Abstract
Background
The aim of this study was to investigate the association between hsCRP (high‐sensitivity C‐reactive protein) and prognosis over time after stroke onset.
Methods and Results
In this prespecified prospective substudy of the Third China National Stroke Registry, a total of 9438 patients with acute ischemic stroke or transient ischemic attack and measured hsCRP were included. Patients were categorized into 3 groups according to the sampling time after index onset (<24 hours, 24–72 hours, 72 hours–8 days). The outcomes consisted of stroke recurrence and combined vascular events within 1 year, and dependence or death defined as modified Rankin Scale score of 3 to 6 at 1 year. The associations between hsCRP and outcomes in different groups were analyzed by using Cox proportional hazards and logistic regression models. The median levels of hsCRP within 24 hours, between 24 and 72 hours and between 72 hours and 8 days were 2.01, 1.72, and 1.72 mg/L, respectively (P < 0.05). Compared with the bottom quartile, patients in the top quartile measured within 72 hours were at increased risk of recurrent stroke (<24 hours: adjusted hazard ratio [HR], 1.57 [95% CI, 1.05–2.35], P = 0.03; 24–72 hours: adjusted HR, 1.60 [95% CI, 1.18–2.17], P = 0.003). Association was attenuated after further adjusting for the Org 10 172 test in the Treatment of Acute Stroke classification (<24 hours: adjusted HR, 1.51 [95% CI, 1.01–2.27]; P = 0.05; 24–72 hours: adjusted HR, 1.55 [95% CI, 1.14–2.10]; P = 0.01). The association only existed in patients with large‐artery atherosclerosis (adjusted HR, 1.68 [95% CI, 1.06–2.64]; P = 0.03). However, the association was not found in the hsCRP level measured between 72 hours and 8 days. Similar results were found for the outcome of combined vascular events. Additionally, hsCRP levels measured between 24 and 72 hours were associated with an increased risk of poor functional outcomes.
Conclusions
Elevated levels of hsCRP measured in the first 72 hours after ischemic stroke or transient ischemic attack but not 72 hours to 8 days, were associated with an increased risk of 1‐year stroke recurrence.
Keywords: high‐sensitivity C‐reactive protein, inflammation, ischemic stroke, TIA
Subject Categories: Inflammation, Ischemic Stroke, Transient Ischemic Attack (TIA)
Nonstandard Abbreviations and Acronyms
- CNSR‐III
Third China National Stroke Registry
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- TOAST
the Org 10 172 test in the Treatment of Acute Stroke
Clinical Perspective.
What Is New?
hsCRP (high‐sensitivity C‐reactive protein) measured within the first 72 hours, but not 72 hours–8 days after index onset, was associated with an increased risk of recurrent stroke events and combined vascular events at 1‐year follow‐up after risk adjustment.
Besides, hsCRP levels measured between 24 and 72 hours were associated with an increased risk of poor functional outcomes.
What Are the Clinical Implications?
The predictive value of hsCRP for 1‐year prognosis may be affected by sampling time in the patients with acute ischemic stroke or transient ischemic attack.
Time‐specific hsCRP measurements may help stratify the risk of new stroke and poor functional outcomes.
A body of evidence has emphasized the crucial role of inflammation in the pathophysiology of atherosclerosis and cardiovascular and cerebrovascular disease. 1 , 2 Most, 3 , 4 , 5 , 6 but not all 7 previous studies indicated that hsCRP (high‐sensitivity C‐reactive protein) one of the most investigated cytokines in cardiovascular research, played a crucial role in stroke recurrence. Variation in sampling time for hsCRP measurement has been shown to affect the association between hsCRP and prognosis of stroke in few prior studies, which might result in these discrepancies. 8 , 9 Di Napoli et al 8 enrolled 128 patients with ischemic stroke with CRP measured within 24 hours after index stroke, 48 to 72 hours, and at hospital discharge and found that CRP at discharge was a better predictor of further cardiovascular events or death. While another study showed that the CRP determination between 12 and 24 hours after symptom onset, not the initial or later CRP measurements best identified patients with a high risk for the unfavorable outcome. 9 However, the sampling time was largely ignored before, and the sample size of the previous study was relatively small. The association between hsCRP at different stages and long‐term recurrent stroke is less established.
Therefore, based on the large‐scale prospective cohort study of the Third China National Stroke Registry (CNSR‐III), we aimed to investigate the association between hsCRP measured at different times after stroke and 1‐year prognosis in patients with acute ischemic stroke or transient ischemic attack (TIA).
Methods
Study Design
The data that support the findings of this study are available from the corresponding author upon reasonable request. The design of CNSR‐III has been described in detail before. In brief, CNSR‐III was a prospective national registry for patients with acute ischemic stroke or TIA between August 2015 and March 2018 from 201 study sites in China. 10 Of these, 171 having prior experience in collecting blood samples voluntarily participated in the prespecified biomarker substudy of the CNSR‐III. Participants were consecutively recruited if meeting the following criteria: (1) age > 18 years; (2) diagnosis within 7 days of ischemic stroke and TIA; and (3) informed consent from the participant or legally authorized representative. Acute ischemic stroke and TIA were diagnosed according to the World Health Organization criteria and confirmed by computed tomography or magnetic resonance imaging. 11 In this study, patients with baseline hsCRP measurement were enrolled.
The protocol of CNSR‐III was approved by the Ethics Committee of Beijing Tiantan Hospital (institutional review board approval number: KY2015‐001‐01) and all participating centers. All patients provided written informed consent.
Baseline Data Collection
Baseline information includes demographic information, medical history of stroke, TIA, hypertension, diabetes, dyslipidemia, atrial fibrillation, coronary artery disease, cigarette smoking, body mass index (calculated as weight in kilograms divided by height in meters squared), prestroke modified Rankin Scale (mRS) score, National Institutes of Health Stroke Scale (NIHSS) score on admission, intravenous recombinant tissue plasminogen activator treatment, and laboratory tests were obtained from medical records by unified trained researchers. 12 All the clinical information was collected through the electronic data capture system. It provided an automatic check for completeness and logical correction of the uploaded data and would enhance the information quality.
Etiologic classification of ischemic stroke was performed according to the Org 10 172 test in the Treatment of Acute Stroke (TOAST) criteria. 13 Central stroke subtyping was conducted by radiologists and neurologists based on standardized phenotypic elements for each subtype as previously defined. 14
Sample Collection and Measurements of hsCRP
Fasting blood samples were obtained from all patients within 24 hours of admission. The sampling time was within 8 days after the onset of the index event. The blood samples were delivered via cold chain to the central laboratory in Beijing Tiantan Hospital. All samples were refrigerated in a cryotube at −80˚ C until assays were performed centrally and blindly. The concentrations of plasma hsCRP were measured on Roche Cobas C501 analyzers.
Follow‐Up and Outcomes
Patients were followed up to obtain clinical outcomes at 12 months by telephone based on standardized interview protocol. 12 We recorded all stroke recurrences, combined vascular events, mortality, and poor functional outcomes during follow‐up. Confirmation of vascular events was sought from the treating hospital. Suspected recurrent stroke without hospitalization was verified by an independent end point judgment committee. Death was confirmed by a death certificate issued by the treated hospital or the local civil registry.
Recurrent stroke was defined as the new occurrence of focal neurological deficits caused by ischemic or hemorrhagic stroke events confirmed by magnetic resonance imaging or computed tomography. Poor functional outcomes were defined by a mRS score of 3 to 6. Combined vascular events were defined as the new occurrence of stroke, myocardial infarction, and cardiovascular death.
Statistical Analysis
Continuous variables were presented as the median and interquartile range, whereas categorical variables as frequencies and proportions. Baseline characteristics were compared among patients in different groups using chi‐square tests for categorical variables and ANOVA or Kruskal‐Wallis tests for continuous variables. According to the onset‐to‐sample collection time, patients were divided into 3 groups (within 24 hours, 24–72 hours, and 72 hours–8 days). We performed Cox proportional hazards models to evaluate the association between hsCRP level and stroke recurrence and combined vascular events in different groups. The assumptions of proportional hazards were validated before these analyses by adding a time‐dependent covariate with the interaction of hsCRP with a logarithmic function of survival time in the model. The Kaplan‐Meier survival curve using the log‐rank univariate test was applied to depict the occurrence of stroke recurrence and combined vascular events. Logistic regression models were used to explore the association of hsCRP with poor functional outcomes. The potential confounders were demographic factors, traditional or clinical risk factors, index event, baseline leukocyte count, thrombolytic therapy, and pre‐mRS score and TOAST classification. Patients were categorized into 4 groups according to the quartiles of hsCRP levels (quartile 1: <0.82 mg/L; quartile 2: 0.82–1.77 mg/L; quartile 3: 1.77–4.71 mg/L; quartile 4: ≥ 4.71 mg/L). Moreover, hsCRP level was further performed by relative risk category (low risk, <1.0 mg/L; average risk, 1–3 mg/L; and high risk, >3 mg/L) recommended by the Centers for Disease Control and Prevention and American Heart Association for cardiovascular disease risk assessment initially. 15 This cut point has been proven to be associated with increased risk of recurrent stroke and poor functional outcomes. 3 Considering that the death is a competing risk of stroke recurrence and combined vascular events, we further used the competing risk analysis of Fine and Gray and subdistribution Cox proportional hazards to test the association between hsCRP measured in different stages after stroke and 1‐year outcomes. We used SAS 9.4 software (SAS Institute, Inc, Cary, NC) to conduct the statistical analyses. A 2‐sided P value of <0.05 was considered significant.
Results
Patients Characteristics
A total of 9438 patients were enrolled in this study (Figure 1). Of all the participants, the median age was 63 (54–70) years, and 6732 (68.48%) patients were men. The median time of sampling after index event onset was 55 hours. The patients included and those excluded in this study were well balanced except for previous dyslipidemia and atrial fibrillation, baseline NIHSS score, index events, and treatment (Table S1). The proportion of patients according to onset‐to‐sample collection time (within 24 hours, 24–72 hours, and 72 hours–8 days) were 20.58%, 41.88%, and 37.54%, respectively. The median levels of hsCRP among the 3 groups were 2.01, 1.72, and 1.72 mg/L, respectively (P < 0.05). Aging, medical history of coronary heart disease and atrial fibrillation, baseline NIHSS score, index event, recombinant tissue plasminogen activator treatment, baseline leukocyte count, and medication during follow‐up differed among the 3 groups (Table 1). There were 222 (2.35%) patients lost to follow‐up at 1 year.
Figure 1. Flowchart of the study population.
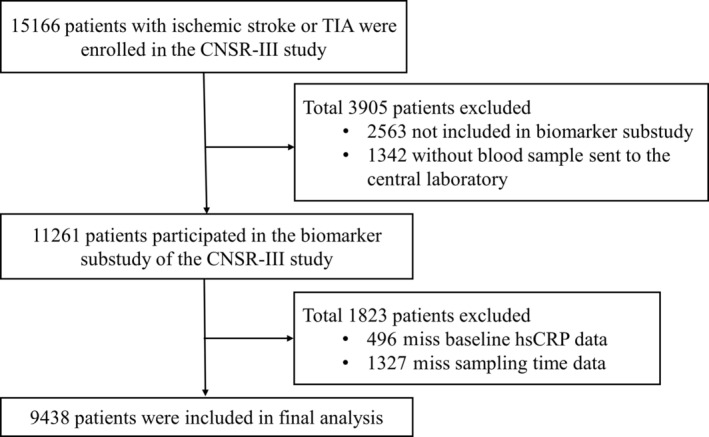
CNSR‐III indicates the Third China National Stroke Registry; hsCRP, high‐sensitivity C‐reactive protein; and TIA, transient ischemic attack.
Table 1.
Baseline Characteristics of Study Population
|
Total n = 9438 |
Onset‐to‐sample collection time | P value | |||
|---|---|---|---|---|---|
|
<24 h n = 1942 |
24–72 h n = 3953 |
72 h–8 d n = 3543 |
|||
| Age, y | 63 (54–70) | 63 (55–71) | 63 (54–71) | 62 (54–69) | <0.001 |
| Male | 6732 (68.48) | 1315 (67.71) | 2681 (67.82) | 2455 (69.29) | 0.19 |
| Body mass index, kg/m2 | 24.49 (22.58–26.57) | 24.47 (22.49–26.42) | 24.47 (22.50–26.56) | 24.49 (22.66–26.67) | 0.21 |
| Current smoking | 2954 (31.30) | 613 (31.57) | 1211 (30.63) | 1194 (32.32) | 0.20 |
| Medical history | |||||
| Stroke | 2149 (22.77) | 470 (24.20) | 905 (22.89) | 774 (21.85) | 0.13 |
| Hypertension | 5955 (63.10) | 1171 (60.33) | 2523 (63.82) | 2261 (63.82) | 0.03 |
| Diabetes | 2262 (23.97) | 436 (22.45) | 945 (23.91) | 881 (24.87) | 0.13 |
| Dyslipidemia | 814 (8.62) | 159 (8.19) | 336 (8.50) | 319 (9.00) | 0.55 |
| Coronary artery disease | 1082 (11.01) | 255 (13.13) | 425 (10.75) | 347 (9.79) | <0.001 |
| Atrial fibrillation | 694 (7.35) | 197 (10.14) | 267 (6.75) | 230 (6.49) | <0.001 |
| NIHSS at admission | <0.001 | ||||
| ≤3 | 5000 (52.98) | 974 (50.15) | 2064 (52.21) | 1962 (55.38) | |
| 3–10 | 3702 (39.22) | 772 (39.75) | 1581 (39.99) | 1349 (38.08) | |
| >10 | 736 (7.80) | 196 (10.09) | 308 (7.79) | 232 (6.55) | |
| Prestroke mRS score 2–5 | 814 (8.62) | 185 (9.53) | 341 (8.63) | 288 (8.13) | 0.35 |
| Index event | <0.001 | ||||
| TIA | 667 (7.07) | 184 (9.47) | 301 (7.61) | 182 (5.14) | |
| Ischemic stroke | 8771 (92.93) | 1758 (90.53) | 3652 (92.39) | 3361 (94.86) | |
| rt‐PA treatment | 966 (10.24) | 444 (22.86) | 330 (8.35) | 192 (5.42) | <0.001 |
| Baseline leukocyte count, 109/L | 5.71 (8.41–4.01) | 7.15 (5.81–8.74) | 6.84 (5.67–8.40) | 6.89 (5.70–8.25) | <0.001 |
| LDL, mmol/L | 2.32 (1.73–2.98) | 2.44 (1.81–3.15) | 2.43 (1.85–3.10) | 2.11 (1.61–2.73) | <0.001 |
| Medication during hospitalization | |||||
| Antiplatelet | 9105 (96.94) | 1863 (96.38) | 3822 (97.13) | 3420 (97.05) | 0.26 |
| Anticoagulants | 962 (10.24) | 235 (12.16) | 384 (9.76) | 343 (9.73) | 0.005 |
| Antihypertensive | 4429 (47.16) | 853 (44.13) | 1857 (47.19) | 1719 (48.78) | 0.007 |
| Antidiabetic | 2430 (25.87) | 454 (23.49) | 1003 (25.49) | 973 (27.61) | 0.003 |
| Statin | 9099 (99.76) | 1865 (99.79) | 3821 (99.74) | 3413 (99.77) | 0.94 |
| hsCRP, mg/L | 1.77 (0.82–4.71) | 2.01 (0.89–5.02) | 1.72 (0.82–4.60) | 1.72 (0.80–4.63) | 0.002 |
| TOAST classification | <0.001 | ||||
| Large‐artery atherosclerosis | 2336 (24.75) | 481 (24.77) | 942 (23.83) | 913 (25.77) | |
| Cardioembolism | 633 (6.71) | 171 (8.81) | 263 (6.65) | 199 (5.62) | |
| Small‐artery occlusion | 1985 (21.03) | 369 (19.00) | 890 (22.51) | 726 (20.49) | |
| Other determined etiology | 106 (1.12) | 22 (1.13) | 43 (1.09) | 41 (1.16) | |
| Undetermined etiology | 4378 (46.39) | 899 (46.29) | 1815 (45.91) | 1664 (46.97) | |
| Days from onset to recurrence | 37 (3–145) | 16 (2–115) | 48 (3–157) | 48 (6–153) | <0.001 |
| 1‐y stroke recurrence | 931 (9.86) | 221 (11.38) | 367 (9.28) | 343 (9.68) | 0.04 |
Variables are presented as median (interquartile range) or number (%).
hsCRP indicates high‐sensitivity C‐reactive protein; LDL, Low‐density lipoprotein cholesterol; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; rt‐PA, recombinant tissue plasminogen activator; TIA, transient ischemic attack; and TOAST, Trial of Org 10 172 in Acute Stroke Treatment.
hsCRP and Recurrent Vascular Events
There were 931 (9.86%) patients with recurrent stroke during 1 year. For all patients, increased hsCRP levels were associated with recurrent stroke (Table 1). The rates of stroke recurrence in patients according to quartiles of hsCRP were 8.17%, 8.35%, 9.97%, and 12.95%, respectively. The hsCRP level measured within 24 hours and between 24 and 72 hours was associated with an increased risk of stroke recurrence (<24 hours: unadjusted HR, 1.86 [95% CI, 1.27–2.74]; P = 0.002; 24 to 72 hours: unadjusted HR, 1.93 [95% CI, 1.44–2.58]; P < 0.001, 72 hours–8 days: unadjusted HR, 1.30 [95% CI, 0.97–1.75]) (Figure 2). After adjustment for age; sex; body mass index; smoking; index event; medical histories of atrial fibrillation, coronary heart disease, ischemic stroke, diabetes, hypertension, and hypercholesterolemia; baseline NIHSS score and baseline leukocyte count; recombinant tissue plasminogen activator treatment; and pre‐mRS score, such associations remained. The risk of recurrent stroke for the patients in the top quartile of hsCRP measured within 24 hours and between 24 and 72 hours, respectively, increased by 57% and 60% (Table 2). However, the association was not found in the hsCRP level measured between 72 hours and 8 days (Table 2). The association was slightly attenuated after further adjusting for TOAST classification (Table 2). Similar results were seen when hsCRP was divided into 3 groups by relative risk category. When hsCRP was evaluated as a continuous variable, we also found the association between hsCRP measured within 72 hours and 1‐year stroke recurrence in the unadjusted model (HR, 1.004 [95% CI, 1.002–1.006]; P < 0.001), and the association was partially weakened in the adjusted model (adjusted HR, 1.002 [95% CI, 1.000–1.005]; P = 0.07). The relationship between hsCRP and combined vascular events also only existed within 72 hours (Figure 3, Table 3). This association persisted when hsCRP was used as a continuous variable (adjusted HR, 1.004 [95% CI, 1.001–1.006]; P = 0.001). All of the assumptions of proportional hazards were met (P > 0.05 for all). In addition, analysis of outcomes including stroke recurrence and combined vascular events, using death as a competing risk, yielded completely similar results (Table S2 and Table S3).
Figure 2. Cumulative incidence of 1‐ year stroke recurrence, according to hsCRP levels.
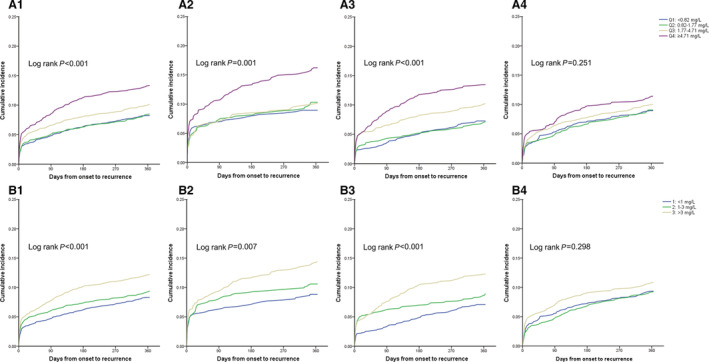
A, Patients were categorized into 4 groups according to hsCRP quartiles. B, Patients were performed by relative risk category (low risk, <1.0 mg/L; average risk, 1–3 mg/L; and high risk, >3 mg/L). A1, A2, A3, and A4, respectively, indicated total population, patients with onset‐to‐sample collection time within 24 hours, 24–72 hours, and 72 hours–8 days. Similarly, B1, B2, B3, B4 indicated total population, patients with onset‐to‐sample collection time within 24 hours, 24–72 hours, and 72 hours–8 days, respectively. Q1 indicates quartile 1; Q2, quartile 2; Q3, quartile 3; and Q4, quartile 4.
Table 2.
Association Between hsCRP Measured in Different Stage and Recurrent Stroke Within 1 Year
| hsCRP. mg/L | Events, n (%) | HR (95% CI)* | P value | HR (95% CI)† | P value |
|---|---|---|---|---|---|
| <24 h | |||||
| Quartile 1 | 38 (8.92) | Reference | … | Reference | … |
| Quartile 2 | 49 (10.21) | 1.10 (0.72–1.68) | 0.67 | 1.08 (0.7–1.65) | 0.74 |
| Quartile 3 | 51 (9.98) | 1.03 (0.67–1.59) | 0.88 | 1.01 (0.65–1.56) | 0.97 |
| Quartile 4 | 83 (15.81) | 1.57 (1.05–2.35) | 0.03 | 1.51 (1.01–2.27) | 0.05 |
| <1 | 48 (8.76) | Reference | … | Reference | … |
| 1–3 | 67 (10.47) | 1.11 (0.76–1.63) | 0.58 | 1.08 (0.74–1.58) | 0.7 |
| >3 | 106 (14.06) | 1.44 (1.00–2.06) | 0.049 | 1.38 (0.96–1.99) | 0.08 |
| 24–72 h | |||||
| Quartile 1 | 71 (7.21) | Reference | … | Reference | … |
| Quartile 2 | 71 (6.98) | 0.94 (0.67–1.31) | 0.72 | 0.92 (0.66–1.29) | 0.64 |
| Quartile 3 | 99 (10.05) | 1.32 (0.97–1.81) | 0.08 | 1.3 (0.95–1.77) | 0.1 |
| Quartile 4 | 126 (13.04) | 1.60 (1.18–2.17) | 0.003 | 1.55 (1.14–2.10) | 0.01 |
| <1 | 90 (7.04) | Reference | … | Reference | … |
| 1–3 | 114 (8.71) | 1.24 (0.93–1.64) | 0.14 | 1.22 (0.92–1.62) | 0.17 |
| >3 | 163 (11.94) | 1.53 (1.17–2.01) | 0.002 | 1.49 (1.13–1.95) | 0.004 |
| 72 h–8 d | |||||
| Quartile 1 | 81 (8.86) | Reference | … | Reference | … |
| Quartile 2 | 79 (8.93) | 0.97 (0.71–1.32) | 0.83 | 0.96 (0.7–1.31) | 0.77 |
| Quartile 3 | 86 (9.86) | 1.04 (0.77–1.42) | 0.78 | 1.01 (0.74–1.38) | 0.93 |
| Quartile 4 | 97 (11.12) | 1.07 (0.78–1.47) | 0.69 | 1.02 (0.74–1.4) | 0.92 |
| <1 | 108 (9.26) | Reference | … | Reference | … |
| 1–3 | 102 (9.08) | 0.93 (0.70–1.22) | 0.58 | 0.91 (0.69–1.19) | 0.49 |
| >3 | 133 (10.61) | 0.99 (0.76–1.30) | 0.94 | 0.95 (0.73–1.25) | 0.73 |
| Total | |||||
| Quartile 1 | 190 (8.17) | Reference | … | Reference | … |
| Quartile 2 | 199 (8.35) | 1.01 (0.82–1.23) | 0.95 | 0.99 (0.81–1.21) | 0.92 |
| Quartile 3 | 236 (9.97) | 1.18 (0.97–1.43) | 0.1 | 1.14 (0.94–1.39) | 0.18 |
| Quartile 4 | 306 (12.95) | 1.44 (1.19–1.74) | <0.001 | 1.37 (1.13–1.66) | 0.001 |
| <1 | 246 (8.22) | Reference | … | Reference | … |
| 1–3 | 283 (9.21) | 1.10 (0.92–1.31) | 0.29 | 1.08 (0.91–1.28) | 0.41 |
| >3 | 402 (11.92) | 1.33 (1.13–1.57) | <0.001 | 1.28 (1.08–1.51) | 0.004 |
HR indicates hazard ratio; mRS, modified Rankin Scale; and rt‐PA, recombinant tissue plasminogen activator.
Adjusted for age, sex, body mass index, current smoking, index event, medical histories of atrial fibrillation, coronary heart disease, stroke, diabetes, hypertension and hypercholesterolemia, baseline National Institutes of Health Stroke Scale score and baseline leukocyte count, rt‐PA treatment, and pre‐mRS score.
Adjusted for age, sex, body mass index, current smoking, index event, medical histories of atrial fibrillation, coronary heart disease, stroke, diabetes, hypertension and hypercholesterolemia, baseline National Institutes of Health Stroke Scale score and baseline leukocyte count, rt‐PA treatment, pre‐mRS score, and the Org 10 172 test in the Treatment of Acute Stroke (TOAST) classification.
Figure 3. Cumulative incidence of 1‐year combined vascular events, according to hsCRP levels.
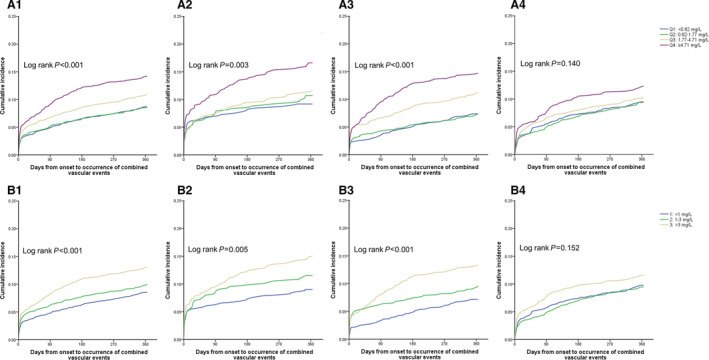
A, Patients were categorized into 4 groups according to hsCRP (high‐sensitivity C‐reactive protein) quartiles. B, Patients were performed by relative risk category (low risk, <1.0 mg/L; average risk, 1–3 mg/L; and high risk, >3 mg/L). A1, A2, A3, and A4, respectively, indicated total population, patients with onset‐to‐sample collection time within 24 hours, 24–72 hours, and 72 hours–8 days. Similarly, B1, B2, B3, B4 indicated total population, patients with onset‐to‐sample collection time within 24 hours, 24–72 hours, and 72 hours–8 days, respectively. Q1 indicates quartile 1; Q2, quartile 2; Q3, quartile 3; and Q4, quartile 4.
Table 3.
Association Between hsCRP Measured in Different Stage and Combined Vascular Events Within 1 Year
| hsCRP, mg/L | Events, n (%) | HR (95% CI)* | P value | HR (95% CI)† | P value |
|---|---|---|---|---|---|
| <24 h | |||||
| Quartile 1 | 39 (9.15) | Reference | … | Reference | … |
| Quartile 2 | 51 (10.63) | 1.11 (0.73–1.69) | 0.62 | 1.09 (0.72–1.67) | 0.68 |
| Quartile 3 | 58 (11.35) | 1.14 (0.75–1.73) | 0.55 | 1.11 (0.73–1.69) | 0.61 |
| Quartile 4 | 85 (16.19) | 1.53 (1.02–2.27) | 0.04 | 1.48 (0.99–2.21) | 0.06 |
| <1 | 49 (8.94) | Reference | … | Reference | … |
| 1–3 | 73 (11.41) | 1.18 (0.82–1.71) | 0.38 | 1.15 (0.8–1.67) | 0.45 |
| >3 | 111 (14.72) | 1.44 (1.01–2.05) | 0.04 | 1.4 (0.98–2.00) | 0.07 |
| 24–72 h | |||||
| Quartile 1 | 72 (7.31) | Reference | … | Reference | … |
| Quartile 2 | 73 (7.18) | 0.96 (0.69–1.33) | 0.79 | 0.93 (0.67–1.3) | 0.68 |
| Quartile 3 | 109 (11.07) | 1.45 (1.07–1.96) | 0.02 | 1.41 (1.04–1.91) | 0.03 |
| Quartile 4 | 138 (14.29) | 1.74 (1.29–2.35) | <0.001 | 1.67 (1.24–2.26) | <0.001 |
| <1 | 91 (7.11) | Reference | … | Reference | … |
| 1–3 | 123 (9.40) | 1.33 (1.01–1.75) | 0.04 | 1.3 (0.99–1.71) | 0.06 |
| >3 | 178 (13.04) | 1.67 (1.28–2.17) | <0.001 | 1.6 (1.23–2.09) | <0.001 |
| 72 h–8 d | |||||
| Quartile 1 | 86 (9.41) | Reference | … | Reference | … |
| Quartile 2 | 82 (9.27) | 0.94 (0.70–1.28) | 0.71 | 0.94 (0.69–1.27) | 0.67 |
| Quartile 3 | 88 (10.09) | 0.99 (0.74–1.35) | 0.99 | 0.97 (0.72–1.32) | 0.86 |
| Quartile 4 | 105 (12.04) | 1.06 (0.78–1.44) | 0.71 | 1.02 (0.75–1.39) | 0.91 |
| <1 | 113 (9.69) | Reference | … | Reference | … |
| 1–3 | 105 (9.35) | 0.91 (0.69–1.19) | 0.48 | 0.89 (0.68–1.17) | 0.41 |
| >3 | 143 (11.40) | 0.99 (0.77–1.30) | 0.99 | 0.97 (0.74–1.26) | 0.79 |
| Total | |||||
| Quartile 1 | 197 (8.47) | Reference | … | Reference | … |
| Quartile 2 | 206 (8.65) | 1.01 (0.83–1.22) | 0.96 | 0.99 (0.81–1.2) | 0.91 |
| Quartile 3 | 255 (10.77) | 1.22 (1.01–1.48) | 0.04 | 1.19 (0.99–1.44) | 0.07 |
| Quartile 4 | 328 (13.88) | 1.47 (1.22–1.77) | <0.001 | 1.4 (1.17–1.69) | <0.001 |
| <1 | 253 (8.45) | Reference | … | Reference | … |
| 1–3 | 301 (9.80) | 1.13 (0.96–1.34) | 0.14 | 1.11 (0.94–1.32) | 0.22 |
| >3 | 432 (12.81) | 1.38 (1.17–1.62) | <0.001 | 1.33 (1.13–1.56) | <0.001 |
HR indicates hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; mRS, modified Rankin Scale; and rt‐PA recombinant tissue plasminogen activator.
Adjusted for age, sex, body mass index, current smoking, index event, medical histories of atrial fibrillation, coronary heart disease, stroke, diabetes, hypertension and hypercholesterolemia, baseline National Institutes of Health Stroke Scale score and baseline leukocyte count, rt‐PA treatment; and pre‐mRS score.
Adjusted for age, sex, body mass index, current smoking, index event, medical histories of atrial fibrillation, coronary heart disease, stroke, diabetes, hypertension and hypercholesterolemia, baseline National Institutes of Health Stroke Scale score and baseline leukocyte count, rt‐PA treatment, pre‐mRS score, and the Org 10 172 test in the Treatment of Acute Stroke (TOAST) classification.
After further adding TOAST classification in the above multivariable model, the association was attenuated (<24 hours: adjusted HR, 1.51 [95% CI, 1.01–2.27]; P = 0.05; 24–72 hours: adjusted HR, 1.55 [95% CI, 1.14–2.10]; P = 0.01), especially when analyzed using the relative risk category (<24 hours: adjusted HR, 1.38 [95% CI, 0.96–1.99]; P = 0.08; 24–72 hours: adjusted HR, 1.49 [95% CI, 1.13–1.95]; P = 0.004) (Table 2). We therefore performed subsequent exploratory analysis by classifying patients according to the TOAST classification, the association between hsCRP measured within 72 hours and recurrent stroke only existed in patients with large‐artery atherosclerosis (adjusted hazard ratio (HR), 1.68 [95% CI, 1.06–2.64]; P = 0.03), and undetermined etiology subtypes (adjusted HR, 1.76 [95% CI, 1.18–2.63]; P = 0) (Table 4).
Table 4.
Association Between hsCRP Measured Within 72 Hours and 1‐Year Stroke Recurrence Stratified by TOAST Classification
| TOAST classification | Events, n (%) | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Large‐artery atherosclerosis | Quartile 1 | 28 (10.89) | Reference | … | Reference | … |
| Quartile 2 | 36 (10.53) | 0.98 (0.60–1.60) | 0.93 | 0.98 (0.6–1.62) | 0.95 | |
| Quartile 3 | 41 (11.20) | 1.04 (0.65–1.69) | 0.86 | 0.99 (0.61–1.63) | 0.98 | |
| Quartile 4 | 87 (19.00) | 1.87 (1.22–2.86) | 0.004 | 1.68 (1.06–2.64) | 0.03 | |
| <1 | 34 (10.30) | Reference | … | Reference | … | |
| 1–3 | 52 (11.11) | 1.11 (0.72–1.71) | 0.64 | 1.11 (0.71–1.73) | 0.64 | |
| >3 | 106 (16.96) | 1.74 (1.18–2.56) | 0.01 | 1.61 (1.07–2.43) | 0.02 | |
| Cardioembolism | Quartile 1 | 8 (10.81) | Reference | … | Reference | … |
| Quartile 2 | 9 (9.57) | 0.9 (0.35–2.33) | 0.83 | 0.87 (0.33–2.3) | 0.78 | |
| Quartile 3 | 14 (12.73) | 1.21 (0.51–2.89) | 0.66 | 1.24 (0.51–3.01) | 0.64 | |
| Quartile 4 | 27 (17.31) | 1.73 (0.79–3.81) | 0.17 | 1.8 (0.77–4.19) | 0.17 | |
| <1 | 12 (11.54) | Reference | … | Reference | … | |
| 1–3 | 13 (10.32) | 0.92 (0.42–2.02) | 0.84 | 0.95 (0.42–2.14) | 0.9 | |
| >3 | 33 (16.18) | 1.49 (0.77–2.88) | 0.24 | 1.56 (0.76–3.19) | 0.22 | |
| Small‐artery occlusion | Quartile 1 | 33 (8.3) | Reference | … | Reference | … |
| Quartile 2 | 20 (5.67) | 0.67 (0.38–1.17) | 0.16 | 0.6 (0.34–1.06) | 0.08 | |
| Quartile 3 | 27 (8.85) | 1.07 (0.64–1.77) | 0.8 | 0.94 (0.55–1.61) | 0.83 | |
| Quartile 4 | 18 (8.78) | 1.08 (0.61–1.91) | 0.81 | 0.83 (0.45–1.55) | 0.56 | |
| <1 | 41 (8.18) | Reference | … | Reference | … | |
| 1–3 | 29 (6.64) | 0.8 (0.5–1.29) | 0.37 | 0.75 (0.46–1.22) | 0.24 | |
| >3 | 28 (8.72) | 1.09 (0.67–1.76) | 0.73 | 0.92 (0.54–1.54) | 0.74 | |
| Other determined etiology | Quartile 1 | 1 (6.25) | Reference | … | Reference | … |
| Quartile 2 | 0 | … | … | … | … | |
| Quartile 3 | 3 (15.0) | 2.37 (0.25–22.79) | 0.45 | … | … | |
| Quartile 4 | 1 (6.67) | 1.04 (0.07–16.6) | 0.98 | … | … | |
| <1 | 1 (5.26) | Reference | … | Reference | … | |
| 1–3 | 2 (8.33) | 1.63 (0.15–17.98) | 0.69 | 0.22 (0–16.84) | 0.49 | |
| >3 | 2 (9.09) | 1.68 (0.15–18.5) | 0.67 | 1.05 (0.01–83.41) | 0.98 | |
| Undetermined etiology | Quartile 1 | 39 (5.84) | Reference | … | Reference | … |
| Quartile 2 | 55 (7.93) | 1.37 (0.91–2.07) | 0.13 | 1.36 (0.9–2.06) | 0.14 | |
| Quartile 3 | 65 (9.35) | 1.65 (1.11–2.45) | 0.01 | 1.54 (1.03–2.3) | 0.04 | |
| Quartile 4 | 76 (11.57) | 2.1 (1.42–3.08) | <0.001 | 1.76 (1.18–2.63) | 0.006 | |
| <1 | 50 (5.73) | Reference | … | Reference | … | |
| 1 to 3 | 85 (9.51) | 1.72 (1.21–2.43) | 0.003 | 1.61 (1.13–2.3) | 0.008 | |
| >3 | 100 (10.56) | 1.93 (1.37–2.71) | <0.001 | 1.65 (1.16–2.34) | 0.005 | |
HR indicates hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; mRS, modified Rankin Scale; rt‐PA recombinant tissue plasminogen activator; and TOAST, the Org 10 172 test in the Treatment of Acute Stroke.
Adjusted for: age, sex, body mass index, current smoking, index event, medical histories of atrial fibrillation, coronary heart disease, ischemic stroke, diabetes, hypertension and hypercholesterolemia, baseline National Institutes of Health Stroke Scale score and baseline leukocyte count, rt‐PA treatment, and pre‐mRS score.
hsCRP and Functional Outcome
A total of 1232 (12.53%) patients had poor functional outcomes at 1 year. The hsCRP level measured between 24 and 72 hours was associated with an increased risk of poor functional outcomes (Table 5). After adjusting for age; sex; body mass index; smoking; index event; medical histories of atrial fibrillation, coronary heart disease, ischemic stroke, diabetes, hypertension, and hypercholesterolemia; baseline NIHSS score and baseline leukocyte count; recombinant tissue plasminogen activator treatment; pre‐mRS score; and 1‐year stroke recurrence, such association remained (Table 5). Besides, we did not observe the association in the hsCRP level measured within 24 hours and between 72 hours and 8 days (Table 5). We obtained similar results when hsCRP was further divided into 3 groups according to relative risk category. We also found the association between hsCRP measured between 24 and 72 hours as a continuous variable and poor functional outcome in the adjusted model (adjusted HR, 1.003 [95% CI, 1.000–1.006]; P = 0.02).
Table 5.
Association Between hsCRP Measured in Different Stage and Poor Functional Outcome Within 1 Year
| hsCRP, mg/L | <24 h | 24–72 h | 72 h–8 d | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events, n (%) | OR (95% CI) * | P value | Events, n (%) | OR (95% CI) * | P value | Events, n (%) | OR (95% CI) * | P value | Events, n (%) | OR (95% CI) * | P value | |
| Quartile 1 | 35 (8.39) | Reference | … | 65 (6.69) | Reference | … | 82 (9.09) | Reference | … | 182 (7.94) | Reference | … |
| Quartile 2 | 46 (9.85) | 1.07 (0.64–1.8) | 0.79 | 89 (8.94) | 1.30 (0.90–1.89) | 0.16 | 87 (10.10) | 1.00 (0.71–1.43) | 0.98 | 222 (9.55) | 1.13 (0.9–1.42) | 0.28 |
| Quartile 3 | 69 (13.83) | 1.27 (0.78–2.08) | 0.34 | 115 (11.97) | 1.51 (1.05–2.16) | 0.02 | 104 (12.18) | 1.01 (0.72–1.41) | 0.98 | 288 (12.45) | 1.26 (1.02–1.57) | 0.04 |
| Quartile 4 | 117 (23.12) | 1.41 (0.88–2.27) | 0.16 | 226 (24.17) | 2.15 (1.53–3.03) | <0.001 | 197 (23.29) | 1.23 (0.88–1.72) | 0.22 | 540 (23.61) | 1.68 (1.37–2.07) | <0.001 |
| <1 | 42 (7.85) | … | … | 87 (6.89) | … | … | 107 (9.31) | … | … | 236 (8.01) | … | … |
| 1–3 | 81 (13.02) | 1.44 (0.94–2.19) | 0.15 | 132 (10.34) | 1.44 (1.05–1.98) | 0.03 | 108 (9.86) | 0.87 (0.64–1.19) | 0.38 | 321 (10.73) | 1.18 (0.97–1.43) | 0.1 |
| >3 | 144 (19.67) | 1.45 (0.97–2.19) | 0.07 | 276 (20.81) | 1.98 (1.48–2.65) | <0.001 | 255 (20.92) | 1.26 (0.95–1.68) | 0.12 | 675 (20.60) | 1.62 (1.36–1.93) | <0.001 |
hsCRP indicate high‐sensitivity C‐reactive protein; mRS, modified Rankin Scale; OR, odds ratio; and rt‐PA, recombinant tissue plasminogen activator.
Adjusted for age, sex, body mass index, current smoking, index event, medical histories of atrial fibrillation, coronary heart disease, stroke, diabetes, hypertension, and hypercholesterolemia, baseline National Institutes of Health Stroke Scale score and baseline leukocyte count, rt‐PA treatment, pre‐mRS score, and 1‐year stroke recurrence.
Discussion
In this study, we found that hsCRP was a statistically significant predictor of 1‐year recurrent stroke or combined vascular events after acute ischemic stroke or TIA. When further exploring the influence of serial measurement of hsCRP, we found that the predictive role of hsCRP existed only within 72 hours after stroke onset. The highest hsCRP level was associated with an ≈60% increased risk of recurrent stroke. However, we did not observe this association at 72 hours to 8 days after index onset. In addition, we also found higher levels of hsCRP measured at 24 to 72 hours were associated with poor functional outcomes.
Inflammation is increasingly recognized to play an important role in atherosclerosis and stroke. CRP is a nonspecific acute‐phase protein, produced in the liver in response to interleukin‐6 expression. Previous studies focus on the association between hsCRP and first stroke. 16 However, relatively limited data are available for the prognostic utility of hsCRP in patients after stroke with conflicting results. 3 , 5 , 7 We speculated one of the reasons for the discrepancies might be the inconsistency of the measurement time of hsCRP, in addition to the study population. For the patients with acute ischemic stroke, the CHANCE (Clopidogrel in High‐Risk Patients With Acute Non‐disabling Cerebrovascular Events) trial found hsCRP levels measured within 48±12 hours after stroke predicted increased risk of recurrent stroke. 3 However, the data from the NOMAS (Northern Manhattan Stroke Study) suggested that levels of hsCRP measured in subacute phase were not associated with risk of recurrent stroke. 7 Consistent with the previous research, hsCRP levels were associated with recurrent stroke, especially when detected within 72 hours in the current study. Several explanations might be given for the different impacts of hsCRP on recurrence after acute ischemic stroke. The inflammation response after acute stroke is known to be related to not only brain damage but also stress, plaque instability, embolism, and coagulation, 17 which could be critical mechanisms for the exacerbated atheroprogression and major source of recurrent vascular events. 18 In our study, the hsCRP levels measured within 72 hours may help to reflect individual inflammatory response and identify patients predisposed to an intensive activation of the vascular inflammatory system, and therefore were more closely associated with recurrence. These hypotheses were supported by our findings that the association between hsCRP levels measured within 72 hours and recurrent stroke was more apparent in the patients with larger‐artery atherosclerosis subtype. The proportion of each stroke subtype in our study was similar to those reported previously. 19 However, it has been shown that a centralized strict application of the TOAST classification criteria can lead to the designation of a significant number of strokes as an undetermined cause subtype when facing competing evidence of different etiology. 20 Intracranial artery stenosis is common in Asian patients, contributing to the high proportion of the larger‐artery atherosclerosis subtype relative to the other subtypes. 21 However, patients with larger‐artery atherosclerosis might have comorbidities such as atrial fibrillation and vice versa. It has been shown that around 30% of patients with cardioembolic stroke had cerebral arterial stenosis ≥50%. 22 Therefore, we speculated that one likely reason for the association between hsCRP within 72 hours and subsequent stroke in the patients with undermined etiology subtype might be a substantial portion of patients in this group having intracranial artery stenosis.
The increased levels of hsCRP after stroke might also be attributable to in‐hospital acquired inflammatory processes, such as infection. We therefore adjusted for leukocyte counts to exclude the effect of secondary complications on functional outcome. We observed a statistically significant association between hsCRP levels measured between 24 and 72 hours and increased risk of poor functional outcomes. Identical with a prior study, we found that hsCRP levels slightly decreased after subacute phases of stroke. 23 It is conceivable that an increased hsCRP level after stroke could be attributable to inflammation related to the pathophysiology of ischemic stroke and might reflect the extent of the ischemic area. 24 Moreover, it has been shown that the peak level of hsCRP was reached after 24 hours of symptom onset, 23 , 25 , 26 and a correlation between CRP measured within 4±2 days after symptom onset and infarct size has been described before. 27 The time course of acute‐phase response might explain the absent relationship of hsCRP detected within 24 hours with functional outcome in our study.
Taken together, our findings discovered that higher hsCRP levels within 72 hours after index onset are strongly associated with poor stroke prognosis. From a clinical point of view, it might help stratify risk of new stroke and poor functional outcomes and identify patients with potential benefits from anti‐inflammatory therapy. Further randomized clinical trials evaluating the effect of early anti‐inflammatory therapy in patients with ischemic stroke or TIA were needed.
Strength and Limitation
The strength of this study is that the data for this study derived from a prospective, multicenter, hospital‐based, and large‐sample‐size cohort. The blood samples from all subcenters were assayed centrally. However, our study also had several limitations. First, the blood sample was drawn from peripheral venous blood. The peripheral inflammatory or acute‐phase responses motivated by ischemic stroke may overlap with preexisting inflammatory processes. Moreover, though it is well established that local inflammation in the brain is associated with peripheral systemic inflammation, 28 the concentration of inflammatory markers in peripheral blood may only partially reflect the inflammatory response at the infarct site. Second, only 1 time point biomarker measurement is available, which precluded us from assessing the contribution of changes in these markers over time. Third, hsCRP is not measured continuously but at different times. The patients measured at each period may be different. It is unclear if the selection effect versus timing of hsCRP is driving the finding. Finally, hospital arrival time has been shown to affect the prognosis of stroke. Absence of this element impeded assessment of its influence on the correlation between hsCRP and outcomes in the current study. It should be considered in further research.
Conclusions
In conclusion, elevated hsCRP measured within 72 hours and at 24 to 72 hours statistically significantly predicted increased risk of recurrent stroke and poor functional outcome within 1 year, respectively, in patients with ischemic stroke or TIA.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (81 870 905, U20A20358), grants from Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019‐I2M‐5‐029) and grants from Capital's Funds for Health Improvement and Research (2020–1‐2041).
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
The authors thank all the study participants and their relatives for supporting the CNSR‐III study. We also thank all the relevant clinicians, statisticians, coordinators, laboratory staff, and imaging technicians who participated in the study for their hard work and dedication.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025464
For Sources of Funding and Disclosures, see page 11.
References
- 1. Kelly PJ, Lemmens R, Tsivgoulis G. Inflammation and stroke risk: a new target for prevention. Stroke. 2021;52:2697–2706. doi: 10.1161/STROKEAHA.121.034388 [DOI] [PubMed] [Google Scholar]
- 2. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Eng J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 3. Li J, Zhao X, Meng X, Lin J, Liu L, Wang C, Wang A, Wang Y, Wang Y, Investigators C. High‐sensitive C‐reactive protein predicts recurrent stroke and poor functional outcome: subanalysis of the clopidogrel in high‐risk patients with acute nondisabling cerebrovascular events trial. Stroke. 2016;47:2025–2030. doi: 10.1161/STROKEAHA.116.012901 [DOI] [PubMed] [Google Scholar]
- 4. Segal HC, Burgess AI, Poole DL, Mehta Z, Silver LE, Rothwell PM. Population‐based study of blood biomarkers in prediction of subacute recurrent stroke. Stroke. 2014;45:2912–2917. doi: 10.1161/STROKEAHA.114.005592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elkind MS, Luna JM, McClure LA, Zhang Y, Coffey CS, Roldan A, Del Brutto OH, Pretell EJ, Pettigrew LC, Meyer BC, et al. C‐reactive protein as a prognostic marker after lacunar stroke: levels of inflammatory markers in the treatment of stroke study. Stroke. 2014;45:707–716. doi: 10.1161/STROKEAHA.113.004562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coveney S, Murphy S, Belton O, Cassidy T, Crowe M, Dolan E, de Gaetano M, Harbison J, Horgan G, Marnane M, et al. Inflammatory cytokines, high‐sensitivity c‐reactive protein, and risk of one‐year vascular events, death, and poor functional outcome after stroke and transient ischemic attack. Int J Stroke. 2021:17:163‐171. DOI: 10.1177/1747493021995595 [DOI] [PubMed] [Google Scholar]
- 7. Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High‐sensitivity C‐reactive protein, lipoprotein‐associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med. 2006;166:2073–2080. doi: 10.1001/archinte.166.19.2073 [DOI] [PubMed] [Google Scholar]
- 8. Di Napoli M, Papa F, Bocola VJS. C‐reactive protein in ischemic stroke: an independent prognostic factor. Stroke. 2001;32:917–924. doi: 10.1161/01.STR.32.4.917 [DOI] [PubMed] [Google Scholar]
- 9. Winbeck K, Poppert H, Etgen T, Conrad B, Sander DJS. Prognostic relevance of early serial C‐reactive protein measurements after first ischemic stroke. Stroke. 2002;33:2459–2464. doi: 10.1161/01.STR.0000029828.51413.82 [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Liao X, Wang C, Zhang N, Zuo L, Yang Y, Pan Y, Xiang X, Jing J, Meng X, et al. Impairment of cognition and sleep after acute ischaemic stroke or transient ischaemic attack in chinese patients: design, rationale and baseline patient characteristics of a nationwide multicentre prospective registry. Stroke Vasc Neurol. 2020;6:139–144. doi: 10.1136/svn-2020-000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stroke‐‐1989 . Recommendations on stroke prevention, diagnosis, and therapy. Report of the who task force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.STR.20.10.1407 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Jing J, Meng X, Pan Y, Wang Y, Zhao X, Lin J, Li W, Jiang Y, Li Z, et al. The third China national stroke registry (CNSR‐III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol. 2019;4:158–164. doi: 10.1136/svn-2019-000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 14. Suo Y, Jing J, Meng X, Li Z, Pan Y, Jiang Y, Yang X, Liu H, Yan H, Liu L, et al. Inconsistent centralised versus non‐centralised ischaemic stroke aetiology. Stroke Vasc Neurol. 2020;5:337–347. doi: 10.1136/svn-2020-000576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the american heart association. Circulation. 2003;107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- 16. Lp PLASC, Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, Ballantyne C, Cannon CP, Criqui M, et al. Lipoprotein‐associated phospholipase a(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gussekloo J, Schaap M, Frölich M, Blauw G, Westendorp RJA. C‐reactive protein is a strong but nonspecific risk factor of fatal stroke in elderly persons. Arterioscler Thromb Vasc Biol. 2000;20:1047–1051. doi: 10.1161/01.ATV.20.4.1047 [DOI] [PubMed] [Google Scholar]
- 18. Roth S, Singh V, Tiedt S, Schindler L, Huber G, Geerlof A, Antoine DJ, Anfray A, Orset C, Gauberti M, et al. Brain‐released alarmins and stress response synergize in accelerating atherosclerosis progression after stroke. Sci Transl Med. 2018;10:eaao1313. doi: 10.1126/scitranslmed.aao1313 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Xu J, Zhao X, Wang D, Wang C, Liu L, Wang A, Meng X, Li H, Wang Y. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke. 2013;44:1232–1237. doi: 10.1161/STROKEAHA.111.000302 [DOI] [PubMed] [Google Scholar]
- 20. Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence‐based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. doi: 10.1002/ana.20617 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, Wang Y, Zou X, Leung TW, Cai Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663–669. doi: 10.1161/STROKEAHA.113.003508 [DOI] [PubMed] [Google Scholar]
- 22. Kim YD, Cha MJ, Kim J, Lee DH, Lee HS, Nam CM, Nam HS, Heo JH. Increases in cerebral atherosclerosis according to CHADS2 scores in patients with stroke with nonvalvular atrial fibrillation. Stroke. 2011;42:930–934. doi: 10.1161/STROKEAHA.110.602987 [DOI] [PubMed] [Google Scholar]
- 23. Worthmann H, Tryc AB, Goldbecker A, Ma YT, Tountopoulou A, Hahn A, Dengler R, Lichtinghagen R, Weissenborn K. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc Dis. 2010;30:85–92. doi: 10.1159/000314624 [DOI] [PubMed] [Google Scholar]
- 24. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/S0166-2236(99)01401-0 [DOI] [PubMed] [Google Scholar]
- 25. Di Napoli M. Early inflammatory response in ischemic stroke. Thromb Res. 2001;103:261–264. doi: 10.1016/S0049-3848(01)00290-0 [DOI] [PubMed] [Google Scholar]
- 26. Montaner J, Alvarez‐Sabín J, Barberá G, Anglés A, Molina C, Abilleira S, Arenillas J, Chacón P, Monasterio J. Correlation between the expression of proinflammatory cytokines and matrix metalloproteinases in the acute phase of an ischemic stroke. Rev Neurol. 2001;33:115–118. doi: 10.33588/rn.3302.2001142 [DOI] [PubMed] [Google Scholar]
- 27. Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin‐6 and interleukin‐1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:800–805. doi: 10.1002/ana.410370614 [DOI] [PubMed] [Google Scholar]
- 28. Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


