Abstract
Background
COVID‐19 is an infectious illness, featured by an increased risk of thromboembolism. However, no standard antithrombotic therapy is currently recommended for patients hospitalized with COVID‐19. The aim of this study was to evaluate safety and efficacy of additional therapy with aspirin over prophylactic anticoagulation (PAC) in patients hospitalized with COVID‐19 and its impact on survival.
Methods and Results
A total of 8168 patients hospitalized for COVID‐19 were enrolled in a multicenter‐international prospective registry (HOPE COVID‐19). Clinical data and in‐hospital complications, including mortality, were recorded. Study population included patients treated with PAC or with PAC and aspirin. A comparison of clinical outcomes between patients treated with PAC versus PAC and aspirin was performed using an adjusted analysis with propensity score matching. Of 7824 patients with complete data, 360 (4.6%) received PAC and aspirin and 2949 (37.6%) PAC. Propensity‐score matching yielded 298 patients from each group. In the propensity score‐matched population, cumulative incidence of in‐hospital mortality was lower in patients treated with PAC and aspirin versus PAC (15% versus 21%, Log Rank P=0.01). At multivariable analysis in propensity matched population of patients with COVID‐19, including age, sex, hypertension, diabetes, kidney failure, and invasive ventilation, aspirin treatment was associated with lower risk of in‐hospital mortality (hazard ratio [HR], 0.62; [95% CI 0.42–0.92], P=0.018).
Conclusions
Combination PAC and aspirin was associated with lower mortality risk among patients hospitalized with COVID‐19 in a propensity score matched population compared to PAC alone.
Keywords: anticoagulation, antiplatelet therapy, aspirin, COVID‐19, prognosis, risk prediction
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- COVID‐19
Coronavirus disease‐19
- PAC
prophylactic anticoagulation
Clinical Perspective
What Is New?
In a propensity score–matched population among patients hospitalized with COVID‐19, a combination of prophylactic anticoagulation and aspirin was associated with lower mortality risk compared with prophylactic anticoagulation alone.
Aspirin treatment was associated with lower risk of in‐hospital mortality at multivariable analysis including age, sex, hypertension, diabetes, renal failure, and invasive ventilation.
What Are the Clinical Implications?
Patients hospitalized with COVID‐19 may benefit from aspirin and prophylactic anticoagulation treatment in terms of reduction of mortality risk.
Randomized multicenter clinical trials are needed to confirm these preliminary findings.
COVID‐19 is an infectious illness, 1 with an increased risk of thromboembolism. The overall rate of venous thromboembolism and arterial thromboembolism among patients with COVID‐19 is 21% and 2%, respectively. 2
Therefore, this complication represents an important therapeutic target that could be prevented by antithrombotic therapy. Data on anticoagulation therapy in patients with COVID‐19 are still debatable. Randomized trials showed no benefit for therapeutic anticoagulation in critically ill patients, 3 , 4 while in non‐critically ill patients there are contrasting data on optimal anticoagulation strategy. 5 , 6 On the other side, antiplatelet therapy in patients with COVID‐19 could provide some benefit in terms of mortality reduction 7 and a large randomized trial (RECOVERY [Randomized evaluation of COVID‐19 Therapy]) showed that aspirin therapy is associated with a 1.2% absolute increase in survival. 8
The aim of this study was therefore to evaluate the impact on survival of prophylactic anticoagulation (PAC) with aspirin over PAC alone in patients hospitalized with COVID‐19 in an observational multicenter study after propensity score matching.
METHODS
The data that support the findings of this study are available upon reasonable request.
Study Design and Population
A total of 8168 patients were included in the HOPE COVID‐19 registry. After excluding patients with insufficient or not reliable data for the purposes of this analysis (n=344), 7824 patients were assessed and followed up in the multicenter international registry (HOPE COVID‐19, https://hopeprojectmd.com, NCT04334291).
The protocol was established by a scientific board from Italy, Spain, Ecuador, and Germany. Patients were enrolled from 7 countries (Spain, Italy, Ecuador, Cuba, Germany, China, Canada). 9
Detailed information about participating hospitals, investigators, collaborators, and the protocol are available on the website of the project (https://hopeprojectmd.com). All patients were diagnosed with COVID‐19 according to World Health Organization interim guidance, through polymerase chain reaction testing. All patients discharged (deceased or alive) from any hospital center were included in the Registry. For this report, patients discharged between January 16, 2020 and May 30, 2020 were considered for the analysis.
All patients gave a written informed consent for the participation in the registry. The local ethics committee approved this study and was consistent with the guidelines of Helsinki. All local principal investigators reviewed the draft and checked for the accuracy and veracity of data.
Data Extraction
Epidemiological, clinical, and outcome data were manually extracted from electronic medical records and evaluated by medical researchers.
Each definition of clinical outcomes was recorded and checked by at least 2 medical doctors in each hospital. Patient’s data were anonymized, and the electronic data were stored and/or filled in an encrypted, password‐protected computer/website.
Throat swab samples were obtained from all patients at admission and tested using real‐time reverse transcriptase–polymerase chain reaction assays according to the World Health Organization recommendation. Additionally, patient’s clinical and laboratory data and imaging test were extracted. All drugs at admission and previous to hospitalization were recorded. Additional information on data records have been provided in a previous article from this research group. 10
Anticoagulation therapy: patients were included if treated during hospitalization with therapeutic or prophylactic dose including oral, subcutaneous, or IV forms. Therapeutic dose included oral anticoagulants, unfractionated or low molecular weight heparin (1 mg/kg daily); Prophylactic dose included patients receiving low molecular weight heparin 40 mg daily.
Aspirin therapy: patients were included if treated during all the hospitalization, since admission, with aspirin 100 mg (either oral or IV). Patients who started aspirin after 24 hours since admission were excluded from the analysis. Additional data on other antiplatelets drugs as clopidogrel, ticlopidine, prasugrel, ticagrelor were also recorded. Patients receiving other antiplatelets drugs than aspirin were excluded from this analysis.
Major bleeding was defined as (1) bleeding associated with hemoglobin <7 g/dL or any red blood cell transfusion, or (2) bleeding associated with at least 2 units of red blood cell transfusion within 48 hours. 11
Outcome and End Point
We considered as primary end point all‐cause mortality during hospitalization. Other events were recorded, such as invasive mechanical ventilation, non‐invasive mechanical ventilation, respiratory insufficiency, heart failure, renal failure, bleeding, sepsis, and embolic events. Events were allocated following Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID‐19). 12
Statistical Analysis
Group statistics are presented as means±SD for continuous variables and as frequency (%) for categorical variables. For group comparisons, Student t test or the Mann‐Whitney U‐test were used for continuous variables with normal and non‐normal distributions, respectively; Pearson chi‐squared‐test or Fisher's exact test were used for categorical variables.
A propensity score for receiving aspirin and PAC was calculated from the overall sample based on variables deemed as clinically relevant for the outcome that were available from the registry: age, sex, hypertension, diabetes, renal failure, and invasive ventilation. A 1:1 propensity matched sample was selected of patients who had prophylactic anticoagulation only with patients who had prophylactic anticoagulation and aspirin therapy. A 0.025% score discordance was considered as acceptable for propensity matching and coupling; the nearest neighbor matching method was used for propensity matching.
Survival was plotted on Kaplan‐Meier curves and assessed with Log‐rank test. Relative risk with 95% CI was calculated. Factors included in propensity matching (statistically significant at univariable analysis or clinically relevant) were also entered into Cox’ multivariable regression analysis model to define the risk of in‐hospital mortality.
Statistical analysis was performed with SPSS statistics 26.0. A P<0.05 was considered as statistically significant, all tests were 2‐sided.
RESULTS
Baseline
Between January 16, 2020 until May 30, 2020, 8168 patients were included in the HOPE COVID‐19 registry. After excluding patients with insufficient or not reliable data for the purposes of this analysis (n=344), 7824 patients were assessed for the present manuscript.
Mean (and SD) age of patients was 64±16 years, 58% (n=4578) were male. Mean (and SD) hospital stay was 11±9 days and follow‐up duration was 19±17 days. Several comorbidities were prevalent as hypertension (48%), diabetes (19%), and obesity with a body mass index >30 (19%). Moreover, 22% of patients had history of heart disease and 18% of lung disease. History of cancer had a prevalence of 13% (Table 1).
Table 1.
Baseline Clinical Features of Patients Hopitalized With COVID‐19, Overall Population, Patients Receiving Prophylactic Anticoagulation With or Without Aspirin
| General population | Prophylactic anticoagulation | Prophylactic anticoagulation and aspirin | P value | |
|---|---|---|---|---|
| No. patients | 7824 | 2949 | 360 | |
| Age,y (means+SD) | 64±16 | 64±16 | 73±11 | <0.01* |
| Male | 58% | 58% | 69% | <0.01* |
| Baseline clinical profile | ||||
| Hypertension | 48% | 45% | 81% | <0.01* |
| Diabetes | 19% | 19% | 38% | <0.01* |
| Obesity (BMI >30) | 19% | 20% | 23% | 0.18 |
| Chronic kidney disease (Cl‐Cr <30 mL/min) | 6%* | 5% | 13% | <0.01* |
| History of lung disease | 18% | 17% | 26% | <0.01* |
| History of heart disease | 22% | 13% | 66% | <0.01* |
| History of cancer | 13% | 12% | 17% | <0.01* |
| In‐hospital complications | ||||
| Thromboembolic events | 2.6% | 2% | 3% | 0.52 |
| Bleeding | 2.3% | 2% | 2% | 0.57 |
| Invasive ventilation | 8.4% | 10% | 9% | 0.85 |
| Death | 18% | 17% | 18% | 0.86 |
BMI indicates body mass index; Cl‐Cr, clearance of creatinine; and PAC, prophylactic anticoagulation.
Indicates statistically significant.
Antithrombotic Therapy During Hospitalization
A total of 4503 (57.5%) patients received anticoagulation therapy, 3327 (73.8%) prophylactic anticoagulation, 1016 (22.5%) therapeutic anticoagulation, and 149 (3.3%) oral anticoagulation (98 warfarin, 51 novel oral anticoagulants).
A total of 730 (9.3%) patients received antiplatelet therapy during hospitalization, and 680 patients (93%) received single antiplatelet drug (645 pts aspirin, 33 pts clopidogrel and one ticlopidine and ticagrelor respectively). Fifty patients (7%) received dual antiplatelet drug (35 pts aspirin and clopidogrel, 10 pts aspirin and ticagrelor, 5 pts aspirin and prasugrel).
Aspirin and Prophylactic Anticoagulation Versus Prophylactic Anticoagulation
After the exclusion of patients who did not receive any anticoagulation and/or aspirin, and those patients who received therapeutic anticoagulation, 3309 patients were left. Three hundred and sixty (4.6%) patients received PAC and aspirin and 2949 (37.6%) patients PAC.
Patients treated with PAC and aspirin were older (73±11 versus 64±16 years, P=0.01), had higher prevalence of male sex (69% versus 58%, P=0.01), of comorbidities as diabetes (38% versus 19%, P=0.01), hypertension (81% versus 45% P=0.01), and history of cardiovascular disease (66% versus 13% P=0.01) (Table 1).
Through propensity score matching, the analysis was restricted to 596 patients, 298 (out of 360, 83%) treated with PAC and aspirin and 298 (out of 2949, 10%) with PAC. Mean age was 73±11 years, 66% to 67% were male, prevalence of hypertension and diabetes was 79% and 33% to 35%, respectively, and 7% underwent invasive ventilation (Tables S1 through S3). Cumulative mortality incidence was lower at hospital discharge in the PAC and aspirin group compared to PAC alone (15% versus 21%, Log Rank P=0.012, Figure).
Figure 1. Survival curves according to prophylactic anticoagulation only vs prophylactic anticoagulation and aspirin (HR, 0.62; [95% CI 0.42–0.95], log rank test, P=0.01).
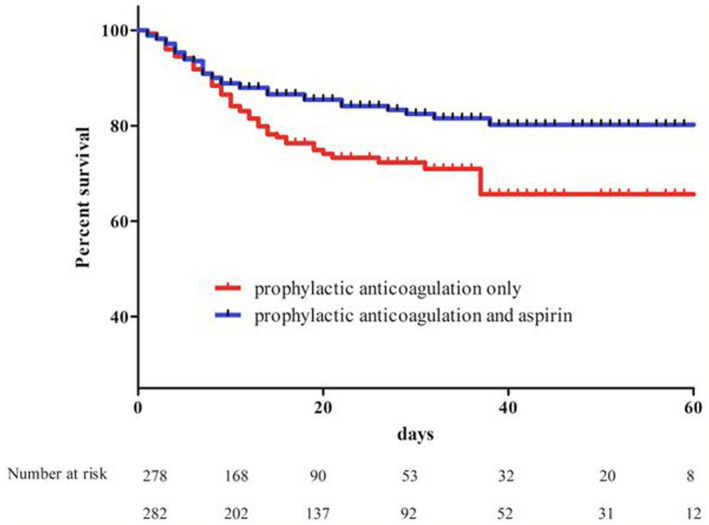
HR indicates hazard ratio.
At multivariable Cox’ regression analysis in propensity matched population of patients with COVID‐19 including age, sex, aspirin treatment, hypertension, diabetes, renal failure, and invasive ventilation, aspirin treatment was associated with lower risk of in‐hospital mortality (hazard ratio [HR], 0.62; [95% CI 0.42–0.95], P=0.018, Table 2).
Table 2.
Predictors of All‐Cause Death at Multivariable Cox Regression Analysis in Propensity‐Matched Population of Patients with COVID‐19 Receiving Prophylactic Anticoagulation With or Without Aspirin
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Aspirin treatment | 0.62 | 0.42–0.92 | 0.02 |
| Age | 1.07 | 1.05–1.10 | <0.01 |
| Male | 2.65 | 1.65–4.23 | <0.01 |
| Hypertension | 1.18 | 0.70–1.98 | 0.53 |
| Diabetes | 0.70 | 0.45–1.08 | 0.11 |
| Renal failure | 1.43 | 0.76–2.67 | 0.26 |
| Invasive ventilation | 3.65 | 2.07–6.44 | <0.01 |
DISCUSSION
We report data about aspirin and anticoagulation therapy from a large multi‐center international registry of patients hospitalized with COVID‐19. We found that:
Approximately 4.6% patients received prophylactic anticoagulation and aspirin.
In a propensity score matched population of patients hospitalized with COVID‐19, additional therapy with aspirin over only PAC treatment was associated with lower cumulative incidence in‐hospital mortality rates.
At multivariable analysis including age, sex, and cardiovascular risk factors aspirin treatment was associated with lower risk of in‐hospital mortality.
COVID‐19 is a multi‐system disorder featured by respiratory insufficiency, endothelial cell dysfunction and hypercoagulability. 13 Coronavirus enters human cells mainly by binding the angiotensin‐converting enzyme 2, which is expressed in lung alveolar cells, vascular endothelial cells, cardiac myocytes, and other cells. 14
Autopsy studies found thromboembolic lesions in several organs with platelet‐fibrin rich thrombi in the pulmonary, renal, hepatic, and cardiac micro‐vasculature. 15 Thrombi were mainly located in pulmonary arteries and arterioles and in micro‐vessels. Moreover, in heart autopsy examination, myocyte necrosis was mainly due to microthrombi with higher prevalence of fibrin and c5b‐9 complement. 16 Therefore, COVID‐19 has been also defined an endothelial disease. 17
In this context, anticoagulation therapy with heparin/low molecular weight heparin, additionally to potential benefit for thrombosis prevention, could have some potential benefits. First reducing viral entry, interacting with SARS‐CoV‐2 spike glycoprotein; second inhibiting heparanase activity, which is associated with disease severity; Third neutralizing the biological effect of chemokines, and cytokines. Through this action it may impact on leukocyte recruitment and trafficking to sites of inflammation, either via neutralization of chemokine, and cytokines or through direct interaction with leukocyte cell surface ligands. 18
Due to this finding several trials have been performed to evaluate the efficacy of anticoagulation therapy in different setting of patients with COVID‐19. Regarding severe illness, therapeutic doses of anticoagulation did not show benefit in term of mortality reduction in randomized trials. 3 , 4 However, among moderately ill patient with COVID‐19, the role of anticoagulation is still debatable. 5 , 6
Lopes et al enrolled 615 patients hospitalized with COVID‐19 with elevated D‐dimer concentration that were randomly assigned (1:1) to either therapeutic or prophylactic anticoagulation. Therapeutic anticoagulation was in‐hospital oral rivaroxaban (20 or 15 mg daily) for stable patients, or unfractionated or low molecular weight heparin for clinically unstable patients, followed by rivaroxaban to day 30. In‐hospital therapeutic anticoagulation did not improve clinical outcomes and was associated with increased risk of bleeding compared to prophylactic anticoagulation. 6
Lawer et al enrolled 2219 patients hospitalized with COVID‐19 with moderate disease (defined as increased levels of D‐dimers, 2 times higher than normal values, and no need for intensive care unit‐level care) and randomized to therapeutic (unfractionated or low molecular weight heparin) or prophylactic anticoagulation. About 12% of both groups were taking concomitant antiplatelet therapy. Authors found that an initial strategy of therapeutic‐dose anticoagulation with heparin was associated with reduced use of cardiovascular or respiratory organ support when compared to usual‐care thromboprophylaxis. However, major bleeding occurred in 1.9% of the patients treated with therapeutic‐dose anticoagulation and in 0.9% with those receiving thromboprophylaxis.
On the other side antiplatelet therapy could be an additional therapeutic target to prevent or treat microthrombi in patients with COVID‐19. Additionally, aspirin irreversibly inhibits cyclooxygenase, has anti‐viral properties and attenuates the pro‐inflammatory effects of the nuclear transcription factor kappa B, which has been implicated as having a critical role in the cytokine storm that occurs with COVID‐19. 19 It may also have pleiotropic effects on the endothelium which could target potential development of endotheliitis, a potential complication for severe COVID‐19. 20
Antiviral properties of antiplatelet therapy are mainly related with their potential activity of suppressing platelet activation. Indeed, in several models of tissue injury during virus infection (influenza A virus, Dengue, HIV‐1, SARS), severe inflammation is driven by unsuppressed platelet activation. 21
A randomized trial (RECOVERY) evaluated the efficacy of aspirin in COVID‐19, enrolling 7351 patients. Authors found that aspirin therapy was associated with a 1.2% increase in survival, with a 0.6% reduction in thrombotic events and an increase in major bleeding events of 0.6%. 8 Our research group in an observational registry of 7824 patients, found that antiplatelet treatment was associated with lower mortality risk and shorter duration of mechanical ventilation. 7 Additionally in the same study when excluding anticoagulated patients, there was a significant benefit of antiplatelet therapy for mortality reduction. 7
Clinical data on prophylactic heparin and aspirin therapy among patients with COVID‐19 have been published only among critically ill patients. Pavoni et al evaluated 42 patients with COVID‐19 admitted to intensive care unit that were treated with aspirin and PAC if D‐dimer were <3000 ng/mL or aspirin and therapeutic heparin if D‐dimer serum levels were higher than 3000 ng/mL. Authors didn’t find any difference in term of survival between these therapeutic approaches. 22
In the present study we evaluate a subset of frail patients, with mean age of 73 years, higher prevalence of male sex (67%) and cardiovascular risk factors as hypertension (79%) and diabetes (33%).
Actually, in the study analysis population all patients were taking prophylactic anticoagulation and at multivariable analysis aspirin therapy was associated with a 38% risk reduction of mortality.
Combination of PAC and aspirin, with aspirin early started during admission, when compared to PAC alone, showed benefit in term of mortality reduction during hospitalization. Therefore, PAC and aspirin may represent a possible therapeutic approach for patients hospitalized with COVID‐19. A careful evaluation of thromboembolic and bleeding risk is needed. Due to the high rate of mayor cardiovascular adverse events following COVID‐19 infection, 23 randomized, double blinded, adequately powered trials, are surely needed to finally address the question.
CONCLUSIONS
Aspirin combined with prophylactic anticoagulation when compared to prophylactic anticoagulation was associated with lower mortality risk. Randomized trials are needed to confirm these preliminary data.
Limitations
The present study is a multi‐center international prospective registry and patients were included during the first months of pandemic; thus, the type of SARS‐Coronavirus and the therapeutic protocols are probably different form current variants and standard therapies.
No patient was randomized to different alternative therapies. Combination of Aspirin and PAC were left to operator choice; Therefore, evaluation of potential benefit was performed with a retrospective analysis.
Most of the patients have 28‐days follow‐up and no conclusion can be done for long‐term benefit.
Cause of death was not specified and analyzed, so cardiovascular deaths cannot be split from any cause deaths.
Sources of Funding
This work was supported by an unconditioned grant (Fundacion Interhospitalaria para la Investigacion Cardiovascular [FIC] Madrid, Spain). This nonprofit institution had no role in the study design; in the collection, analysis, interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication. This paper has been published with the financial support of the Dept. of Medical and Surgical Sciences of the University of Foggia, Foggia, Italy.
Disclosures
None.
Supporting information
Tables S1–S3
For Sources of Funding and Disclosures, see page 7.
References
- 1. Zhu NA, Zhang D, Wang W, Li X, Yang BO, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID‐19 is high and associated with a higher risk of mortality: a systematic review and meta‐analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazloomzadeh S, Khaleghparast S, Ghadrdoost B, Mousavizadeh M, Baay MR, Noohi F, Sharifnia H, Ahmadi A, Tavan S, Malekpour Alamdari N, et al. Effect of intermediate‐dose vs standard‐dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID‐19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, Carrier M, Reynolds HR, Kumar A, Turgeon AF, et al.; REMAP‐CAP Investigators; ACTIV‐4a Investigators; ATTACC Investigators . Therapeutic anticoagulation in critically ill patients with Covid‐19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, Gong MN, Carrier M, Rosenson RS, Reynolds HR, et al.; The ATTACC, ACTIV‐4a, and REMAP‐CAP Investigators* Therapeutic anticoagulation in non‐critically ill patients with Covid‐19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopes RD, de Barros e Silva PGM, Furtado RHM, Macedo AVS, Bronhara B, Damiani LP, Barbosa LM, de Aveiro Morata J, Ramacciotti E, de Aquino Martins P, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID‐19 and elevated D‐dimer concentration (ACTION): an open‐label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santoro F, Nuñez‐Gil IJ, Vitale E, Viana‐Llamas MC, Reche‐Martinez B, Romero‐Pareja R, Feltez Guzman G, Fernandez Rozas I, Uribarri A, Becerra‐Muñoz VM, et al. Antiplatelet therapy and outcome in COVID‐19: the Health Outcome Predictive Evaluation Registry. Heart. 2022;108:130–136. doi: 10.1136/heartjnl-2021-319552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. RECOVERY Collaborative Group . Aspirin in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet. 2022;399:143–151. doi: 10.1016/S0140-6736(21)01825-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Núñez‐Gil IJ, Estrada V, Fernández‐Pérez C, Feltes G, Vedia O, Vergara‐Uzcategui CE, Moreno‐Menguía VH, Cerrato E, D'Ascenzo F, Raposeiras‐Roubin S, et al. Health Outcome Predictive Evaluation for COVID 19 international registry (HOPE COVID‐19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. doi: 10.1016/j.conctc.2020.100654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santoro F, Núñez‐Gil IJ, Viana‐Llamas MC, Maroun Eid C, Romero R, Fernández Rozas I, Aparisi A, Becerra‐Muñoz VM, García Aguado M, Huang J, et al. Anticoagulation therapy in patients with coronavirus disease 2019: results from a multicenter international prospective registry (Health Outcome Predictive Evaluation for Corona Virus Disease 2019 [HOPE‐COVID19]). Crit Care Med. 2021;49:e624–e633. doi: 10.1097/CCM.0000000000005010 [DOI] [PubMed] [Google Scholar]
- 11. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 12. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID‐19). Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazzeffi MA, Chow JH, Tanaka K. COVID‐19 associated hypercoagulability: manifestations, mechanisms, and management. Shock. 2021;55:465–471. doi: 10.1097/SHK.0000000000001660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, et al. Megakaryocytes and platelet‐fibrin thrombi characterize multi‐organ thrombosis at autopsy in COVID‐19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, Nasr A, Kutys R, Guo L, Cornelissen A, et al. Microthrombi as a major cause of cardiac injury in COVID‐19: a pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828 [DOI] [PubMed] [Google Scholar]
- 17. Mei H, Luo L, Hu Y. Thrombocytopenia and thrombosis in hospitalized patients with COVID‐19. J Hematol Oncol. 2020;13:161. doi: 10.1186/s13045-020-01003-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buijsers B, Yanginlar C, Maciej‐Hulme ML, de Mast Q, van der Vlag J. Beneficial non‐anticoagulant mechanisms underlying heparin treatment of COVID‐19 patients. EBioMedicine. 2020;59:102969. doi: 10.1016/j.ebiom.2020.102969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kopp E, Ghosh S. Inhibition of NF‐kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854 [DOI] [PubMed] [Google Scholar]
- 20. Florêncio FKZ, Tenório MO, Macedo Júnior ARA, Lima SG. Aspirin with or without statin in the treatment of endotheliitis, thrombosis, and ischemia in coronavirus disease. Rev Soc Bras Med Trop. 2020;53:e20200472. doi: 10.1590/0037-8682-0472-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller‐Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393 [DOI] [PubMed] [Google Scholar]
- 22. Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Venous thromboembolism and bleeding in critically ill COVID‐19 patients treated with higher than standard low molecular weight heparin doses and aspirin: a call to action. Thromb Res. 2020;196:313–317. doi: 10.1016/j.thromres.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie Y, Xu E, Bowe B, Al‐Aly Z. Long‐term cardiovascular outcomes of COVID‐19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


