Abstract
Background
Rheumatic heart disease (RHD) takes a heavy toll in low‐ and middle‐income countries. We aimed to present worldwide estimates for the burden of the RHD during 1990 to 2019 using the GBD (Global Burden of Disease) study.
Methods and Results
Sociodemographic index (SDI) and age‐period‐cohort analysis were used to assess inequity. The age‐standardized death, disability‐adjusted life years, incidence, and prevalence rates of RHD were 3.9 (95% uncertainty interval, 3.3–4.3), 132.9 (95% uncertainty interval, 115.0–150.3), 37.4 (28.6–46.7), and 513.7 (405.0–636.3) per 100 000 in 2019, respectively. The age‐standardized incidence and prevalence rates increased by 14.4% and 13.8%, respectively. However, disability‐adjusted life years and death rates decreased by 53.1% and 56.9%, respectively. South Asia superregion had the highest age‐standardized disability‐adjusted life years and deaths. Sub‐Saharan Africa had the highest age‐standardized incidence and prevalence rates. There was a steep decline in RHD burden among higher‐SDI countries. However, only age‐standardized deaths and disability‐adjusted life years rates decreased in lower‐SDI countries. The age‐standardized years of life lost and years lived with disability rates for RHD significantly declined as countries' SDI increased. The coefficients of birth cohort effect on the incidence of RHD showed an increasing trend from 1960 to 1964 to 2015 to 2019; however, the birth cohort effect on deaths attributable to RHD showed unfailingly decreasing trends from 1910 to 1914 to 2015 to 2019.
Conclusions
There was a divergence in the burden of RHD among countries based on SDI levels, which calls for including RHD in global assistance and funding. Indeed, many countries are still dealing with an unfinished infectious disease agenda, and there is an urgency to act now to prevent an increase in future RHD burden.
Keywords: disability‐adjusted life years, global burden of disease, heart, rheumatic fever, rheumatic heart disease
Subject Categories: Epidemiology, Primary Prevention, Race and Ethnicity, Women, Secondary Prevention
Nonstandard Abbreviations and Acronyms
- ASDR
age‐standardized death rate
- ASIR
age‐standardized incidence rate
- ASPR
age‐standardized prevalence rate
- GBD
Global Burden of Disease
- RHD
rheumatic heart disease
- SDI
sociodemographic index
- WHO
World Health Organization
- YLDs
years lived with disability
- YLLs
years of life lost
Clinical Perspective
What Is New?
The incidence and prevalence rates of rheumatic heart disease (RHD) increased globally, whereas death rates showed declining trend during the study period; RHD continues to take a heavy toll on far too many people in lower‐income countries.
Quantifying the burden of RHD with epidemiologic measures in this study, we provided a clearer picture of the inequity patterns in the RHD status by age, sex, country, and socioeconomic status, which have mainly been underestimated by the health authorities.
There are fundamental disparities among countries and territories with disparate socioeconomic status on RHD, making it among the most neglected diseases relative to burden worldwide.
What Are the Clinical Implications?
Until there is an effective vaccine for RHD, a multilevel approach is suggested, which includes primordial prevention via improving the socioeconomic status of populations at risk, primary prevention via treating patients with strep throat using benzathine–penicillin‐G, secondary prevention via antibiotic prophylaxis among patients with previous rheumatic fever or RHD, and tertiary prevention via medical/surgical treatment of RHD complications.
In settings with limited resources, the physicians' task for an antibiotic prescription could be shifted to community health workers, who could play a key role in delivering preventive medications via practical clinical algorithms, diagnostic tools, availability of appropriate antibiotics, and supportive supervision.
Rheumatic heart disease (RHD), a known complication following acute rheumatic fever, 1 remains to be the most acquired heart disease among people aged <25 years. 2 RHD is usually associated with overcrowding, poor housing conditions, and improper health literacy. 3 High sociodemographic index (SDI) countries have managed to reduce RHD incidence via significant elimination of acute rheumatic fever occurrence 4 and its recurrence. 5 Nevertheless, RHD still affects older adults, immigrants, marginalized, and underprivileged populations in high‐SDI countries. The achievements in lowering RHD incidence are known to be attributable to improvements in socioeconomic status, 4 better performance of health care systems, implementation of control programs, 6 and the widespread use of penicillin G benzathine to treat streptococcal pharyngitis. 7 Despite improvements in high‐SDI countries, low and low‐middle SDI countries have been less successful in lowering the burden of RHD. 8
The World Health Organization (WHO) proposed prevention and treatment guidelines for RHD >6 decades ago. 9 Previous calls for advocacy, action, and investment to reduce the RHD burden have been neglected. 3 Moreover, there was a sharp decrease in focus on RHD after apparent RHD elimination in high‐SDI countries, 10 and even the Global Rheumatic Heart Disease Control Program, coordinated by WHO since 1984, ended in the early 2000s. 11 Nevertheless, novel regional and international initiatives against RHD have emerged. The World Heart Federation has set a 2025 RHD reduction target, some low‐ and middle‐SDI countries implemented national programs for RHD prevention and control, 12 , 13 and the World Health Assembly endorsed a global resolution on RHD in 2018. 14
Prioritizing investment and providing proper action plans require accurate global, regional, and national burden estimates. The objective of this study was to present worldwide estimates of RHD prevalence, incidence, death, years of life lost (YLLs), years lived with disability (YLDs), and disability‐adjusted life years (DALYs) from 1990 to 2019 by age groups and SDI quintiles using GBD (Global Burden of Disease) 2019. 15
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Data Source
The data of GBD were used in the study, which includes high‐quality estimations on vital epidemiological measures, including prevalence, incidence, death, YLLs, YLDs, and DALYs for 286 causes of death, 369 diseases and injuries, and 87 risk factors in 204 countries and territories. GBD classified countries and territories into 7 superregions and 21 regions. The 7 superregions are high income; Latin America and Caribbean; Sub‐Saharan Africa; North Africa and Middle East; Southeast Asia, East Asia, and Oceania; South Asia; and Central Europe, Eastern Europe, and Central Asia. 16 RHD data were extracted from GBD 2019: GBD code: B.2.1; International Classification of Diseases, Tenth Revision (ICD‐10), codes I01 to I01.9, I02.0, and I05 to I09.9 were used for mapping mortality and new cases. 17 Sources used in the study are presented in Table S1.
Fatal Estimates
The vital registration system and surveillance data were transformed and modeled to provide the cause‐specific death estimates. Furthermore, data were mapped to the GBD list of disease causes. To enhance the comparability of death data sources, reclassification and redistribution of codes that are nonspecific or unspecific were performed. In addition, a regression analysis using Bayesian geospatial regression software (Cause of Death ensemble model) was considered to model deaths from RHD. Eight selected covariates for Cause of Death ensemble models of RHD were defined, including RHD summary exposure value scalar (+1 direction), improved water (−1 direction), malnutrition (+1 direction), sanitation (−1 direction), health care access and quality index (−1 direction), lag‐distributed income per capita (−1 direction), SDI (−1 direction), and education via years per capita (−1 direction). Finally, the single cause death estimates were adjusted by applying CoDCorrect algorithm. YLLs were calculated using normative global life expectancy and the number of deaths by age.
Nonfatal Estimates
To provide estimates on the incidence and prevalence of RHD, data were collected using the existing scientific reports on cohorts, registries, population surveys, health system administrative data, and inpatient/outpatient claims data. RHD was defined by clinical diagnosis, and estimates of RHD included cases identified by clinical history and physical examination, including auscultation or standard echocardiographic criteria. Consistent disease estimates were produced by using epidemiologic state‐transition disease modeling software, DisMod‐MR, and Bayesian meta‐regression software, MR‐BRT. Two covariates were selected, including RHD summary exposure value scalar and lag‐distributed income per capita. The survival of RHD was modeled using death/incidence ratios across various geographical locations and age groups. The 10‐year prevalence was then calculated for each incidence cohort. YLDs were calculated by multiplying each sequela's prevalence by its disability weight (0.049; 95% CI, 0.031–0.072) and by adding the procedure‐related morbidity associated with RHD treatment. RHD DALYs were calculated by summing YLDs and YLLs.
Decomposition Investigation
The contribution of population growth, aging, and variations in age‐specific incidence rates to the witnessed new cases changes was investigated. In the first step, the age structure and age‐specific incidence rate of RHD in 1990 were applied to the population size of 2019. In the second step, the age structure and age‐specific incidence rate of RHD in 2019 were applied to the population size of 1990. The difference between the new cases, as calculated in the first and second steps, was considered the contribution of age structure changes from 1990 to 2019. The difference between the new cases in the first step and the actual new cases in 1990 was attributed to the population growth. The differences between the value of the second step and the actual new cases in 2019 were attributed to the changes in age‐specific incidence rates. 18
Inequity Pattern
Countries were categorized using SDI to investigate the burden of RHD based on development status. 19 SDI of countries was basically calculated using 3 factors: (1) average income per person, (2) educational attainment, and (3) total fertility rate. SDI scores were categorized into 5 quintiles, including high SDI, high‐middle SDI, middle SDI, low‐middle SDI, and low SDI.
Age‐period‐cohort analysis with the intrinsic estimator method was performed to decompose the effects of 3 collinear factors named age, period, and cohort. Data were stratified in 22 birth cohorts starting from 1910 to 1914, 6 5‐year calendar periods starting from 1990, and 17 5‐year age groups starting from age <5 years to age ≥80 years. 20
Statistical Analysis
Age standardization was conducted using the direct method, applying a global age structure from the year 2019. Age‐standardized rates of RHD for countries were calculated using the GBD world population standard and reported per 100 000 individuals. The 95% uncertainty intervals (95% UIs) were reported for each metric using 2.5% and 97.5% quintiles across 1000 draws. The comparison for the differences in values of each metric from 1990 to 2019 was computed to calculate the total and percentage of change. All essential data analysis, tables, and illustrations were performed using R statistical package v3.4.3 (http://www.r‐project.org, RRID: SCR_001905). The "apc‐ie" command in STATA software was used for age‐period‐cohort effect analysis.
Ethical Approval and Consent to Participate
Not applicable.
Results
Global and Superregional Burden of RHD
On global scale, RHD caused 305 651 (95% UI, 259 220–340 486) deaths in 2019: 173 933 (140 652–208 089) among women and 131 717 (113 445–159 904) among men. Between 1990 and 2019, there was a 15.6% (−30.5% to −2.1%) decrease in the number of deaths attributable to RHD: 17.2% (−34.2% to 3.1%) decrease among women and 13.4% (−30.9% to 8.0%) among men (Table 1). The age‐standardized death rate (ASDR) of RHD at the global level decreased from 8.9 (8.0–10.1) per 100 000 population in 1990 to 3.9 (3.3–4.3) per 100 000 population in 2019. Although the trend of ASDRs of RHD from 1990 to 2019 was decreasing among both men and women, the rates were slightly higher among women than men (Figure 1 and Table 1). Considering the GBD superregions, the ASDRs of RHD were unfailingly highest among countries in South Asia superregion with 11.2 (8.6–13.4) in 100 000 population in 2019, and lowest among countries in Latin America and Caribbean, with 1.1 (1.0–1.2) in 100 000 population in 2019 (Table 2).
Table 1.
Global Burden of RHD in 1990 and 2019
| Measure | Age (metric) | Year | % Change (1990–2019) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 2019 | |||||||||
| Both | Women | Men | Both | Women | Men | Both | Women | Men | ||
| Incidence | All ages, n | 1 863 318 (1 438 465 to 2 308 707) | 1 000 723 (778 585 to 1 235 154) | 862 595 (663 211 to 1 075 583) | 2 789 443 (2 153 319 to 3 454 256) | 1 490 770 (1 159 323 to 1 837 879) | 1 298 673 (995 189 to 1 617 204) | 49.7 (47.1 to 52.2) | 49.0 (46.1 to 52.0) | 50.6 (47.9 to 53.2) |
| Age‐standardized, rate per 100 000 | 32.7 (25.8 to 40.0) | 35.5 (28.2 to 43.4) | 29.8 (23.4 to 36.7) | 37.4 (28.6 to 46.7) | 40.6 (31.1 to 50.4) | 34.3 (26.3 to 42.9) | 14.4 (11.2 to 17.0) | 14.1 (10.6 to 17.1) | 15.1 (12.1 to 17.7) | |
| Prevalence | All ages, n | 23 756 847 (18 791 683 to 29 295 709) | 13 099 934 (10 421 455 to 16 035 909) | 10 656 912 (8 363 711 to 13 199 602) | 40 502 345 (32 052 904 to 50 062 426) | 22 519 239 (17 895 086 to 27 713 122) | 17 983 106 (14 198 981 to 22 406 039) | 70.5 (66.6 to 74.2) | 71.9 (67.5 to 76.1) | 68.7 (65.1 to 72.7) |
| Age‐standardized, rate per 100 000) | 451.6 (363.3 to 552.5) | 501.6 (405.5 to 609.7) | 400.5 (321.4 to 492.4) | 513.7 (405.0 to 636.3) | 572.2 (450.7 to 705.5) | 455.2 (359.2 to 566.2) | 13.8 (11.0 to 16.0) | 14.1 (10.9 to 16.7) | 13.7 (11.2 to 15.8) | |
| Deaths | All ages, n | 362 160 (326 259 to 408 222) | 210 057 (185 677 to 238 266) | 152 103 (125 111 to 184 940) | 305 651 (259 220 to 340 486) | 173 933 (140 652 to 208 089) | 131 717 (113 445 to 159 904) | −15.6 (−30.5 to −2.1) | −17.2 (−34.2 to 3.1) | −13.4 (−30.9 to 8.0) |
| Age‐standardized, rate per 100 000 | 8.9 (8.0 to 10.1) | 9.7 (8.5 to 11.0) | 8.2 (6.7 to 10.0) | 3.9 (3.3 to 4.3) | 4.1 (3.3 to 4.8) | 3.6 (3.1 to 4.4) | −56.9 (−64.7 to −49.8) | −58 (−67.1 to −47.5) | −55.8 (−65 to −44.9) | |
| DALYs | All ages, n | 13 168 339 (11 896 460 to 14 634 663) | 7 420 707 (6 565 305 to 8 275 336) | 5 747 632 (4 827 059 to 6 847 812) | 10 673 882 (9 207 379 to 12 121 608) | 5 840 425 (4 813 977 to 6 963 022) | 4 833 457 (4 121 949 to 5 729 105) | −18.9 (−30.6 to −7.7) | −21.3 (−34.3 to −5.2) | −15.9 (−29.9 to 0.2) |
| Age‐standardized, rate per 100 000 | 283.3 (255.9 to 315.2) | 312.7 (276.9 to 349.8) | 253.7 (211.8 to 304) | 132.9 (115.0 to 150.3) | 142.0 (117.5 to 168.6) | 123.5 (105.8 to 145.9) | −53.1 (−60.0 to −46.4) | −54.6 (−62.2 to −45.3) | −51.3 (−59.8 to −41.8) | |
| YLLs | All ages, n | 12 010 791 (10 869 595 to 13 434 295) | 6 783 119 (5 961 779 to 7 613 484) | 5 227 672 (4 260 508 to 6 297 315) | 8 683 950 (7 431 179 to 9 774 672) | 4 733 750 (3 803 664 to 5 787 570) | 3 950 201 (3 400 008 to 4 706 404) | −27.7 (−39.4 to −16.8) | −30.2 (−43.8 to −13.7) | −24.4 (−37.8 to −7.2) |
| Age‐standardized, rate per 100 000 | 261.2 (235.9 to 292.9) | 288.1 (253.9 to 323.4) | 234.0 (190.1 to 284.8) | 107.7 (92.7 to 120.9) | 113.9 (92.1 to 138.8) | 101.1 (87.4 to 120.1) | −58.8 (−65.6 to −52.5) | −60.5 (−68.3 to −51.1) | −56.8 (−64.7 to −46.9) | |
| YLDs | All ages, n | 1 157 548 (692 048 to 1 779 160) | 637 587 (385 648 to 979 760) | 519 960 (309 324 to 802 264) | 1 989 931 (1 200 919 to 3 044 823) | 1 106 675 (671 449 to 1 687 184) | 883 256 (528 946 to 1 359 110) | 71.9 (67.9 to 76.0) | 73.6 (69.1 to 78.0) | 69.9 (66.1 to 74.0) |
| Age‐standardized, rate per 100 000 | 22.1 (13.3 to 33.7) | 24.5 (14.9 to 37.2) | 19.7 (11.8 to 30.0) | 25.2 (15.2 to 38.7) | 28 (17 to 42.9) | 22.4 (13.4 to 34.6) | 13.9 (11.1 to 16.0) | 14.4 (11.1 to 16.9) | 13.7 (11.2 to 15.9) | |
DALYs indicates disability‐adjusted life year; RHD, rhematic heart disease; YLDs, years lived with disability; and YLLs, years of life lost.
Figure 1. Age‐standardized epidemiological measures (incidence, prevalence, deaths, and disability‐adjusted life years [DALYs] of rheumatic heart disease at global scale across years from 1990 to 2019 by sex).

Table 2.
Age‐Standardized Rates (95% Uncertainty Intervals) Attributable to RHD in 1990 and 2019
| GBD superregion | Measure | Age‐standardized rate (per 100 000) | % Change (1990 to 2019) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 2019 | |||||||||
| Both | Women | Men | Both | Women | Men | Both | Women | Men | ||
| Central Europe, Eastern Europe, and Central Asia | Incidence | 19.3 (17.2 to 21.7) | 20.7 (18.4 to 23.3) | 17.7 (15.6 to 20.1) | 17.4 (14.4 to 20.7) | 18.5 (15.4 to 21.8) | 16.1 (13.2 to 19.4) | −9.9 (−17.1 to −3.3) | −10.7 (−17.6 to −4.1) | −8.6 (−16.3 to −1.2) |
| Prevalence | 264.1 (235.3 to 297.4) | 292.1 (260.6 to 329.1) | 224.2 (198.4 to 255.6) | 254.2 (217.1 to 299.7) | 279.8 (239.4 to 329.5) | 222.6 (187.9 to 263.6) | −3.7 (−10.8 to 3.3) | −4.2 (−11 to 2.8) | −0.7 (−8.8 to 6.9) | |
| Deaths | 5.6 (5.4 to 5.9) | 5.8 (5.6 to 6.1) | 5.3 (5 to 5.7) | 1.8 (1.6 to 1.9) | 1.9 (1.7 to 2) | 1.6 (1.5 to 1.8) | −68.4 (−71.6 to −65.4) | −68.1 (−71.1 to −64.5) | −69.4 (−73.3 to −65.9) | |
| DALYs | 198 (188.2 to 207.1) | 201.3 (191.9 to 211.1) | 191.1 (178 to 203.2) | 66.3 (59.2 to 74.1) | 68.4 (60.1 to 77.4) | 62.9 (56.5 to 71) | −66.5 (−69.7 to −62.9) | −66 (−69.6 to −62.3) | −67.1 (−71 to −62.9) | |
| YLLs | 185.4 (177 to 193.1) | 187.4 (179.4 to 195.7) | 180.4 (167.8 to 192) | 54 (48.4 to 59.5) | 54.9 (48.6 to 61.1) | 52.2 (46.7 to 58) | −70.9 (−73.9 to −67.9) | −70.7 (−73.8 to −67.2) | −71.1 (−74.8 to −67.4) | |
| YLDs | 12.6 (8 to 18.1) | 13.9 (8.9 to 20) | 10.7 (6.7 to 15.5) | 12.3 (7.5 to 18.3) | 13.5 (8.2 to 20) | 10.8 (6.6 to 16.1) | −2.4 (−9.5 to 4.3) | −3 (−9.9 to 4) | 0.8 (−7.6 to 8.6) | |
| High‐income | Incidence | 8.1 (7.2 to 9.1) | 8.9 (8 to 10) | 7.2 (6.4 to 8) | 7.2 (6.5 to 8) | 7.6 (6.9 to 8.4) | 6.8 (6.1 to 7.6) | −11.1 (−15.3 to −6.7) | −14.9 (−19.3 to −10.3) | −5 (−9.6 to 0.2) |
| Prevalence | 98.1 (86.8 to 109.4) | 108.5 (96.3 to 120.7) | 85.6 (75.4 to 95.9) | 93.1 (83.7 to 103.5) | 100.4 (90.1 to 111.1) | 85.4 (76.8 to 95.1) | −5.1 (−10.5 to 0.4) | −7.5 (−12.9 to −1.6) | −0.2 (−6.2 to 5.9) | |
| Deaths | 2.7 (2.5 to 2.8) | 3 (2.8 to 3.2) | 2.2 (2.1 to 2.3) | 1.2 (1.1 to 1.3) | 1.3 (1.1 to 1.5) | 1.1 (1 to 1.1) | −55 (−58.6 to −51.7) | −56.6 (−60.9 to −52.8) | −52.2 (−55.1 to −49.3) | |
| DALYs | 61.4 (58.8 to 64) | 68.9 (65.7 to 71.9) | 51.6 (49.4 to 54.1) | 24.6 (22.3 to 27) | 26.2 (23.5 to 28.9) | 22.4 (20.6 to 24.6) | −60 (−62.3 to −57.5) | −61.9 (−64.5 to −59.3) | −56.6 (−59 to −53.8) | |
| YLLs | 56.2 (54 to 57.9) | 63 (60.4 to 65.2) | 47.1 (45.6 to 48.9) | 19.7 (18 to 21.1) | 20.9 (18.8 to 22.8) | 18 (16.9 to 19.1) | −64.9 (−67 to −62.8) | −66.8 (−69.2 to −64.5) | −61.8 (−63.8 to −59.7) | |
| YLDs | 5.2 (3.5 to 7.5) | 5.8 (3.9 to 8.4) | 4.5 (2.9 to 6.5) | 4.9 (3.2 to 6.9) | 5.3 (3.5 to 7.6) | 4.4 (2.8 to 6.3) | −6.6 (−11.6 to −1.4) | −8.8 (−13.8 to −3.3) | −1.8 (−7.4 to 4.4) | |
| Latin America and Caribbean | Incidence | 39.2 (29.9 to 49) | 41.5 (31.6 to 51.7) | 36.9 (28 to 46.4) | 39 (29.5 to 48.9) | 41.5 (31.6 to 52.2) | 36.5 (27.7 to 45.9) | −0.6 (−1.8 to 0.5) | 0 (−1.7 to 1.7) | −1.1 (−2.8 to 0.4) |
| Prevalence | 652.9 (511.1 to 807.9) | 731.1 (574.6 to 900.2) | 571 (446.6 to 715.1) | 654.9 (511.5 to 812.1) | 738.8 (580.1 to 910.7) | 566.5 (441.6 to 708.4) | 0.3 (−0.9 to 1.6) | 1.1 (−0.8 to 2.8) | −0.8 (−2.3 to 0.9) | |
| Deaths | 3.1 (2.9 to 3.3) | 3.7 (3.4 to 4) | 2.4 (2.2 to 2.6) | 1.1 (1 to 1.2) | 1.3 (1.1 to 1.4) | 0.8 (0.8 to 0.9) | −65 (−69.3 to −61.6) | −65.6 (−70.2 to −61.4) | −64.8 (−69.7 to −60.3) | |
| DALYs | 135.1 (121.4 to 152.1) | 161.8 (144.9 to 181.3) | 107 (94.2 to 122.5) | 64.7 (51.3 to 81) | 75.3 (59.7 to 93.9) | 53.2 (41.7 to 68.2) | −52.1 (−58.3 to −45.9) | −53.4 (−59.8 to −46.9) | −50.2 (−57.3 to −43.2) | |
| YLLs | 104.1 (99.1 to 110.4) | 127.2 (117.5 to 136.5) | 79.7 (74.7 to 86.3) | 33.6 (29.7 to 38) | 40.3 (34.7 to 47) | 26.2 (23.2 to 29.6) | −67.8 (−71.7 to −63.9) | −68.3 (−72.5 to −63.7) | −67.2 (−71.5 to −62.4) | |
| YLDs | 31 (18.4 to 47.7) | 34.6 (20.6 to 52.7) | 27.2 (16.1 to 42.2) | 31.2 (18.4 to 48.2) | 35 (20.8 to 54) | 27.1 (15.9 to 42.3) | 0.6 (−0.8 to 2) | 1.3 (−0.8 to 3.4) | −0.5 (−2.5 to 1.4) | |
| North Africa and Middle East | Incidence | 23.8 (18.6 to 29.6) | 24.5 (19.2 to 30.4) | 23 (17.9 to 28.8) | 25.6 (19.7 to 31.9) | 26.5 (20.4 to 32.9) | 24.8 (19 to 31) | 7.8 (3.5 to 11.6) | 8.3 (3.8 to 12.3) | 7.5 (3 to 11.7) |
| Prevalence | 368.8 (293.2 to 455.9) | 395.7 (313.7 to 488.5) | 343.2 (271.8 to 429.3) | 388.9 (304.7 to 483.5) | 420 (328.6 to 521) | 360.4 (282.5 to 449) | 5.4 (1.3 to 8.5) | 6.1 (1.6 to 9.8) | 5 (0.9 to 8.8) | |
| Deaths | 4.5 (3.4 to 6.7) | 5.3 (3.8 to 7.5) | 3.7 (2.3 to 6.3) | 1.6 (1.4 to 2) | 1.9 (1.5 to 2.3) | 1.4 (1 to 1.8) | −63.4 (−74.6 to −52.5) | −63.5 (−74 to −46.8) | −62.9 (−77.5 to −43.9) | |
| DALYs | 173.1 (139.6 to 223.6) | 209.4 (157.4 to 256.5) | 138.2 (94.4 to 205.2) | 67.1 (54.2 to 82.4) | 77.1 (60.9 to 94.4) | 57.7 (42.2 to 74.8) | −61.3 (−70.2 to −52.1) | −63.2 (−71 to −48.7) | −58.2 (−71.4 to −40.2) | |
| YLLs | 155.2 (123.3 to 205) | 190.4 (139.9 to 235.6) | 121.5 (76.7 to 184.8) | 48.2 (38 to 60.8) | 56.9 (43.2 to 71.3) | 40.2 (27 to 54.2) | −68.9 (−77.1 to −59.4) | −70.1 (−77 to −55.6) | −66.9 (−78.4 to −49.5) | |
| YLDs | 17.9 (10.5 to 27.4) | 19 (11.3 to 29) | 16.7 (9.8 to 25.8) | 18.8 (11.1 to 29.2) | 20.2 (12 to 31.3) | 17.6 (10.3 to 27.1) | 5.5 (1.1 to 9.1) | 6.1 (1.2 to 10.7) | 5 (0.3 to 9.5) | |
| South Asia | Incidence | 42.4 (31.9 to 53.2) | 49.2 (36.7 to 61.3) | 36.1 (27.3 to 45.6) | 43 (32.2 to 54.2) | 49.2 (36.6 to 62) | 37.1 (27.9 to 47.1) | 1.3 (−1.7 to 4.3) | 0 (−4.1 to 3.9) | 2.7 (−0.6 to 5.6) |
| Prevalence | 623.9 (481.5 to 781.4) | 737.2 (565.6 to 923.1) | 519.5 (404.4 to 659.7) | 645.1 (498.2 to 811.7) | 752.7 (579.1 to 945.3) | 540.7 (415.8 to 684.7) | 3.4 (0.5 to 6.2) | 2.1 (−1.6 to 5.8) | 4.1 (1.3 to 6.9) | |
| Deaths | 20.7 (16.9 to 26.4) | 20.7 (16.2 to 25.4) | 20.7 (14.6 to 30.7) | 11.2 (8.6 to 13.4) | 12.2 (8.6 to 16.4) | 10.1 (7.7 to 14.5) | −45.9 (−59.6 to −30.6) | −41 (−59.1 to −17.5) | −51 (−64.9 to −35.3) | |
| DALYs | 630.7 (525.2 to 772.2) | 650 (519.8 to 782.7) | 613.1 (456.2 to 852.3) | 348.5 (272.4 to 412.2) | 382.2 (278.6 to 511.1) | 314.9 (246.9 to 417.6) | −44.7 (−56.5 to −32.2) | −41.2 (−55.4 to −23.3) | −48.6 (−60.3 to −33.7) | |
| YLLs | 600.4 (495.2 to 739.6) | 614.4 (483.9 to 743.1) | 587.6 (431.3 to 824.7) | 317 (243.6 to 381) | 345.7 (243.6 to 470) | 288.3 (222.1 to 388.9) | −47.2 (−58.9 to −34.1) | −43.7 (−58.9 to −24.9) | −50.9 (−62.8 to −35.7) | |
| YLDs | 30.3 (18.4 to 46.3) | 35.5 (21.8 to 54) | 25.5 (15.4 to 39) | 31.5 (19 to 48) | 36.5 (22.2 to 55.5) | 26.6 (15.9 to 40.8) | 3.9 (1 to 6.5) | 2.7 (−1 to 6.4) | 4.4 (1.1 to 7.5) | |
| Southeast Asia, East Asia, and Oceania | Incidence | 26 (20.5 to 32.2) | 27.4 (21.6 to 33.7) | 24.7 (19.4 to 30.6) | 21.9 (17.2 to 27.1) | 23.3 (18.3 to 28.9) | 20.6 (16.1 to 25.7) | −15.9 (−17.7 to −14.3) | −15 (−17 to −13.1) | −16.3 (−18.3 to −14.3) |
| Prevalence | 397.1 (316 to 491.9) | 431 (345.5 to 533.1) | 364.1 (288.5 to 452) | 355.3 (283.6 to 436.6) | 394.4 (316.3 to 484.5) | 317.2 (253.7 to 391.2) | −10.5 (−12.4 to −8.6) | −8.5 (−10.7 to −6.4) | −12.9 (−15.2 to −10.3) | |
| Deaths | 14.3 (12.4 to 16.8) | 16.8 (13.9 to 20.4) | 11.6 (9.4 to 13.8) | 3.5 (3 to 4) | 3.6 (2.9 to 4.3) | 3.5 (2.8 to 4.3) | −75.4 (−81.2 to −69.1) | −78.8 (−84.1 to −72) | −69.9 (−78.5 to −57.3) | |
| DALYs | 356 (310.9 to 405.9) | 430.5 (361.4 to 514.7) | 280.1 (231 to 329.1) | 91.8 (79.6 to 103.9) | 96.4 (81.9 to 112.3) | 87.5 (72 to 103.8) | −74.2 (−79.2 to −69) | −77.6 (−82.7 to −71.6) | −68.8 (−76.6 to −58.1) | |
| YLLs | 335.9 (293.2 to 385.1) | 408.5 (340.3 to 491.5) | 261.8 (213.4 to 309) | 73.5 (62.9 to 83.4) | 75.8 (62.7 to 89.7) | 71.3 (57.8 to 86) | −78.1 (−82.7 to −73) | −81.4 (−85.8 to −75.8) | −72.8 (−80.2 to −62) | |
| YLDs | 20.2 (12.1 to 30.9) | 22 (13.4 to 33.4) | 18.4 (11.1 to 28.4) | 18.3 (11 to 27.8) | 20.5 (12.4 to 31) | 16.2 (9.7 to 24.9) | −9 (−11.2 to −6.6) | −6.6 (−9.2 to −3.8) | −11.9 (−14.6 to −8.9) | |
| Sub‐Saharan Africa | Incidence | 67.3 (50.6 to 85.6) | 69.4 (52 to 88.2) | 65.1 (48.9 to 82.4) | 70 (52.4 to 88.8) | 72.4 (54 to 91.9) | 67.6 (50.4 to 85.9) | 4.1 (2.6 to 5.8) | 4.4 (2.6 to 6.3) | 3.7 (2 to 5.8) |
| Prevalence | 984.2 (766.4 to 1237.4) | 1028.3 (799.3 to 1287.9) | 938.7 (730.1 to 1179.7) | 1030.9 (799 to 1297.8) | 1080 (833.7 to 1349.2) | 978.4 (757.7 to 1232.7) | 4.8 (3.4 to 6.3) | 5 (3.3 to 6.8) | 4.2 (2.5 to 6.2) | |
| Deaths | 5.7 (4.6 to 6.8) | 6 (4.4 to 7.4) | 5.3 (3.7 to 7.6) | 2.6 (2.1 to 3) | 2.5 (2 to 3.2) | 2.6 (2.1 to 3.3) | −54.9 (−63.8 to −44.7) | −58.1 (−66.4 to −47.1) | −50.8 (−64.3 to −32.4) | |
| DALYs | 216.8 (178.4 to 259.6) | 233.6 (180.6 to 281.5) | 199.5 (151 to 258.7) | 119.8 (94.9 to 148.8) | 121.3 (94.6 to 151.7) | 118.2 (91.5 to 149.4) | −44.7 (−54.6 to −34.7) | −48.1 (−56.9 to −36.6) | −40.8 (−54.9 to −25.2) | |
| YLLs | 169.8 (138.2 to 205.9) | 184.7 (136.7 to 226.4) | 154.4 (111.1 to 216.4) | 70.4 (58 to 85.3) | 69.8 (54.3 to 88.1) | 71.1 (56.2 to 90.7) | −58.5 (−67.5 to −48.8) | −62.2 (−69.4 to −51.5) | −54 (−67.4 to −37.1) | |
| YLDs | 47 (27.9 to 73.5) | 48.9 (29 to 76.4) | 45 (26.6 to 70.7) | 49.4 (29.4 to 76.9) | 51.5 (30.8 to 80.2) | 47.1 (27.8 to 73.8) | 5.1 (3.7 to 6.8) | 5.3 (3.3 to 7.2) | 4.7 (2.7 to 6.7) | |
DALY indicates disability‐adjusted life year; GBD, Global Burden of Disease; RHD, rheumatic heart disease; YLDs, years lived with disability; and YLLs, years of life lost.
RHD led to nearly 10.7 million (9.2–12.1) DALYs in 2019 globally, which showed a decrease from just over 13.2 million (11.9–14.6) in 1990. Compatible with total trend in all‐age DALYs, the age‐standardized DALYs showed 54.6% (−62.2% to −45.3%) and 51.3% (−59.8% to −41.8%) decrease among women and men, respectively (Figure 1). Considering the age‐standardized rates in 2019, RHD contributed to 132.9 (115.0–150.3) DALYs in 100 000 population, which was almost half of the age‐standardized rate in 1990 (Table 1). During the past 30 years, the age‐standardized DALY rate of RHD was consistently lowest among the high‐income superregion, with 24.6 (22.3–27.0) in 100 000 population in 2019, and highest among South Asia superregion, with 348.5 (272.4–412.2) in 100 000 population in 2019 (Table 2). Consistent with the ASDRs, the age‐standardized DALY rate decreased during the past 30 years; however, the rates were higher among women (Figure 1). Although the declining trend in age‐standardized DALYs of RHD was consistent with trends in YLLs, with 58.8% (−65.6% to −52.5%) decrease since 1990, the age‐standardized YLD rate increased by 13.9% (11.1%–16.0%) in the study period. In addition, YLDs contributed to 7.8% and 19.0% of rates in DALYs in 1990 and 2019, respectively. However, the contribution of YLLs decreased from 91.2% in 1990 to 81.4% in 2019 (Table 1).
Nearly 2.8 (2.2–3.5) million new cases and 40.5 (32.1–50.1) million prevalent cases of RHD were identified in 2019, worldwide, showing a 1.5‐ and 1.7‐fold increase since 1990, respectively (Figure 1). The increase in the absolute values of new cases since 1990 was mainly attributable to population growth (44.6%), and 17.9% of the current trend was attributable to an increase in incidence rate of RHD (Table S2).
The global age‐standardized incidence rate (ASIR) and age‐standardized prevalence rate (ASPR) of RHD were 37.4 (28.6–46.7) and 513.7 (405.0–636.3) per 100 000 population in 2019, respectively. The corresponding rates in 1990 were 32.7 (25.8–40.0) and 451.6 (363.3–552.5) in 100 000 population for ASIR and ASPR, respectively. The ASIRs and ASPRs among women increased by 14.1% (10.6%–17.1%) and 14.1% (10.9%–16.7%) from 1990 to 2019, respectively (Table 1 and Figure 1). Among men, the corresponding rate increased by 15.1% (12.1%–17.7%) and 13.7% (11.2%–15.8%) from 1990 to 2019. Considering the GBD superregions, Sub‐Saharan Africa and the high‐income superregion showed the highest and lowest ASIRs and ASPRs for all the years from 1990 to 2019 (Tables 1 and 2).
Regional and National Burden of RHD
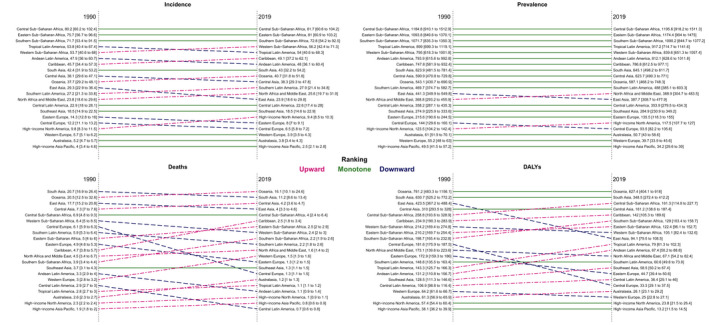
At the regional level, Oceania had the highest ASDRs attributable to RHD in 2019 among women (15.5 [9.5– 23.4] in 100 000 population), men (16.7 [7.8–31.1] per 100 000 population), and both sexes combined (16.1 [10.1–24.6] in 100 000 population). Concurrently, Oceania had the highest age‐standardized DALY rate for women, men, and both sexes combined, with the rates of 639.8 (403.5–976.8), 615.5 (321.9–1065.4), and 627.4 (404.1–918.1) per 100 000 population, respectively. South, Central, and East Asia were the regions with the next highest ASDRs attributable to RHD for both women and men in 2019, with rates of 11.2 (8.6–13.4), 4.2 (3.6–4.7), and 4.0 (3.3–4.6) per 100 000 population, respectively.
South Asia, Central Sub‐Saharan Africa, and Central Asia ranked the next highest age‐standardized DALY rate in 2019. Central Sub‐Saharan Africa had the fifth‐highest ASDRs (4.0 [2.4–6.0] per 100 000 population in 2019) attributable to RHD; however, it was consistently the top region in terms of ASIRs (81.7 [60.6–104.2] per 100 000 population in 2019) and ASPRs (1195.6 [918.2–1511.3] per 100 000 population in 2019) in all years from 1990 to 2019.
The lowest ASDRs attributable to RHD were among the countries in Central Latin America region, with the rate of 0.7 (0.6–0.8) per 100 000 population in 2019. High‐income Asia Pacific had the second lowest ASDRs (0.8 [0.6–0.9] per 100 000 population), whereas the lowest ASIR (2.5 [2.1–2.8] per 100 000 population), ASPR (34.2 [29.6–39.0] per 100 000 population), and age‐standardized DALY (13.2 [11.5–14.5] per 100 000 population) were in this region in 2019.
From 1990 to 2019, the trends of ASDR and age‐standardized DALY rate attributable to RHD were decreasing for all regions, with the highest decrease for Central Europe, followed by East Asia. The lowest decrease in ASDRs and DALYs during the study period was for Oceania, with 21.5% (−38.2% to −1.4%) and 17.6% (−35.4% to −4.6%) decrease, respectively.
The trends in ASIRs and ASPRs attributable to RHD varied markedly among various regions from 1990 to 2019. The ASIRs and ASPRs of 13 of 21 regions increased from 1990 to 2019, with the most increase in Oceania, with 8.1% (3.6%–12.7%) increase in ASIR and 8.1% (3.3%–13.0%) increase in ASPR. The most decrease in ASIRs and ASPRs was among countries of Central Europe and Eastern Europe, with 47.0% (−49.6% to −44.3%) and 37.1% (−40.4% to −33.8%) decrease in rates, respectively (Figure 2). Considering the trends in the absolute numbers of new cases of RHD, the overall changes of absolute numbers in all regions were increasing, except for Central Europe, East Asia, and Eastern Europe regions, which showed 39.8%, 26.5%, and 38.0% decrease in the number of new cases from 1990 to 2019. Table S2 presents the contribution of population growth, aging, and variations in age‐specific incidence rates of RHD in the witnessed trends.
Figure 2. Regional age‐standardized incidence, prevalence, deaths, and disability‐adjusted life years (DALYs) of rheumatic heart disease in 1990 and 2019.
Across countries, Solomon Islands had the highest ASDR of RHD in all years from 1990 (30.9 [18.1–46.1] per 100 000 population) to 2019 (21.1 [10.9–31.2] per 100 000 population), despite a 31.8% (−54.2% to −4.6%) decrease in ASDRs since 1990. The second highest ASDR attributable to RHD was in Kiribati in 1990 and 2019 (Figure 3A). Commensurate with the ASDRs, the highest age‐standardized DALY rate in 2019 was at the Solomon Islands, followed by Kiribati (Figure 3B). Finland and the Republic of Korea had the lowest ASDRs and age‐standardized DALY rate for RHD in 2019.
Figure 3. Age‐standardized rheumatic heart disease epidemiological rates in both sexes in 204 countries or territories in 1990 and 2019: death (A), disability‐adjusted life years (DALYs) (B), incidence (C), and prevalence (D).
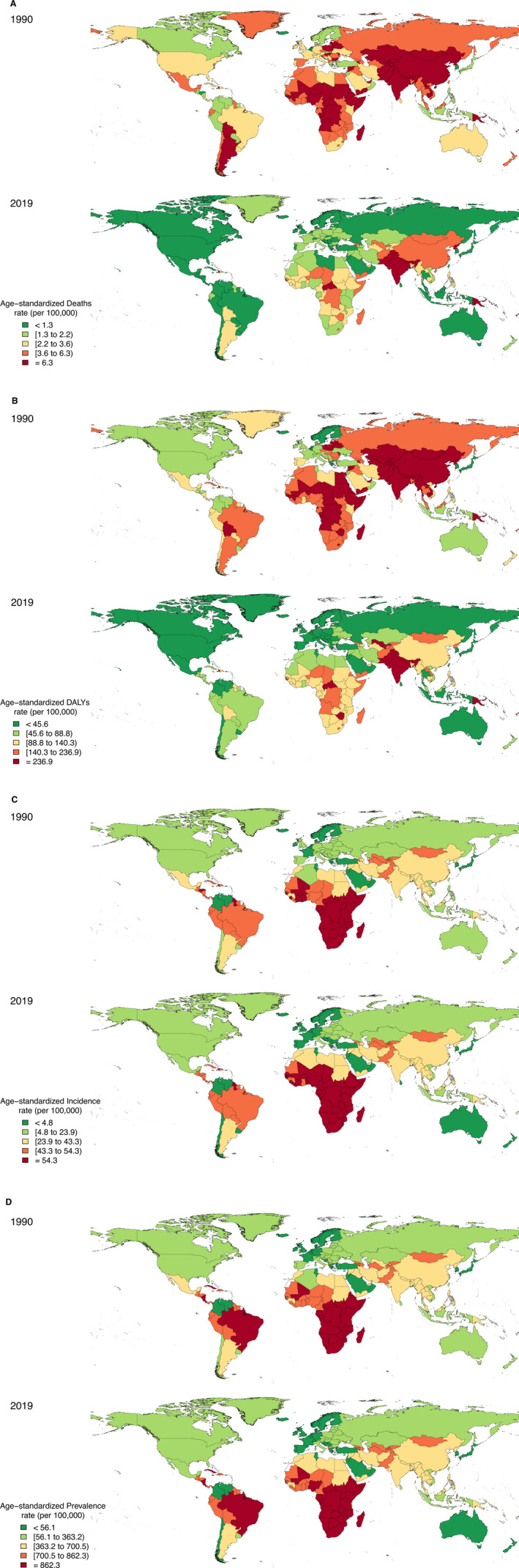
The highest and lowest ASIR of RHD were in Uganda and Finland in 2019, with rates of 94.0 (68.4–128.3) and 1.5 (1.3–1.7) per 100 000 population, respectively (Figure 3C). The highest and lowest ASPR of RHD were among Eritrea (1370.1 [1054.6–1735.7] per 100 000 population) and Finland (18.0 [14.7–21.7] per 100 000 population) in 2019 (Figure 3D).
From 1990 to 2019, the ASDRs and age‐standardized DALY rate of RHD decreased among all countries, except for 3 countries. The most decrease for ASDRs and age‐standardized DALYs during the study period has been among Thailand (89.8% [−93.0% to −86.1%]) and Poland (86.7% [−88.7% to −84.6%]), respectively. The difference between the highest and lowest age‐standardized rates for deaths and DALYs attributable to RHD decreased from 30.1 and 1177.2 in 1990 to 20.7 and 801.8 in 2019, respectively (Data S1).
Inequity Pattern in RHD Burden
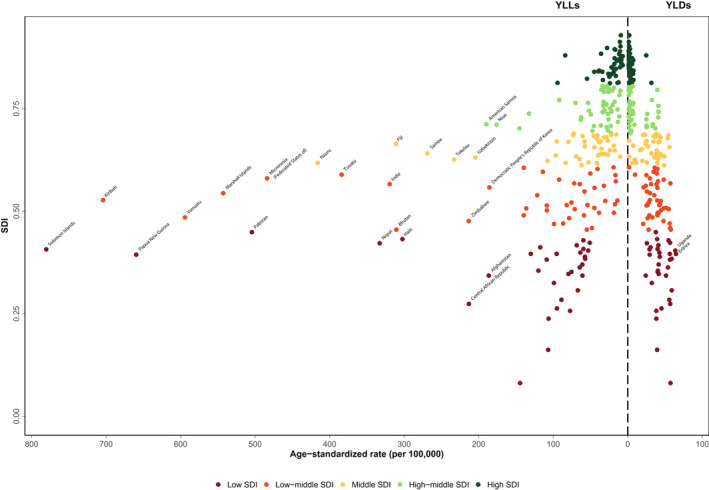
In 2019, the age‐standardized rates of RHD were higher among lower SDI countries. The low‐SDI countries had ASIR, ASDR, and age‐standardized DALY rates of 58.9 (44.4–74.2), 8.5 (7.0–10.2), and 275.5 (22.8–324.6) per 100 000 population, respectively, which were 10.0‐, 7.7‐, and 12.1‐fold more than corresponding rates among high‐SDI region. From 1990 to 2019, all age‐standardized rates of RHD have shown a steep decline among countries of high‐SDI, high‐middle‐SDI, and middle‐SDI regions; however, only ASDRs and age‐standardized DALY rate of RHD showed decreases in low‐middle‐SDI and low‐SDI regions (Figure S1).
Considering the composition of DALYs attributable to RHD, expected age‐standardized YLL rates for RHD declined profoundly as SDI increased. At the same time, age‐standardized YLD rates for RHD demonstrate relatively little changes by SDI. At higher SDIs, the composition of disease burden shifted toward YLDs (Figure 4).
Figure 4. Expected relationship between age‐standardized years of life lost (YLLs) and years lived with disability (YLDs) rates and sociodemographic index (SDI) for 204 countries.
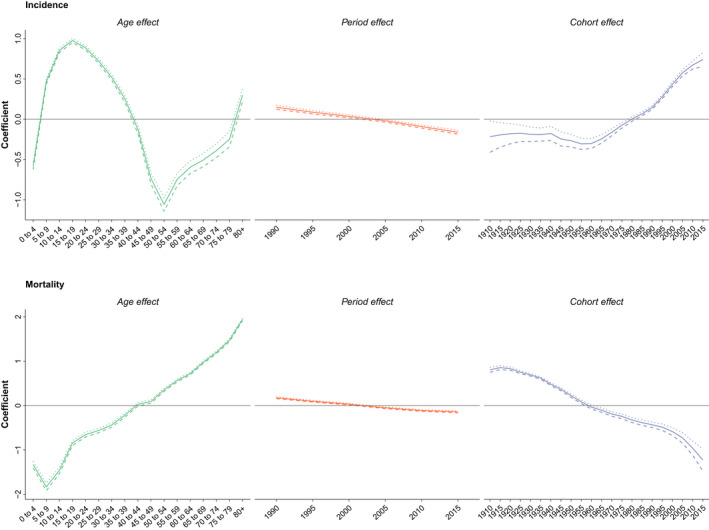
The analysis results on the age‐period‐cohort effect on incidence and deaths attributable to RHD worldwide are presented in Figure 5. The coefficient of age group effect on the incidence of RHD peaked in the age group of 15 to 19 years, with the rate of 0.98 (0.95–1.00), and started to decrease to the values as low as −1.06 (−1.14 to −0.97) in the age group of 50 to 54 years. The coefficient of age effect on deaths attributable to RHD showed increasing trends from the age group of 5 to 9 years (−1.83 [−1.92 to −1.75]) to the age group of ≥80 years (1.94 [1.91–1.97]). RHD had incessantly increasing trends in coefficients of period effect on incidence and mortality from 1990 to 1994 to 2015 to 2019. The coefficients of birth cohort effect on incidence of RHD showed a significantly increasing trend from 1960 to 1964 to 2015 to 2019; however, the birth cohort effect on deaths attributable to RHD showed unfailingly decreasing trends from 0.81 (0.75–0.86) in 1910 to 1914 to −1.23 (−1.48 to −0.98) in 2015 to 2019 (Table S3).
Figure 5. Age‐period‐cohort effect on rheumatic heart disease incidence and death.
Age Pattern of RHD
Patterns in global rates of RHD by age were similar between men and women and across years from 1990 to 2019 and were consistently higher among women than rates among men. From 1990 to 2019, the death rate of RHD showed an increasing pattern by age and peaked at >85 years in both sexes. The DALY rate of RHD showed the current increasing trend by age at both 1990 and 2019. However, the DALY rate peaked at 75 to 79 years among women and 80 to 84 years in 1990. The age of the peaked DALY rate was at >85 years at both sexes. The global peak of incidence rate among women and men was 15 to 19 and 10 to 14 years, respectively. Nevertheless, the trends in the incidence rate of RHD rocketed up from the age group of 45 to 49 years at both sexes and peaked at the age group of ≥85 years again. The current dual trend was observed at age patterns of ASPRs of RHD for men and women in 1990 and 2019.
Age patterns in various rates of RHD showed an increasing trend by age in the high‐SDI region and peaked at ≥85 years in all years from 1990 to 2019. Although the increasing trend by age is also witnessed at the death rate of all SDI regions and the peak age group of death rate was at ≥85 years for both sexes, and in both 1990 and 2019, the trend of disparate measures varies markedly across various SDI regions. The age trend of DALY rate among various SDI regions is similar to the death rate trend; however, the age pyramid base is wider than the death rate. Considering the pattern of incidence and prevalence rates of RHD by age, the lower the SDI, the wider the age pyramid base in both men and women in both 1990 and 2019, as the peak of incidence rate in the low‐SDI region was at the age group of 10 to 14 years among men and women in 2019 (Figure S2).
Discussion
In this study, multiple data sources and epidemiologic modeling techniques were used to estimate the global burden of RHD over the past 3 decades. The health‐related burden of RHD has dramatically declined in affluent countries. Nevertheless, RHD continues to take a heavy toll on far too many people in lower‐income countries, making it among the most neglected diseases relative to its burden worldwide. In this sense, the study results could be used by health care authorities in countries for resources allocation and hopefully drive efforts to reduce the disease burden further worldwide.
Globally, the ASIR and ASPR of RHD increased; however, the ASDR decreased during the study period. The increase in the new cases of RHD was majorly attributable to population growth and an increase in the incidence rate. Although YLLs and DALYs of RHD dramatically decreased, YLDs increased from 1990 to 2019. The burden of RHD was much higher among lower SDI countries. The age‐standardized YLL and YLD rates for RHD significantly declined as countries' SDI increased.
The age‐standardized DALY rate, although decreasing, has been higher among women than men. Although acute rheumatic fever is equally common among men and women, RHD occurs more commonly among women than men. 21 The reasons for this inequity in burden are yet to be understood; however, the roles of greater autoimmune susceptibility, exposure to infection, unequal access to primary and secondary acute rheumatic fever prophylaxis, and hemodynamic changes during pregnancy have been underscored. 22
The burden of RHD varied significantly among countries. The age‐standardized DALY, ASIR, and ASPR were lowest among the high‐income superregion and highest among Southeast Asia, East Asia, and Oceania superregion, which also had the highest age‐standardized death rates attributable to RHD. Central Sub‐Saharan Africa was the first region regarding age‐standardized incidence rate during the study period, consistent with other studies. 23
Looking closer at Southeast Asia, East Asia, and Oceania superregion, the Oceania region had the highest age‐standardized DALYs and death rates attributable to RHD, which could be fueled by overcrowding, poor housing conditions, and improper health literacy. There is evidence that the actual number of deaths attributable to RHD could even be up to twice the records in vital registration systems, which could impose a burden much more than estimated. 24 Eradication of RHD calls for a centrally planned approach with a particular focus on prevention, early detection, and follow‐up. Australia and New Zealand have spent millions to eliminate the burden of RHD among indigenous families and immigrants. Although there may be some evidence that genetic susceptibility may have a role in the significantly higher burden of RHD among Indigenous populations in the Oceania region, 25 socioeconomic and environmental conditions are the overwhelming significant modifiable determinants of RHD prevention. 26 The New Zealand government launched a program for the primordial and primary prevention of RHD in 2011, spending >$13 per capita, to increase awareness of rheumatic fever, reduce household crowding, and improve access to timely and effective treatment for strep throat infections. 27 The Australian government has invested $2 per capita in Rheumatic Fever Strategy during 2009 to 2021, resulting in no measurable reductions in the rates of rheumatic fever and RHD. Thus, they have recently launched RHD Endgame Strategy with a larger investment targeting all areas of control from primordial prevention to tertiary prevention to eliminate RHD in Australia by 2031. 28
Improvements in housing and hygiene education have lessened the burden of RHD worldwide. Nevertheless, RHD remains the most acquired heart disease among people aged <25 years. 29 Given that the age group 15 to 19 years had the highest coefficient for age effect on RHD incidence, prompt medical interventions could substantially alleviate RHD mortality. 29 The burden of RHD was related to the socioeconomic development status of countries. Although both YLLs and YLDs attributable to RHD decreased as countries' SDI improved, the heterogeneities across countries based on SDI was more evident in YLLs. Higher YLLs attributable to RHD in low‐SDI countries could be inadequate health infrastructure and poor health care services and management. The gaps between high‐SDI countries and low‐SDI countries were also reflected in the burden of RHD across various age groups. The DALYs attributable to RHD had an increasing trend by age in the high‐SDI region and peaked at ≥85 years, which could show the cohort effect, 2 as reflected in the age‐period‐cohort effect in this study. Nevertheless, the incidence rate of RHD for the low‐SDI region was at the age group of 10 to 14 years among men and women in 2019.
RHD, a complex consequence of acute rheumatic fever, is a febrile illness caused by group A streptococcus infection. The antibodies against the infection slowly damage the heart valves in RHD pathogenesis. The rheumatic fever per se is not likely to be fatal; however, untreated RHD could result in mortality. 30 RHD diagnosis still relies on a clinical diagnostic algorithm with no gold standard confirmatory test, which leaves the potential for imperfect specificity and sensitivity. This calls for further research to improve case detection, especially for use in moderate‐/high‐risk settings with limited resources. 31 Improvements in diagnosis in low‐SDI countries could help policy makers better understand the problem severity, as there are many undiagnosed cases in such countries. 24
The witnessed gaps and inequities call for concerted efforts to lessen the burden of RHD in areas with limited resources. Although low‐SDI countries are witnessing a global shift of disease burden from communicable diseases to noncommunicable diseases, 32 , 33 they are still dealing with RHD, a neglected consequence of a communicable disease, which could add to their existing challenges.
Thus, there is an urgent need for investment and resource allocation to lessen RHD's burden. Given the level of technology, expenses, and the expertise required for tertiary prevention of RHD, the prevention of rheumatic fever recurrence among patients with previous rheumatic fever or RHD seems to be the most feasible measure among the mentioned prevention levels. In 2018, the World Health Assembly launched a coordinated global response to RHD and acute rheumatic fever and called for concerted efforts in prevention, treatment, and care, 14 the work plan of which is being developed. In the meantime, the WHO Benzathine Penicillin Technical Working Group endeavors to address global supply and demand issues for benzathine penicillin G for rheumatic fever prevention. 34 However, the disruptive effect of the COVID‐19 pandemic on any progress is concerning.
The WHO emphasizes improved access to primary health care and extensive investment in a community and primary health care workforce trained in the prevention, diagnosis, and evidence‐based management of group A β‐hemolytic streptococcal pharyngitis and acute rhematic fever. 14 In this sense, public health authorities and donors need to use the existing evidence to allocate resources proportionate to disease burden and witnessed inequalities and support countries through exchange of expertise and training. As the required expertise and training are also currently lacking in low‐SDI countries, the physicians' task for an antibiotic prescription could be shifted to community health workers, who could play a key role in delivering preventive medications via practical clinical algorithms, diagnostic tools, availability of appropriate antibiotics, and supportive supervision. 35
Until there is an effective vaccine for RHD prevention, the WHO suggests following a multilevel approach against RHD: primordial prevention, including improving the socioeconomic status of populations at risk; primary prevention, including treating patients with strep throat using benzathine penicillin G; secondary prevention, including antibiotic prophylaxis among patients with previous rheumatic fever or RHD; and tertiary prevention, including medical/surgical treatment of RHD complications. 23 , 29
Strengths and Limitations
This study presents estimates for incidence, prevalence, deaths, YLLs, YLDs, and DALYs attributable to RHD based on GBD 2019. It provides the opportunity to investigate the current situation and time trends of health metrics and measures worldwide during the past 3 decades to fill information gaps and implement action to address this avoidable disease. Nevertheless, the overall quality of GBD estimates fundamentally relies on the accuracy of data sources used in the modeling. The availability and reliability of registry data and the paucity of other RHD‐specific data among countries with limited resources could be questionable, resulting in a need for modeling and data extrapolation. In this sense, the GBD study includes various modeling processes to overcome this limitation and presents metrics with 95% UIs. Moreover, the GBD does not provide subnational data estimates for all countries and thus makes it unfeasible to pick up within‐country inequities by location and subpopulations.
Conclusions
Our study shows a divergence in the burden of RHD among countries based on SDI levels, which calls for including RHD in global assistance and funding. Indeed, many countries are still dealing with an unfinished infectious disease agenda, and there is an urgency to act now to prevent an increase in future burden from RHD.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S2
Data S1
Acknowledgments
The authors would like to express their profound gratitude to the Institute for Health Metrics and Evaluation and all the staff of Non‐Communicable Diseases Research Center of Tehran University of Medical Sciences, Tehran, Iran.
Author contributions: Study concept and design: Dr Majnoon and Dr Farzadfar; acquisition of data: S. Saeedi Moghaddam, Dr Ghamari, and Dr Abbasi‐Kangevari; analysis and interpretation of data: S. Saeedi Moghaddam, Z. Esfahani, Dr Shobeiri, A. Ghanbari, Dr Ghamari, Dr Abbasi‐Kangevari, Dr Keykhaei, Dr Naderian, and Dr Mokdad; drafting of the manuscript: Dr Ghamari and Dr Abbasi‐Kangevari; critical revision of the manuscript for important intellectual content: S. Saeedi Moghaddam, Dr Aminorroaya, Dr Negar Rezaei, Dr Malekpour, Dr Keykhaei, Dr Naderian, Dr Nazila Rezaei, Dr Larijani, Dr Majnoon, Dr Farzadfar, and Dr Mokdad; statistical analysis: S. Saeedi Moghaddam, A. Ghanbari, Z. Esfahani, and Dr Shobeiri; administrative, technical, or material support: Dr Negar Rezaei, Dr Farzadfar, Dr Larijani, Dr Majnoon, Dr Nazila Rezaei, and Dr Mokdad; study supervision: Dr Farzadfar, Dr Majnoon, and Dr Mokdad. All authors have read and approved the manuscript before submission.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025284
For Sources of Funding and Disclosures, see page 16.
Contributor Information
Mohsen Abbasi‐Kangevari, Email: mohsen.abbasi@sbmu.ac.ir.
Nazila Rezaei, Email: nazila_R@yahoo.com.
Mohamad Taghi Majnoon, Email: 107majnoon@gmail.com.
Farshad Farzadfar, Email: f-farzadfar@tums.ac.ir.
References
- 1. Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379:953–964. doi: 10.1016/S0140-6736(11)61171-9 [DOI] [PubMed] [Google Scholar]
- 2. Bennett J, Zhang J, Leung W, Jack S, Oliver J, Webb R, Wilson N, Sika‐Paotonu D, Harwood M, Baker MG. Rising ethnic inequalities in acute rheumatic fever and rheumatic heart disease, New Zealand, 2000–2018—Volume 27, Number 1—January 2021—emerging infectious diseases journal—CDC. Emerg Infect Dis. 2021;27:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nascimento BR, Beaton AZ. Rheumatic heart disease and socioeconomic development. Lancet Glob Health. 2019;7:e1297–e1299. doi: 10.1016/S2214-109X(19)30369-9 [DOI] [PubMed] [Google Scholar]
- 4. Kang K, Chau K, Howell E, Anderson M, Smith S, Davis TJ, Starmer G, Hanson J. The temporospatial epidemiology of rheumatic heart disease in Far North Queensland, tropical Australia 1997–2017; impact of socioeconomic status on disease burden, severity and access to care. PLoS Negl Trop Dis. 2021;15:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonald M, Brown A, Noonan S, Carapetis JR. Preventing recurrent rheumatic fever: the role of register based programmes. Heart. 2005;91:1131. doi: 10.1136/hrt.2004.057570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nordet P, Lopez R, Dueñas A, Sarmiento L. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience (1986–1996–2002). Cardiovasc J Afr. 2008;19:135–140. doi: 10.10520/EJC23128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 8. Ordunez P, Martinez R, Soliz P, Giraldo G, Mujica OJ, Nordet P. Rheumatic heart disease burden, trends, and inequalities in the Americas, 1990‐2017: a population‐based study. Lancet Glob Health. 2019;7:e1388–e1397. doi: 10.1016/S2214-109X(19)30360-2 [DOI] [PubMed] [Google Scholar]
- 9. Expert committee on rheumatic diseases, first report. World Health Organ Tech Rep Ser. 1954;78:1–25. [PubMed] [Google Scholar]
- 10. Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med. 2007;357:439–441. doi: 10.1056/NEJMp078039 [DOI] [PubMed] [Google Scholar]
- 11. Shawar YR, Shiffman J. Generating global priority for addressing rheumatic heart disease: a qualitative policy analysis. J Am Heart Assoc. 2020;9:e014800. doi: 10.1161/JAHA.119.014800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Regmi PR, Upadhyaya AB. Rheumatic Fever (RF) and Rheumatic Heart Disease (RHD) Prevention and COntrol Program in Nepal. Nepal. Heart J. 2017;6:88–93. doi: 10.3126/njh.v6i1.18603 [DOI] [Google Scholar]
- 13. Hugo‐Hamman C, Forster N. National advisory committee for the prevention and control of rheumatic fever and rheumatic heart disease in Namibia: cardio news. Cardiovasc J Afr. 2015;26:251. [PubMed] [Google Scholar]
- 14. White A. WHO Resolution on rheumatic heart disease. Eur Heart J. 2018;39:4233. doi: 10.1093/eurheartj/ehy764 [DOI] [PubMed] [Google Scholar]
- 15. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi‐Kangevari M, Abbastabar H, Abd‐Allah F, Abdelalim A, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi‐Kangevari M, Abbastabar H, Abd‐Allah F, Abdelalim A, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. 2021 ICD‐10‐CM Codes I05‐I09: chronic rheumatic heart diseases. Available at: https://www.icd10data.com/ICD10CM/Codes/I00‐I99/I05‐I09. Accessed August 21, 2021.
- 18. Azadnajafabad S, Saeedi Moghaddam S, Mohammadi E, Rezaei N, Ghasemi E, Fattahi N, Aminorroaya A, Azadnajafabad R, Aryannejad A, Rezaei N, et al. Global, regional, and national burden and quality of care index (QCI) of thyroid cancer: a systematic analysis of the Global Burden of Disease Study 1990–2017. Cancer Med. 2021;10:2496–2508. doi: 10.1002/cam4.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mokdad AH, Mensah GA, Krish V, Glenn SD, Miller‐Petrie MK, Lopez AD, Murray CJL. Global, regional, national, and subnational big data to inform health equity research: perspectives from the Global Burden of Disease Study 2017. Ethn Dis. 2019;29:159–172. doi: 10.18865/ed.29.S1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Y, Fu WJ, Land KC. A Methodological comparison of age‐period‐cohort models: the intrinsic estimator and conventional generalized linear models. Sociol Methodol. 2004;34:75–110. [Google Scholar]
- 21. Lilyasari O, Prakoso R, Kurniawati Y, Roebiono PS, Rahajoe AU, Sakidjan I, Harimurti GM. Clinical profile and management of rheumatic heart disease in children and young adults at a tertiary cardiac center in Indonesia. Front Surg. 2020;7:47. doi: 10.3389/fsurg.2020.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, Sable C, Steer A, Wilson N, Wyber R, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:1–24. doi: 10.1038/nrdp.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noubiap JJ, Agbor VN, Bigna JJ, Kaze AD, Nyaga UF, Mayosi BM. Prevalence and progression of rheumatic heart disease: a global systematic review and meta‐analysis of population‐based echocardiographic studies. Sci Rep. 2019;9:1–14. doi: 10.1038/s41598-019-53540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parks T, Kado J, Miller AE, Ward B, Heenan R, Colquhoun SM, Bärnighausen TW, Mirabel M, Bloom DE, Bailey RL, et al. Rheumatic heart disease‐attributable mortality at ages 5–69 years in Fiji: a five‐year, national, population‐based record‐linkage cohort study. PLoS Negl Trop Dis. 2015;9:5–69. doi: 10.1371/JOURNAL.PNTD.0004033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horwood PF, Tarantola A, Goarant C, Matsui M, Klement E, Umezaki M, Navarro S, Greenhill AR. Health challenges of the pacific region: insights from history, geography, social determinants, genetics, and the microbiome. Front Immunol. 2019;10:2184. doi: 10.3389/fimmu.2019.02184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis. 2018;12:e0006577. doi: 10.1371/JOURNAL.PNTD.0006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rheumatic Fever Prevention Programe | Ministry of Health NZ. Available at: https://www.health.govt.nz/our‐work/diseases‐and‐conditions/rheumatic‐fever. Accessed April 19, 2022.
- 28. Wyber R, Noonan K, Halkon C, Enkel S, Cannon J, Haynes E, Mitchell AG, Bessarab DC, Katzenellenbogen JM, Bond‐Smith D, et al. Ending rheumatic heart disease in Australia: the evidence for a new approach. Med J Aust. 2020;213(suppl 10):S3–S31. doi: 10.5694/mja2.50853 [DOI] [PubMed] [Google Scholar]
- 29. Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol. 2013;10:284–292. doi: 10.1038/nrcardio.2013.34 [DOI] [PubMed] [Google Scholar]
- 30. McCall C. Rheumatic heart disease in the Pacific island nations. Lancet. 2018;391:926. doi: 10.1016/S0140-6736(18)30615-9 [DOI] [PubMed] [Google Scholar]
- 31. Beaton A, Carapetis J. The 2015 revision of the Jones criteria for the diagnosis of acute rheumatic fever: implications for practice in low‐income and middle‐income countries. Heart Asia. 2015;7:7. doi: 10.1136/heartasia-2015-010648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Danaei G, Farzadfar F, Kelishadi R, Rashidian A, Rouhani OM, Ahmadnia S, Ahmadvand A, Arabi M, Ardalan A, Arhami M, et al. Iran in transition. Lancet. 2019;393:1984–2005. doi: 10.1016/S0140-6736(18)33197-0 [DOI] [PubMed] [Google Scholar]
- 33. Farzadfar F, Naghavi M, Sepanlou SG, Saeedi Moghaddam S, Dangel WJ, Davis Weaver N, Aminorroaya A, Azadnajafabad S, Koolaji S, Mohammadi E, et al. Health system performance in Iran: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2022;399:1625–1645. doi: 10.1016/s0140-6736(21)02751-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rheumatic heart disease. Available at: https://www.who.int/news‐room/fact‐sheets/detail/rheumatic‐heart‐disease. Accessed August 21, 2021.
- 35. Graham K, Sinyangwe C, Nicholas S, King R, Mukupa S, Källander K, Counihan H, Montague M, Tibenderana J, Hamade P. Rational use of antibiotics by community health workers and caregivers for children with suspected pneumonia in Zambia: a cross‐sectional mixed methods study. BMC Public Health. 2016;16:897. doi: 10.1186/s12889-016-3541-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2
Data S1


