Abstract
Background
In patients with ductal‐dependent pulmonary blood flow, initial palliation includes catheter‐based patent ductus arteriosus (PDA) stent or surgical aortopulmonary shunt (APS). This meta‐analysis aimed to compare outcomes between PDA stent and APS.
Methods and Results
A comprehensive literature search yielded six retrospective observational studies. Pooled adjusted hazard ratios (HR) were included to control for covariates and assess time to event analysis. Of 757 patients, 243 (32.1%) underwent PDA stent and 514 (67.9%) underwent APS. Pulmonary atresia with intact ventricular septum and expected biventricular repair were more common with PDA stent compared with APS (39.6% versus 21.2%, P<0.001 and 57.9% versus 46.6%, P=0.007, respectively). There was no statistically significant difference in mortality between PDA stent and APS (HR, 0.71; [95% CI, 0.26–1.93]; P=0.50). PDA stent was associated with lower risk of postprocedural complications (odds ratio [OR], 0.45; [95% CI, 0.25–0.81]; P=0.008), mechanical circulatory support (OR, 0.27; [95% CI, 0.09–0.79]; P=0.02), and shorter intensive care unit length of stay (−4.03 days; [95% CI, −5.99 to −2.07]; P<0.001), hospital length of stay (−5.54 days; [95% CI, −9.20 to −1.88]; P=0.003), and duration of mechanical ventilation (−3.41 days; [95% CI, −5.29 to −1.52]; P<0.001). There was no difference in pulmonary artery growth or hazard of unplanned reintereventions.
Conclusions
PDA stent has a similar hazard of mortality compared with APS. Benefits to PDA stent include shorter duration of mechanical ventilation, shorter hospital length of stay, and fewer complications. Differences in patient characteristics exist with more patients with pulmonary atresia with intact ventricular septum and expected biventricular repair undergoing PDA stent.
Keywords: aortopulmonary shunt, congenital heart disease, ductal‐dependent pulmonary blood flow, mortality, patent ductus arteriosus stent, single ventricle, tetralogy of Fallot
Subject Categories: Meta Analysis, Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- APS
aortopulmonary shunt
- DDPBF
ductal‐dependent pulmonary blood flow
- PA‐IVS
pulmonary atresia with intact ventricular septum
- SMD
standardized mean difference
Clinical Perspective.
What Is New?
In cyanotic congenital heart disease with ductal‐dependent pulmonary blood flow, patent ductus arteriosus stent is associated with fewer complications and shorter length of stay.
There is no significant difference in mortality or unplanned reinterventions to treat cyanosis after patent ductus arteriosus stent compared with aortopulmonary shunt.
What Are the Clinical Implications?
Patent ductus arteriosus stent may be favorable to surgical aortopulmonary shunt secondary to similar mortality and reintervention rates and multiple benefits in postintervention care.
Randomized control trials are needed to further determine which strategy is superior in different types of congenital heart disease with ductal‐dependent pulmonary blood flow.
Establishing a stable source of pulmonary blood flow is crucial in patients with cyanotic congenital heart disease (CHD) and ductal‐dependent pulmonary blood flow (DDPBF). Historically, this was achieved through a surgical aortopulmonary shunt (APS). 1 , 2 APS may promote pulmonary artery growth and thus allow for the next stage palliation or corrective procedure to be performed in the future. However, there remains a significant risk of morbidity and mortality with this surgical procedure. 3 , 4 , 5 , 6 , 7 Over the last decade, PDA stent has emerged as a viable alternative to APS. 8 , 9 Ductal stent is a less invasive approach compared with APS and avoids the need for cardiopulmonary bypass in the vulnerable neonatal period which allows for faster recovery. 8 , 10 , 11 Nevertheless, PDA stent also carries risks of procedural complications and increased need for reinterventions. 8 , 11
Comparisons between the PDA stent and APS have been limited primarily to single‐center studies and only a few recent multicenter retrospective studies. 1 Limitations in statistical power may limit the conclusions of many studies. Additionally, the management of DDPBF is evolving at many institutions towards using PDA stent in select patients and there is a critical need for an objective appraisal of the current evidence. 12 A meta‐analysis increases the number of observations resulting in better statistical power and objectively assesses the level of evidence. Thus, this study aimed to conduct a systematic review and meta‐analysis to compare mortality risk and clinical outcomes after PDA stent and APS in cyanotic CHD with DDPBF.
Methods
This systematic review and meta‐analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement. 13 The data that support the findings of this study are available from the corresponding author upon reasonable request. This systematic review followed a protocol that was developed a priori. We performed a comprehensive search from PubMed and Embase databases. Keywords and detailed vocabulary were used to search for studies that evaluated mortality, procedural outcomes, and postprocedural complications after PDA stent and APS in patients with cyanotic CHD and DDPBF. APS procedures included Blalock‐Taussig‐Thomas shunts and other systemic‐pulmonary artery shunts. Inclusion criteria were as follows: (1) comparative study design between PDA stent and APS, (2) subjects with single ventricle physiology or biventricular CHD (i.e. tetralogy of Fallot) and DDPBF, and (3) at least 10 subjects who had undergone PDA stent. Only studies published between 2005 and 2020 were included.
Definitions of Outcomes
Outcomes were defined as mortality, postprocedural extracorporeal mechanical oxygenation (ECMO), intensive care unit (ICU) length of stay (LOS), total hospital LOS, duration of mechanical ventilation, and procedural complications. Procedural complications were defined as injuries that were directly related to the PDA stent or APS procedure. Additionally, rates of unplanned reintervention to treat cyanosis were compared. Unplanned reinterventions included unplanned surgical or catheter‐based procedures focused on the APS, PDA stent, or pulmonary arteries to increase pulmonary blood flow because of the development of clinically concerning cyanosis. Planned reinterventions in the absence of cyanosis, such as routine catheterizations for surveillance or hemodynamic assessment, were not evaluated because of the lack of consistent reporting. Lastly, pulmonary artery size and symmetry after PDA stent or APS were evaluated by assessing the measured Nakata index and symmetry index, respectively. The Nakata index is defined as the sum of the cross‐sectional area of the pulmonary arteries divided by the body surface area. The pulmonary artery symmetry index is defined as the ratio of the area of the smaller pulmonary artery to the larger pulmonary artery. Expected biventricular repair was defined as a subsequent definitive surgical repair that consisted of anatomic repair. 14 Single‐ventricle physiology was defined as subsequent surgical repair that consisted of a palliative superior cavopulmonary anastomosis. 14
When multiple studies from the same authors or institutions reported the same outcome, the study with the greatest number of subjects was included in the data analysis. This allowed for the inclusion of the maximal number of patients for each outcome measure.
Data Extraction
Five authors (S.T., D.P., S.K., S.B., T.A.) separately and independently screened all identified studies using study title, abstract, and full‐length articles to determine whether the study met the screening criteria. Data were extracted independently by 2 authors (S.T. and S.K.). Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used to allow for screening by multiple reviewers simultaneously and for data extraction. Discrepancies between 2 reviewers about study eligibility were reviewed by a separate author (T.A.) and discrepancies between 2 reviewers about data extraction were reviewed by a separate author (D.P.).
Methodological Quality and Risk of Bias Assessment
No randomized trials were identified. Risk of bias of the observational studies was evaluated using the Newcastle‐Ottawa tool. 15 This tool included an assessment of how participants were selected from the population of interest, how comparable study subjects were, how the outcome was assessed, and the length and adequacy of follow‐up when applicable. Studies were classified as having high‐risk (1–3 points), intermediate‐risk (4–5 points), or low‐risk of bias (6–9 points). All discrepancies were resolved by a second reviewer (T.A.).
Statistical Analysis
All statistical analyses were performed using R software, version 4.0.3 (The R Foundation, Vienna, Austria). For studies that reported median and the first and third quartiles, the sample mean and SD were determined according to Cochrane Handbook for Systematic Reviews of Interventions. 16 Common diagnoses of tetralogy of Fallot and pulmonary atresia with intact ventricular septum (PA‐IVS) and biventricular and single ventricle repair were compared with Chi‐square test with Yates' continuity correction. Continuous variables were analyzed as mean differences with 95% CI. For event rate data, odds ratio (OR) with 95% CI was calculated using the total number of events and patients as reported in the individual studies. For mortality outcome and risk of unplanned reinterventions, hazard ratios (HR) were measured to account for the timing of events. 17 Furthermore, while not all studies adjusted for covariates, adjusted HR that controlled for other important covariates in multivariable survival analysis were applied to minimize bias. As we anticipated the presence of heterogeneity among studies, random‐effects models were applied to pool effect sizes. The DerSimonian‐Laird estimator was performed to estimate the between‐study variance and the Jackson method was used to calculate 95% CI. 18 Further, fix‐effects models were performed to assess the robustness of the results. Sensitivity analyses were also implemented to assess the robustness of the results: the restricted maximum likelihood estimator was applied for continuous outcome data and the Paule‐Mandel estimator was performed for binary outcome data. Q‐profile method was used to estimate a CI for the between‐study variance. 19 Statistical significance was defined as P<0.05.
Publication bias was assessed using a visual funnel plot and the Egger tests. Further, the “trim and fill” method was used to examine whether hypothetical missing studies substantially changed the estimates. 20
Results
The initial search yielded 2312 studies and all abstracts were reviewed. The full text was reviewed for 16 articles and 6 studies met inclusion criteria for the analysis (Figure 1). 14 , 21 , 22 , 23 , 24 , 25 Included studies are summarized in Table 1. In total, 757 patients had DDPBF, 243 (32.1%) patients underwent PDA stent, and 514 (67.9%) patients underwent surgical placement of APS. The average age at initial intervention was 20 days for PDA stent and 27 days for APS (P=0.18). PA‐IVS was more common in the PDA stent group compared with the APS group (39.6% versus 21.2%, P<0.001). There was no significant difference in the proportion of patients with the diagnosis of tetralogy of Fallot between the 2 groups (8.9% versus 14.2%, P=0.196). Two studies categorized a total of 594 patients based on expected future biventricular versus single ventricle repair. There was a higher proportion of expected biventricular repair in the PDA stent group compared with the APS group (57.9% versus 46.6%, P=0.007). Additionally, 2 studies categorized patients based on the presence of antegrade pulmonary blood flow. Patients with PDA stent were more likely to have antegrade pulmonary blood flow compared with patients with APS (52.3% versus 38.2%, P<0.001).
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram for study selection.
Table 1.
Characteristics of Included Studies in Meta‐Analysis
| Author, Year, and Study type | Inclusion period | No. of patients | Age (days) at intervention | Cardiac diagnoses | Outcomes reported |
|---|---|---|---|---|---|
|
Amoozgar 23 2012 Multicenter |
2009–2011 |
PDA stent=15 APS=20 |
PDA stent Median 20 days (range 4–180) APS Median 37 days (range 6–330) |
PDA stent
APS
|
1. Mortality 2. Complications 3. LOS, total 4. Pulmonary artery growth: dimensions, Nakata index |
|
Bentham 24 2018 Multicenter |
2012–2015 |
PDA stent=83 APS=171 |
PDA stent Median 8 days (25th–75th percentile: 4–13) APS Median 8 days (25th–75th percentile: 5–15) |
PDA stent
APS
|
1. Mortality/Survival 2. Reinterventions 3. ECMO 4. LOS, total 5. LOS, ICU 6. Mechanical ventilation 7. Pulmonary artery growth: dimensions, Nakata index |
|
Glatz 14 2018 Multicenter |
2008–2015 (PDA stent) 2012–2015 (APS) |
PDA stent=106 APS=251 |
PDA stent Median 9 days (25th–75th percentile: 5–15) APS Median 6 days (25th–75th percentile: 4–15) |
PDA stent
APS
|
1. Mortality 2. Complications 3. Reinterventions 4. ECMO 5. LOS, total 6. LOS, ICU 7. Mechanical ventilation 8. Pulmonary artery growth: Nakata index, pulmonary artery symmetry index |
|
Mallula 22 2015 Single center |
2006–2013 |
PDA stent=13 APS=16 |
PDA stent Median 7 days (range 2–13) APS Median 5 days (range 2–28) |
PDA stent
APS
|
1. Mortality 2. Complications 3. Reinterventions 4. LOS, total 5. Mechanical ventilation |
|
McMullan 21 2014 Single center |
2002–2011 |
PDA stent=13 APS=42 |
PDA stent Median 13 days (range 4–43) APS Median 12 days (range 2–218) |
PDA stent
APS
|
1. Survival 2. Complications 3. Reinterventions |
|
Santoro 25 2009 Single center |
2003–2009 |
PDA stent=13 APS=14 |
PDA stent Mean 22 ± 39 days (range 1–84) APS Mean 21 ± 30 days (range 7–76) |
PDA stent
APS
|
1. Pulmonary artery growth: Nakata index, pulmonary artery dimension z‐scores |
APS indicates aortopulmonary shunt; ASD, atrial septal defect; AVSD, atrioventricular septal defect; CHD, congenital heart disease; DILV, double inlet left ventricle; DORV, double outlet right ventricle; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IVS, intact ventricular septum; LOS, length of stay; MA, mitral atresia; PA, pulmonary atresia; PDA, patent ductus arteriosus; PS, pulmonary stenosis; TA, tricuspid atresia; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; and VSD, ventricular septal defect.
Procedural Complications
Procedural success for PDA stent was reported in 3 studies with a pooled success rate of 85%. Common procedural complications after APS included perioperative bleeding, surgical wound exploration, and arrhythmia. 14 , 21 , 22 , 23 Less commonly reported complications included stroke, thrombosis, early reoperation, ventricular dysfunction, mediastinitis, multiorgan dysfunction, seizure, lung collapse, chylothorax, and pulmonary congestion and hemorrhage. 14 , 21 , 22 , 23 For patients who underwent PDA stent, procedural complications included ductal spasm and access‐related vascular injury. 14 Arrhythmias were less commonly noted (1.6%) and reported by one study. 14 Rare complications associated with PDA stent were stent migration, bacteremia, right ventricular perforation, and duct dissection, all of which were reported as single instances. Four studies compared procedural complications. The rate of complications was 10.9% in the PDA stent group and 21.3% in the APS group. PDA stent was associated with a lower risk of procedural complications when compared with APS (OR, 0.45; [95% CI, 0.25–0.81]; P=0.008; I 2=0%) (Figure 2A), which was consistent in the fixed‐effects model (OR, 0.43; [95% CI, 0.24–0.76]; P=0.004) and the sensitivity analysis (OR, 0.45; [95% CI, 0.25–0.81]; P=0.008).
Figure 2. Forest plot showing results of meta‐analysis of complications and hazard of unplanned reinterventions between patent ductus arteriosus stent and aortopulmonary shunt.
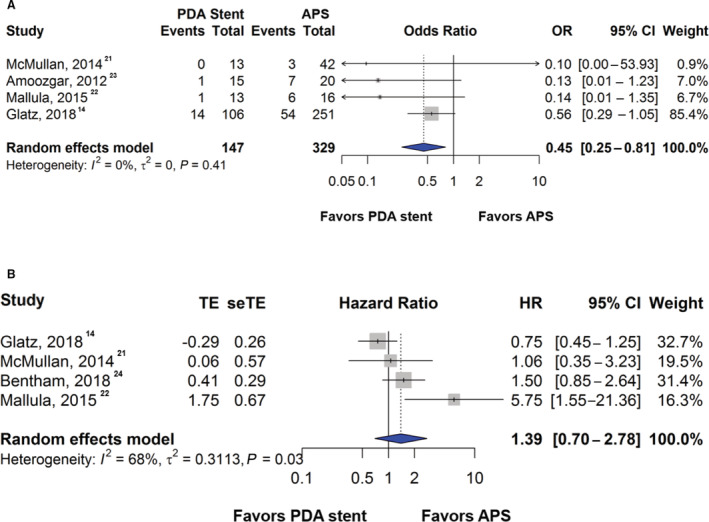
A, Comparison of the odds of complications between patent ductus arteriosus stent and aortopulmonary shunt. Complications include perioperative bleeding, surgical wound exploration, and arrhythmias after aortopulmonary shunt and ductal spasm and access‐related vascular injury after patent ductus arteriosus stent. B, Comparison of the hazard of unplanned reinterventions to treat cyanosis between patent ductus arteriosus stent and aortopulmonary shunt. APS indicates aortopulmonary shunt; HR, hazard ratio; OR, odds ratio; PDA, patent ductus arteriosus; seTE, standard error of treatment estimate; and TE, treatment estimate.
Reinterventions
Four studies compared unplanned reinterventions to treat cyanosis after PDA stent or APS. The pooled proportion of unplanned reintervention rate was 22.7% in the APS group compared with 25.6% in the PDA stent group. PDA stent was associated with a higher hazard for unplanned reinterventions to treat cyanosis when compared with APS although this did not reach statistical significance (HR, 1.39; [95% CI, 0.70–2.78]; P=0.35; I 2=68%) (Figure 2B). Similar findings were observed in the fixed‐effects model (HR, 1.16; [95% CI, 0.82–1.64]; P=0.40) and in the sensitivity analysis (HR, 1.44; [95% CI, 0.65–3.19]; P=0.37).
Length of Stay and Duration of Mechanical Ventilation
Two studies reported data on ICU LOS and 4 studies reported data on total hospital LOS. Patients with a PDA stent had an ICU LOS that was 4.03 days shorter than those with APS (95% CI, −5.99 to −2.07; P<0.001; I 2=66%) (Figure 3A), which was similar in the fixed‐effects model (mean difference: −4.08; [95% CI, −5.22 to −2.94]; P<0.001) and in the sensitivity analysis (mean difference: −4.03; [95% CI, −5.99 to −2.07]; P<0.001). PDA stent was associated with shorter total hospital LOS by 5.54 days (95% CI, −9.20 to −1.88; P=0.003; I 2=78%) (Figure 3B), confirmed by the fixed‐effects model (mean difference: −4.36; [95% CI, −5.85 to −2.87]; P<0.001) and sensitivity analysis (mean difference: −7.21; [95% CI, −14.01 to −0.41]; P=0.038). Additionally, 3 studies reported the duration of postprocedural mechanical ventilation. There was a mean difference of 3.41 days (95% CI, −5.29 to −1.52; P<0.001; I 2=88%) in the duration of postprocedural mechanical ventilation, favoring PDA stent (Figure 3C). A consistent finding was observed in the fixed‐effects model (mean difference: −2.26; [95% CI, −2.67 to −1.85]; P<0.001). In the sensitivity analysis, PDA stent demonstrated shorter postprocedural mechanical ventilation but did not reach statistical significance (mean difference: −5.09; [95% CI, −10.70 to 0.51]; P=0.07).
Figure 3. Forest plot showing results of meta‐analysis of length of stay and duration of mechanical ventilation between patent ductus arteriosus stent and aortopulmonary shunt.
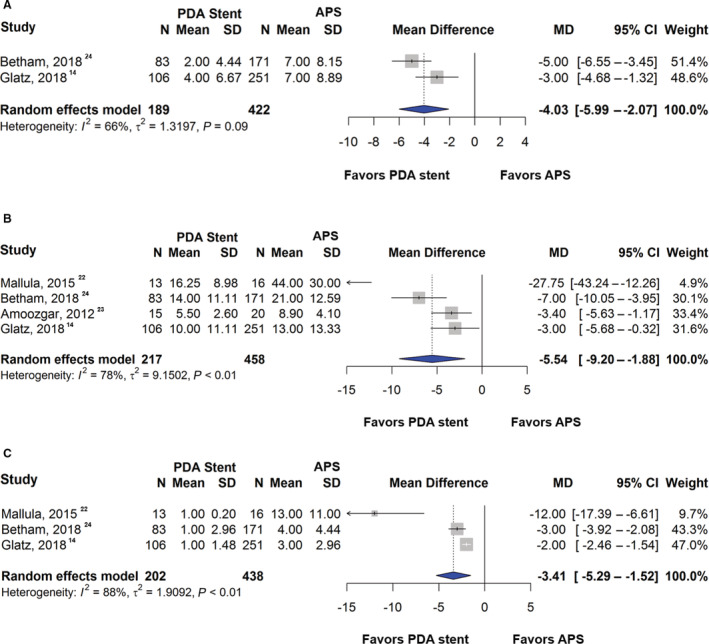
A, Comparison of the intensive care unit length of stay, (B) total hospital length of stay, and (C) duration of mechanical ventilation between patent ductus arteriosus stent and aortopulmonary shunt. APS indicates aortopulmonary shunt; MD, mean difference; and PDA, patent ductus arteriosus.
Mortality and ECMO Support
The pooled mortality rate was 8.2% in the PDA stent group compared with 11.8% in the APS group. There was no statistically significant difference in mortality hazard between PDA stent or APS groups although the hazard was lower in the PDA stent group (HR, 0.71; [95% CI, 0.26–1.93]; P=0.50; I 2=54%) (Figure 4A). Two studies reported outcomes of postprocedural ECMO support. ECMO support was less frequent after PDA stent compared with APS (OR, 0.27; [95% CI, 0.09–0.79]; P=0.02; I 2=0%) (Figure 4B). Sensitivity analyses showed similar findings (HR, 0.71; [95% CI, 0.27–1.91]; P=0.49 for mortality and OR, 0.27; [95% CI, 0.09–0.79]; P=0.02 for ECMO support). The fixed‐effect models demonstrated consistent findings as well (HR, 0.80; [95% CI, 0.44–1.43]; P=0.49 for mortality and OR, 0.27; [95% CI, 0.09–0.79]; P=0.02 for ECMO support).
Figure 4. Forest plot showing results of meta‐analysis of mortality and extracorporeal membrane oxygenation support between patent ductus arteriosus stent and aortopulmonary shunt.
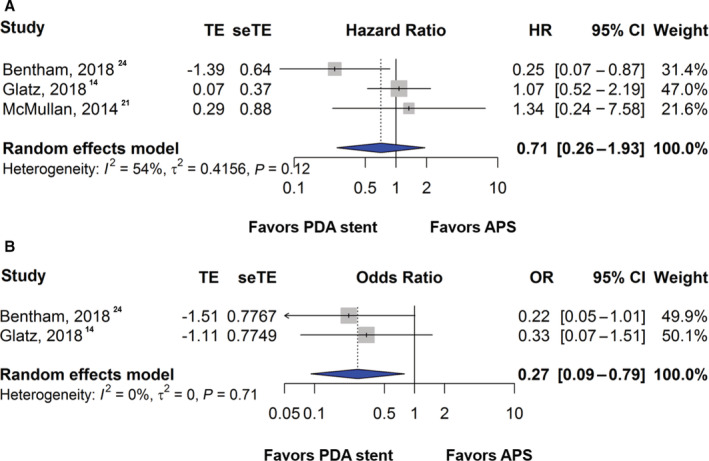
Comparison of the (A) hazard of mortality and (B) rate of extracorporeal membrane oxygenation support between patent ductus arteriosus stent and aortopulmonary shunt. APS indicates aortopulmonary shunt; HR, hazard ratio; OR, odds ratio; PDA, patent ductus arteriosus; seTE, standard error of treatment estimate; and TE, treatment estimate.
Pulmonary Artery Size and Symmetry
No significant difference was found in the Nakata index between PDA stent and APS groups (standardized mean difference [SMD], 0.09; [95% CI, −0.20 to 0.37]; P=0.55) (Figure 5A). There was also no difference in pulmonary artery symmetry as measured by the symmetry index between the 2 groups (SMD, 0.38; [95% CI, −0.26 to 1.03]; P=0.25) (Figure 5B). Further, fixed‐effects models showed consistent results (SMD, 0.14; [95% CI, −0.07 to 0.35]; P=0.20 for the Nakata index and SMD, 0.17; [95% CI, −0.03 to 0.37]; P=0.10 for the symmetry index). Similar findings were observed in the sensitivity analysis (SMD, 0.08; [95% CI, −0.21 to 0.38]; P=0.57 for the Nakata index and SMD, 0.46; [95% CI, −0.53 to 1.45]; P=0.36 for the symmetry index).
Figure 5. Forest plot showing results of meta‐analysis of pulmonary artery growth between patent ductus arteriosus stent and aortopulmonary shunt.
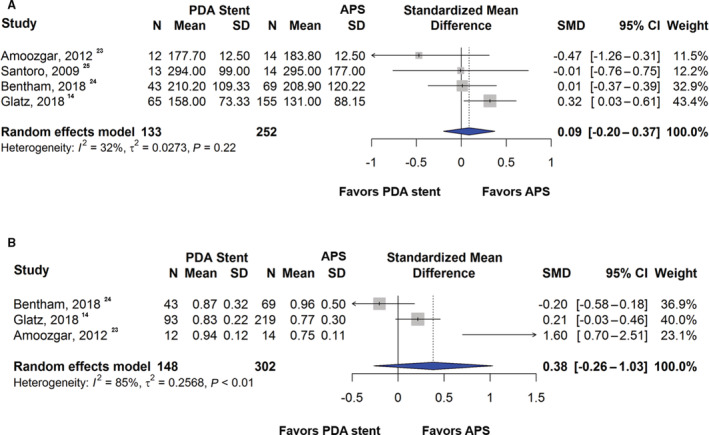
Comparison of pulmonary artery growth using the (A) Nakata index and (B) pulmonary artery symmetry index between patent ductus arteriosus stent and aortopulmonary shunt. APS indicates aortopulmonary shunt; SMD, standardized mean difference; and PDA, patent ductus arteriosus.
Quality Assessment and Publication Bias
All included studies were of low risk of bias with scores ≥6 points on the Newcastle Ottawa scale (Figure S1). Publication bias was illustrated in the funnel plots (Figure S2). The additional analyses using the “trim and fill” method suggested that hypothetical “missing” studies did not substantially change our pooled estimates (Figure S3).
Discussion
This meta‐analysis evaluated a large body of published literature and 6 studies were included for the analysis of outcomes after PDA stent and APS in patients with cyanotic CHD and DDPBF (Figure 6). Additionally, this analysis also adjusted for covariates when possible. This study demonstrated no statistically significant difference in risk of mortality or unplanned reinterventions to treat cyanosis between the 2 approaches. Numerically the hazard for mortality was higher in APS while the unplanned reintervention hazard was higher in the PDA stent group. PDA stent was associated with fewer procedural complications, shorter ICU and hospital LOS, and fewer days of mechanical ventilation. Importantly, there was no statistically significant difference in pulmonary artery size and symmetry between the 2 groups. Furthermore, this study also demonstrates that the diagnosis of PA‐IVS, expected future biventricular repair, and antegrade pulmonary blood flow were more common in the PDA stent group compared with APS group.
Figure 6. Summary of meta‐analysis results.
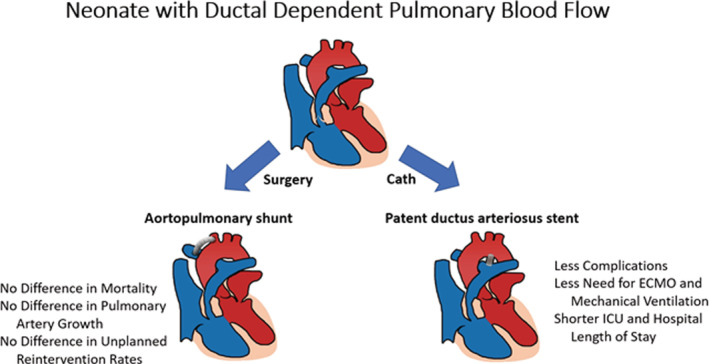
ECMO indicates extracorporeal membrane oxygenation; and ICU, intensive care unit.
Although the analysis showed a numerically lower hazard for death with the PDA stent, this did not reach statistical significance. Additionally, PDA stent was associated with less procedural complications, postprocedural ECMO support, mechanical ventilation and shorter hospital LOS. The APS procedure carried up to a 21% rate of procedural complications compared with 11% after PDA stent. Procedural complications are typically less catastrophic and life‐threatening in the PDA stent group. Thus, it was not surprising that there was less need for ECMO support and mechanical ventilation after PDA stent along with a shorter ICU and hospital LOS. Other studies have also shown that the PDA stent approach is associated with lower cost of care compared with APS. 26 The effect of the initial palliation strategy on longer‐term outcomes including neurodevelopmental outcomes is yet to be determined.
In this meta‐analysis, there was no significant difference in the rate of unplanned reintervention to treat cyanosis between the groups, although it was numerically higher in the PDA stent group. The increased rate of reinterventions after PDA stent described in the literature is secondary to the inclusion of planned reintervention procedures. As many studies did not include separate planned intervention rates in the comparison, we could not perform a meta‐analysis on the rate of planned interventions. The timing of reinterventions may also be different between the groups. Bentham et al. described that the majority of interventions in their APS group occurred in the early interstage period (need for early shunt revision or change to another source of pulmonary blood flow) and late interstage period (need for APS stent). 24 Reinterventions after PDA stent were predominantly late, comprising of catheter‐based procedures to re‐stent or balloon dilate the existing PDA stent as neointimal proliferation within the stent may occur. Reinterventions are typically planned to prolong the time between initial palliation and the next palliative procedure or definitive surgery and may account for a proportion of late‐occurring procedures.
It is important to note that this study found that PDA stent was more common in patients with PA‐IVS, with expected biventricular repair, and with antegrade pulmonary flow. Patients with PA‐IVS tend to have a “straight” ductus arteriosus making it easier to stent. 27 Expected biventricular repair and antegrade pulmonary flow offers the advantage of having a dual pulmonary blood flow source resulting in more stable hemodynamics during PDA stent procedure and perceived fewer procedural complications. 12 , 14
With the increased experience with PDA stents, placement of a PDA stent is feasible and safe in more types of CHD anatomy; some centers have started performing PDA stent for all cyanotic infants with DDPBF. 12 , 28 , 29 Furthermore, a PDA stent may be the preferred option in neonates with multiple comorbidities or those who may be high risk for cardiac surgery. However, it is important to recognize that a surgical APS may be the best option for palliation when PDA stent is not achievable, particularly in complex PDA morphologies that prohibit stent placement. 30 Ultimately, the patient's clinical history, ductus arteriosus anatomy, and surgical risk factors should be carefully assessed and the decision between APS and PDA stent should be individualized, taking institutional experience into consideration.
Recently, another meta‐analysis by Alsagheir and colleagues pooled the data from 6 studies to assess whether PDA stent was associated with better outcomes compared with APS. 31 The data from our meta‐analysis was consistent with Alsagheir et al., showing better postoperative morbidities after the PDA stent including shorter postprocedural hospitalization and fewer procedural complications. The midterm mortality was better in the PDA stent group in the previous meta‐analysis as opposed to our study where there was no statistically significant difference in mortality. However, Alsagheir et al. pooled relative risk ratios and unadjusted relative risk for mortality, which does not adjust for covariates and does not account for the time to event. To address this, we only reported hazard ratios to account for time to event and adjusted ratios to account for covariates. Our findings are consistent with Glatz et al., which was the largest multicenter study to date included in this meta‐analysis and showed no statistically significant difference in adjusted hazard of mortality. 14 Our meta‐analysis also differs from the previous meta‐analysis in that pulmonary artery size and symmetry were evaluated with the Nakata index and the pulmonary artery symmetry index. This further supports the finding that pulmonary artery growth may not differ between intervention groups. Additionally, this meta‐analysis also demonstrated group differences in common cardiac diagnoses and in the proportion of expected biventricular or single ventricular repair in this population.
Limitations
Given the nature of this clinical question, all studies included in this meta‐analysis were retrospective studies. Retrospective studies are prone to confounders and covariates may not have been considered in some studies, but we have attempted to decrease the effect of confounders and covariates in our analysis by using adjusted HRs and ORs. Additionally, although PDA stent has become more prevalent, the majority of patients included in this study underwent surgical APS. This may be affected by center variability and preference to perform APS versus PDA stent, which would introduce selection bias to the included studies. Lastly, the cohorts are not equally representative of all cyanotic CHD with DDPBF patients. Not all types of CHD with DDPBF were evaluated, given the low incidence of certain diagnoses and the heterogeneity in data reporting. However, we were able to compare rates of APS and PDA stent among patients with 2 common types of CHD with DDPBF, with expected biventricular repair, and with antegrade pulmonary blood flow to provide insight into how anatomy may influence decision making. Given limitations inherent to observational studies, the COMPASS trial (Comparison of Methods of Pulmonary Blood Flow Augmentation in Neonates: Shunt Versus Stent) is being performed by the Pediatric Heart Network.
Conclusions
This meta‐analysis demonstrated that PDA stent has a similar hazard of mortality compared with APS. There are benefits to PDA stent, including shorter duration of mechanical ventilation support, shorter hospital and ICU LOS, and fewer procedural complications. The existing literature supports differences in patient characteristics with more patients with PA‐IVS and expected biventricular repair undergoing PDA stent.
Disclosures
Dr Goldstein is a consultant for W.L Gore & Associates, Medtronic, Mezzion Pharma and PECA Labs. The remaining authors have no disclosures to report.
Sources of Funding
None.
Supporting information
Figures S1–S3
For Sources of Funding and Disclosures, see page 11.
References
- 1. Boucek DM, Qureshi AM, Goldstein BH, Petit CJ, Glatz AC. Blalock‐Taussig shunt versus patent ductus arteriosus stent as first palliation for ductal‐dependent pulmonary circulation lesions: a review of the literature. Congenit Heart Dis. 2019;14:105–109. doi: 10.1111/chd.12707 [DOI] [PubMed] [Google Scholar]
- 2. Kiran U, Aggarwal S, Choudhary A, Uma B, Kapoor PM. The Blalock and taussig shunt revisited. Ann Card Anaesth. 2017;20:323–330. doi: 10.4103/aca.ACA_80_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bove T, Vandekerckhove K, Panzer J, De Groote K, De Wolf D, Francois K. Disease‐specific outcome analysis of palliation with the modified Blalock‐Taussig shunt. World J Pediatr Congenit Heart Surg. 2015;6:67–74. doi: 10.1177/2150135114558690 [DOI] [PubMed] [Google Scholar]
- 4. Sasikumar N, Hermuzi A, Fan CS, Lee KJ, Chaturvedi R, Hickey E, Honjo O, Van Arsdell GS, Caldarone CA, Agarwal A, et al. Outcomes of Blalock‐Taussig shunts in current era: a single center experience. Congenit Heart Dis. 2017;12:808–814. doi: 10.1111/chd.12516 [DOI] [PubMed] [Google Scholar]
- 5. Petrucci O, O'Brien SM, Jacobs ML, Jacobs JP, Manning PB, Eghtesady P. Risk factors for mortality and morbidity after the neonatal Blalock‐Taussig shunt procedure. Ann Thorac Surg. 2011;92:642–651; discussion 651–652. doi: 10.1016/j.athoracsur.2011.02.030 [DOI] [PubMed] [Google Scholar]
- 6. Dirks V, Pretre R, Knirsch W, Valsangiacomo Buechel ER, Seifert B, Schweiger M, Hubler M, Dave H. Modified Blalock Taussig shunt: a not‐so‐simple palliative procedure. Eur J Cardiothorac Surg. 2013;44:1096–1102. doi: 10.1093/ejcts/ezt172 [DOI] [PubMed] [Google Scholar]
- 7. Dorobantu DM, Pandey R, Sharabiani MT, Mahani AS, Angelini GD, Martin RP, Stoica SC. Indications and results of systemic to pulmonary shunts: results from a national database. Eur J Cardiothorac Surg. 2016;49:1553–1563. doi: 10.1093/ejcts/ezv435 [DOI] [PubMed] [Google Scholar]
- 8. Aggarwal V, Petit CJ, Glatz AC, Goldstein BH, Qureshi AM. Stenting of the ductus arteriosus for ductal‐dependent pulmonary blood flow‐current techniques and procedural considerations. Congenit Heart Dis. 2019;14:110–115. doi: 10.1111/chd.12709 [DOI] [PubMed] [Google Scholar]
- 9. Gibbs JL, Rothman MT, Rees MR, Parsons JM, Blackburn ME, Ruiz CE. Stenting of the arterial duct: a new approach to palliation for pulmonary atresia. Br Heart J. 1992;67:240–245. doi: 10.1136/hrt.67.3.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santoro G, Gaio G, Giugno L, Capogrosso C, Palladino MT, Iacono C, Caianiello G, Russo MG. Ten‐years, single‐center experience with arterial duct stenting in duct‐dependent pulmonary circulation: early results, learning‐curve changes, and mid‐term outcome. Catheter Cardiovasc Interv. 2015;86:249–257. doi: 10.1002/ccd.25949 [DOI] [PubMed] [Google Scholar]
- 11. Udink Ten Cate FE, Sreeram N, Hamza H, Agha H, Rosenthal E, Qureshi SA. Stenting the arterial duct in neonates and infants with congenital heart disease and duct‐dependent pulmonary blood flow: a multicenter experience of an evolving therapy over 18 years. Catheter Cardiovasc Interv. 2013;82:E233–E243. doi: 10.1002/ccd.24878 [DOI] [PubMed] [Google Scholar]
- 12. Ratnayaka K, Nageotte SJ, Moore JW, Guyon PW, Bhandari K, Weber RL, Lee JW, You H, Griffin DA, Rao RP, et al. Patent ductus arteriosus stenting for all ductal‐dependent cyanotic infants: waning use of Blalock‐Taussig shunts. Circ Cardiovasc Interv. 2021;14:e009520. doi: 10.1161/CIRCINTERVENTIONS.120.009520 [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glatz AC, Petit CJ, Goldstein BH, Kelleman MS, McCracken CE, McDonnell A, Buckey T, Mascio CE, Shashidharan S, Ligon RA, et al. Comparison between patent ductus arteriosus stent and modified Blalock‐Taussig shunt as palliation for infants with ductal‐dependent pulmonary blood flow: insights from the congenital catheterization research collaborative. Circulation. 2018;137:589–601. doi: 10.1161/CIRCULATIONAHA.117.029987 [DOI] [PubMed] [Google Scholar]
- 15. Norris JM, Simpson BS, Ball R, Freeman A, Kirkham A, Parry MA, Moore CM, Whitaker HC, Emberton M. A modified Newcastle‐Ottawa scale for assessment of study quality in genetic urological research. Eur Urol. 2021;79:325–326. doi: 10.1016/j.eururo.2020.12.017 [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane; 2022. Available at: www.training.cochrane.org/handbook. Accessed February 10, 2022. [Google Scholar]
- 17. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 19. Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D, Salanti G. Methods to estimate the between‐study variance and its uncertainty in meta‐analysis. Res Synth Methods. 2016;7:55–79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 21. McMullan DM, Permut LC, Jones TK, Johnston TA, Rubio AE. Modified Blalock‐Taussig shunt versus ductal stenting for palliation of cardiac lesions with inadequate pulmonary blood flow. J Thorac Cardiovasc Surg. 2014;147:397–401. doi: 10.1016/j.jtcvs.2013.07.052 [DOI] [PubMed] [Google Scholar]
- 22. Mallula K, Vaughn G, El‐Said H, Lamberti JJ, Moore JW. Comparison of ductal stenting versus surgical shunts for palliation of patients with pulmonary atresia and intact ventricular septum. Catheter Cardiovasc Interv. 2015;85:1196–1202. doi: 10.1002/ccd.25870 [DOI] [PubMed] [Google Scholar]
- 23. Amoozgar H, Cheriki S, Borzoee M, Ajami G, Soltani M, Ahmadipour M, Peiravian F, Amirghofran A. Short‐term result of ductus arteriosus stent implantation compared with surgically created shunts. Pediatr Cardiol. 2012;33:1288–1294. doi: 10.1007/s00246-012-0304-x [DOI] [PubMed] [Google Scholar]
- 24. Bentham JR, Zava NK, Harrison WJ, Shauq A, Kalantre A, Derrick G, Chen RH, Dhillon R, Taliotis D, Kang SL, et al. Duct stenting versus modified Blalock‐Taussig shunt in neonates with duct‐dependent pulmonary blood flow: associations with clinical outcomes in a multicenter National Study. Circulation. 2018;137:581–588. doi: 10.1161/CIRCULATIONAHA.117.028972 [DOI] [PubMed] [Google Scholar]
- 25. Santoro G, Capozzi G, Caianiello G, Palladino MT, Marrone C, Farina G, Russo MG, Calabro R. Pulmonary artery growth after palliation of congenital heart disease with duct‐dependent pulmonary circulation: arterial duct stenting versus surgical shunt. J Am Coll Cardiol. 2009;54:2180–2186. doi: 10.1016/j.jacc.2009.07.043 [DOI] [PubMed] [Google Scholar]
- 26. Goldstein BH, O'Byrne ML, Petit CJ, Qureshi AM, Dai D, Griffis HM, France A, Kelleman MS, McCracken CE, Mascio CE, et al. Differences in cost of care by palliation strategy for infants with ductal‐dependent pulmonary blood flow. Circ Cardiovasc Interv. 2019;12:e007232. doi: 10.1161/CIRCINTERVENTIONS.118.007232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roggen M, Cools B, Brown S, Boshoff D, Heying R, Eyskens B, Gewillig M. Can ductus arteriosus morphology influence technique/outcome of stent treatment? Catheter Cardiovasc Interv. 2020;95:1149–1157. doi: 10.1002/ccd.28725 [DOI] [PubMed] [Google Scholar]
- 28. Santoro G, Capozzi G, Giordano M, Gaio G, Palladino MT, Iacono C, Mahmoud HT, Russo MG. Fate of duct‐dependent, discontinuous pulmonary arteries after arterial duct stenting. Pediatr Cardiol. 2017;38:1370–1376. doi: 10.1007/s00246-017-1672-z [DOI] [PubMed] [Google Scholar]
- 29. Baspinar O, Sahin DA. Bilateral ductal stenting for discontinuity of the pulmonary artery via the femoral and carotid arteries in an infant. Case Rep Cardiol. 2015;2015:619653. doi: 10.1155/2015/619653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alwi M. Stenting the ductus arteriosus: case selection, technique and possible complications. Ann Pediatr Cardiol. 2008;1:38–45. doi: 10.4103/0974-2069.41054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alsagheir A, Koziarz A, Makhdoum A, Contreras J, Alraddadi H, Abdalla T, Benson L, Chaturvedi RR, Honjo O. Duct stenting versus modified Blalock‐Taussig shunt in neonates and infants with duct‐dependent pulmonary blood flow: a systematic review and meta‐analysis. J Thorac Cardiovasc Surg. 2021;161:379–390.e8. doi: 10.1016/j.jtcvs.2020.06.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S3


