Abstract
Background
The sympathetic cotransmitter, neuropeptide Y (NPY), is released into the coronary sinus during ST‐segment–elevation myocardial infarction and can constrict the coronary microvasculature. We sought to establish whether peripheral venous (PV) NPY levels, which are easy to obtain and measure, are associated with microvascular obstruction, myocardial recovery, and prognosis.
Methods and Results
NPY levels were measured immediately after primary percutaneous coronary intervention and compared with angiographic and cardiovascular magnetic resonance indexes of microvascular function. Patients were prospectively followed up for 6.4 (interquartile range, 4.1–8.0) years. PV (n=163) and coronary sinus (n=68) NPY levels were significantly correlated (r=0.92; P<0.001) and associated with multiple coronary and imaging parameters of microvascular function and infarct size (such as coronary flow reserve, acute myocardial edema, left ventricular ejection fraction, and late gadolinium enhancement 6 months later). We therefore assessed the prognostic value of PV NPY during follow‐up, where 34 patients (20.7%) developed heart failure or died. Kaplan‐Meier survival analysis demonstrated that high PV NPY levels (>21.4 pg/mL by binary recursive partitioning) were associated with increased incidence of heart failure and mortality (hazard ratio, 3.49 [95% CI, 1.65–7.4]; P<0.001). This relationship was maintained after adjustment for age, cardiovascular risk factors, and previous myocardial infarction.
Conclusions
Both PV and coronary sinus NPY levels correlate with microvascular function and infarct size after ST‐segment–elevation myocardial infarction. PV NPY levels are associated with the subsequent development of heart failure or mortality and may therefore be a useful prognostic marker. Further research is required to validate these findings.
Keywords: biomarker, cardiovascular magnetic resonance imaging, microvasculature, percutaneous coronary intervention, prognosis, sympathetic cotransmitter
Subject Categories: Autonomic Nervous System, Biomarkers, Coronary Circulation, Ischemia, Risk Factors, Heart Failure, Myocardial Infarction, Angiography, Magnetic Resonance Imaging (MRI), Prognosis, Percutaneous Coronary Intervention, Revascularization, Acute Coronary Syndromes, Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- CS
coronary sinus
- NPY
neuropeptide Y
- PPCI
primary percutaneous coronary intervention
- PV
peripheral venous
Clinical Perspective.
What Is New?
Neuropeptide‐Y levels, when measured from a peripheral vein at the time of primary percutaneous coronary intervention, correlate with coronary microvascular dysfunction, greater myocardial injury, reduced left ventricular ejection fraction 6 months after ST‐segment–elevation myocardial infarction, and subsequent heart failure and mortality over a median follow‐up of 6.4 years, even after adjustment for age and cardiovascular risk factors.
We provide a definition of high peripheral venous neuropeptide Y that is associated with subsequent heart failure and mortality.
What Are the Clinical Implications?
Neuropeptide Y, which can easily and safely be measured from a peripheral vein at a single time point after primary percutaneous coronary intervention, may be a useful biomarker to guide prognosis.
It may also be a useful theranostic biomarker to guide the use of neuropeptide‐Y receptor antagonists given previous observations of the ability of such drugs to reduce infarct size in an animal model.
In patients with acute ST‐segment–elevation myocardial infarction (STEMI), the immediate aim is to restore coronary perfusion by expeditious revascularization of the infarct‐related epicardial vessel using primary percutaneous coronary intervention (PPCI). Despite this, one third of patients do not regain satisfactory myocardial reperfusion, experiencing a phenomenon known as “no‐reflow.” This involves an ongoing flow restriction in the microcirculation and is associated with prolonged ST‐segment elevation, larger infarct volume, lower left ventricular ejection fraction (LVEF), recurrent heart failure admission, and death. 1 The cause for this remains unclear but is likely multifaceted. 2 Downstream embolization of fragments of clot or thrombus into the microvasculature is thought to contribute, but during PPCI, only low volumes of these embolic particles have been observed, and clinical trials suggest intraprocedural thrombectomy to be of little benefit. 3 , 4 Ischemia‐reperfusion–related tissue swelling may lead to microvascular compression, direct endothelial damage, widespread platelet and neutrophil activation, and the formation of platelet plugs. 5 , 6 Functional vasoconstriction of the microcirculation is also emerging as a potentially important mechanism in the pathogenesis of no‐reflow and occurs in response to locally released vasoactive compounds that occur during STEMI. 7 , 8 , 9
Neuropeptide Y (NPY) is a cotransmitter that is released alongside norepinephrine from sympathetic nerve terminals, particularly during conditions of sympathetic hyperactivity, such as myocardial infarction. 10 NPY is known to be the most abundant neuropeptide in the heart and is significantly increased at the time of PPCI for STEMI, remaining high for at least 48 hours following revascularization. 11 Clinical studies before the development of PPCI demonstrated higher peripheral levels of “NPY‐like activity” during myocardial infarction correlated with a higher incidence of mortality at 1 year. 12 NPY is a potent vasoconstrictor, and earlier studies have shown coronary artery infusion of NPY in humans led to typical ischemic ECG changes and chest pain, without significant epicardial artery vasoconstriction. 13 A recent study by our group suggests that this may be a result of NPY causing selective constriction of the coronary microvasculature via the Y1 receptor. 14 This study also demonstrated that high coronary sinus (CS) NPY levels in 45 patients undergoing PPCI for STEMI correlated with increased microcirculatory dysfunction at the time of PPCI. However, CS blood sampling is challenging and requires a further invasive catheter procedure. It remains unclear whether a similar relationship exists for other measures of circulating NPY and whether this can be used to guide prognosis.
Accordingly, we examine a large cohort of patients to see whether peripheral venous (PV) NPY or the transcardiac NPY gradient (CS‐arterial difference) is closely associated with severe microvascular obstruction and reduced myocardial recovery, as seen with CS NPY. Given that the most pragmatic measurement to obtain clinically is PV NPY, we sought to ascertain whether this was associated with the development of heart failure or death in the OxAMI (Oxford Acute Myocardial Infarction) Study.
Methods
See Data S1 Supplement for expanded methods. Local research ethics committee (REC 10/H0408/24) and institutional review board committee approval was granted, and the study complied with the Declaration of Helsinki. All study participants gave written informed consent. Patients were prospectively enrolled as part of the OxAMI Study. All data are available on reasonable request.
Results
A total of 164 patients with STEMI were recruited and underwent PV (n=163) and/or CS and coronary arterial (n=68) blood sampling immediately after PPCI. The baseline clinical characteristics of these patients are summarized in Table 1. Most patients (76.8%) were men, with a mean age of 62.4 years, and experienced predominantly left anterior descending artery infarcts (53.7%). Overall PV and CS NPY levels were similar (20.5 [interquartile range, 10.1–34.0] versus 28.7 [interquartile range, 19.0–48.5] pg/mL) and significantly positively correlated with one another (r=0.92; n=67; P<0.001). There was no significant correlation between the CS‐A difference (−0.4 [interquartile range, −4.5 to 4.1] pg/mL) and CS NPY levels (r=0.08; n=68; P=0.52).
Table 1.
Patient Characteristics
| Baseline characteristics | PV blood sampling (n=163) | CS and aortic blood sampling (n=68) | Total (n=164) |
|---|---|---|---|
| Age, y | 62.4±11.9 | 62.8±12.7 | 62.4±11.9 |
| Men | 125 (76.7) | 51 (75.0) | 126 (76.8) |
| Cardiovascular risk factors | |||
| Previous myocardial infarction | 13 (9.6) | 4 (5.9) | 14 (10.2) |
| Hypertension | 73 (44.8) | 27 (39.7) | 74 (45.1) |
| Diabetes | 18 (11.0) | 7 (10.3) | 18 (10.9) |
| Hypercholesterolemia | 71 (43.6) | 25 (36.8) | 72 (43.9) |
| Smoking history | 109 (66.9) | 54 (79.4) | 110 (67.1) |
| Family history of coronary disease | 57 (35.8) | 27 (42.2) | 57 (35.6) |
| On‐admission medications | |||
| β‐Blocker | 26 (15.9) | 7 (10.3) | 26 (15.8) |
| ACE inhibitor/At II receptor blocker | 34 (20.8) | 10 (14.7) | 34 (20.7) |
| Statin | 36 (22.1) | 13 (19.1) | 36 (22.0) |
| Observations | |||
| Systolic blood pressure, mm Hg | 133.9±25.8 | 131.9±27.2 | 133.8±25.7 |
| Diastolic blood pressure, mm Hg | 81.4±17.6 | 82.4±19.4 | 81.4±17.5 |
| Heart rate, bpm | 77.7±19.0 | 79.8±19.7 | 77.6±18.9 |
| Peak troponin I, mg/L | 42.7±25.9 | 41.4±15.6 | 42.5±26.0 |
| Pain‐to‐balloon time, min | 174.0 (120.0–282.0) | 180.0 (120.0–313.5) | 174.5 (120.0–279.0) |
| Infarct artery | |||
| LAD | 86 (53.4) | 55 (82.1) | 87 (53.7) |
| LCx/Int | 20 (12.3) | 12 (17.9) | 20 (12.2) |
| RCA | 56 (34.6) | 0 (0) | 56 (34.4) |
Values are mean±SD, number (percentage), or median (interquartile range). ACE indicates angiotensin‐converting enzyme; At II, angiotensin II; bpm, beats per minute; CS, coronary sinus; Int, intermediate artery; LAD, left anterior descending artery; LCx, left circumflex artery; PV, peripheral venous; and RCA, right coronary artery.
Correlations of PV, CS, and Transcardiac Gradient NPY Levels With Invasive and Imaging Measures of Microvascular Function and Imaging Measures of Left Ventricular Functional Recovery
Like CS NPY levels, PV NPY levels (but not a CS‐A difference) correlated significantly with a lower coronary flow reserve measured via coronary flow wire (Table 2). PV NPY levels also correlated with several cardiac magnetic resonance imaging parameters of myocardial injury and subsequent recovery following PPCI, as observed with CS NPY levels. These include a significant positive correlation with the extent of myocardial edema observed at 2 days, a positive correlation with late gadolinium enhancement (LGE) extent, and inverse correlation with LVEF 6 months after PPCI for STEMI, as illustrated in Table 2. No significant correlations were observed for the CS‐A difference in NPY levels for any invasive or imaging parameters.
Table 2.
Correlations Between Arterial NPY Levels, Coronary Hemodynamics, and Cardiac Magnetic Resonance Measurements
| Variable | CS (n=68) | PV (n=163) | CS‐A (n=68) | |||
|---|---|---|---|---|---|---|
| R | P value | R | P value | R | P value | |
| Coronary hemodynamics | ||||||
| Coronary flow reserve | −0.24 | <0.05* | −0.23 | <0.01* | −0.13 | 0.30 |
| Index of microcirculatory resistance | 0.09 | 0.50 | 0.03 | 0.72 | −0.14 | 0.26 |
| Post‐PPCI cardiac MRI | ||||||
| Ejection fraction | −0.18 | 0.22 | −0.26 | 0.01* | −0.06 | 0.66 |
| Microvascular obstruction | 0.49 | <0.001* | 0.09 | 0.42 | −0.14 | 0.35 |
| Edema (% LV) | 0.49 | <0.001* | 0.25 | 0.02* | −0.03 | 0.82 |
| Late gadolinium enhancement | 0.36 | 0.01* | 0.07 | 0.52 | −0.12 | 0.41 |
| End‐diastolic volume | 0.12 | 0.42 | 0.12 | 0.23 | 0.09 | 0.53 |
| End‐systolic volume | 0.15 | 0.35 | 0.20 | 0.05* | 0.18 | 0.28 |
| 6‐mo Cardiac MRI | ||||||
| Ejection fraction | −0.43 | 0.01* | −0.26 | 0.02* | 0.29 | 0.08 |
| End‐diastolic volume | 0.08 | 0.61 | 0.09 | 0.43 | −0.07 | 0.69 |
| End‐systolic volume | 0.23 | 0.16 | 0.23 | 0.03* | −0.19 | 0.25 |
| Late gadolinium enhancement | 0.57 | <0.001* | 0.27 | 0.01* | −0.11 | 0.52 |
CS indicates coronary sinus; LV, left ventricle; MRI, magnetic resonance imaging; NPY, neuropeptide Y; PPCI, primary percutaneous coronary intervention; and PV, peripheral venous.
indicates statistical significance.
Survival Analysis
Patients were followed up for a period of 6.4 (interquartile range, 4.1–8.0) years following the index event. During follow‐up, 20 patients (12.2%) developed heart failure, 20 patients (12.2%) died, and 34 patients (20.7%) in total experienced events, reaching the composite primary end point of heart failure or mortality. Patients in the events group were more likely to experience hypertension, diabetes, or hypercholesterolemia, and admission heart rate was significantly higher (Table 3). TIMI (Thrombolysis in Myocardial Infarction) flow at presentation and pain‐to‐balloon time were similar in both groups. Coronary flow reserve was significantly lower in those experiencing events, and index of microcirculatory resistance was higher (Table 3). The cardiovascular magnetic resonance scan at 6 months, however, revealed significantly larger infarct size (as measured by LGE extent) in patients experiencing heart failure or death, as well as a trend toward a lower LVEF. PV NPY was significantly higher in those patients who sustained adverse events compared with those who did not (28.6 versus 18.5 pg/mL; P=0.03) (Table 3).
Table 3.
Patient Characteristics by Clinical Outcome
| Baseline characteristics | Heart failure/mortality (n=34) | Event‐free survival (n=130) | P value |
|---|---|---|---|
| Age, y | 71.3±10.5 | 60.1±11.2 | <0.000001* |
| Men | 27 (79.4) | 99 (76.2) | 0.69 |
| Cardiovascular risk factors | |||
| Hypertension | 22 (64.7) | 52 (40) | 0.01* |
| Diabetes | 11 (32.4) | 7 (5.4) | <0.00001* |
| Hypercholesterolemia | 21 (61.8) | 51 (39.2) | 0.02* |
| Smoking history | 21 (61.8) | 89 (68.5) | 0.46 |
| Previous myocardial infarction | 4 (12.9) | 10 (9.5) | 0.59 |
| Family history of coronary disease | 11 (32.4) | 46 (36.5) | 0.65 |
| Observations | |||
| Systolic blood pressure, mm Hg | 140.3±24.1 | 132.2±26.0 | 0.11 |
| Diastolic blood pressure, mm Hg | 80.6±14.0 | 81.6±18.3 | 0.73 |
| Heart rate, bpm | 88 (72–100) | 71 (63–84.3) | <0.01* |
| Peak troponin I, mg/L | 50 (43.6–50) | 50 (25.4–50) | 0.16 |
| Pain‐to‐balloon time, min | 175 (130–300) | 174 (120–268) | 0.53 |
| Infarct artery | |||
| LAD | 20 (58.8) | 67 (52.3) | 0.50 |
| LCx/Int | 7 (20.6) | 13 (10.1) | 0.10 |
| RCA | 7 (20.6) | 49 (38.0) | 0.06 |
| TIMI flow at presentation | |||
| 0 | 26 (86.7) | 80 (72.7) | 0.21 |
| 1 | 1 (3.2) | 9 (8.2) | 0.34 |
| 2 | 1 (3.2) | 14 (12.7) | 0.13 |
| 3 | 3 (9.7) | 6 (5.4) | 0.61 |
| Coronary hemodynamics | |||
| CFR | 1.3 (1.0–1.6) | 1.6 (1.2–2.2) | 0.01* |
| IMR | 39.6 (27.9–96.4) | 26.4 (18.0–42.0) | <0.01* |
| Cardiac MRI | |||
| MVO, % | 1.3 (0–6.3) | 1.0 (0–3.8) | 0.66 |
| Ejection fraction at 48 h, % | 45.3±11.4 | 48.4±8.9 | 0.22 |
| LGE at 48 h, % | 30.6±19.5 | 40.0±13.5 | 0.95 |
| Edema, % | 36.8±20.2 | 42.7±13.0 | 0.28 |
| Ejection fraction at 6 mo, % | 49.0 (43.3–58.5) | 57 (47.5–61.3) | 0.06 |
| LGE at 6 mo, % | 29.0±13.2 | 19.0±12.3 | <0.01* |
| Peripheral venous NPY, pg/mL | 28.6 (13.5–49.0) | 18.5 (9.5–32.2) | 0.03* |
Values are mean±SD, number (percentage), or median (interquartile range). Bpm indicates beats per minute; CFR, coronary flow reserve; IMR, index of microvascular resistance; Int, intermediate artery; LAD, left anterior descending artery; LCx, left circumflex artery; LGE, late gadolinium enhancement; MRI, magnetic resonance imaging; MVO, microvascular obstruction; NPY, neuropeptide Y; RCA, right coronary artery; and TIMI, thrombolysis in myocardial infarction.
inidicates statistical significance.
PV NPY as a continuous variable was associated with heart failure or death, with an estimated hazard ratio (HR) of 1.014 (95% CI, 1.006–1.022; P<0.001). A multivariable Cox proportional hazard model, adjusting for age, sex, smoking, hypertension, hypercholesterolemia, diabetes, family history of cardiovascular disease, and previous myocardial infarction, did not affect the association of PV NPY with the combined end point (HR, 1.011 [95% CI, 1.001–1.021]; P=0.03). We then used binary recursive partitioning analysis to define a PV NPY threshold that best identifies patients reaching the primary outcome. This cutoff (21.4 pg/mL) had a C statistic of 0.62 (95% CI, 0.52–0.73; P=0.03) for the combined end point (heart failure or death), whereas heart failure diagnosis as an independent end point had a C statistic of 0.67 (95% CI, 0.56–0.79; P=0.01). Patients with high PV NPY were older, but otherwise the 2 groups were well matched in terms of cardiovascular risk factors (Table 4). Patients with high PV NPY had similar pain‐to‐balloon times and TIMI flow at presentation, but lower coronary flow reserve and a trend toward greater myocardial edema. Cardiovascular magnetic resonance imaging in patients with high PV NPY revealed a significantly higher percentage of LGE and lower LVEF at 6 months. Kaplan‐Meier survival analysis demonstrated that high PV NPY levels are associated with an increased incidence of death (HR, 3.07 [95% CI, 1.18–7.97]; P=0.02), heart failure (HR, 6.16 [95% CI, 2.04–18.6]; P<0.001), or both (HR, 3.49 [95% CI, 1.65–7.4]; P<0.001) (Figure – Panels A, C, and E). Adjusting for age, sex, smoking, hypertension, hypercholesterolemia, diabetes, family history of cardiovascular disease, and previous myocardial infarction did not affect the association of high PV NPY with the combined end point (HR, 3.31 [95% CI, 1.28–8.53]; P=0.01) or heart failure (HR, 30.1 [95% CI, 3.23–280.42]; P=0.003), but PV NPY was not a significant independent risk factor for death alone following adjustment (P=0.66), as shown in Figure – Panels B, D, and F.
Table 4.
Patient Characteristics by High Versus Low PV NPY Levels
| Baseline characteristics |
High NPY (≥21.4 pg/mL; n=78) |
Low NPY (<21.4 pg/mL; n=85) | P value |
|---|---|---|---|
| Age, y | 65.8±12.4 | 59.2±10.7 | <0.001* |
| Men | 55 (70.5) | 70 (82.4) | 0.08 |
| Cardiovascular risk factors | |||
| Hypertension | 40 (51.2) | 33 (38.9) | 0.11 |
| Diabetes | 8 (10.3) | 10 (11.8) | 0.76 |
| Hypercholesterolemia | 37 (47.4) | 34 (40.0) | 0.34 |
| Smoking history | 53 (67.9) | 56 (65.9) | 0.78 |
| Previous myocardial infarction | 6 (9.0) | 7 (10.3) | 0.79 |
| Family history of coronary disease | 27 (36.0) | 30 (35.7) | 0.97 |
| Observations | |||
| Systolic blood pressure, mm Hg | 136.4±26.8 | 131.7±24.7 | 0.25 |
| Diastolic blood pressure, mm Hg | 81.9±18.7 | 81.0±16.5 | 0.77 |
| Heart rate, bpm | 78 (63–90) | 72 (66–85) | 0.57 |
| Peak troponin I, mg/L | 50 (38.7–50) | 50 (21.9–50) | 0.31 |
| Pain‐to‐balloon time, min | 166 (120–270) | 176 (121.8–282.5) | 0.61 |
| Infarct artery | |||
| LAD | 48 (63.2) | 38 (44.7) | 0.02* |
| LCx/Int | 10 (13.0) | 10 (11.7) | 0.81 |
| RCA | 19 (24.6) | 37 (43.5) | 0.01* |
| TIMI flow at presentation | |||
| 0 | 44 (75.9) | 62 (74.7) | 0.88 |
| 1 | 4 (6.9) | 6 (7.2) | 0.94 |
| 2 | 7 (12) | 8 (9.6) | 0.65 |
| 3 | 3 (5.2) | 7 (8.4) | 0.46 |
| Coronary hemodynamics | |||
| CFR | 1.3 (1.1–1.9) | 1.7 (1.3–2.2) | 0.04* |
| IMR | 31 (20.2–52.2) | 29.6 (18.9–43.5) | 0.54 |
| Cardiac MRI | |||
| MVO, % | 1 (0–5.2) | 1 (0–3.5) | 0.30 |
| Ejection fraction at 48 h, % | 46.0±10.0 | 49.3±8.6 | 0.09 |
| LGE at 48 h, % | 32.7±14.9 | 29.9±13.9 | 0.35 |
| Edema, % | 44.8±13.9 | 39.6±14.6 | 0.08 |
| Ejection fraction at 6 mo, % | 53 (43–57) | 58 (50–64) | 0.01* |
| LGE at 6 mo, % | 24.3±13.4 | 17.7±11.9 | 0.02* |
Values are mean±SD, number (percentage), or median (interquartile range). Bpm indicates beats per minute; CFR, coronary flow reserve; IMR, index of microvascular resistance; Int, intermediate artery; LAD, left anterior descending artery; LCx, left circumflex artery; LGE, late gadolinium enhancement; MRI, magnetic resonance imaging; MVO, microvascular obstruction; NPY, neuropeptide Y; PV, peripheral venous; RCA, right coronary artery; and TIMI, thrombolysis in myocardial infarction.
indicates statistical significance.
Figure 1. Kaplan‐Meier survival analysis, illustrating event‐free survival following ST‐segment–elevation myocardial infarction, according to peripheral venous neuropeptide‐Y (NPY) levels before (A, C, and E) and after (B, D, and F) adjustment for age, sex, hypertension, diabetes, hypercholesterolemia, family history of coronary artery disease, smoking history, and previous myocardial infarction.
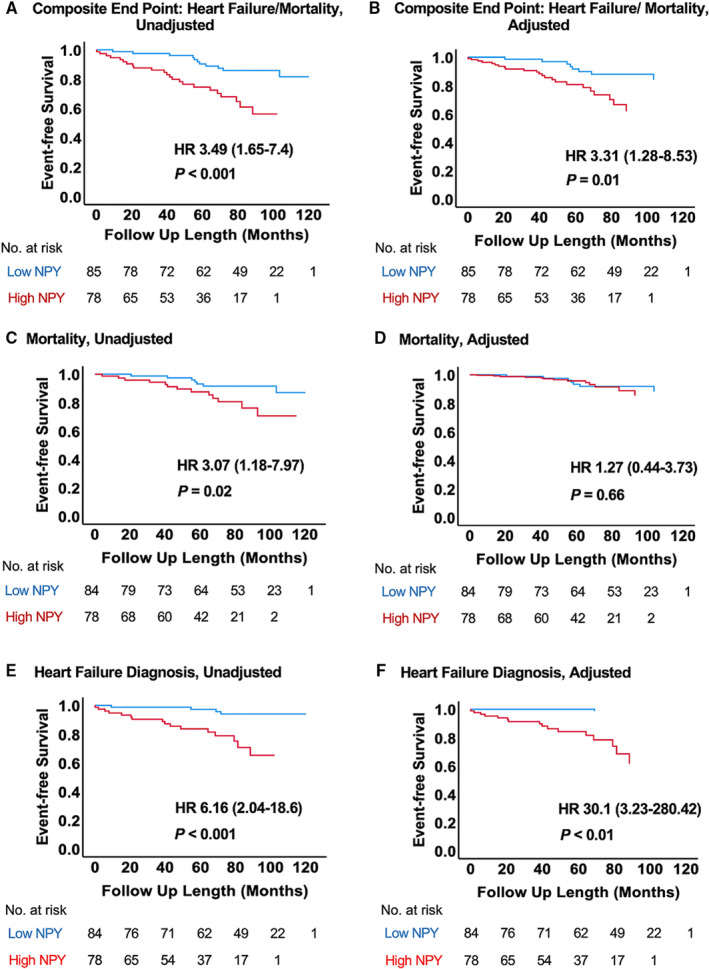
Binary recursive partitioning analysis was used to derive a cutoff for high and low NPY (≥21.4 and <21.4 pg/mL, respectively). A and B, Events are of a composite of death and heart failure diagnosis (n=34). C and D, Events are all‐cause mortality (n=20). E and F, Events are heart failure diagnosis (n=20). HR indicates hazard ratio.
Discussion
In this study, we show that high peripheral venous NPY levels, analyzed immediately after PPCI, correlate with coronary microvascular dysfunction, greater myocardial injury, and reduced LVEF 6 months after STEMI. Moreover, high PV NPY levels are associated with subsequent heart failure and mortality, even after adjustment for age and cardiovascular risk factors.
These findings build on our previous observations in a small translational study, where we showed that CS NPY levels are elevated during STEMI and are associated with increased microvascular dysfunction. The present study confirms that CS NPY has the strongest relationship with indexes of reperfusion and left ventricular functional recovery, probably because it more accurately reflects local cardiac NPY levels that the coronary microcirculation is exposed to. PV NPY levels also correlate well with these parameters, despite additional contributions from hepatic and mesenteric release. 15 Interestingly, there was no significant relationship between indexes of reperfusion and the transcardiac NPY gradient, which was small in magnitude. Peripheral and cardiac NPY levels are extremely high during STEMI, and it is likely that they will have equilibrated by the time of PPCI, often several hours from the onset of chest pain and heightened sympathetic drive.
We have recently shown that CS NPY levels are associated with adverse clinical outcomes in patients with stable chronic heart failure who are undergoing implantation of cardiac resynchronization devices. 16 However, CS NPY is not practical to obtain routinely, and requires a second central venous cannulation procedure that introduces additional risk. In contrast, NPY can be measured easily and safely from a peripheral vein, and the major finding of the present study is that high levels of PV NPY are associated with the development of heart failure or death after reperfusion. This relationship is maintained even after adjustment for age, sex, and major cardiovascular risk factors, including hypertension, diabetes, high cholesterol, family history, smoking status, and previous myocardial infarction. Thus, a simple and readily accessible PV NPY measurement is a potentially useful biomarker in this cohort that could offer incremental information on other known prognosticators. Indeed, NPY at the time of PPCI for STEMI correlates strongly with infarct size, as measured by LGE and ejection fraction at 6 months, and has a similar relationship with events on univariable and multivariable analysis.
NPY has previously been shown to induce myocardial ischemia, demonstrable through ECG ST‐T wave changes, reduction of intramyocardial pH, and LVEF in dogs. 17 Exogenous administration of NPY in humans with microvascular angina induced transient myocardial ischemia despite minimal vasospasm of the epicardial coronary arteries. 18 We have shown that NPY, which has a long plasma half‐life, is significantly elevated during PPCI for STEMI and remains high for at least 48 hours. 11 In rats, we have explored the mechanism by which NPY constricts the microcirculation via a Y1 receptor pathway and demonstrated that this receptor is also expressed on vascular smooth muscle cells in the media of human coronary microarteries. 14 The Y1 receptor is also expressed on ventricular myocytes and can lead to calcium loading and increased propensity to arrhythmia, even in the presence of β‐blockade. 19 In a rat model of STEMI, we have provided proof of principle that antagonism of the Y1 receptor can reduce infarct size and the incidence of ventricular arrhythmia after subsequent reperfusion. 14 , 19
Also, there are other ways in which NPY plays an important mechanistic role in the pathophysiology of atherosclerosis, STEMI, and ischemic heart failure. For example, genetic polymorphisms in the NPY gene and those of several of its receptors are associated with early‐onset atherosclerosis. 10 NPY can be taken up into megakaryocytes; and following plaque rupture, activated platelets may release NPY locally. Although in the short‐term, NPY may cause microvascular constriction, in the longer‐term, an elevation in dipeptidyl peptidase‐4 expression within the endothelium may increase cleavage of NPY1–36 to NPY3–36, which has a higher binding affinity for Y2 and Y5 receptors, promoting angiogenesis. Dipeptidyl peptidase‐4 inhibitors used to treat type 2 diabetes are associated with a significant increase in the risk of serious heart failure events in several large clinical trials. 20 In the short‐term, NPY may reduce vagal acetylcholine release via the Y2 receptor and directly maintain cardiac contraction and inotropy, but in the longer‐term, it promotes ventricular myocyte hypertrophy. 10 , 21 , 22 It is interesting to note that with our data, the relationship between high PV NPY and heart failure or mortality is lost during multivariable Cox regression analysis after adjustment for coronary flow reserve, LGE extent, and LVEF at 6 months (P=0.41). Mechanistically, this is consistent with the hypothesis that NPY contributes to microvascular dysfunction and subsequent infarct size, leading to heart failure and mortality following STEMI, rather than having other independent effects outside that of influencing infarct size.
Measuring PV NPY levels may identify patients in whom closer monitoring and more aggressive interventions are required. It is cheaper and more practical to measure PV NPY than routinely perform coronary flow wire measurements of microvascular function or undertake cardiovascular magnetic resonance at both 2 days and 6 months following the event, which also offer valuable prognostic information but are not readily available in all centers. 23 , 24 , 25 , 26 In addition to standard pharmacotherapy, antagonizing NPY Y1 receptors may also have the potential to mitigate the effects of no‐reflow following STEMI, leading to improved outcomes in selected patients with high initial PV NPY. In this way, high PV NPY has the potential to also be a theranostic biomarker.
Study Limitations
Conceivably, CS NPY could more strongly correlate with events, given its stronger association with microvascular resistance, and a further study powered to evaluate this association would be informative. In addition to this, more precise temporal dynamics of circulating NPY following PPCI for STEMI may be more strongly correlated with poorer outcomes, although this would be more challenging to obtain clinically compared with a test at a single time point when NPY levels are at their highest.
Conclusions
Both PV and CS NPY levels significantly correlate with microvascular function and infarct size after STEMI, whereas the CS‐A difference in NPY levels does not, presumably as both cardiac and peripheral NPY release is extremely high and equilibrated by the time of PPCI. High PV NPY levels, which are easy to obtain and measure following coronary intervention, are independently associated with subsequent heart failure and death and could prove to be a useful biomarker in risk stratifying these patients.
Appendix
OxAMI (Oxford Acute Myocardial Infarction) Study Investigators
Robin P. Choudhury, DM; Rajesh K. Kharbanda, MBChB, PhD; Adrian P. Banning, MBBS, MD; Jeremy P. Langrish, MB BCh, PhD; Andrew Lucking, MBChB, PhD; Sam Dawkins, MBBS, DPhil; Giovanni Luigi De Maria, MD, PhD; Vanessa M. Ferreira, MD, DPhil; Keith M. Channon, MD, PhD.
Sources of Funding
The study was supported by a British Heart Foundation Senior Clinical Research Fellowship (FS/SCRF/20/32005) to Dr Herring, a BHF Chair award (CH/16/1/32013) to Dr Channon, the BHF Oxford Centre of Research Excellence (RE/13/1/30181), and the National Institute for Health Research Oxford Biomedical Research Centre.
Disclosures
None.
Supporting information
Acknowledgments
This study would also not have been possible without the tireless support of the coronary care unit and catheter laboratory staff of the Oxford Heart Centre at the John Radcliffe Hospital. The contributions of colleagues in the Oxford Acute Vascular Imaging Centre are also acknowledged. We are also grateful to the patients who participated.
The OxAMI (Oxford Acute Myocardial Infarction) Study Investigators are listed in the Appendix.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024850
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Neil Herring, Email: neilherring@doctors.org.uk.
OxAMI (Oxford Acute Myocardial Infarction) Study:
Keith M. Channon, Vanessa M. Ferreira, Giovanni Luigi De Maria, Sam Dawkins, Andrew Lucking, Jeremy P. Langrish, Adrian P. Banning, Rajesh K. Kharbanda, and Robin P. Choudhury
References
- 1. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no‐reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054 [DOI] [PubMed] [Google Scholar]
- 2. Heusch G. Coronary microvascular obstruction: the new Frontier in cardioprotection. Basic Res Cardiol. 2019;114:45. doi: 10.1007/s00395-019-0756-8 [DOI] [PubMed] [Google Scholar]
- 3. Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, Kedev S, Thabane L, Stankovic G, Moreno R, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389–1398. doi: 10.1056/NEJMoa1415098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhindi R, Kajander OA, Jolly SS, Kassam S, Lavi S, Niemela K, Fung A, Cheema AN, Meeks B, Alexopoulos D, et al. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention‐alone in ST‐segment elevation myocardial infarction: the optical coherence tomography sub‐study of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Eur Heart J. 2015;36:1892–1900. doi: 10.1093/eurheartj/ehv176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kloner RA, Ganote CE, Jennings RB. The "no‐reflow" phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 7. Jeong YH, Kim WJ, Park DW, Choi BR, Lee SW, Kim YH, Lee CW, Hong MK, Kim JJ, Park SW, et al. Serum B‐type natriuretic peptide on admission can predict the 'no‐reflow' phenomenon after primary drug‐eluting stent implantation for ST‐segment elevation myocardial infarction. Int J Cardiol. 2010;141:175–181. doi: 10.1016/j.ijcard.2008.11.189 [DOI] [PubMed] [Google Scholar]
- 8. Niccoli G, Giubilato S, Russo E, Spaziani C, Leo A, Porto I, Leone AM, Burzotta F, Riondino S, Pulcinelli F, et al. Plasma levels of thromboxane A2 on admission are associated with no‐reflow after primary percutaneous coronary intervention. Eur Heart J. 2008;29:1843–1850. doi: 10.1093/eurheartj/ehn325 [DOI] [PubMed] [Google Scholar]
- 9. Niccoli G, Lanza GA, Shaw S, Romagnoli E, Gioia D, Burzotta F, Trani C, Mazzari MA, Mongiardo R, De Vita M, et al. Endothelin‐1 and acute myocardial infarction: a no‐reflow mediator after successful percutaneous myocardial revascularization. Eur Heart J. 2006;27:1793–1798. doi: 10.1093/eurheartj/ehl119 [DOI] [PubMed] [Google Scholar]
- 10. Tan CMJ, Green P, Tapoulal N, Lewandowski AJ, Leeson P, Herring N. The role of neuropeptide Y in cardiovascular health and disease. Front Physiol. 2018;9:1281. doi: 10.3389/fphys.2018.01281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuculi F, Herring N, De Caterina AR, Banning AP, Prendergast BD, Forfar JC, Choudhury RP, Channon KM, Kharbanda RK. Relationship of plasma neuropeptide Y with angiographic, electrocardiographic and coronary physiology indices of reperfusion during ST elevation myocardial infarction. Heart. 2013;99:1198–1203. doi: 10.1136/heartjnl-2012-303443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ullman B, Hulting J, Lundberg JM. Prognostic value of plasma neuropeptide‐Y in coronary care unit patients with and without acute myocardial infarction. Eur Heart J. 1994;15:454–461. doi: 10.1093/oxfordjournals.eurheartj.a060526 [DOI] [PubMed] [Google Scholar]
- 13. Clarke JG, Davies GJ, Kerwin R, Hackett D, Larkin S, Dawbarn D, Lee Y, Bloom SR, Yacoub M, Maseri A. Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet. 1987;1:1057–1059. doi: 10.1016/s0140-6736(87)90483-1 [DOI] [PubMed] [Google Scholar]
- 14. Herring N, Tapoulal N, Kalla M, Ye X, Borysova L, Lee R, Dall'Armellina E, Stanley C, Ascione R, Lu CJ, et al. Neuropeptide‐Y causes coronary microvascular constriction and is associated with reduced ejection fraction following ST‐elevation myocardial infarction. Eur Heart J. 2019;40:1920–1929. doi: 10.1093/eurheartj/ehz115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morris MJ, Cox HS, Lambert GW, Kaye DM, Jennings GL, Meredith IT, Esler MD. Region‐specific neuropeptide Y overflows at rest and during sympathetic activation in humans. Hypertension. 1997;29:137–143. doi: 10.1161/01.hyp.29.1.137 [DOI] [PubMed] [Google Scholar]
- 16. Ajijola OA, Chatterjee NA, Gonzales MJ, Gornbein J, Liu K, Li D, Paterson DJ, Shivkumar K, Singh JP, Herring N. Coronary sinus neuropeptide Y levels and adverse outcomes in patients with stable chronic heart failure. JAMA Cardiol. 2020;5:318–325. doi: 10.1001/jamacardio.2019.4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maturi MF, Greene R, Speir E, Burrus C, Dorsey LM, Markle DR, Maxwell M, Schmidt W, Goldstein SR, Patterson RE. Neuropeptide‐Y. A peptide found in human coronary arteries constricts primarily small coronary arteries to produce myocardial ischemia in dogs. J Clin Invest. 1989;83:1217–1224. doi: 10.1172/JCI114004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosano GMC, Tousoulis D, McFadden E, Clarke J, Davies GJ, Kaski JC. Effects of neuropeptide Y on coronary artery vasomotion in patients with microvascular angina. Int J Cardiol. 2017;238:123–127. doi: 10.1016/j.ijcard.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 19. Kalla M, Hao G, Tapoulal N, Tomek J, Liu K, Woodward L; Oxford Acute Myocardial Infarction S , Dall'Armellina E, Banning AP, Choudhury RP, et al. The cardiac sympathetic co‐transmitter neuropeptide Y is pro‐arrhythmic following ST‐elevation myocardial infarction despite beta‐blockade. Eur Heart J. 2020;41:2168–2179. doi: 10.1093/eurheartj/ehz852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Packer M. Do DPP‐4 inhibitors cause heart failure events by promoting adrenergically mediated cardiotoxicity? Clues from laboratory models and clinical trials. Circ Res. 2018;122:928–932. doi: 10.1161/CIRCRESAHA.118.312673 [DOI] [PubMed] [Google Scholar]
- 21. Herring N, Cranley J, Lokale MN, Li D, Shanks J, Alston EN, Girard BM, Carter E, Parsons RL, Habecker BA, et al. The cardiac sympathetic co‐transmitter galanin reduces acetylcholine release and vagal bradycardia: implications for neural control of cardiac excitability. J Mol Cell Cardiol. 2012;52:667–676. doi: 10.1016/j.yjmcc.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herring N, Lokale MN, Danson EJ, Heaton DA, Paterson DJ. Neuropeptide Y reduces acetylcholine release and vagal bradycardia via a Y2 receptor‐mediated, protein kinase C‐dependent pathway. J Mol Cell Cardiol. 2008;44:477–485. doi: 10.1016/j.yjmcc.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 23. Carrick D, Haig C, Ahmed N, Carberry J, Yue May VT, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Hood S, et al. Comparative prognostic utility of indexes of microvascular function alone or in combination in patients with an acute ST‐segment‐elevation myocardial infarction. Circulation. 2016;134:1833–1847. doi: 10.1161/CIRCULATIONAHA.116.022603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rein M, Eitel I, Reinstaer SJ. Role of cardiac magnetic resonance to improve risk prediction following acute ST‐elevation myocardial infarction. J Clin Med. 2020;9:1041. doi: 10.3390/jcm9041041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Waha S, Desch S, Eitel I, Fuernau G, Zachrau J, Leuschner A, Gutberlet M, Schuler G, Thiele H. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long‐term outcome after ST‐elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J. 2010;31:2660–2668. doi: 10.1093/eurheartj/ehq247 [DOI] [PubMed] [Google Scholar]
- 26. Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli‐Ducci C, Kansal P, Carr JC, Holly TA, Lloyd‐Jones D, Klocke FJ, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end‐systolic volume index: prospective cohort study. Heart. 2008;94:730–736. doi: 10.1136/hrt.2007.122622 [DOI] [PubMed] [Google Scholar]
- 27. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Group ESD . Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2018;40:237–269. doi: 10.1093/eurheartj/ehy462 [DOI] [PubMed] [Google Scholar]
- 28. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 29. Gilard M, Mansourati J, Etienne Y, Larlet JM, Truong B, Boschat J, Blanc JJ. Angiographic anatomy of the coronary sinus and its tributaries. Pacing Clin Electrophysiol. 1998;21:2280–2284. doi: 10.1111/j.1540-8159.1998.tb01167.x [DOI] [PubMed] [Google Scholar]
- 30. Cuculi F, Dall'Armellina E, Manlhiot C, De Caterina AR, Colyer S, Ferreira V, Morovat A, Prendergast BD, Forfar JC, Alp NJ, et al. Early change in invasive measures of microvascular function can predict myocardial recovery following PCI for ST‐elevation myocardial infarction. Eur Heart J. 2014;35:1971–1980. doi: 10.1093/eurheartj/eht434 [DOI] [PubMed] [Google Scholar]
- 31. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


