Arrhythmic risk stratification is essential in cardiac sarcoidosis (CS) but remains challenging because of the disease's unpredictable dynamic inflammatory nature. 1
Therefore, our goal was to describe the arrhythmia burden and outcomes in a large prospective cohort of patients from the CSC (Cardiac Sarcoidosis Consortium).
The CSC, an international collaborative research group committed to deepening the understanding of CS, has more than 25 participating sarcoidosis centers in the United States, India, Japan, United Kingdom, Sweden, and the Netherlands. Data are collected annually in a prospective secure, web‐based registry managed at the University of Michigan. The data that support the findings of this study are available from the corresponding author upon reasonable request. Institutional review board approval was obtained from each institution and informed written consent was obtained from each patient.
A total of 587 patients with CS based on Heart Rhythm Society Consensus Guidelines criteria or Japanese Diagnostic criteria were enrolled from 2011 to the present. 2 A total of 164 patients (28%; male 62%, age 54±11 years) had at least one 24‐hour Holter. We analyzed the prevalence of the following arrhythmias on 24‐hour Holter: nonsustained ventricular tachycardia (NSVT), sustained ventricular tachycardia, atrioventricular block, and premature ventricular contraction (PVC). NSVT (34%) and PVC ≥5% (22.0%) were the most common findings (Figure). A high PVCs burden was common: 15% had ≥10% PVCs, and 6% had ≥20%. High‐degree atrioventricular block occurred in 5% and sustained ventricular tachycardia in 4%. NSVT occurred significantly more often in patients with left ventricular ejection fraction <50% (38/77 versus 15/78, P<0.001). Men had significantly higher burden of PVCs than women: ≥5% (28/103 versus 8/61, P=0.03); ≥10% (20/103 versus 4/61, P=0.02). Of these 587 patients (male 60%, age 53±12 years, left ventricular ejection fraction ≤35% in 34.1% patients), 168 (28.6%) experienced an adverse outcome defined as any of the following: death, left ventricular assistance device implantation, heart transplantation, and appropriate implantable cardioverter‐defibrillator shock or pacing. In the entire enrolled population, we analyzed arrhythmias reported from Holters, implantable devices/monitors, or clinically documented.
Figure . Arrhythmia burden on 24‐hour Holter monitoring in 164 patients with cardiac sarcoidosis.
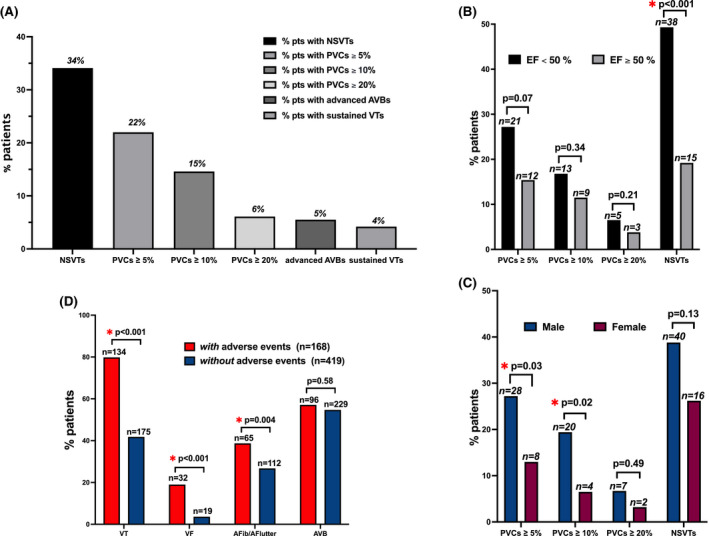
In the Cardiac Sarcoidosis Consortium, arrhythmias were common in patients with cardiac sarcoidosis, with NSVT occurring in one third of patients (A). On 24‐hour Holter monitoring in 164 patients with cardiac sarcoidosis, NSVT occurred more frequently in patients with left ventricular ejection fraction <50% (B), and PVCs occurred more commonly in male patients (C). Arrhythmia outcomes in 587 patients with cardiac sarcoidosis enrolled in the Cardiac Sarcoidosis Consortium, divided into 2 groups according to the presence or absence of adverse events, defined as death, left ventricular assistance device implantation, heart transplantation, and appropriate implantable cardioverter‐defibrillator shock or pacing (D). AFib indicates atrial fibrillation; AFlutter, atrial flutter; AVB, atrioventricular block; EF, ejection fraction; NSVT, nonsustained ventricular tachycardia; PVC, premature ventricular contraction; and VT, ventricular tachycardia.
Ventricular tachycardia, either sustained or NSVT, was associated with a higher risk of adverse events (odds ratio [OR], 2.8; 95% CI, 1.7–4.7; P<0.001); ventricular fibrillation was associated with almost 4‐fold increased risk of those outcomes (OR, 3.9;95% CI, 1.7–8.7; P<0.001). In addition, patients with CS and adverse outcomes had a higher incidence of atrial fibrillation/flutter than those without events (38.7% versus 26.7%, P=0.004), whereas no significant differences were appreciated between the 2 groups according to the incidence of atrioventricular block (57.1% versus 54.7%, P=0.58).
In the CSC registry, we found a higher risk of adverse events in patients with ventricular arrhythmias or atrial fibrillation/flutter and a complex arrhythmic load on 24‐hour Holter monitoring in patients with CS. NSVTs were the most common arrhythmia documented in one third of patients and occurring more often in patients with left ventricular ejection fraction <50%. Male patients had significantly higher burdens of PVCs. The association between arrhythmias on 24‐hour Holter and disease evolution is undetermined in CS. 1 , 2 , 3 The erratic behavior of the inflammatory process and the heterogenic response to nonstandardized therapeutic regimens make arrhythmia control challenging in CS. A small study recently demonstrated a 3‐fold increase in daily PVCs count in patients with clinically manifest CS during corticosteroid treatment; likewise, steroid therapy seems less efficacious in patients with reduced left ventricular ejection fraction. 3 In this analysis, male patients had a higher incidence of PVCs than female patients, agreeing with the results of the US Nationwide Inpatient Sample of 308 064 patients with sarcoidosis, where men were more likely to have ventricular arrhythmias (55% versus 45%) and higher rates of implantable cardioverter‐defibrillator and cardiac resynchronization therapy defibrillator implantation. However, despite the greater incidence of ventricular arrhytmias in men, women with CS seem to develop an enhanced fibrotic reaction and face worse outcomes. 4
In this multicenter international registry, we describe arrhythmia outcomes along with the key role of 24‐hour Holter monitoring in detecting arrhythmic burden in patients with CS, identifying features warranting potential concern. Nevertheless, we observed that 24‐hour Holter is still an underused diagnostic tool as only 28% of patients with CS underwent Holter monitoring, and it could deserve wider application in everyday clinical practice.
This analysis has a potential selection bias, as arrhythmias were not evaluated in the same way within the enrolled population, and the patients who received Holter monitors might have a different arrhythmia propensity than those who were not monitored. Moreover, this analysis did not disclose the relationship between arrhythmias and patients' inflammatory milieu detected by imaging or laboratory tests and the potential influence of therapeutic strategies (eg, immunosuppressors). However, these data are hypothesis generating and encourage the research in CS to produce high‐quality evidence on arrhythmias' hallmarks and their parallelism with disease course. Ongoing randomized controlled trials such as CHASM CS (Cardiac Sarcoidosis Multi‐Center Randomized Controlled Trial; NCT03593759), evaluating the efficacy and safety of a combined methotrexate/prednisone therapy, and MAGIC ART (Interleukin‐1 Blockade for Treatment of Cardiac Sarcoidosis; NCT04017936), testing the effects and safety of interleukin‐1 blockade with anakinra, are leading the way forward to find more answers for arrhythmia management in this yet mysterious disease. 5 Our data from the international prospective Cardiac Sarcoidosis Consortium add meaningful information from a large population with, shedding light on the association of arrhythmias with clinical outcomes in patients with CS.
Appendix
Cardiac Sarcoidosis Consortium investigators list: Edoardo Bressi, MD, Virginia Commonwealth University, Pauley Heart Center, Division of Cardiology, Department of Internal Medicine, Richmond, VA; Jordana Kron, MD, Virginia Commonwealth University, Pauley Heart Center, Division of Cardiology, Department of Internal Medicine, Richmond, VA; Kenneth A. Ellenbogen, MD, Virginia Commonwealth University, Pauley Heart Center, Division of Cardiology, Department of Internal Medicine, Richmond, VA; Thomas C. Crawford, MD, Department of Cardiology, University of Michigan Health System, Ann Arbor, MI; Frank Bogun, MD, Department of Cardiology, University of Michigan Health System, Ann Arbor, MI; Eric Puroll, MD, Department of Cardiology, University of Michigan Health System, Ann Arbor, MI; Xiaokui Gu, MA; Department of Cardiology, University of Michigan Health System, Ann Arbor, MI; Khaled Nour, MD, Henry Ford Hospital, Division of Cardiology, Detroit, MI; Alexandra Chicos, MD; Division of Cardiology, Department of Medicine, and the Bluhm Cardiovascular Institute, Northwestern Memorial Hospital, Northwestern University, Chicago, IL; Henri Roukoz, MD, Cardiovascular Division, Department of Medicine, University of Minnesota Medical School, Minneapolis, MN; Peter Zimetbaum, MD, Beth Israel Deaconess Medical Center, Boston, MA; Steven Kalbfleisch, MD, Division of Cardiovascular Medicine, The Ohio State University Wexner Medical Center, Columbus, OH; Muhammad Afzal, MD; Division of Cardiovascular Medicine, The Ohio State University Wexner Medical Center, Columbus, OH; Francis Murgatroyd, MD, Department of Cardiology King's College Hospital NHS Foundation Trust London, UK; Katherine Martin, RN, Department of Cardiology King's College Hospital NHS Foundation Trust London, UK; David Steckman, MD, Division of Cardiology, Albany Medical Center, Albany, NY; Mikhail Torosoff, MD, Division of Cardiology, Albany Medical Center, Albany, NY; Marc Judson, MD; Division of Cardiology, Albany Medical Center, Albany, NY; Lynda Rosenfeld, MD, Section of Cardiovascular Medicine, Yale University School of Medicine, New Haven, CT; Ann Garlitski, MD, The New England Cardiac Arrhythmia Center, Tufts Medical Center, Tufts University School of Medicine, Boston, MA; Kyoko Soejima, MD, Kyorin University School of Medicine, Tokyo, Japan; Adarsh Bhan, MD, Advocate Christ Medical Center, Oak Lawn, IL; Vasanth Vedantham, MD, University of California‐San Francisco, San Francisco, CA; Timm‐Michael Dickfeld, MD, University of Maryland School of Medicine, Baltimore, MD; David De Lurgio, MD, Emory University, St. Joseph's Hospital, Atlanta, GA; Pyotr Platonov, MD, Department of Cardiology, Institution for Clinical Sciences, Lund University, Lund, Sweden; Matthew Zipse, MD, Division of Cardiology, University of Colorado Anschutz Medical Campus, Aurora, CO; Suguru Nishiuchi, MD, Division of Cardiology Tenri Hospital, Tenri Japan; Matthew Ortman, MD, Division of Cardiology, Cooper Medical School of Rowan University, Camden, NJ; Calambur Narasimhan, MD, Department of Electrophysiology, AIG Hospitals, Hyderabad, India; Kris Patton, MD, Department of Medicine, University of Washington, Seattle, WA; David Rosenthal, MD, University of California‐San Francisco, San Francisco, CA; Siddharth Mukerji, MD, Memorial Hermann Heart & Vascular Institute, Houston, TX; Jarieke Hoogendoorn, MD, Department of Cardiology, Willem Einthoven Center of Arrhythmia Research and Management, Leiden University Medical Center, The Netherlands; Katja Zeppenfeld, MD, Department of Cardiology, Willem Einthoven Center of Arrhythmia Research and Management, Leiden University Medical Center, The Netherlands; William Sauer, MD, Division of Cardiovascular Medicine, Brigham and Women's Hospital, Boston, MA; Scott Feitell, DO, Rochester Regional Health, NY.
Sources of Funding
Dr Kron was supported by CTSA award No. UL1TR002649 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Dr Platonov’s cardiac sarcoid research program is funded by The Swedish Heart‐Lung Foundation and the Skåne University Hospital.
Disclosures
All authors have reported that they have no relationships relevant to the contents of this research letter to disclose.
For Sources of Funding and Disclosures, see page 4.
Contributor Information
Edoardo Bressi, Email: edo.bressi@gmail.com.
for the Cardiac Sarcoidosis Consortium:
Edoardo Bressi, Thomas C. Crawford, Frank Bogun, Xiaokui Gu, Kenneth Ellenbogen, Alexandra Chicos, Henri Roukoz, Peter Zimetbaum, Steven Kalbfleisch, Francis Murgatroyd, David Steckman, Lynda Rosenfeld, Ann Garlitski, Kyoko Soejima, Adarsh Bhan, Vasanth Vedantham, Timm‐Michael Dickfeld, David De Lurgio, Pyotr Platonov, Matthew Zipse, Suguru Nishiuchi, Matthew Ortman, Calambur Narasimhan, Kris Patton, David Rosenthal, Siddharth Mukerji, Jarieke Hoogendoorn, Katja Zeppenfeld, William Sauer, and Jordana Kron
References
- 1. Rosenfeld LE, Chung MK, Harding CV, Spagnolo P, Grunewald J, Appelbaum J, Sauer WH, Culver DA, Joglar JA, Lin BA, et al. Arrhythmias in cardiac sarcoidosis bench to bedside: a case‐based review. Circ Arrhythm Electrophysiol. 2021;14:e009203. doi: 10.1161/CIRCEP.120.009203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 3. Medor MC, Spence S, Nery PB, Beanlands R, Promislow S, Juneau D, Kemp R, Ha AC, Rivard L, Gula L, et al. Treatment with corticosteroids is associated with an increase in ventricular arrhythmia burden in patients with clinically manifest cardiac sarcoidosis: insights from implantable cardioverter‐defibrillator diagnostics. J Cardiovasc Electrophysiol. 2020;31:2751–2758. doi: 10.1111/jce.14689 [DOI] [PubMed] [Google Scholar]
- 4. Kalra R, Malik S, Chen K‐H, Ogugua F, Athwal PSS, Elton AC, Velangi PS, Ismail MF, Chhikara S, Markowitz JS, et al. Sex differences in patients with suspected cardiac sarcoidosis assessed by cardiovascular magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2021;14:e009966. doi: 10.1161/CIRCEP.121.009966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birnie D, Beanlands RSB, Nery P, Aaron SD, Culver DA, DeKemp RA, Gula L, Ha A, Healey JS, Inoue Y, et al. Cardiac Sarcoidosis multi‐center randomized controlled trial (CHASM CS‐ RCT). Am Heart J. 2020;220:246–252. doi: 10.1016/j.ahj.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


