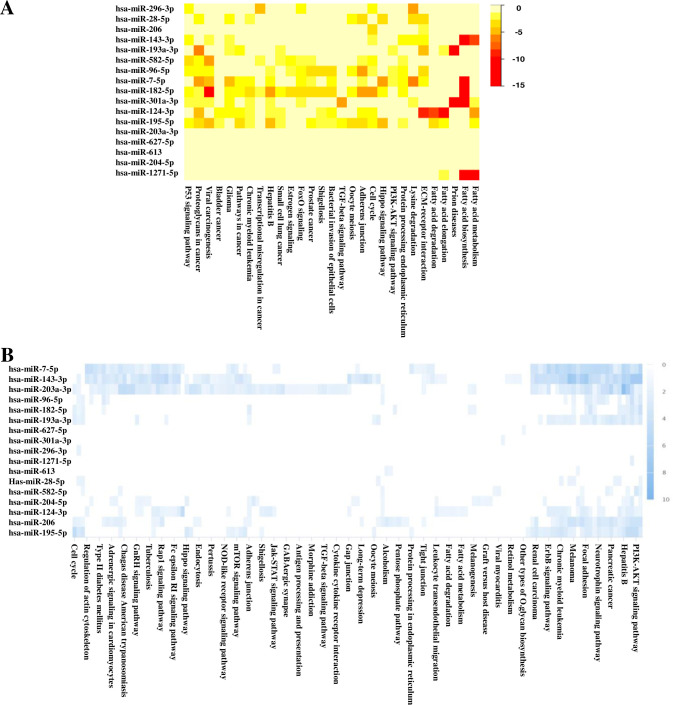
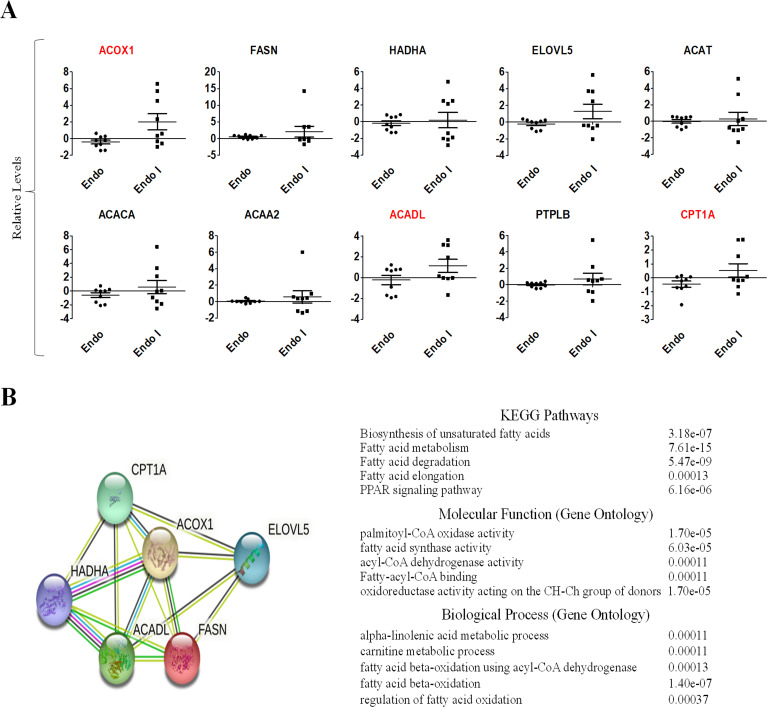
Previous studies discovered that elevated UCA1 can act as a miRNA sponge to change the miRNA profiles, thus control disease development and progress (S6 Table in S1 File) [37, 38]. We therefore wanted to know the possible impacts on miRNA network by elevated levels of UCA1 variants through structural stabilization. To achieve this goal, microarray data of GEO dataset (GSE120103) was used to confirm that patients with endometriosis expressed higher UCA1 levels as compared to healthy controls (p-value = 0.0083) (S4 Fig in S1 File). In addition, a miRNA network analysis associated with elevated UCA1 was done by DIANA (S7 Table in S1 File), and the resultant data indicated several candidate genes involved in fatty acid metabolism, biosynthesis and degradation (Fig 5A). Similar findings were also found by searching mirPath data bank (Fig 5B). To further confirm the results, we analyzed the gene expression levels of those candidate genes by using microarray data of GEO dataset (GSE120103). The most significantly relevant genes were found, including FASN, PTPLB, ACACA, ACADL, ACAT1, ACAA2, ACOX1 and HADHA, which can be elevated in endometriosis patients, especially in patients with infertility (Fig 6A). STRING protein-protein interaction analysis of those validated genes revealed their involvement in biosynthesis of unsaturated fatty acids, fatty acid metabolism/degradation/elongation, PPAR signaling pathway and fatty acid beta-oxidation pathway (Fig 6B).
Fig 5. UCA1 functions as a miRNA sponge to remodel the miRNA networks.
The miRNA networks associated with UCA1 elevation were predicted by (A) DIANA Tools mirPath and (B) miRPathDB v2.0. Heat map analyses indicate the statistic significances (-log p-values) of major down-stream pathways regulated by the altered miRNA networks.
Fig 6. Expression levels of key effector genes of UCA1 as being a miRNA sponge in endometrial patients with/without infertility.
(A) Expression levels of UCA1 down-stream genes involved in fatty acid metabolism pathways were analyzed by using microarray data of GEO databank (GSE120103). Endo: patients without infertility; Endo-I: patients with infertility. (B) Key pathways associated with altered down-stream effectors were analyzed by STRING protein-protein interactome databank.
UCA1 knockdown down-regulated enhanced lipogenesis in endometrial cells
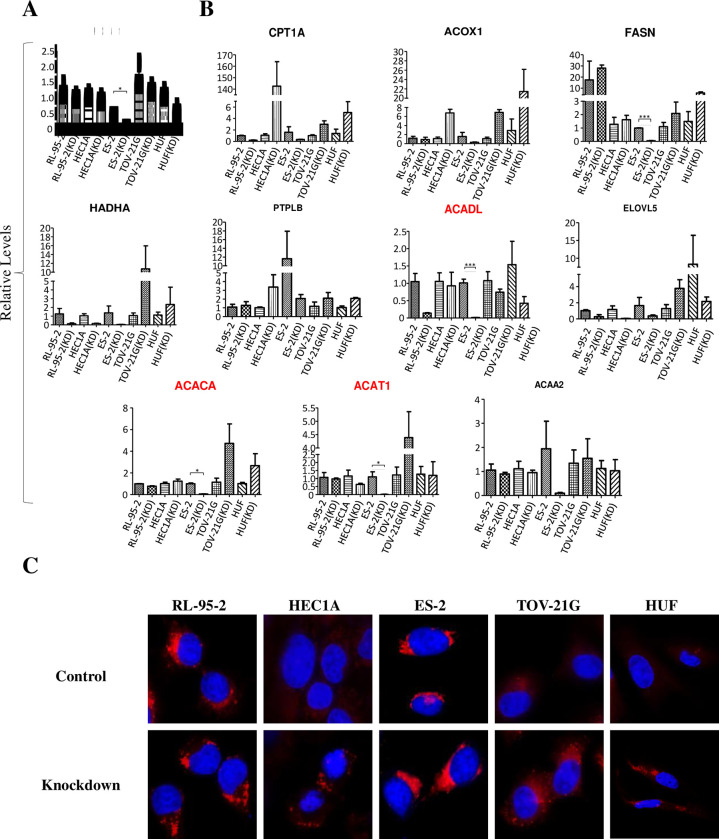
To further validate the possible functions of UCA1 in fatty acid metabolism in endometrial cells, UCA1 knockdown was performed by co-transfecting cells with pDECKO_UCA1 and lentiCas9 vectors. The selected cell lines included endometrial cancer cells (RL-95-2, HEC1A), clear cell type ovarian cancer cells (endometriosis-origin; ES-2, TOV-21G) and human uterus fibroblast (HUF, as the normal cell control). After drug selection and cell enrichment, RNA samples were extracted from the transfected cells and subjected to RT-qPCR analyses. Our qPCR results indicate that most of the transfected cells showed reduced UCA1 levels (Fig 7A). In particular, UCA1 was downregulated massively in ES-2 cells. Consistent with our previous findings, ACADL, ACAT1, and ACAA2 genes, the major regulators in fatty acid degradation and beta-oxidation pathways, were significantly down-regulated (Fig 7B). Lipid droplet staining by LipidTOX-Red further confirmed accumulated lipids in treated cells after UCA1 knockdown (Fig 7C). With those results described above, we can conclude that elevated UCA1 in endometriosis patients may act as a miRNA sponge to alter miRNA profiles which favor gene expression of key regulators in fatty acid metabolism.
Fig 7. Functional impacts of UCA1 knockdown on fatty acid metabolism in endometrial cells.
(A) UCA1 knockdown in different endometrial cell lines (RL-95-2, HEC1A, ES-2, TOV-21G, and HUF). (B) Relative gene expression levels of key genes in fatty acid metabolism (CPT1A, ACOX1, FASN, HADHA, ACADL, ELOVL5, ACACA, ACAT1, PTPLB, ACAA2) were detected by RT-qPCR in UCA1-knockdown and control cells. (C) Lipid droplet staining in treated cells was performed by using LipidTOX-Red.
Discussion
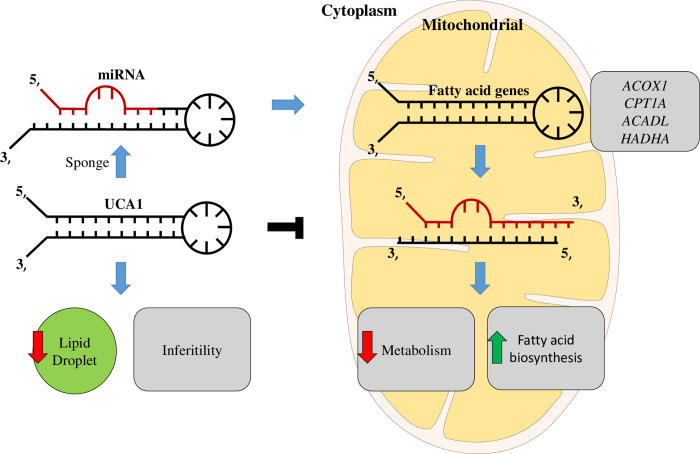
In this study, we discovered the involvement of novel haplotypes of UCA1 gene in endometriosis development and the associated clinical outcomes, including long-term pain and infertility (Tables 1 to 3 and Fig 1). Those risk haplotypes are composed by the genetic combinations among three functional SNPs at rs113579010 (allele G), rs73003273 (allele A) and rs73005445 (allele G). Most of patients who carry those risk haplotypes show the tendency to harbor cul-de-sac infiltration (Fig 2), which can partially explain their associations with long-term pain and infertility. Since those functional SNPs were selected due to their ability to alter thermo-stability, we further investigated the changes in optimal net energy and RNA structures of those UCA1 variants by RNA folding prediction. Our data confirmed that most of the UCA1 variants with those risk haplotypes showed reduced free-energy/enhanced stability (Fig 3), which can subsequently alter their full-length RNA structures (Fig 4), suggesting elevated UCA1 in endometriotic lesions. Considering the potent roles of UCA1 as a miRNA sponge, we further defined several pathways involved in fatty acid metabolism as the key down-stream signaling networks induced by altered miRNA profiles after UCA1 up-regulation (S5 Fig and S6 Table in S1 File). Transcriptome analysis by using dataset from GSE120103 confirmed increased levels of UCA1 in patients with endometriosis (S4 Fig in S1 File). Interestingly, several key regulatory genes involved in fatty acid metabolism were also found up-regulated in patients, especially for the ones with infertility (Fig 6). Functional knockdown of UCA1 in endometrial cells can significantly suppressed expression levels of key genes in lipogenesis (ACADL, ACAT1, and ACAA2 genes), resulting in accumulation of lipid droplets in the treated cells (Fig 7). Our study therefore reveals a novel role of UCA1 in endometriosis development and the associated infertility through regulating genes involved in fatty acid metabolism and lipogenesis (Fig 8).
Fig 8. Proposed pathogenesis model in the development of endometriosis and the associated infertility based on the findings of this study.
Elevated UCA1 in endometriosis patients via RNA stabilization by functional SNPs can disrupt lipogenesis regulation by sponging key miRNAs with target genes involved in lipid metabolism. Such epigenetic regulation can further lead to reduced biosynthesis of triacyglycerol for oocyte future maturation and increased lipotoxicity, resulting in infertility.
UCA1 overexpression has been reported in different types of cancer, such as breast cancer, ovarian cancer, bladder cancer, and hepatocellular carcinoma [26, 27, 39, 40]. In addition to its potent roles in tumorigenesis, UCA1 overexpression was also frequently linked with advanced cancerous behaviors, including metastasis [28, 30], drug resistance [26, 40] and anti-immune surveillance [41]. As endometriosis shares several similar phenotypes with cancer including uncontrolled cell proliferation and long-term inflammation, this study reported a novel mechanism in endometriosis development by upregulating UCA1 through stabilizing RNA structures of the risk haplotype variants. Based on UCA1 sponging miRNA profile analysis (Fig 4), cell cycle up-regulation is one of downstream pathways associated with UCA1 elevation, suggesting the advantages for lesion growth. In addition, patients with those risk haplotypes also showed higher pain scores, which may also indicate long-term inflammation associated with UCA1 elevation, probably due to consistent lesion growth.
Another interesting finding in this study is the involvement of fatty acid metabolism associated with increased UCA1 levels (Figs 5 to 7). Although a previous study has revealed a possible connection between fatty acids (e.g. palmitate acid) and UCA1 [42], no solid evidence was provided to confirm the direct relationship between UCA1 and fatty acid metabolism pathway. Through miRNA network analysis, we have found that UCA1 can sponges certain miRNAs that target genes involved in fatty acid metabolism, including FADS2, ELOVL5, ACOX1, CPT1A, ACADL and HADHA (Fig 5). Among those genes, ACOX1, CPT1A, ACADL and HADHA which participate in fatty acid beta-oxidation can be further validated by clinical samples (Fig 6) and cell-based study (Fig 7). This new finding provides a new insight on the molecular link between UCA1 and fatty acid metabolism. Those findings may explain and support the involvement of UCA1 in palmitate acid-induced cancer aggressiveness [42].
In our study, we also found that endometriosis patients with higher UCA1 were more susceptible to being infertile, part of the reason may be due to unhealthy conditions of the oocyte. Oocyte maturation is an essential process to make women fertile, but this process could be easily affected by the energy statuses of cumulus cells surrounding the oocyte [43]. Cumulus cells are supportive cells that can produce lipid droplets to protect and supply energy to oocytes [44]. One possible scenario associated with increased infertility in patients with risk haplotypes of UCA1 would be that elevated UCA1 can enhance beta-oxidation in cumulus cells, resulting in the shortage of lipid droplet storage. Such energy-exhausted condition may limit the cells to supply sufficient energy to oocytes during oocyte maturation, thus reduce the quality of oocytes in patients (Fig 8). It has been known that women with low BMI, especially Asian women, are at higher risk to become infertile than those with normal or higher BMI [45]. Clinical observations also revealed that lower BMI is a risk factor for endometriosis development and such association is higher in patients with infertility [46–48].
Although our data indicated the involvement of UCA1 genetic variants in endometriosis development and the associated infertility, limitations in this study should be addressed here. Firstly, this study should be considered as a pilot study due to a relatively small sample size. A validation study by using another cohort with a bigger sample size can further elucidate the potent impacts of those functional SNPs and risk haplotypes on this disease. Secondly, we currently only confirmed the functional links between UCA1 levels and lipid droplet storage by in vitro cell line-based studies. In vivo study by using UCA1 gene knock-out mice may be able to further confirm how UCA1 influences fatty acid metabolism in the reproductive system and infertility development.
Materials and methods
Clinical samples and patient information
Blood samples were collected from study subjects who were recruited at China Medical University Hospital, Taiwan. Both patients and healthy women were confirmed by laparotomy or laparoscopy examines in this prospective study. This study was approved by the Institutional Review Board (IRB) at the CMUH (CMUH106-REC1-138) with informed consent from each participant. For the control group, we selected a healthy cohort with matched age profiles as the patient group. Clinical information of the study subjects was collected from clinical notes. Endometriosis stages were classified according to the guidelines of the American Society of Reproductive Medicine (ASRM) as stage 1, 2, 3, and 4, respectively [49].
Genome typing by MassARRAY
DNA samples were extracted from blood samples of study subjects by using Genomic DNA isolation kit (Qiagen, Valencia, CA, USA). MassARRAY system (S5A Fig in S1 File) were used to verify genetic variations of the selected functional SNPs in different lncRNA genes. In this MassARRAY system (Agena Bioscience, San Diego, CA, USA), PCR primers were designed (as shown in S3 Table in S1 File) to amplify the target regions followed by shrimp alkaline phosphatase (SAP) treatment to dephosphorylate excess nucleotides. The treated samples were further subjected to iPLEX extension reaction with extension primers that can anneal into the specified SNP sites of the amplified DNA fragments and the reactions can be terminated by terminator nucleotides. The final extension products (analytes) were desalted and transferred onto a SpectroCHIP® Array with an automated nano-dispenser. MALDI-TOF-MS was used to perform matrix assisted laser desorption ionization that can differentiate the sizes of SNP variations (S5B Fig in S1 File).
RNA structure and thermo-stability prediction
To study the structure and thermo-stability of the lncRNAs with genetic substitutions at the defined SNP sites, local sequences (total 121bp, the SNP site with the sequences of upstream 60 bp and downstream 60bp) were submitted to MaxExpect platform (rna.urmc.rochester.edu/RNAstructureWeb/Servers/Predict1/Predict1.html) to analyze the RNA stability and structure. As for the UCA1 variants with different haplotypes, thermo stability of the selected region (total 2001bp, the rs113579010 SNP site with the sequences of upstream 1000 bp and downstream 1000 bp which can also cover the other two SNP sites) were predicted by UNAfold platform (www.unafold.org/mfold/applications/rna-folding-form-v2.php). The full structures of UCA1 variants were predicted by RNAfold platform (rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi). The RNA stability was estimated for alterations in the overall free energy change (ΔΔG).
Statistical analysis
Distributions of allelic and genotypic frequencies of selected SNPs were compared between healthy controls and endometriosis patients by 2X2 chi-square analysis (vassarstats.net/odds2x2.html). The total numbers and frequencies of allelic types, genotypes and haplotypes were compared between controls and patients and presented in different contingency tables with 95% confidence intervals (95% CIs) and odds ratio (ORs). The dominant alleles were utilized as the references.
Pathway analysis
DIANA Tools mirPath (snf-515788.vm.okeanos.grnet.gr/index.php?r = mirpath) was utilized to analyze multi miRNA networks and the altered Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways according to expression levels of downstream target genes. On the other hand, miRPathDB v2.0 heatmap calculator (mpd.bioinf.uni-sb.de/heatmap_calculator.html?organism = hsa) was used to calculate miRNA network. The p-values of major altered pathways were calculated by Fisher’s exact test. Differences of the hypergeometric distributions were considered statistically significant when p-values < 0.050. STRING database (string-db.org/cgi/input.pl?sessionId=DdLKh4m0pH4j&input_page_show_search=on) was used to predict protein-protein interactions between different effector genes.
Cell culture & UCA1 knockdown
Ovarian clear cancer cells (TOV-21G and ES-2) and endometrial cells (RL95-2 and HEC1A) were purchased from Bioresource Collection and Research center (BRCR), Taiwan. Human uterine fibroblast (HUF) cell line was purchased from ScienCell Research Laboratories (SC-7040; Carlsbad, CA, USA). The cells were maintained in DMEM medium containing 10% FBS and 1% penicillin/streptomycin (Gibco/Thermo Fisher Scientific, Waltham, MA, USA). Vector pDECKO_UCA1 (Addgene, Watertown, MA) which expresses two gRNAs targeting UCA1 promoter was used to knockdown UCA1 by co-transfection with lentiCas9-Blast (Addgene, Watertown, MA) into cells. The effected cells were further enriched by 5 μg/ml puromycin in the culture medium.
Supporting information
(RAR)
Acknowledgments
The authors thank Dr. Kuan-Hao Tsui (Kaohsiung Veterans General Hospital, Taiwan) for his critical comments on this study. The authors also thank Dr. Senthilkumar Ravichandran (National Sun Yatsen University, Taiwan) for his technical assistance.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by grants from Ministry of Science and Technology, Taiwan (110-2320-B-039-033 and 111-2320-B-039-030-MY3), Ministry of Health and Welfare, Taiwan (MHW 11017), China Medical University Hospital, Taiwan (DMR111-120), and National Sun Yat-sen University- Kaohsiung Medical University (110-P019). This study was also partially supported by grants from National Health and Family Planning Commission of Henan Province, China (2018020215 and LHGJ20200448). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sun PR, Jia SZ, Lin H, Leng JH, Lang JH. Genome-wide profiling of long noncoding ribonucleic acid expression patterns in ovarian endometriosis by microarray. Fertil Steril. 2014;101(4):1038–46 e7. doi: 10.1016/j.fertnstert.2013.12.035 [DOI] [PubMed] [Google Scholar]
- 2.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. doi: 10.1038/s41572-018-0008-5 [DOI] [PubMed] [Google Scholar]
- 3.Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39(4):535–49. doi: 10.1016/j.ogc.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertil Steril. 2019;111(2):327–40. doi: 10.1016/j.fertnstert.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 5.Mikhaleva LM, Radzinsky VE, Orazov MR, Khovanskaya TN, Sorokina AV, Mikhalev SA, et al. Current Knowledge on Endometriosis Etiology: A Systematic Review of Literature. Int J Womens Health. 2021;13:525–37. doi: 10.2147/IJWH.S306135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riman T, Nilsson S, Persson IR. Review of epidemiological evidence for reproductive and hormonal factors in relation to the risk of epithelial ovarian malignancies. Acta Obstet Gynecol Scand. 2004;83(9):783–95. doi: 10.1111/j.0001-6349.2004.00550.x [DOI] [PubMed] [Google Scholar]
- 7.Huang T, Townsend MK, Wentzensen N, Trabert B, White E, Arslan AA, et al. Reproductive and Hormonal Factors and Risk of Ovarian Cancer by Tumor Dominance: Results from the Ovarian Cancer Cohort Consortium (OC3). Cancer Epidemiol Biomarkers Prev. 2020;29(1):200–207. doi: 10.1158/1055-9965.EPI-19-0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sainz de la Cuesta R, Eichhorn JH, Rice LW, Fuller AF Jr., Nikrui N, Goff BA. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol. 1996;60(2):238–44. doi: 10.1006/gyno.1996.0032 [DOI] [PubMed] [Google Scholar]
- 9.Anglesio MS, Yong PJ. Endometriosis-associated Ovarian Cancers. Clin Obstet Gynecol. 2017;60(4):711–27. doi: 10.1097/GRF.0000000000000320 [DOI] [PubMed] [Google Scholar]
- 10.Butova R, Vychytilova-Faltejskova P, Souckova A, Sevcikova S, Hajek R. Long Non-Coding RNAs in Multiple Myeloma. Noncoding RNA. 2019;5(1). doi: 10.3390/ncrna5010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–93. doi: 10.1126/science.1192002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–14. doi: 10.1016/j.molcel.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Zhu J, Wang F, Guan Z, Ge Y, Yang X, et al. LncRNAs and their role in cancer stem cells. Oncotarget. 2017;8(66):110685–92. doi: 10.18632/oncotarget.22161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini ES, Meryet-Figuiere M, Sabzalipoor H, Kashani HH, Nikzad H, Asemi Z. Dysregulated expression of long noncoding RNAs in gynecologic cancers. Mol Cancer. 2017;16(1):107. doi: 10.1186/s12943-017-0671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang WT, Sun YM, Huang W, He B, Zhao YN, Chen YQ. Genome-wide Long Non-coding RNA Analysis Identified Circulating LncRNAs as Novel Non-invasive Diagnostic Biomarkers for Gynecological Disease. Sci Rep. 2016;6:23343. doi: 10.1038/srep23343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee GH, Choi YM, Hong MA, Yoon SH, Kim JJ, Hwang K, et al. Association of CDKN2B-AS and WNT4 genetic polymorphisms in Korean patients with endometriosis. Fertil Steril. 2014;102(5):1393–7. doi: 10.1016/j.fertnstert.2014.07.1237 [DOI] [PubMed] [Google Scholar]
- 17.Bhan A, Soleimani M, Mandal SS. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017;77(15):3965–81. doi: 10.1158/0008-5472.CAN-16-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long X, Song K, Hu H, Tian Q, Wang W, Dong Q, et al. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J Exp Clin Cancer Res. 2019;38(1):345. doi: 10.1186/s13046-019-1329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Yang C, Li Y, Chen A, Li L, You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Biosci Rep. 2018;38(2). doi: 10.1042/BSR20171150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Liu M, Zou Y, Mao M, Shen T, Zhang C, et al. Long non-coding RNA growth arrest-specific transcript 5 is involved in ovarian cancer cell apoptosis through the mitochondria-mediated apoptosis pathway. Oncol Rep. 2015;34(6):3212–21. doi: 10.3892/or.2015.4318 [DOI] [PubMed] [Google Scholar]
- 21.Qiu YR, Zhao MY, Sun L, Yang BC, Hei KW, Du X, et al. Expression of IncRNA UCA1 in ovarian cancer and its clinical significance. Eur J Gynaecol Oncol. 2017;38(2):191–5. [PubMed] [Google Scholar]
- 22.Zhang L, Cao X, Zhang L, Zhang X, Sheng H, Tao K. UCA1 overexpression predicts clinical outcome of patients with ovarian cancer receiving adjuvant chemotherapy. Cancer Chemother Pharmacol. 2016;77(3):629–34. doi: 10.1007/s00280-016-2963-4 [DOI] [PubMed] [Google Scholar]
- 23.Ma N, Li S, Zhang Q, Wang H, Qin H, Wang S. Long non-coding RNA GAS5 inhibits ovarian cancer cell proliferation via the control of microRNA-21 and SPRY2 expression. Exp Ther Med. 2018;16(1):73–82. doi: 10.3892/etm.2018.6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo LL, Wang SF. Downregulated Long Noncoding RNA GAS5 Fails to Function as Decoy of CEBPB, Resulting in Increased GDF15 Expression and Rapid Ovarian Cancer Cell Proliferation. Cancer Biother Radiopharm. 2019;34(8):537–46. doi: 10.1089/cbr.2019.2889 [DOI] [PubMed] [Google Scholar]
- 25.Li J, Huang H, Li Y, Li L, Hou W, You Z. Decreased expression of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion, and indicates a poor prognosis in ovarian cancer. Oncol Rep. 2016;36(6):3241–50. doi: 10.3892/or.2016.5200 [DOI] [PubMed] [Google Scholar]
- 26.Pan J, Li X, Wu W, Xue M, Hou H, Zhai W, et al. Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 2016;382(1):64–76. doi: 10.1016/j.canlet.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 27.Xiao JN, Yan TH, Yu RM, Gao Y, Zeng WL, Lu SW, et al. Long non-coding RNA UCA1 regulates the expression of Snail2 by miR-203 to promote hepatocellular carcinoma progression. J Cancer Res Clin Oncol. 2017;143(6):981–90. doi: 10.1007/s00432-017-2370-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Shi X, Li C, Wang X, Hou K, Li Z, et al. Long non-coding RNA UCA1 upregulation promotes the migration of hypoxia-resistant gastric cancer cells through the miR-7-5p/EGFR axis. Exp Cell Res. 2018;368(2):194–201. doi: 10.1016/j.yexcr.2018.04.030 [DOI] [PubMed] [Google Scholar]
- 29.Li J, Wang M, Chen X. Long non-coding RNA UCA1 modulates cell proliferation and apoptosis by regulating miR-296-3p/Myc axis in acute myeloid leukemia. Cell Cycle. 2020;19(12):1454–65. doi: 10.1080/15384101.2020.1750814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang C, Yang Y, Guan J, Lv T, Qu S, Fu Q, et al. LncRNA UCA1 sponges miR-204-5p to promote migration, invasion and epithelial-mesenchymal transition of glioma cells via upregulation of ZEB1. Pathol Res Pract. 2018;214(9):1474–81. doi: 10.1016/j.prp.2018.07.036 [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Xiong JB, Zhang GY, Liu Y, Jie ZG, Li ZR. Long Noncoding RNA UCA1 Regulates PRL-3 Expression by Sponging MicroRNA-495 to Promote the Progression of Gastric Cancer. Mol Ther Nucleic Acids. 2020;19:853–64. doi: 10.1016/j.omtn.2019.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Li ZY, Wang XL, Dang Y, Zhu XZ, Zhang YH, Cai BX, et al. Long non-coding RNA UCA1 promotes the progression of paclitaxel resistance in ovarian cancer by regulating the miR-654-5p/SIK2 axis. Eur Rev Med Pharmacol Sci. 2020;24(2):591–603. doi: 10.26355/eurrev_202001_20035 [DOI] [PubMed] [Google Scholar]
- 33.Chang CY, Lai MT, Chen Y, Yang CW, Chang HW, Lu CC, et al. Up-regulation of ribosome biogenesis by MIR196A2 genetic variation promotes endometriosis development and progression. Oncotarget. 2016;7(47):76713–25. doi: 10.18632/oncotarget.11536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan Y, Qu K, Zhang QC, Flynn RA, Manor O, Ouyang Z, et al. Landscape and variation of RNA secondary structure across the human transcriptome. Nature. 2014;505(7485):706–9. doi: 10.1038/nature12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams C, Hoang L, Yosef A, Alotaibi F, Allaire C, Brotto L, et al. Nerve Bundles and Deep Dyspareunia in Endometriosis. Reprod Sci. 2016;23(7):892–901. doi: 10.1177/1933719115623644 [DOI] [PubMed] [Google Scholar]
- 36.Tanbo T, Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet Gynecol Scand. 2017;96(6):659–67. doi: 10.1111/aogs.13082 [DOI] [PubMed] [Google Scholar]
- 37.Ghafouri-Fard S, Taheri M. UCA1 long non-coding RNA: An update on its roles in malignant behavior of cancers. Biomed Pharmacother. 2019;120:109459. doi: 10.1016/j.biopha.2019.109459 [DOI] [PubMed] [Google Scholar]
- 38.Neve B, Jonckheere N, Vincent A, Van Seuningen I. Epigenetic Regulation by lncRNAs: An Overview Focused on UCA1 in Colorectal Cancer. Cancers (Basel). 2018;10(11). doi: 10.3390/cancers10110440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li GY, Wang W, Sun JY, Xin B, Zhang X, Wang T, et al. Long non-coding RNAs AC026904.1 and UCA1: a "one-two punch" for TGF-beta-induced SNAI2 activation and epithelial-mesenchymal transition in breast cancer. Theranostics. 2018;8(10):2846–61. doi: 10.7150/thno.23463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, Niu H, Qin Q, Yang S, Wang Q, Yu C, et al. lncRNA UCA1 Mediates Resistance to Cisplatin by Regulating the miR-143/FOSL2-Signaling Pathway in Ovarian Cancer. Mol Ther Nucleic Acids. 2019;17:92–101. doi: 10.1016/j.omtn.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CJ, Zhu CC, Xu J, Wang M, Zhao WY, Liu Q, et al. The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol Cancer. 2019;18(1):115. doi: 10.1186/s12943-019-1032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan J, Dai Q, Zhang T, Li C. Palmitate acid promotes gastric cancer metastasis via FABP5/SP1/UCA1 pathway. Cancer Cell Int. 2019;19:69. doi: 10.1186/s12935-019-0787-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robker RL, Hennebold JD, Russell DL. Coordination of Ovulation and Oocyte Maturation: A Good Egg at the Right Time. Endocrinology. 2018;159(9):3209–18. doi: 10.1210/en.2018-00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aardema H, van Tol HTA, Wubbolts RW, Brouwers J, Gadella BM, Roelen BAJ. Stearoyl-CoA desaturase activity in bovine cumulus cells protects the oocyte against saturated fatty acid stress. Biol Reprod. 2017;96(5):982–92. doi: 10.1095/biolreprod.116.146159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cong J, Li P, Zheng L, Tan J. Prevalence and Risk Factors of Infertility at a Rural Site of Northern China. PLoS One. 2016;11(5):e0155563. doi: 10.1371/journal.pone.0155563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):94–8. doi: 10.1016/j.ejogrb.2004.11.019 [DOI] [PubMed] [Google Scholar]
- 47.Shah DK, Correia KF, Vitonis AF, Missmer SA. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum Reprod. 2013;28(7):1783–92. doi: 10.1093/humrep/det120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi KW, Shin JH, Park MS, Kim T, Kim SH, Hur JY. Association of body mass index with severity of endometriosis in Korean women. Int J Gynaecol Obstet. 2009;105(1):39–42. doi: 10.1016/j.ijgo.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 49.Schenken RS. Modern concepts of endometriosis. Classification and its consequences for therapy. J Reprod Med. 1998;43(3 Suppl):269–75. [PubMed] [Google Scholar]