Abstract
Background
Several studies investigated the role of selective serotonin reuptake inhibitors (SSRIs) in improving poststroke recovery; thus, we have decided to conduct this systematic review and meta‐analysis to investigate the efficacy and safety of SSRIs in poststroke recovery.
Methods and Results
In this meta‐analysis we searched the following databases: PubMed, Cochrane, Scopus, and Google Scholar. The studies were included if they were placebo‐controlled trials in design and reported SSRIs’ effects on poststroke depression, anxiety, disability, dependence, motor abilities, and cognitive functions. The quality of the included studies was assessed using the revised Cochrane risk‐of‐bias tool for randomized trials. The search yielded 44 articles that included 16 164 patients, and about half of the participants were treated with SSRIs. Our results showed that SSRIs had a significant effect on preventing depression (weighted mean difference [WMD], −7.05 [95% CI, −11.78 to −2.31]), treating depression according to the Hamilton Rating Scale for Depression score (WMD, −1.45 [95% CI, −2.77 to −0.14]), anxiety (relative risk, 0.23 [95% CI, 0.09–0.61]), dependence (WMD, 8.86 [95% CI, 1.23–16.48]), motor abilities according to National Institutes of Health Stroke Scale score (WMD, −0.79 [95% CI, −1.42 to −0.15]), and cognitive functions (WMD, 1.00 [95% CI, 0.12–1.89]). On the other hand, no significant effect of SSRIs on disability was observed. Additionally, we found that treating with SSRIs increased the risk of seizures (relative risk, 1.44 [95% CI, 1.13–1.83]), whereas there was no difference in the incidence of gastrointestinal symptoms or bleeding between SSRIs and a placebo.
Conclusions
Our study showed that SSRIs are effective in preventing and treating depression, and improving anxiety, motor function, cognitive function, and dependence in patients after stroke. These benefits were only reproducible with the citalopram subanalysis but not fluoxetine. Further well‐conducted placebo‐controlled trials are needed to investigate the safety and efficacy of citalopram among patients after stroke.
Registration
URL: www.crd.york.ac.uk/prospero/; Unique identifier: CRD42021285766.
Keywords: human, recovery, selective serotonin reuptake inhibitors, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Intracranial Hemorrhage, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- NIHSS
National Institutes of Health Stroke Scale
- WMD
weighted mean difference
Clinical Perspective
What Is New?
Our study is the first systematic review and meta‐analysis to show that selective serotonin reuptake inhibitors are effective in improving poststroke recovery.
Our study showed that selective serotonin reuptake inhibitors are effective in preventing and treating depression, and improving anxiety, motor function, cognitive function, and dependence in patients after stroke.
Our positive findings on selective serotonin reuptake inhibitors were mainly driven by citalopram and not fluoxetine.
What Are the Clinical Implications?
Further well‐conducted placebo‐controlled trials are needed to investigate the safety and efficacy of citalopram among patients after stroke.
Every year, about 13.7 million individuals are affected by stroke globally, 1 and about half of the stroke survivors are suffering from disability. 2 Two meta‐analyses estimated that the prevalence of poststroke depression among stroke survivors was 30%. 3 , 4 Moreover, these poststroke sequelae were associated with a higher risk for subsequent stroke mortality. 5 Despite the fact that considerable advances have been made in treating the acute form of stroke, there is a constant need to find new and improved methods of treatment. Specifically, those treatments revolve around the long‐term recovery aspect of stroke regardless of eligibility for acute treatments. 6 Many interventions that involve monoaminergic drugs, including selective serotonin reuptake inhibitors (SSRIs), were shown to improve the neurological deficit and disability of patients with stroke. 7 , 8 , 9
Several studies evaluated the role of SSRIs in several aspects of stroke recovery. However, the results of these studies were contradictory, with some studies concluding that SSRIs improved poststroke recovery, whereas others indicated that SSRIs did not provide any benefits for patients with stroke. Moreover, 2 Cochrane systematic reviews in 2012 and 2018 showed that SSRIs failed to improve poststroke recovery. 10 , 11 However, these reviews highlighted that the included studies had several limitations and heterogeneity. In response to this conclusion, an international collaboration developed a core protocol for 3 trials of fluoxetine for recovery after stroke. 12 , 13 The aforementioned Cochrane reviews, conducted before the development of this protocol, did not evaluate the role of SSRIs in treating mental disorders among patients with stroke and did not investigate the efficacy of each drug in the SSRIs family solely. Additionally, several trials were completed since the last Cochrane review in 2018. This necessitates a more updated systematic review and meta‐analysis that accounts for the mentioned issues; hence, we decided to conduct this study to evaluate the role of SSRIs in poststroke recovery.
Methods
Registration
The data that support the findings of this study are available from the corresponding author upon reasonable request. In this meta‐analysis, we followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. This study was prospectively registered in the International Prospective Register of Systematic Reviews (CRD42021285766). The institutional review board at our institution approved the conductance of this research.
Search Strategy
The search was conducted on October 20, 2021 and November 20, 2021 by A.A.T. and F.H.A. independently, using the following databases; PubMed, Cochrane, Google Scholar, and Scopus. The following keywords, selective serotonin reuptake inhibitors AND stroke, and their related Medical Subject Headings terms were used. Afterward, the search results were cross‐matched, and any discrepancies were resolved by discussion. The search results, after cross‐matching, were imported to Rayyan (www.rayyan.ai), and duplicates were removed.
Selection Process
The inclusion criteria of selecting the studies were if they were placebo‐controlled trials in design and reported SSRIs’ effects on poststroke depression, anxiety, disability, dependence, motor abilities, and cognitive functions. Any studies that did not meet these criteria were excluded from our analysis. The exposure of interest was using SSRIs among stroke patients, and the outcomes of interest were poststroke depression, anxiety, disability, dependence, motor abilities, and cognitive functions as well as side effects of using SSRIs. Depression was measured using the Hamilton Rating Scale for Depression (HAM‐D), Beck Depression Inventory, and Patient Health Questionnaire 9, whereas anxiety was measured using the Hamilton Anxiety Scale. Cognitive function was assessed by the Montreal Cognitive Assessment and the Mini‐Mental State Examination (MMSE), whereas motor functions were measured using the National Institutes of Health Stroke Scale (NIHSS) and Fugl‐Meyer Assessment of Motor Recovery. Additionally, dependence was measured using the Functional Independence Measure Score and Barthel Index, whereas disability was measured by modified Rankin Scale score. Any study that used tools different from the aforementioned scales were included in the systematic review but not in the meta‐analysis to prevent large heterogeneity in the analysis. The following SSRIs side effects were assessed: gastrointestinal symptoms including abdominal pain, nausea, vomiting and diarrhea, seizures, and bleeding. These side effects were chosen because seizures and bleeding can result in risks that outweigh any benefit from using SSRIs among stroke patients. 10 Also, the gastrointestinal side effects were selected because of their high frequency and their significant association with noncompliance. 14 Additionally, all the mentioned outcomes were assessed as binary variables and continuous variables. The study selection was done by A.A.T. and F.H.A. independently, and any discrepancies were resolved by discussion.
Data Extraction and Quality Assessment
The variables of interest were extracted by A.A.T. and F.H.A. independently, then checked by Y.Y.O. and L.M.A.‐H., and any discrepancies were resolved by discussion. After the data were extracted, the quality of the included studies was assessed using the revised Cochrane risk‐of‐bias tool for randomized trials, which was also done by A.A.T. and F.H.A. independently, then checked by Y.Y.O. and L.M.A.‐H, and any differences in the scoring were resolved by discussion.
Statistical Analysis
After the data were extracted, the relative risk (RR) and its corresponding 95% CI were calculated for binary outcomes using the Altman equation, and if any 0 had been encountered in the outcomes, 0.5 was added to all cells. 15 The RR and its 95% CI were used as the effect size for the binary outcomes. For continuous outcomes, the mean and standard deviation were used as the measure of effect in the data analysis. Whenever median and interquartile range were encountered in the extracted data, they were converted to mean and standard deviation using the method described by Hozo et al. 16 Finally, the mean and standard deviation for the continuous outcomes were used to measure the differences in the outcomes between the intervention groups using the weighted mean difference (WMD) and its related CI. The analysis was done by creating a model for each outcome by pooling the studies that assessed the same outcome using the same measurement tool. The studies were pooled using the random‐effects model when I² was >50%, whereas they were pooled using the fixed‐effects model when I² was ≤50%. We used the Cochran Q heterogeneity test and I² statistic to assess statistical heterogeneity. Meta XL version 5.3 (EpiGear International, Queensland, Australia) was used in the data analysis.
Results
Search Results
Our search yielded 1629 articles, and of them 350 were duplicates. The remaining 1279 articles were screened using the title and abstract, of which 1047 articles were excluded for having a nonexperimental design, using a different intervention, or assessing a different population. The 232 remaining articles were tested against the inclusion criteria using the full‐text form. Of them, 188 articles were excluded because they were non–placebo‐controlled trials or no data about the outcomes of interest, including studies, investigated the effect of SSRIs on pathological crying, muscle strength measurements, visual improvements, and brain activity using imaging such as functional magnetic resonance imaging. Finally, 44 articles were included in the quantitative and qualitative data synthesis. The detailed study selection process is described in Figure 1.
Figure 1. PRISMA flowchart.
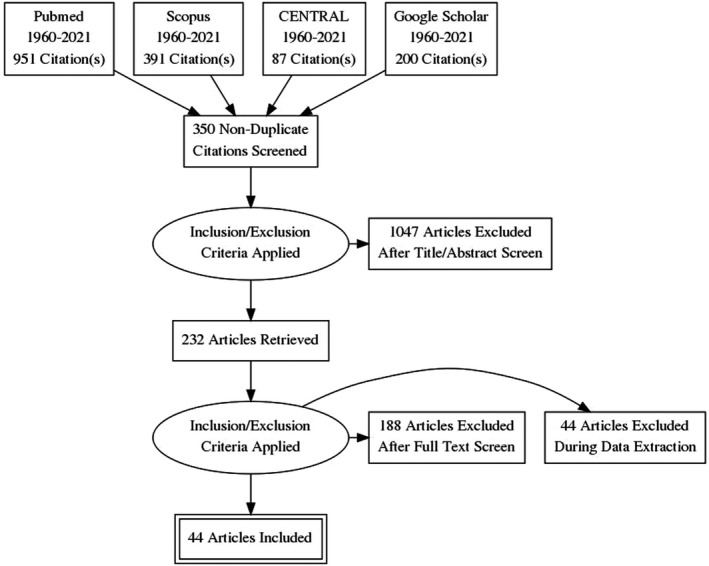
PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. CENTRAL, Cochrane.
General Characteristics
The total number of the included patients was 16 164 patients from 44 studies. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 Of them, 50.5% were treated using SSRIs (8137/16 164), whereas 49.5% were treated using a placebo (8027/16 164). The majority of the studies were conducted in Asia and Europe. The most common comorbidities included in the studies were diabetes and hypertension; 50.0% and 43.2%, respectively, of the included articles included patients with these comorbidities. Furthermore, the most common outcome assessed by the included studies was the efficacy of SSRIs in treating depression; 47.7% of the included articles evaluated this outcome. This was followed by dependence and disability; 38.6% of the included studies evaluated each of these outcomes. Depression and anxiety were most commonly assessed using the HAM‐D and Hamilton Anxiety Scale, respectively. In addition, the majority of the studies used the MMSE, NIHSS, and Barthel Index in assessing cognitive deficit, motor function, and dependence, respectively. Most of the studies assessed disability as a binary variable. Moreover, 58.8% of the included studies evaluated the efficacy of fluoxetine, whereas 38.6% of them evaluated citalopram. The summary of the characteristics of the included studies are described in the Table.
Table 1.
Characteristics of the Included Studies
| Study | Country | No. of participants | No. of placebo patients | No. of treatment patients | Age, mean±SD/median (range) | Comorbidities | Funding status | Outcomes (tools) |
|---|---|---|---|---|---|---|---|---|
| Nct 18 | France | 102 | 55 | 57 | Fluoxetine: 66.4±11.7, placebo: 62.9±13.4 | Diabetes, hypertension, hyperlipidemia, smoker, cardiac disease, and stroke | Not funded | Disability (mRS) and depression (HAM‐D) and motor function (NIHSS and FMMS) |
| Gong et al 19 | China | 126 | 62 | 64 | Fluoxetine: 56.68±17.59, placebo: 57.79±17.54 | Hypertension and diabetes | Not funded | Motor disability (NIHSS and FMMS) and disability (mRS) |
| Savadi Oskouie et al 20 | Iran | 144 | 72 | 72 | Citalopram: 65±10.90, placebo: 66.20±11.37 | Diabetes, hypertension, hyperlipidemia, smoker, coronary artery disease, and stroke | Not funded | Disability (binary) |
| Effects trial 21 | Sweden | 1500 | 750 | 750 | Fluoxetine: 70.6±11.3, placebo: 71.0±10.5 | Coronary artery diseases, stroke, diabetes, and depression | Not funded | Motor functions (NIHSS) and disability (mRS) |
| Bembenek et al 22 | Poland | 61 | 30 | 31 | Fluoxetine: 66.60±12.60, placebo: 66.35±12.46 | Depression, diabetes, coronary artery diseases, hypertension, smoking, alcohol, obesity, hyperlipidemia, stroke, and intracerebral hemorrhage | Not funded | Motor deficit (MRC and NIHSS), disability (mRS), and dependence (BI) |
| Marquez‐Romero et al 23 | Mexico | 30 | 14 | 16 | Fluoxetine: 54±10, placebo: 60.5±18 | Diabetes, hyperlipidemia, smoker, and hypertension | Funded | Disability (mRS) and motor function (FMMS and NIHSS) |
| Kraglund et al 24 | Denmark | 641 | 323 | 319 | Citalopram: 68 (24–97), placebo: 68 (19–99) | Peripheral arterial disease, hypertension, smoker, coronary artery disease, and diabetes | Not funded | Disability (mRS) |
| Bonin Pinto et al 25 | USA | 18 | 10 | 8 | Fluoxetine: 50.5±16.57, placebo: 57.38 | N/A | Not funded | Motor function (JHFT) |
| Cao et al 26 | China | 100 | 47 | 53 | N/A | N/A | Not funded | Depression (HAM‐D), dependence (BI), cognitive functions (MMSE), and motor functions (NIHSS) |
| Dennis et al 27 | UK | 3106 | 1553 | 1553 | Fluoxetine: 71.24±12.35, placebo: 71.48±12.06 | Coronary artery disease, stroke, diabetes, hyponatremia, intracerebral hemorrhage, fractures, depression, and gastrointestinal bleeding | Not funded | Motor function (NIHSS) and disability (mRS) |
| Rampello et al 28 | Italy | 68 | 34 | 34 | Citalopram: 73.13±4.00, placebo: 74.71±4.66 | Hypertension, diabetes, and hyperlipidemia | Not funded | Depression (HAM‐D and BDI) |
| Asadollahi et al 29 | Iran | 90 | 30 | 60 | Fluoxetine: 60.2±8.52, citalopram: 58.7±8.56, placebo: 61.7±9.6 | Hypertension, hyperlipidemia, smoking, and coronary artery disease | Not funded | Motor function (FMMS) |
| Cao et al 30 | China | 97 | 48 | 49 | Citalopram: 62±10.9, placebo: 63±9.7 | N/A | Not funded | Motor functions (NIHSS), cognitive function (MMSE), dependence (BI), and depression (HAM‐D) |
| Choi‐Kwon et al 31 | South Korea | 83 | 43 | 40 | Fluoxetine: 57.28±8.3, placebo: 56.48±8.4 | Hypertension, diabetes, coronary artery disease, smoking, and hyperlipidemia | Not funded | Depression (binary) |
| Li et al 32 | China | 90 | 30 | 60 | Fluoxetine: 69.2±3.50, placebo: 67.8±3.90 | N/A | Not funded | Depression (HAM‐D), dependence (BI) |
| Kim et al 33 | South Korea | 405 | 195 | 210 | Citalopram: 63.6±12.6, placebo: 63.5±12.0 | Hypertension, diabetes, hyperlipidemia, coronary artery disease, smoking, and alcohol | Funded | Depression (HAM‐D), disability (mRS), cognitive function (MOCA), and motor functions (NIHSS) |
| He et al 34 | China | 374 | 187 | 187 | Fluoxetine: 60.46±10.35, placebo: 62.66±11.69 | Hypertension, diabetes, and smoking | Not funded | Motor (binary) |
| Mikami et al 35 | USA | 98 | 47 | 51 | Citalopram: 60.8±14.0, placebo: 62.7±13.3 | Hypertension, hyperlipidemia, diabetes, coronary artery disease, and heart failure | Not funded | Cognitive functions (RBANS) and dependence (FIMS) |
| Chan et al 36 | USA | 19 | 13 | 6 | N/A | Mental disorders and alcohol | Not funded | Anxiety (HAMA) and depression (HAM‐D) |
| Choi‐Kwon et al 37 | Korea | 125 | 64 | 61 | Fluoxetine: 58.41±8.92, placebo: 58.18±8.85 | Hypertension, diabetes, coronary artery disease, smoker, and hyperlipidemia | Not funded | Depression (binary) |
| Robinson et al 56 | USA and Argentina | 33 | 17 | 16 | Fluoxetine: 65±14, placebo: 73±8 | None | Not funded | Dependence (FIMS), cognitive function (MMSE), anxiety (HAMA), and depression (HAM‐D) |
| Andersen et al 17 | Denmark | 16 | 16 | 16 | N/A | None | Funded | Depression (HAM‐D) |
| Fruehwald et al 38 | Switzerland | 50 | 24 | 26 | Fluoxetine: 64.8±13.8, placebo: 64.0±14.3 | None | Not funded | Depression (HAM‐D and BDI) |
| Robinson et al 39 | USA | 117 | 58 | 59 | Citalopram: 61.3±13.7, placebo: 63.9±13.3 | Mental disorders, hypertension, hyperlipidemia, diabetes, coronary artery disease, heart failure, and chronic obstructive lung disease | Not funded | Depression (binary) |
| Hankey et al 40 | Australia, New Zealand, and Vietnam | 1221 | 615 | 606 | N/A | N/A | Funded | Depression (PHQ9) |
| Choi‐Kwon et al 41 | Korea | 152 | 76 | 76 | Fluoxetine: 58.41±8.92, placebo: 58.18±8.85 | N/A | Not funded | Depression (binary) |
| Jorge et al 42 | USA | 88 | 45 | 43 | Citalopram: 60.8±14.4, placebo: 64.2±13.9 | N/A | Not funded | Cognitive function (RABNS) |
| Mikami et al 43 | USA | 61 | 29 | 32 | Fluoxetine: 65.7±12.4, placebo: 72.5±9.4 | N/A | Funded | Disability (mRS) |
| Wiart et al 44 | France | 31 | 15 | 16 | Fluoxetine: 66.3±7.1, placebo: 68.9±11.6 | N/A | Funded | Depression (HAM‐D), dependence (FIMS), and cognitive function (MMSE) |
| Gou et al 45 | China | 182 | 92 | 90 | Fluoxetine: 59.52±10.52, placebo: 60.51±11.69 | Smoking, hypertension, and diabetes | Not funded | Motor function (NIHSS) |
| Gao et al 46 | China | 182 | 91 | 91 |
Citalopram: 46±50.5, placebo: 48±52.7 |
Coronary artery disease, hypertension, diabetes, smoker, and alcohol | Not funded |
Dependence (BI and FIMS) and depression (HAM‐D) |
| Mikami et al 47 | USA | 96 | 49 | 47 | Citalopram: 61.5±13.7, placebo: 64.8±13.5 | Hypertension, coronary artery disease, diabetes, congestive heart failure, and atrial fibrillation | Not funded | Anxiety and depression (binary) |
| Acler et al 49 | Italy | 20 | 10 | 10 | N/A | N/A | Not funded | Depression (HAM‐D and BDI), dependence (BI), motor function (NIHSS) |
| Brown et al 50 | UK | 19 | 10 | 9 | N/A | N/A | Not funded | Depression (HAM‐D) |
| Narushima et al 51 | USA | 33 | 16 | 17 | N/A | N/A | Not funded | Depression (HAM‐D) |
| Simis et al 52 | Brazil | 93 | 57 | 36 | N/A | N/A | Funded | Depression (HAM‐D) |
| Andersen et al 53 | Denmark | 66 | 33 | 33 | Citalopram: 68.2±4.2, placebo: 65.8±9.0 | N/A | Funded | Depression (HAM‐D and MMSE) |
| Rasmussen et al 54 | Denmark | 137 | 67 | 70 | Sertraline: 72.0±9.0, placebo: 68.0±11.0 | N/A | Funded | Depression (binary) |
| Andersen et al 55 | Denmark | 16 | 8 | 8 | N/A | N/A | Funded | Depression (HAM‐D) |
| Lundstorm et al 57 | Sweden | 1500 | 750 | 750 | Fluoxetine: 70.6±11.3, placebo: 71.0±10.5 | Coronary artery disease, stroke, diabetes, intracranial bleeding, gastrointestinal bleeding, fractures, and depression | Not funded | Cognitive function (MOCA), motor function (NIHSS) |
| Focus Collaboration 58 | UK | 3127 | 1563 | 1564 | Fluoxetine: 71.5±12.1, placebo: 71.5±12.1 | Coronary artery disease, stroke, diabetes, hyponatremia, intracranial bleeding, gastrointestinal bleeding, fractures, and depression | Not funded | Mental health (MHI) and disability (mRS) |
| Krishnan et al 59 | India | 168 | 84 | 84 | N/A | Diabetes, hypertension, and coronary artery disease | Funded | Dependence (BI) |
| He et al 34 | China | 30 | 15 | 15 | Fluoxetine: 59.07±15.17, placebo: 60.54±14.02 | Diabetes, hyperlipidemia, and hypertension | Not funded | Depression (PHQ9) |
| Almeida et al 60 | Australia and New Zealand | 1280 | 638 | 642 | N/A | N/A | Not funded | Depression (PHQ9) |
BDI indicates Beck Depression Inventory; BI, Barthel Index; FIMS, Functional Independence Measure Score; FMMS, Fugl‐Meyer Assessment of Motor Recovery; HAMA, Hamilton Anxiety Scale; HAM‐D, Hamilton Rating Scale for Depression; JHFT, Jebsen Hand Function Test; MHI, mental health inventory; MMSE, Mini‐Mental State Exam; MOCA, Montreal Cognitive Assessment; MRC, medical research council; mRS, modified Rankin Scale; N/A, not available; NIHSS, National Institutes of Health Stroke Scale; PHQ9, Patient Health Questionnaire 9; and RABNS, repeatable battery for the assessment of neuropsychological status.
Quality Assessment of the Included Studies
The revised Cochrane risk‐of‐bias tool for randomized trials quality assessment of the included studies 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 showed that 59.1% of the included studies had low risk of bias. On the other hand, 38.6% and 2.3% of the included studies had moderate and high risk of bias, respectively (Figure S1). The detailed revised Cochrane risk‐of‐bias tool for randomized trials quality assessment of the included studies 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 is illustrated in Figure S2.
Main Outcomes
Preventing Depression
The model that evaluated the effect of SSRIs in preventing poststroke depression according to HAM‐D score included 2 articles. 26 , 30 This model showed no significant difference in depression HAM‐D scores between the SSRI group and the placebo group at baseline. This model showed insignificant heterogeneity (Figure 2; P=0.95, I 2=0%). At the end of follow‐up, the SSRI group had significantly lower HAM‐D depression score compared with the placebo group (Figure 2; WMD, −7.05 [95% CI, −11.78 to −2.31]). This model showed significant heterogeneity (Figure 2; P=0.00, I 2=96%).
Figure 2. SSRIs for preventing poststroke depression provided by HAM‐D. 26 , 30 .
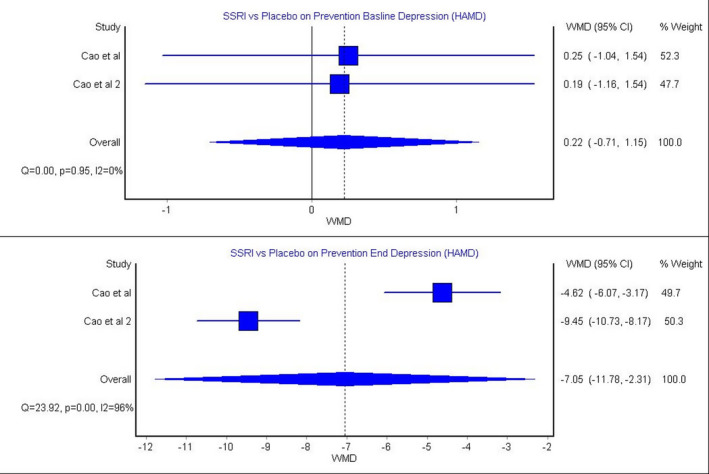
HAMD indicates Hamilton Rating Scale for Depression; SSRIs, selective serotonin reuptake inhibitors; and WMD, weighted mean difference.
Treating Depression
The model that investigated the effect of SSRIs in treating poststroke depression according to HAM‐D score included 8 articles. 32 , 38 , 39 , 46 , 49 , 50 , 52 , 53 The results showed that there was no significant difference between the SSRI group and placebo group at baseline. The heterogeneity of this model was significant (Figure 3; P=0.00, I 2=97%). On the other hand, at the end of treatment, the SSRI group had a significantly lower HAM‐D score compared with a placebo (Figure 3; WMD, −1.45 [95% CI, −2.77 to −0.14]). This model showed significant heterogeneity (Figure 3; P=0.00, I 2=69%). In addition, Andersen et al showed that using SSRIs in treating poststroke depression was significantly effective in reducing depression provided by HAM‐D scores. Furthermore, 2 studies 38 , 49 evaluated the effect of SSRIs in treating poststroke depression according to Beck Depression Inventory scores. The results of these studies conducted by Acler et al 49 and Fruehwald et al 38 showed that there was no significant difference in Beck Depression Inventory scores between the SSRI group and placebo group at baseline or at the end of treatment. In addition, 2 studies 38 , 60 evaluated the efficacy of SSRIs in treating poststroke depression according to Patient Health Questionnaire 9 scores. The study by Almeida et al 60 showed that there was significant improvement in Patient Health Questionnaire 9 scores after treatment, whereas the study by Fruehwald et al 38 showed insignificant difference after the treatment.
Figure 3. SSRIs for treating poststroke depression provided by HAM‐D. 32 , 38 , 39 , 46 , 49 , 50 , 52 , 53 .
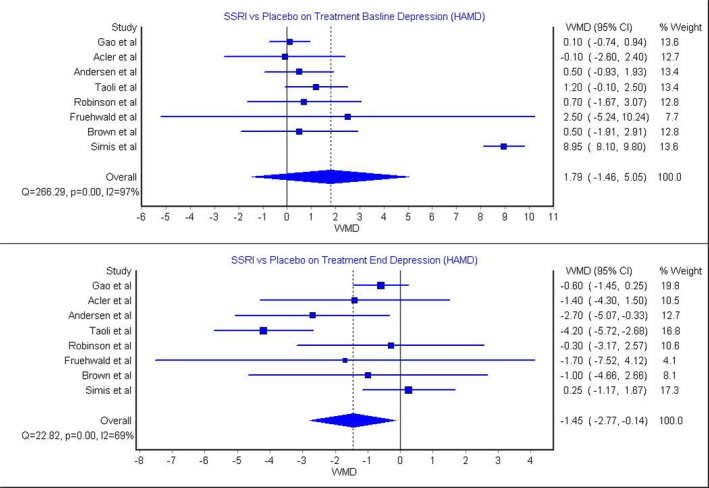
HAMD indicates Hamilton Rating Scale for Depression; SSRIs, selective serotonin reuptake inhibitors; and WMD, weighted mean difference.
Treating Anxiety
Two studies assessed the efficacy of SSRIs in treating poststroke anxiety as a binary variable. 18 , 36 The model that pooled those studies showed significantly lower anxiety among the SSRI group compared with the placebo group (Figure 4; RR, 0.23 [95% CI, 0.09–0.61]). This model showed insignificant heterogeneity (Figure 4; P=0.98, I 2=0%).
Figure 4. SSRIs for treating poststroke anxiety. 18 , 36 .
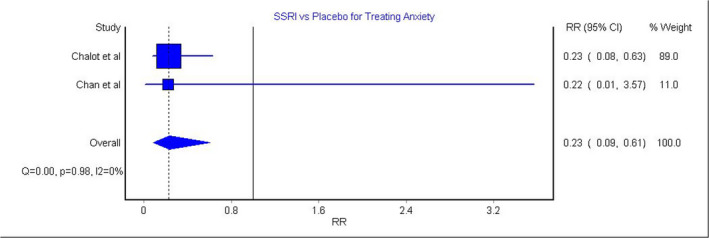
RR indicates relative risk; and SSRIs, selective serotonin reuptake inhibitors.
Dependence
The model that studied the effect of SSRIs on poststroke dependence provided by Barthel Index scores included 10 articles.* This model showed insignificant difference between the SSRI group and placebo group at baseline. This model showed significant heterogeneity (Figure 5; P=0.00, I 2=62%). At the end of the treatment, the analysis revealed that the SSRI group had significantly lower dependence (higher Beck Depression Inventory scores) compared with the placebo group (Figure 5; WMD, 8.86 [95% CI, 1.23–16.48]). This model showed significant heterogeneity (Figure 5; P=0.00, I 2=99%).
Figure 5. SSRIs for poststroke dependence provided by BI.
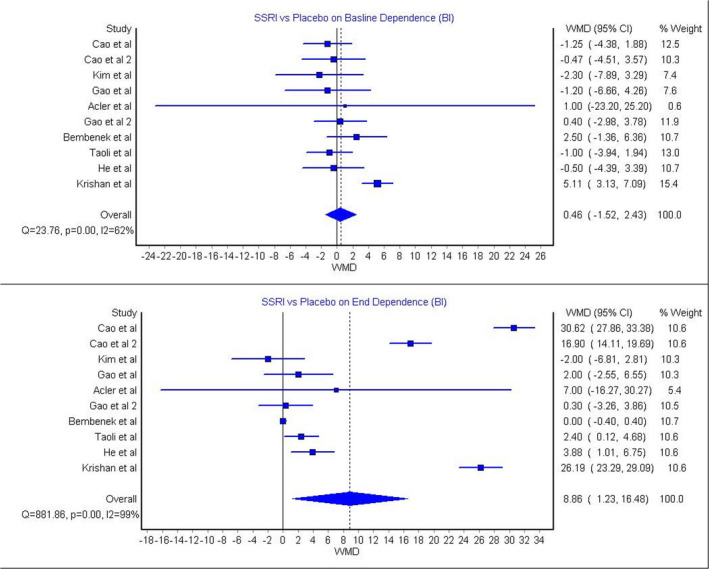
22, 26, 30, 32, 33, 34, 46, 49, 59, 61
BI indicates Barthel Index; SSRIs, selective serotonin reuptake inhibitors; and WMD, weighted mean difference.
Disability
Two studies conducted by Kim et al 33 and Kraglund et al 24 assessed the efficacy of SSRIs in reducing poststroke disability as a continuous variable provided by modified Rankin Scale scores. Both studies showed that SSRIs were not significantly associated with improvement in modified Rankin Scale disability scores. Moreover, the model that assessed disability as a binary variable included 10 articles.† This model showed an insignificant difference between the SSRI group and the placebo group in disability (Figure S3; RR, 0.95 [95% CI, 0.88–1.04;). This model showed significant heterogeneity (Figure S3; P=0.00, I 2=79%). Furthermore, Bembenek et al 22 showed that SSRIs did not significantly reduce poststroke disability provided by the Medical Research Council and Brunstorm tools. On the other hand, Mikami et al 43 showed that SSRIs significantly improved poststroke disability.
Cognitive Function
Four studies evaluated the efficacy of SSRIs in treating poststroke cognitive deficit. 26 , 30 , 39 , 44 The model that included those articles showed insignificant difference at baseline between the SSRI group and the placebo group. This model showed insignificant heterogeneity (Figure S4; P=0.98, I 2=0%). At the end of treatment, the analysis showed significantly higher cognitive functions among the SSRI group compared with the placebo group (Figure S4; WMD, 1.00 [95% CI, 0.12–1.89]). This model showed insignificant heterogeneity (Figure S4; P=0.12, I 2=48%). Furthermore, Jorge et al 42 showed that patients who were treated with fluoxetine had significantly higher cognitive function provided by the Repeatable Battery for the Assessment of Neuropsychological Status scores.
Motor Function
The model that evaluated the efficacy of SSRIs in improving motor function according to the NIHSS score included 11 articles.‡ This model showed insignificant difference in NIHSS scores between the SSRI group and the placebo group at baseline; this model had insignificant heterogeneity (Figure S5; P=0.87, I 2=0%). At the end of treatment, the model showed significantly higher motor functions among the SSRI group (lower NIHSS score) compared with the placebo group (Figure S5; WMD, −0.79 [95% CI, −1.42 to −0.15]). This model had significant heterogeneity (Figure S5; P=0.00, I 2=97%). Moreover, the model that assessed the efficacy of SSRIs in improving motor functions according to the Fugl‐Meyer Assessment of Motor Recovery score included 5 studies. 18 , 19 , 28 , 29 This model showed insignificant difference between the SSRI group and the placebo group at baseline. This model showed insignificant heterogeneity (Figure S6; P=0.72, I 2=0%). However, at the end of the treatment, the model showed significantly higher motor functions (higher Fugl‐Meyer Assessment of Motor Recovery scores) among the SSRI group compared with the placebo group (Figure S6; WMD, 14.67 [95% CI, 3.64–25.69]). This model showed significant heterogeneity (Figure S6; P=0.00, I 2=81%). In addition, the model that assessed the efficacy of SSRIs in improving motor function as a binary variable showed insignificant difference between the SSRI group and the placebo group 20 , 22 , 27 (Figure S7; RR, 1.00 [95% CI, 0.97–1.03]). This model showed significant heterogeneity (Figure S7; P=0.01, I 2=77%). Furthermore, Pinto et al showed that fluoxetine was significantly associated with better motor functions provided by the Jebsen Hand Function Test tool.
Side Effects
Gastrointestinal Side Effects
The occurrence of gastrointestinal symptoms as side effects of SSRIs were assessed by 20 studies.§ The model that pooled those studies showed that patients on SSRIs were not significantly different in the incidence of gastrointestinal symptoms compared with patients on a placebo (Figure S8; RR, 1.29 [95% CI, 1.00–1.67]). This model showed significant heterogeneity (Figure S8; P=0.00, I 2=80%).
Bleeding
The model that evaluated the risk of bleeding from SSRIs included 10 studies.‖ This model revealed that SSRIs were not significantly associated with higher risk of bleeding compared with a placebo (Figure S8; RR, 0.92 [95% CI, 0.76–1.12]). This model had insignificant heterogeneity (Figure S8; P=0.45, I 2=0%).
Seizures
The model that evaluated SSRIs for the risk of seizures included 8 studies. 18 , 21 , 27 , 34 , 40 , 45 , 53 , 58 This model showed that SSRIs were significantly associated with higher risk of seizures compared with a placebo (Figure S8; RR, 1.44 [95% CI, 1.13–1.83]). This model had insignificant heterogeneity (Figure S8; P=0.82, I 2=0%).
Sensitivity Analysis
Fluoxetine
Our models showed that fluoxetine was significantly effective in treating poststroke depression 18 , 31 , 32 , 36 , 37 , 40 , 41 (Figure S9; RR, 0.62 [95% CI, 0.43–0.90]). This model had significant heterogeneity (Figure S9; P=0.01, I 2=67%). On the other hand, the analysis revealed that fluoxetine was not significantly associated with prevention of poststroke depression or treating it provided by HAM‐D score¶ (Figures S10 and S11). In addition, fluoxetine use was not significantly associated with preventing poststroke depression or improvement of poststroke dependence, disability, or motor function # (Figures S12 through S14). Furthermore, 2 studies 39 , 44 evaluated the efficacy of fluoxetine in improving poststroke cognitive functions provided by the MMSE. Both studies showed that fluoxetine was insignificantly associated with improvement in MMSE scores.
Citalopram
Similar to the primary analysis, our models showed that citalopram was significantly associated with poststroke depression prevention 26 , 30 (Figure S15; at end: WMD, −7.05 [95% CI, −11.78 to −2.31], P=0.00, I 2=96%) and treatment 46 , 49 , 53 (Figure S16; at end: WMD, −0.88 [95% CI, −1.65 to −0.11], P=0.25, I 2=29%) provided by HAM‐D score. Also, treatment with citalopram was significantly associated with treating poststroke depression as a binary variable 18 , 43 (Figure S17; RR, 0.23 [95% CI, 0.10–0.54], P=not available, I 2=0%). However, citalopram did not significantly reduce poststroke depression as a binary variable 26 , 33 , 56 (Figure S18). Also, it was not significantly effective in improving disability provided by the modified Rankin Scale score 24 , 33 (Figure S19) or as a binary variable 20 , 24 (Figure S20). In addition, treatment with citalopram was not significantly associated with improvement of dependence provided by Barthel Index scores 30 , 33 , 46 , 49 (Figure S21). Moreover, consistent with the primary analysis, citalopram significantly improved poststroke cognitive deficit as shown in the MMSE scores 26 , 30 (Figure S22; at end: WMD, 1.50 [95% CI, 0.52–2.48], P=0.56, I 2=0%) and motor functions as shown in the NIHSS scores 19 , 20 , 33 , 49 (Figure S23; at end: WMD, −1.37 [95% CI, −2.44 to −0.29], P=0.00, I 2=96%).
Discussion
In this systematic review and meta‐analysis, we included 44 randomized controlled trials that investigated the effect of SSRIs on poststroke recovery (depression, anxiety, disability, dependence, motor abilities, and cognitive functions). Our results showed that SSRIs were significantly effective in preventing and treating poststroke depression. Moreover, the results also showed significant improvement of poststroke anxiety, dependence, motor abilities, and cognitive functions among patients who were treated with SSRIs. There was no significant improvement in disability after treatment with SSRIs. Furthermore, our study demonstrated that treatment with SSRIs was significantly associated with higher risk of seizures compared with a placebo, whereas there was no significant difference in the incidence of gastrointestinal symptoms or bleeding between the SSRI and placebo groups. On the other hand, the Cochrane review showed that SSRIs were significantly effective in treating poststroke depression but not in preventing it. 10 Also, in contrast with our results, the Cochrane review showed that there was no significant improvement in dependence, motor abilities, and cognitive functions among patients who were treated with SSRIs. 10 Additionally, the Cochrane review showed that SSRIs significantly increased the incidence of gastrointestinal symptoms but not seizures compared with a placebo. The deviation in our study findings compared with the Cochrane study results published in 2019 is a consequence of the large heterogeneity in the Cochrane study, because it was conducted before the implementation of the core protocol developed by international collaboration in 2019. 12 , 13 On the other hand, our review included several studies after the implementation of this protocol, which reduces the heterogeneity in our study compared with the Cochrane review. Furthermore, although the Cochrane collaboration updated their review in 2021, and their results contradict our findings, 62 the latest Cochrane review searched the databases up to December 2020, 62 whereas we searched the databases up to November 2021, and we identified 5 trials that were published during 2021 that were not included in the latest Cochrane review. However, the Cochrane review included more studies with wider ethnic variation, because they included studies with different languages and settings, whereas we only included studies that were published in English. In addition, previous studies showed that several genetic polymorphisms affect the efficacy and safety of SSRIs, which might explain the differences between our study and the previously mentioned Cochrane review. 63 , 64 Also, it is important to mention that the investigators of the latest Cochrane review were involved in several trials that assessed SSRIs efficacy and safety in improving poststroke depression outcomes, which might affect the results of their review.
On the safety of SSRIs among patients after stroke, our study demonstrated that SSRIs were significantly associated with higher risk of seizures compared with a placebo. However, this finding can be confounded by many variables including the low sodium and serotonin levels, which were both described in patients after stroke 61 , 65 and can induce seizures. 61 , 66 It is important to mention that none of the included studies accounted for the serotonin or sodium level among their patients before starting the intervention. Furthermore, several observational studies and open‐label trials showed that SSRIs were not associated with seizures, and in contrast, they were associated with reduction in seizure frequency and duration among patients with epilepsy. 67
We conducted a subanalysis to investigate the effect of different SSRI agents on poststroke recovery. Similar to the primary analysis, the subanalysis results showed that citalopram was significantly associated with improving the poststroke recovery of depression, cognitive function, and motor function. However, fluoxetine was only effective in treating poststroke depression. This indicates that the beneficial effects of SSRIs on poststroke recovery that we found in the primary analysis were driven by citalopram not fluoxetine. Similar to our findings, several studies showed that citalopram had higher efficacy and tolerability compared with fluoxetine in treating depression among adults. 68 These differences in the efficacy between fluoxetine and citalopram can be explained by the fact that fluoxetine has an extremely long half‐life compared with citalopram; hence, a much longer duration is required with fluoxetine to reach a steady state concentration and exert its action. 69 Also, it was shown that fluoxetine has active metabolites resulting in prolongation of the SSRIs’ side effects compared with citalopram. 69
This is the most updated systematic review and meta‐analysis after the implementations of the international collaboration core guidelines in conducting trials for the effect of SSRIs on poststroke recovery. In addition, this is the first review to show that citalopram could improve poststroke recovery. Also, this study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses and Cochrane Collaboration guidelines. However, this study had several limitations. First of all, we only included studies that were published in the English language, which might limit the generalizability of our results. Second, a large proportion of the included studies were low‐moderate‐quality studies. Third, different scales were used by the included trials to measure the same outcome, which reduced the number of the trials in several models. This necessitates well‐conducted placebo‐controlled clinical trials that use standardized tools for assessing poststroke recovery outcomes to assess the safety and the efficacy of SSRIs in promoting poststroke recovery. Fourth, there was high heterogeneity across several outcomes, which might be attributed to different times of starting SSRIs after the stroke, different SSRI doses used by the trials, and different characteristics of patients included in the trials.
Conclusions
Our study showed that SSRIs are effective in preventing and treating depression, and improving anxiety, motor function, cognitive function, and dependence in patients after stroke. These benefits were only reproducible with the citalopram subanalysis but not fluoxetine, suggesting that citalopram but not fluoxetine improved the recovery outcomes of patients after stroke. Further well‐conducted placebo‐controlled trials are needed to investigate the safety and efficacy of citalopram among patients after stroke.
Sources of Funding
None.
Disclosures
None.
Supporting information
Figures S1–S23
Acknowledgments
Author contributions: Drs Toubasi, Kalbouneh and Albustanji were involved in conceptualization. Drs Toubasi, Kalbouneh, Al‐Harasis, Obaid, and Albustanji were involved in data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, and writing the original draft. Drs Toubasi and Kalbouneh were involved in supervision and reviewing and editing the article.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025868
For Sources of Funding and Disclosures, see page 14.
Footnotes
References
- 1. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/s1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:2296–2301. doi: 10.1161/01.STR.0000183622.75135.a4 [DOI] [PubMed] [Google Scholar]
- 4. Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta‐analysis. Br J Psychiatry. 2013;202:14–21. doi: 10.1192/bjp.bp.111.107664 [DOI] [PubMed] [Google Scholar]
- 5. Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta‐analysis. Ageing Res Rev. 2019;50:102–109. doi: 10.1016/j.arr.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 6. McCann SK, Irvine C, Mead GE, Sena ES, Currie GL, Egan KE, Macleod MR, Howells DW. Efficacy of antidepressants in animal models of ischemic stroke: a systematic review and meta‐analysis. Stroke. 2014;45:3055–3063. doi: 10.1161/strokeaha.114.006304 [DOI] [PubMed] [Google Scholar]
- 7. Schallert T, Jones T, Weaver M, Shapiro L, Crippens D, Fulton RJPMR. Pharmacologic and anatomic considerations in recovery of function. Phys Med Rehabil. 1992;6:375–389. [Google Scholar]
- 8. Liepert J. Pharmacotherapy in restorative neurology. Curr Opin Neurol. 2008;21:639–643. doi: 10.1097/WCO.0b013e32831897a3 [DOI] [PubMed] [Google Scholar]
- 9. Loubinoux I, Chollet F. Neuropharmacology in stroke recovery. In: Cramer S, Nudo R, eds. Brain Repair After Stroke. Cambridge University Press; 2010:183–193. [Google Scholar]
- 10. Legg LA, Tilney R, Hsieh CF, Wu S, Lundström E, Rudberg AS, Kutlubaev MA, Dennis M, Soleimani B, Barugh A, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev. 2019;2019:CD009286. doi: 10.1002/14651858.CD009286.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mead GE, Hsieh CF, Lee R, Kutlubaev MA, Claxton A, Hankey GJ, Hackett ML. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev. 2012;11:Cd009286. doi: 10.1002/14651858.CD009286.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graham C, Lewis S, Forbes J, Mead G, Hackett ML, Hankey GJ, Gommans J, Nguyen HT, Lundström E, Isaksson E, et al. The FOCUS, AFFINITY and EFFECTS trials studying the effect(s) of fluoxetine in patients with a recent stroke: statistical and health economic analysis plan for the trials and for the individual patient data meta‐analysis. Trials. 2017;18:627. doi: 10.1186/s13063-017-2385-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mead G, Hackett ML, Lundström E, Murray V, Hankey GJ, Dennis M. The FOCUS, AFFINITY and EFFECTS trials studying the effect(s) of fluoxetine in patients with a recent stroke: a study protocol for three multicentre randomised controlled trials. Trials. 2015;16:369. doi: 10.1186/s13063-015-0864-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. David DJ, Gourion D. Antidepressant and tolerance: determinants and management of major side effects. Encephale. 2016;42:553–561. doi: 10.1016/j.encep.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 15. Altman DG. Practical Statistics for Medical Research. CRC Press; 1990. [Google Scholar]
- 16. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andersen G, Vestergaard K, Riis JO. Post‐stroke pathological crying or emotional affect treated with citalopram: a selective serotonin reuptake inhibitor. Acta Neurol Scand. 1994;89:151. [Google Scholar]
- 18. Nct . Effects of 3 months daily treatment with selective serotonin reuptake inhibitor (SSRI, fluoxetine) on motor rehabilitation after ischemic stroke. FLAME Trial. 2008. Available at: http://clinicaltrialsgov/show/nct00657163. Accessed November 1, 2021.
- 19. Gong L, Yang X, Feng Y, Fei Z, Wang M, Qin B, Wang Q, Pan W. The efficacy of integrative anti‐depressive therapy on motor recovery after ischemic stroke—a randomized clinical trial. Eur J Integr Med. 2020;35:101102. doi: 10.1016/j.eujim.2020.101102 [DOI] [Google Scholar]
- 20. Savadi Oskouie D, Sharifipour E, Sadeghi Bazargani H, Hashemilar M, Nikanfar M, Ghazanfari Amlashi S, Abbaszade Z, Sadeghihokmabadi E, Rikhtegar R, Golzari SEJ. Efficacy of citalopram on acute ischemic stroke outcome: a randomized clinical trial. Neurorehabil Neural Repair. 2017;31:638–647. doi: 10.1177/1545968317704902 [DOI] [PubMed] [Google Scholar]
- 21. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2020;19:661–669. doi: 10.1016/s1474-4422(20)30219-2 [DOI] [PubMed] [Google Scholar]
- 22. Bembenek JP, Niewada M, Kłysz B, Mazur A, Kurczych K, Głuszkiewicz M, Członkowska A. Fluoxetine for stroke recovery improvement—the doubleblind, randomised placebo‐controlled FOCUS‐Poland trial. Neurol Neurochir Pol. 2020;54:544–551. doi: 10.5603/PJNNS.a2020.0099 [DOI] [PubMed] [Google Scholar]
- 23. Marquez‐Romero JM, Reyes‐Martínez M, Huerta‐Franco MR, Ruiz‐Franco A, Silos H, Arauz A. Fluoxetine for motor recovery after acute intracerebral hemorrhage, the FMRICH trial. Clin Neurol Neurosurg. 2020;190:105656. doi: 10.1016/j.clineuro.2019.105656 [DOI] [PubMed] [Google Scholar]
- 24. Kraglund KL, Mortensen JK, Damsbo AG, Modrau B, Simonsen SA, Iversen HK, Madsen M, Grove EL, Johnsen SP, Andersen G. Neuroregeneration and vascular protection by citalopram in acute ischemic stroke (TALOS). Stroke. 2018;49:2568–2576. doi: 10.1161/strokeaha.117.020067 [DOI] [PubMed] [Google Scholar]
- 25. Bonin Pinto C, Morales‐Quezada L, de Toledo Piza PV, Zeng D, Saleh Vélez FG, Ferreira IS, Lucena PH, Duarte D, Lopes F, El‐Hagrassy MM, et al. Combining fluoxetine and rTMS in poststroke motor recovery: a placebo‐controlled double‐blind randomized phase 2 clinical trial. Neurorehabil Neural Repair. 2019;33:643–655. doi: 10.1177/1545968319860483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao JX, Liu L, Sun YT, Zeng QH, Yang ZD, Chen JC. Escitalopram improves neural functional prognosis and endothelial dysfunction in patients with acute cerebral infarction. Restor Neurol Neurosci. 2020;38:385–393. doi: 10.3233/rnn-201041 [DOI] [PubMed] [Google Scholar]
- 27. Dennis M, Forbes J, Graham C, Hackett M, Hankey GJ, House A, Lewis S, Lundström E, Sandercock P, Mead G. Fluoxetine to improve functional outcomes in patients after acute stroke: the FOCUS RCT. Health Technol Assess. 2020;24:1–94. doi: 10.3310/hta24220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rampello L, Chiechio S, Nicoletti G, Alvano A, Vecchio I, Raffaele R, Malaguarnera M. Prediction of the response to citalopram and reboxetine in post‐stroke depressed patients. Psychopharmacology. 2004;173:73–78. doi: 10.1007/s00213-003-1698-1 [DOI] [PubMed] [Google Scholar]
- 29. Asadollahi M, Ramezani M, Khanmoradi Z, Karimialavijeh E. The efficacy comparison of citalopram, fluoxetine, and placebo on motor recovery after ischemic stroke: a double‐blind placebo‐controlled randomized controlled trial. Clin Rehabil. 2018;32:1069–1075. doi: 10.1177/0269215518777791 [DOI] [PubMed] [Google Scholar]
- 30. Cao JX, Liu L, Sun YT, Zeng QH, Wang Y, Chen JC. Effects of the prophylactic use of escitalopram on the prognosis and the plasma copeptin level in patients with acute cerebral infarction. Braz J Med Biol Res. 2020;53:e8930. doi: 10.1590/1414-431x20208930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi‐Kwon S, Choi J, Kwon SU, Kang DW, Kim JS. Fluoxetine is not effective in the treatment of post‐stroke fatigue: a double‐blind, placebo‐controlled study. Cerebrovasc Dis. 2007;23:103–108. doi: 10.1159/000097045 [DOI] [PubMed] [Google Scholar]
- 32. Li LT, Wang SH, Ge HY, Chen J, Yue SW, Yu M. The beneficial effects of the herbal medicine Free and Easy Wanderer Plus (FEWP) and fluoxetine on post‐stroke depression. J Altern Complement Med. 2008;14:841–846. doi: 10.1089/acm.2008.0010 [DOI] [PubMed] [Google Scholar]
- 33. Kim JS, Lee E‐J, Chang D‐I, Park J‐H, Ahn SH, Cha J‐K, Heo JH, Sohn S‐I, Lee B‐C, Kim D‐E, et al. Efficacy of early administration of escitalopram on depressive and emotional symptoms and neurological dysfunction after stroke: a multicentre, double‐blind, randomised, placebo‐controlled study. Lancet Psychiatry. 2017;4:33–41. doi: 10.1016/s2215-0366(16)30417-5 [DOI] [PubMed] [Google Scholar]
- 34. He YT, Tang BS, Cai ZL, Zeng SL, Jiang X, Guo Y. Effects of fluoxetine on neural functional prognosis after ischemic stroke: a randomized controlled study in China. J Stroke Cerebrovasc Dis. 2016;25:761–770. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.035 [DOI] [PubMed] [Google Scholar]
- 35. Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, Small SL, Fonzetti P, Hegel MT, Robinson RG. Prevention of poststroke apathy using escitalopram or problem‐solving therapy. Am J Geriatr Psychiatry. 2013;21:855–862. doi: 10.1016/j.jagp.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 36. Chan KL, Campayo A, Moser DJ, Arndt S, Robinson RG. Aggressive behavior in patients with stroke: association with psychopathology and results of antidepressant treatment on aggression. Arch Phys Med Rehabil. 2006;87:793–798. doi: 10.1016/j.apmr.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 37. Choi‐Kwon S, Han SW, Kwon SU, Kang DW, Choi JM, Kim JS. Fluoxetine treatment in poststroke depression, emotional incontinence, and anger proneness: a double‐blind, placebo‐controlled study. Stroke. 2006;37:156–161. doi: 10.1161/01.STR.0000190892.93663.e2 [DOI] [PubMed] [Google Scholar]
- 38. Fruehwald S, Gatterbauer E, Rehak P, Baumhackl U. Early fluoxetine treatment of post‐stroke depression–a three‐month double‐blind placebo‐controlled study with an open‐label long‐term follow up. J Neurol. 2003;250:347–351. doi: 10.1007/s00415-003-1014-3 [DOI] [PubMed] [Google Scholar]
- 39. Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, Fonzetti P, Hegel M, Arndt S. Escitalopram and problem‐solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA. 2008;299:2391–2400. doi: 10.1001/jama.299.20.2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hankey GJ, Hackett ML, Almeida OP, Flicker L, Mead GE, Dennis MS, Etherton‐Beer C, Ford AH, Billot L, Jan S, et al. Twelve‐month outcomes of the AFFINITY trial of fluoxetine for functional recovery after acute stroke: AFFINITY Trial Steering Committee on Behalf of the AFFINITY Trial Collaboration. Stroke. 2021;52:2502–2509. doi: 10.1161/strokeaha.120.033070 [DOI] [PubMed] [Google Scholar]
- 41. Choi‐Kwon S, Choi J, Kwon SU, Kang DW, Kim JS. Fluoxetine improves the quality of life in patients with poststroke emotional disturbances. Cerebrovasc Dis. 2008;26:266–271. doi: 10.1159/000147454 [DOI] [PubMed] [Google Scholar]
- 42. Jorge RE, Acion L, Moser D, Adams HP Jr, Robinson RG. Escitalopram and enhancement of cognitive recovery following stroke. Arch Gen Psychiatry. 2010;67:187–196. doi: 10.1001/archgenpsychiatry.2009.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mikami K, Jorge RE, Adams HP Jr, Davis PH, Leira EC, Jang M, Robinson RG. Effect of antidepressants on the course of disability following stroke. Am J Geriatr Psychiatry. 2011;19:1007–1015. doi: 10.1097/JGP.0b013e31821181b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wiart L, Petit H, Joseph PA, Mazaux JM, Barat M. Fluoxetine in early poststroke depression: a double‐blind placebo‐controlled study. Stroke. 2000;31:1829–1832. doi: 10.1161/01.str.31.8.1829 [DOI] [PubMed] [Google Scholar]
- 45. Guo Y, He Y, Tang B, Ma K, Cai Z, Zeng S, Zhang Y, Jiang X. Effect of using fluoxetine at different time windows on neurological functional prognosis after ischemic stroke. Restor Neurol Neurosci. 2016;34:177–187. doi: 10.3233/rnn-150535 [DOI] [PubMed] [Google Scholar]
- 46. Gao J, Lin M, Zhao J, Bi S, Ni Z, Shang X. Different interventions for post‐ischaemic stroke depression in different time periods: a single‐blind randomized controlled trial with stratification by time after stroke. Clin Rehabil. 2017;31:71–81. doi: 10.1177/0269215515626232 [DOI] [PubMed] [Google Scholar]
- 47. Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, Small SL, Fonzetti P, Hegel MT, Robinson RG. Prevention of post‐stroke generalized anxiety disorder, using escitalopram or problem‐solving therapy. J Neuropsychiatry Clin Neurosci. 2014;26:323–328. doi: 10.1176/appi.neuropsych.11020047 [DOI] [PubMed] [Google Scholar]
- 48. He Y, Deng J, Zhang Y, Cai Z, Zhang H, Guo Y. The effect of fluoxetine on morning blood pressure surge in patients with ischemic stroke: a prospective preliminary clinical study. Blood Press Monit. 2021;26:288–291. doi: 10.1097/mbp.0000000000000538 [DOI] [PubMed] [Google Scholar]
- 49. Acler M, Robol E, Fiaschi A, Manganotti P. A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol. 2009;256:1152–1158. doi: 10.1007/s00415-009-5093-7 [DOI] [PubMed] [Google Scholar]
- 50. Brown KW, Sloan RL, Pentland B. Fluoxetine as a treatment for post‐stroke emotionalism. Acta Psychiatr Scand. 1998;98:455–458. doi: 10.1111/j.1600-0447.1998.tb10119.x [DOI] [PubMed] [Google Scholar]
- 51. Narushima K, Kosier JT, Robinson RG. Preventing poststroke depression: a 12‐week double‐blind randomized treatment trial and 21‐month follow‐up. J Nerv Ment Dis. 2002;190:296–303. doi: 10.1097/00005053-200205000-00005 [DOI] [PubMed] [Google Scholar]
- 52. Simis S, Nitrini R. Cognitive improvement after treatment of depressive symptoms in the acute phase of stroke. Arq Neuropsiquiatr. 2006;64:412–417. doi: 10.1590/s0004-282x2006000300012 [DOI] [PubMed] [Google Scholar]
- 53. Andersen G, Vestergaard K, Lauritzen L. Effective treatment of poststroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke. 1994;25:1099–1104. doi: 10.1161/01.str.25.6.1099 [DOI] [PubMed] [Google Scholar]
- 54. Rasmussen A, Lunde M, Poulsen DL, Sørensen K, Qvitzau S, Bech P. A double‐blind, placebo‐controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics. 2003;44:216–221. doi: 10.1176/appi.psy.44.3.216 [DOI] [PubMed] [Google Scholar]
- 55. Andersen G, Vestergaard K, Riis JO. Citalopram for post‐stroke pathological crying. Lancet. 1993;342:837–839. doi: 10.1016/0140-6736(93)92696-q [DOI] [PubMed] [Google Scholar]
- 56. Robinson RG, Schultz SK, Castillo C, Kopel T, Kosier JT, Newman RM, Curdue K, Petracca G, Starkstein SE. Nortriptyline versus fluoxetine in the treatment of depression and in short‐term recovery after stroke: a placebo‐controlled, double‐blind study. Am J Psychiatry. 2000;157:351–359. doi: 10.1176/appi.ajp.157.3.351 [DOI] [PubMed] [Google Scholar]
- 57. Lundström E, Isaksson E, Greilert Norin N, Näsman P, Wester P, Mårtensson B, Norrving BO, Wallén H, Borg J, Hankey GJ, et al. Effects of fluoxetine on outcomes at 12 months after acute stroke: results from EFFECTS, a randomized controlled trial. Stroke. 2021;52:3082–3087. doi: 10.1161/strokeaha.121.034705 [DOI] [PubMed] [Google Scholar]
- 58. Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double‐blind, randomised, controlled trial. Lancet. 2019;393:265–274. doi: 10.1016/s0140-6736(18)32823-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Krishnan K, K M, K N, Teja YD, Reddy VS, Raju NS, Rathinam KK. Role of fluoxetine in pharmacological enhancement of motor functions in stroke patients: a randomized, placebo‐controlled, single‐blind trial. Contemp Clin Trials Commun. 2021;23:100800. doi: 10.1016/j.conctc.2021.100800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Almeida OP, Hankey GJ, Ford A, Etherton‐Beer C, Flicker L, Hackett M, Mead GE, Dennis MS, Billot L, Jan S, et al. Depression outcomes among patients treated with fluoxetine for stroke recovery: the AFFINITY randomized clinical trial. JAMA Neurol. 2021;78:1072–1079. doi: 10.1001/jamaneurol.2021.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gao HQ, Zhu HY, Zhang YQ, Wang LX. Reduction of cerebrospinal fluid and plasma serotonin in patients with post‐stroke depression: a preliminary report. Clin Invest Med. 2008;31:E351–356. doi: 10.25011/cim.v31i6.4921 [DOI] [PubMed] [Google Scholar]
- 62. Legg LA, Rudberg A‐S, Hua X, Wu S, Hackett ML, Tilney R, Lindgren L, Kutlubaev MA, Hsieh C‐F, Barugh AJ, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev. 2021;11:CD009286. doi: 10.1002/14651858.CD009286.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stein MB, Seedat S, Gelernter J. Serotonin transporter gene promoter polymorphism predicts SSRI response in generalized social anxiety disorder. Psychopharmacology. 2006;187:68–72. doi: 10.1007/s00213-006-0349-8 [DOI] [PubMed] [Google Scholar]
- 64. Zhu J, Klein‐Fedyshin M, Stevenson JM. Serotonin transporter gene polymorphisms and selective serotonin reuptake inhibitor tolerability: review of pharmacogenetic evidence. Pharmacotherapy. 2017;37:1089–1104. doi: 10.1002/phar.1978 [DOI] [PubMed] [Google Scholar]
- 65. Berrut G, Cubillé M. Multimorbidity and epilepsia in the elderly. Geriatr Psychol Neuropsychiatr Vieil. 2019;17:13–19. doi: 10.1684/pnv.2019.0780 [DOI] [PubMed] [Google Scholar]
- 66. Horvath GA, Selby K, Poskitt K, Hyland K, Waters PJ, Coulter‐Mackie M, Stockler‐Ipsiroglu SG. Hemiplegic migraine, seizures, progressive spastic paraparesis, mood disorder, and coma in siblings with low systemic serotonin. Cephalalgia. 2011;31:1580–1586. doi: 10.1177/0333102411420584 [DOI] [PubMed] [Google Scholar]
- 67. Ribot R, Ouyang B, Kanner AM. The impact of antidepressants on seizure frequency and depressive and anxiety disorders of patients with epilepsy: is it worth investigating? Epilepsy Behav. 2017;70:5–9. doi: 10.1016/j.yebeh.2017.02.032 [DOI] [PubMed] [Google Scholar]
- 68. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta‐analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/s0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lochmann D, Richardson T. Selective serotonin reuptake inhibitors. Handb Exp Pharmacol. 2019;250:135–144. doi: 10.1007/164_2018_172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S23


