Abstract
Background
Exercise‐induced high heart rate may impair exercise tolerance by reducing diastolic filling time and ventricular filling in heart failure with preserved ejection fraction (HFpEF). Given the importance of chronotropic response, we hypothesized that reduction in diastolic filling time because of exercise‐induced increased heart rate would not impair cardiac output reserve and exercise capacity. We sought to determine the association between heart rate, diastolic filling time, hemodynamics, and exercise capacity in HFpEF.
Methods and Results
Patients with HFpEF (n=66) and controls without HF (n=107) underwent bicycle exercise echocardiography with simultaneous expired gas analysis to measure oxygen consumption. Diastolic filling time was assessed by the overlap time between mitral E‐ and A‐waves (longer overlap time indicates shorter diastolic filling duration). Overlap time increased (ie, diastolic filling time shortened) in HFpEF and controls as heart rate increased with exercise, and the relationship was similar between the groups. Greater heart rate response correlated with higher cardiac output (r=0.51, P<0.0001) and oxygen consumption (r=0.50, P<0.0001) during peak exercise. Shorter diastolic filling time, as assessed by longer overlap time, was correlated with higher cardiac output (r=0.47, P<0.0001) and peak oxygen consumption (r=0.38, P=0.007), not with E/e′ or right ventricular‐pulmonary artery uncoupling. Longer overlap time was associated with mitral A velocity (r=0.53, P<0.0001) and left atrial booster pump strain (r=0.42, P<0.0001).
Conclusions
Shortening of diastolic filling interval in tandem with increased heart rate during exercise does not limit cardiac output reserve or exercise capacity in HFpEF.
Keywords: chronotropic incompetence, diastolic filling time, exercise, heart failure with preserved ejection fraction
Subject Categories: Echocardiography, Exercise Testing, Heart Failure
Nonstandard Abbreviations and Acronyms
- CO
cardiac output
- HFpEF
heart failure with preserved ejection fraction
- LA
left atrial
- PASP
pulmonary artery systolic pressure
- VO2
oxygen consumption
Clinical Perspective.
What Is New?
We show that the relationship between diastolic filling time and heart rates throughout the exercise is similar between patients with heart failure and preserved ejection fraction and controls and that shorter diastolic filling time is associated with higher cardiac output and greater exercise capacity.
We also demonstrate a moderate association between diastolic filling time and left atrial booster pump function during peak exercise.
The use of β‐blockers lowers peak heart rate but does not affect the relationships of diastolic filling time with heart rates and left atrial booster pump function during exercise.
What Are the Clinical Implications?
These data suggest that shortening the diastolic filling interval in tandem with increased heart rate during exercise does not limit cardiac output reserve or exercise capacity in patients with heart failure with preserved ejection fraction.
Our data indicate a compensatory mechanism for the reduction in the diastolic filling period by enhancing left atrial contractile function.
These data may provide an opportunity to reconsider the use of β‐blockers in patients with heart failure with preserved ejection fraction.
Exercise intolerance is a cardinal manifestation in patients with heart failure (HF) with preserved ejection fraction (HFpEF). 1 , 2 , 3 While multiple cardiac and extracardiac abnormalities are related to reduced exercise capacity, left ventricular (LV) diastolic dysfunction is a primary contributor to this by increasing LV filling pressure and compromising ventricular filling. 4 , 5 Given the importance of diastole to ventricular filling and coronary perfusion, high heart rate during exercise may have adverse effects on exercise tolerance by reducing the diastolic filling time, which limits ventricular filling and increases left atrial (LA) pressure. 6 In addition to the high prevalence of atrial fibrillation (AF), systemic hypertension, and coronary artery disease, frequent use of β‐blockers in patients with HFpEF (≈80%) may be related to the speculation that slowing the heart rate may enhance diastolic filling and exercise capacity. 7 , 8 , 9 , 10 , 11 However, clinical trials have shown no convincing evidence to support the benefits of pharmacological heart rate lowering in patients with HFpEF. 8 , 9 , 10 , 11 , 12
An increase in heart rate is a major driver in augmenting cardiac output (CO) to meet the metabolic demands that increase during exercise, but this is limited in patients with HFpEF (that is, chronotropic incompetence). 2 , 13 , 14 , 15 , 16 , 17 The potential mechanisms remain unclear but may be related to sinus node dysfunction, autonomic dysfunction, or premature cessation of exercise before maximal sinus node activation because of exertional dyspnea and fatigue. 18 , 19 Regardless of the mechanism, chronotropic incompetence contributes to exercise intolerance and poor quality of life in patients with HFpEF. 13 , 14 However, little is known regarding how elevated heart rates during exercise contribute to diastolic filling time, CO augmentation, and exercise tolerance in HFpEF. Based on this background, we hypothesized that reduction in diastolic filling time because of increased heart rate during exercise would not compromise CO reserve and aerobic capacity in patients with HFpEF. Accordingly, we sought to determine the association between heart rate, diastolic filling time, hemodynamics, and exercise capacity during supine bicycle exercise in patients with HFpEF.
Methods
Study Population
This was a retrospective study that assessed the association between diastolic filling time, exercise capacity, and echocardiographic markers of hemodynamics during exercise in patients with HFpEF. Consecutive subjects who were referred to the echocardiographic laboratory of the Gunma University Hospital, Maebashi, Japan for exercise stress echocardiography for the evaluation of exertional dyspnea between October 2019 and September 2021 were enrolled. The study was approved by our institutional review board with a waiver of consent. The data are available from the corresponding author upon reasonable request.
HFpEF was defined by typical symptoms or signs of HF (exertional dyspnea and/or peripheral edema), normal left ventricular EF (≥50%), and objective evidence of elevated left heart filling pressures at rest and/or with exercise (at least 1 of the following: the American Society of Echocardiography/European Association of Cardiovascular Imaging–recommended echocardiographic diastolic dysfunction; the ratio of transmitral E to mitral annular e′ velocities [E/e′] during exercise >15; or invasively measured pulmonary capillary wedge pressure [PCWP] at rest >15 mm Hg and/or with supine ergometry exercise ≥25 mm Hg). 20 , 21 , 22 , 23 Control participants who were also referred to exercise echocardiography because of clinical indication of exertional dyspnea were also included as a comparator group. The controls were required to have no evidence of the cardiac cause of dyspnea, including normal rest and exercise left‐sided filling pressure and pulmonary artery (PA) pressure (criteria above). Patients with EF <50%, age <30 years, significant left‐sided valvular heart disease (>moderate regurgitation, >mild stenosis), infiltrative, restrictive, or hypertrophic cardiomyopathy, and non‐Group II pulmonary artery hypertension or exercise‐induced pulmonary hypertension without elevation in E/e′ (pulmonary artery mean pressure with exercise >30 mm Hg with a total pulmonary resistance [that is, pulmonary artery mean pressure/CO of >3 mm Hg·min/L]) were excluded. 24 , 25 We also excluded patients without mitral A wave, such as AF or supraventricular arrhythmia rhythm, during echocardiography because of the requirement of mitral overlap time measurements, as described later. The probability of HFpEF was assessed using the Heart Failure Association Pre‐test assessment, Echocardiography & natriuretic peptide, Functional testing, Final etiology (HFA‐PEFF) score (0–6 points). 21
Exercise Stress Echocardiography
Two‐dimensional Doppler echocardiography was performed by experienced sonographers using a commercially available ultrasound system (Vivid E95, GE Healthcare, Horten, Norway). LV systolic function was assessed by EF, systolic mitral annular tissue velocity at the septal annulus (mitral s′), and LV longitudinal strain. LV volumes, EF, and LV longitudinal strain were determined using apical 4‐chamber views. 26 LV diastolic function was assessed using the early diastolic mitral inflow velocity (E), early diastolic mitral annular tissue velocity at the septal annulus (e′), and E/e′ ratio. Stroke volume was determined from the LV outflow dimension and pulse Doppler profile, and CO was calculated from the product of the heart rate and stroke volume. Left atrial (LA) deformation analysis was performed to measure LA reservoir, conduit, and booster pump strain. 27 , 28 LA strain was calculated as the average of strain in 6 segments in the apical 4‐chamber views using the QRS as the fiducial point. 29 Myocardial deformation analyses were performed at rest and during peak exercise using a commercially available software (EchoPAC PC; GE, Milwaukee, WI), and strain measurements were presented as absolute values. Right ventricular (RV) systolic function was assessed using systolic tissue velocity at the lateral tricuspid annulus (TV s′). PA systolic pressure (PASP) was determined from peak tricuspid regurgitation and estimated right atrial pressure (RAP). The RAP was estimated from the diameter of the inferior vena cava and its respiratory change (coded as 3, 8, or 15 mm Hg). 30 The pulmonary artery mean pressure was then calculated as 0.61×PASP +2 mm Hg. 31
The diastolic filling period was assessed by the overlap time between mitral E‐ and A‐waves (Figure 1), as previously described. 32 , 33 This index was chosen because of its ease of measurement, low observer variability, and associations with LV filling pressures and clinical outcomes in patients with HF. 32 , 33 A longer overlap time value indicates a shorter diastolic filling duration. If there was no overlap, the distance between the end of the E‐wave and the beginning of the A‐wave was expressed as a negative value (that is, longer diastolic filling time). When E‐ and A‐waves were fused during exercise, the overlap time was defined as the time from the beginning to the end of the fusion waveform.
Figure 1. Measurements of overlap time.
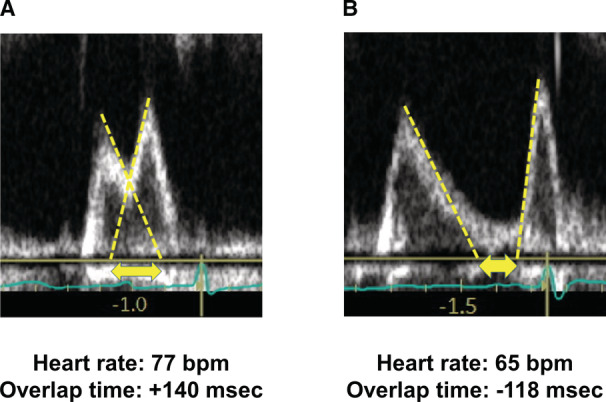
A, Diastolic filling period was assessed by the overlap time between mitral E‐ and A‐waves. A longer overlap time value indicates a shorter diastolic filling duration. B, If there was no overlap, the distance between the end of the E‐wave and the beginning of the A‐wave was expressed as a negative value.
All participants underwent supine ergometry exercise, starting at 20 W for 5 minutes, in 20‐W increments in 3‐minute stages until subject‐reported exhaustion, as previously described. 34 , 35 Echocardiographic images were obtained at baseline and during all stages of exercise. Chronotropic responses were assessed by percent predicted heart rate and heart rate reserve, calculated as the difference between peak exercise and resting heart rates, divided by the difference of age‐predicted maximal heart rate (220−age) and resting heart rate. 36 All Doppler measurements represent the mean of 3 beats. In a subset of participants (n=100), expired gas analysis was performed simultaneously with echocardiography at rest and throughout the exercise to measure breath‐by‐breath oxygen consumption (VO2), carbon dioxide production (VCO2), tidal volume, and minute ventilation (VE). Percent predicted peak VO2 was determined using the Wasserman‐Hansen equation. 37 Ventilatory efficiency was assessed by the slope of VE to VCO2 (VE/VCO2 slope), and objective effort was estimated by the respiratory exchange ratio (VCO2/VO2). 2
Reproducibility Analysis
The reproducibility of the overlap time was assessed in 25 randomly selected patients. Intra‐ and interobserver agreement was evaluated after the same observer and another experienced reader repeated the analysis using intraclass correlation coefficients (ICCs).
Exercise Right Heart Catheterization
A subset of participants (n=18) underwent clinically indicated right heart catheterization at rest and during supine ergometry exercise as confirmatory testing. RAP, PA pressures, and PCWP were measured at end‐expiration (mean of ≥3 beats) using a 7 Fr fluid‐filled catheter. CO was determined from simultaneously measured VO2 and PA and arterial blood gases using the direct Fick method. After resting data were obtained, hemodynamic assessments were repeated during supine ergometry exercise, starting at 20 W for 5 minutes, increasing in 20‐W increments in 3‐minute stages to exhaustion. The diagnosis of HFpEF was defined as elevated PCWP at rest (>15 mm Hg) and/or during exercise (≥25 mm Hg). The reproducibility of the PCWP assessment was tested in a random sample of 10 patients using the ICCs.
Statistical Analysis
Data are reported as mean (SD), median (interquartile range), or number (%) unless otherwise specified. Between‐group differences were compared using the χ2 test, unpaired t test, or Wilcoxon rank‐sum test. To assess relationships between 2 variables of interest, Pearson (for normally distributed data) or Spearman (for non‐normally distributed data) correlation coefficients were used based on the distribution of data, as appropriate. Multivariable regression analyses were used to determine the differences in the interactions or intercepts of the relationship of 2 variables between groups. All tests were 2‐sided, with a significance level of P<0.05. All statistical analyses were performed using JMP 15.2.0 (SAS Institute, Cary, NC).
Results
Baseline Clinical Characteristics
Of the 206 consecutive participants meeting the study criteria, 33 patients (23 HFpEF and 10 controls) did not show A‐wave during the examination, resulting in a final study cohort of 173 patients (66 HFpEF and 107 controls). Of the 66 patients with HFpEF, 18 were diagnosed based on exercise right heart catheterization, and hemodynamic data are presented in Table S1. PCWP and RAP increased dramatically during peak exercise, with increases in PA pressures and CO. The intra‐ and interobserver ICCs for PCWP were 0.97 and 0.93 at rest and 0.88 and 0.85 during exercise.
Compared with control subjects, patients with HFpEF were older and had a higher prevalence of diabetes, systemic hypertension, and coronary artery disease and greater HFA‐PEFF score and natriuretic peptide levels than controls (Table 1). Sex, body mass index, renal function, and prevalence of paroxysmal AF were similar between the groups. Interstitial lung disease was more common in control subjects than in HFpEF. Patients with HFpEF were treated with β‐blockers and mineralocorticoid receptor antagonists more frequently, but other medication use was similar between the groups. The LV mass index and LA volume index were larger in patients with HFpEF than in controls.
Table 1.
Baseline Characteristics
| Controls (n=107) | HFpEF (n=66) | P value | |
|---|---|---|---|
| Age, y | 63±13 | 74±8 | <0.0001 |
| Female, n (%) | 73 (68) | 41 (62) | 0.41 |
| Body mass index, kg/m2 | 23.3±5.5 | 24.0±4.0 | 0.36 |
| HFA‐PEFF score | 3 (2, 4) | 5 (3, 6) | <0.0001 |
| Comorbidities | |||
| Diabetes, n (%) | 12 (11) | 17 (26) | 0.01 |
| Hypertension, n (%) | 71 (66) | 55 (83) | 0.01 |
| Paroxysmal atrial fibrillation, n (%) | 10 (9) | 10 (15) | 0.25 |
| Coronary artery disease, n (%) | 3 (3) | 12 (18) | 0.0005 |
| Interstitial lung disease, n (%) | 27 (28%) | 7 (12%) | 0.02 |
| Medications | |||
| ACEI or ARB, n (%) | 33 (31) | 29 (44) | 0.08 |
| β‐Blocker, n (%) | 12 (7) | 22 (33) | 0.0004 |
| Loop diuretic, n (%) | 15 (14) | 12 (18) | 0.47 |
| MRA, n (%) | 4 (4) | 8 (12) | 0.04 |
| SGLT2 inhibitor, n (%) | 2 (2) | 1 (2) | 0.86 |
| Laboratories | |||
| NT‐proBNP (pg/mL) (n=118) | 99 (66 145) | 247 (117 784) | 0.0001 |
| BNP (pg/mL) (n=69) | 30 (17, 55) | 69 (35 136) | <0.0001 |
| Creatinine, mg/dL | 0.73 (0.62, 0.90) | 0.75 (0.61, 0.95) | 0.69 |
| eGFR, mL/min per 1.73 m2 | 67±21 | 63±23 | 0.34 |
| LV and LA structures | |||
| LV mass index, g/m2 | 79±19 | 91±22 | 0.0004 |
| LA volume index, mL/m2 | 24 (19, 30) | 35 (28, 46) | <0.0001 |
Data are mean±SD, median (interquartile range), or n (%). ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin‐receptor blockers; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; HFA‐PEFF, Heart Failure Association Pre‐test assessment, Echocardiography & natriuretic peptide, Functional testing, Final etiology; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and SGLT2, sodium‐glucose cotransporter‐2.
Resting Echocardiographic Markers
At rest, systolic and diastolic blood pressures and oxygen saturation were similar between the groups, but heart rate was lower in patients with HFpEF than in controls (Table 2). There was no difference in LV end‐diastolic and systolic volumes or EF in patients with HFpEF and controls. Patients with HFpEF had more impaired LV systolic function (lower mitral s′ tissue velocity and LV longitudinal strain), LV diastolic function (lower e′ velocity and higher mitral E wave and E/e′ ratio), and LA function (lower reservoir, conduit, and booster strain) than controls. There was no difference in overlap times between the groups. Patients with HFpEF displayed higher PASP and RAP than controls, while stroke volume, CO, and RV systolic function were similar between the groups.
Table 2.
Vital Signs and Echocardiographic Measures
| Baseline | 20 W exercise | Peak exercise | ||||
|---|---|---|---|---|---|---|
| Controls | HFpEF | Controls | HFpEF | Controls | HFpEF | |
| Vital signs | ||||||
| Heart rate, bpm | 76±14 | 69±11* | 95±13 | 91±14 | 117±21 | 106±21† |
| Percent predicted HR, % | … | … | … | … | 75±13 | 73±14 |
| Heart rate reserve, % | … | … | … | … | 51±23 | 50±23 |
| Systolic BP, mm Hg | 130±23 | 132±20 | 151±30 | 151±26 | 168±33 | 164±31 |
| Diastolic BP, mm Hg | 75±14 | 72±13 | 83±17 | 83±22 | 85±18 | 84±20 |
| Saturation, % | 97±2 | 97±1 | 96±3 | 96±3 | 95±4 | 95±4 |
| Left heart | ||||||
| LV end‐diastolic volume, mL | 66±22 | 67±25 | 71±24 | 75±28 | 73±23 | 75±29 |
| LV end‐systolic volume, mL | 24±10 | 25±12 | 23±10 | 26±13 | 21±9 | 23±13 |
| LV ejection fraction, % | 65±7 | 63±7 | 68±7 | 67±7 | 72±7 | 70±8 |
| E‐wave, cm/s | 63±16 | 74±26† | 89±21 | 111±25* | 112±24 | 130±27* |
| A‐wave, cm/s | 76±21 | 91±27* | 93±23 | 113±32* | 111±27 | 118±41 |
| E/A ratio | 0.87±0.30 | 0.86±0.36 | 1.00±0.35 | 1.05±0.35 | 1.06±0.31 | 1.27±0.53‡ |
| Overlap time, ms | 3±142 | −14±135 | 78±84 | 64±100* | 133±76 | 102±87‡ |
| Mitral e′, cm/s | 7.1±2.1 | 5.2±1.5* | 8.8±2.3 | 6.7±1.5* | 10.1±2.6 | 7.5±1.7* |
| Mitral s′, cm/s | 8.4±1.6 | 7.2±1.6* | 8.4±1.9 | 7.2±1.4* | 9.3±2.2 | 8.0±2.1* |
| E/e′ ratio | 9 (8, 10) | 14 (11, 18)* | 11 (9, 12) | 16 (15, 20)* | 11 (10, 13) | 17 (15, 20)* |
| Stroke volume, mL | 56±16 | 60±18 | 66±17 | 64±19 | 67±18 | 63±18 |
| Cardiac output, L/min | 4.2±1.2 | 4.2±1.1 | 6.2±1.7 | 5.9±1.5 | 7.8±2.2 | 6.8±2.0† |
| Cardiac index, L/min per m2 | 2.6±0.8 | 2.6±0.7 | 3.8±1.0 | 3.6±0.9 | 4.8±1.3 | 4.2±1.2† |
| LV longitudinal strain, % | 17±3 | 16±3‡ | … | … | 21±3 | 18±4* |
| LA reservoir strain, % | 39±13 | 27±11* | … | … | 47±16 | 29±13* |
| LA conduit strain, % | 18±10 | 13±7* | … | … | 14±11 | 10±7‡ |
| LA booster strain, % | 21±8 | 14±7* | … | … | 32±14 | 18±10* |
| Right heart | ||||||
| TV s′, cm/s | 12.8±3.0 | 12.8±2.6 | 14.2±3.1 | 13.1±2.4‡ | 15.4±3.0 | 14.2±3.1‡ |
| PASP, mm Hg | 20±6 | 24±8* | 31±9 | 41±12* | 33±10 | 46±13* |
| RAP, mm Hg | 3 (3, 3) | 3 (3, 3)† | 3 (3, 3) | 3 (3, 8)† | 3 (3, 8) | 8 (3, 8)* |
Data are mean±SD, or median (interquartile range). BP indicates blood pressure; e′, early diastolic mitral annular tissue velocity; E/A ratio, the ratio of early diastolic mitral inflow to mitral inflow by active atrial contraction; E/e′ ratio, the ratio of early diastolic mitral inflow to mitral annular tissue velocities; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LA, left atrial; LV, left ventricular; PASP, pulmonary artery systolic pressure; RAP, right atrial pressure; s′, systolic mitral annular tissue velocity; and TV s′, systolic tissue velocity at the lateral tricuspid annulus.
P<0.001 vs controls.
P<0.01 vs controls.
P<0.05 vs controls.
Effects With Exercise
Peak exercise workload achieved was lower (60 [40, 80] versus 40 [40, 60] W, P=0.0002) and exercise duration was shorter (614±196 versus 532±178 seconds, P=0.006) in patients with HFpEF than in controls. The majority of the patients with HFpEF (75%) achieved a high peak respiratory exchange ratio (≥1.05) and the mean respiratory exchange ratio was similar between groups (1.10±0.17 in controls versus 1.12±0.15 in HFpEF, P=0.56), suggesting that most of the patients exercise to near maximal effort levels. Borg perceived effort and dyspnea scores at peak exercise were similar between the groups (16 [14, 17] in controls versus 16 [14, 18] in HFpEF, P=0.86 and 6 [4, 8] in controls versus 6 [5, 8] in HFpEF, P=0.12, respectively), but effort and dyspnea scores relative to the workload performed were higher in patients with HFpEF than in controls (0.25 [0.20, 0.32] in controls versus 0.32 [0.25, 0.43] in HFpEF, P=0.0004, and 0.09 [0.05, 0.13] in controls versus 0.13 [0.08, 0.18] in HFpEF, P=0.0003, respectively). In a subset of participants with simultaneous expired gas analysis (n=100), peak VO2 was impaired and VE/VCO2 slope was elevated in patients with HFpEF compared with those in controls (13.4±4.1 mL/min per kg in controls versus 11.8±3.5 mL/min per kg in HFpEF, P=0.04; and 35.5±8.1 in controls versus 38.9±7.9 in HFpEF, P=0.04), although percent predicted peak VO2 was similar between groups (45±16% in controls versus 45±12% in HFpEF, P=0.99).
During matched submaximal and peak exercise, heart rate was significantly increased in both groups (Figure 2A). but it was lower at peak exercise in patients with HFpEF than in controls. Overlap time also increased with increased exercise workload in both groups, suggesting that heart rate increased at the expense of the diastolic filling period (Figure 2B). The overlap time during peak exercise was shorter (that is, diastolic filling time was longer) in patients with HFpEF than in controls (Figure 2B). This may be explained by the lower exercise heart rate in the patients given a tied association between heart rate and overlap time during peak exercise (r=0.64, P<0.0001, Figure 2C). The relationship between heart rate and overlap time throughout the exercise was similar between patients with HFpEF and controls (Figure 2D), suggesting that reduction in the diastolic filling duration associated with exercise did not differ between the groups.
Figure 2. Changes in heart rate and overlap time during exercise.
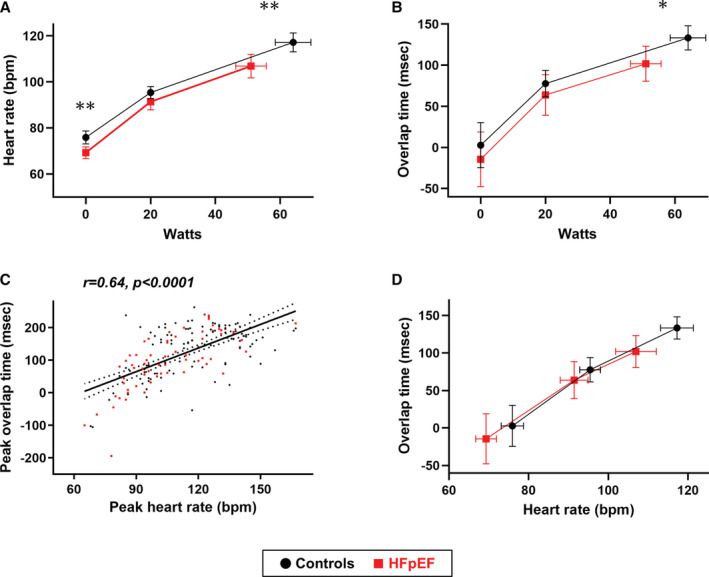
A and B, Heart rate and overlap time as a function of workload in heart failure with preserved ejection fraction (HFpEF) and control subjects. C, There was a moderate correlation between heart rate and overlap time during peak exercise. D, From baseline to peak exercise, the relationship between heart rate and overlap time was similar between patients with HFpEF and control subjects. The vertical and horizontal error bars represent 95% CIs. *P<0.05 and **P<0.01 between groups for single time point comparisons. bpm indicates beats per minute.
During 20 W and peak exercise, end‐diastolic and systolic volumes and EF were similar in patients with HFpEF and controls. As expected, E‐wave increased to a greater extent during submaximal and peak exercise in patients with HFpEF than in controls with less increase in mitral e′ velocity, resulting in a greater increase in the E/e′ ratio during the exercise (Table 2). This was accompanied by more severe RV‐PA uncoupling during peak exercise, manifested by higher PASP and lower TV s′. Increases in CO with peak exercise were depressed in patients with HFpEF compared with those in controls secondary to limitations in LV systolic reserve (lower mitral s′ velocity and LV longitudinal strain) and chronotropic incompetence (lower heart rate). During peak exercise, differences in LA reservoir and booster pump strain between the groups increased further, whereas LA booster pump strain but reservoir strain significantly increased with exercise in patients with HFpEF (P=0.0002 and P=0.42, respectively; paired t test).
Relationships Between Overlap Time, Hemodynamics, and Exercise Tolerance During Exercise
As expected, the ability to enhance heart rate during peak exercise was correlated with higher CO, better ventilatory efficiency (lower VE/VCO2 slope, r=−0.37, P=0.0002), and higher exercise capacity as assessed by peak VO2, peak workload achieved (r=0.38, P<0.0001), and exercise duration (r=0.37, P<0.0001) (Figure 3A and 3B). Similarly, longer overlap time (that is, shorter diastolic filling time) was associated with higher CO, VO2, exercise intensity, and exercise duration as well as lower VE/VCO2 slope during peak exercise (r=0.47, P<0.0001; r=0.38, P=0.007; r=0.26, P=0.0004; r=0.20, P=0.01, and r=−0.36, P=0.0002; respectively). These relationships persisted even in patients with HFpEF only (Figure 3C and 3D), suggesting that reduction in the diastolic filling time during exercise did not compromise CO reserve and exercise capacity in HFpEF. The overlap time was also correlated with higher mitral e′ velocity (r=0.27, P=0.001) as well as higher A‐wave (r=0.53, P<0.0001) and LA booster pump strain (r=0.42, P<0.0001) during exercise in the entire population. The correlations between overlap time and LA contractile function during peak exercise were observed when HFpEF and controls were assessed separately (Figure 4A and 4B). In contrast, there was no or modest correlations of peak overlap time with E/e′ ratio, PASP, and TV s′ during peak exercise when assessed in the whole population (r=−0.14, P=0.10, r=−0.08, P=0.32, and r=0.16, P=0.03, respectively) or in HFpEF only (r=0.03, P=0.85, r=0.07, P=0.54, and r=0.32, P=0.01, respectively).
Figure 3. Correlations between overlap time, cardiac output, and oxygen consumption during peak exercise.
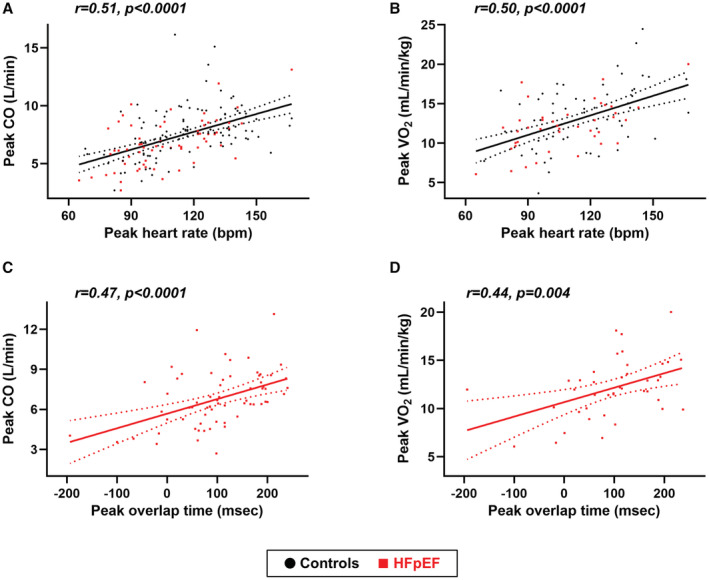
A and B, Higher heart rate during peak exercise was correlated with higher cardiac output (CO) and better exercise capacity as assessed by peak oxygen consumption (VO2) in all participants. C and D, Similarly, longer overlap time (ie, shorter diastolic filling time) was associated with higher CO and VO2 during peak exercise even in patients with HFpEF. bpm indicates beats per minute; and HFpEF, heart failure with preserved ejection fraction.
Figure 4. Correlations between overlap time and left atrial function during peak exercise.
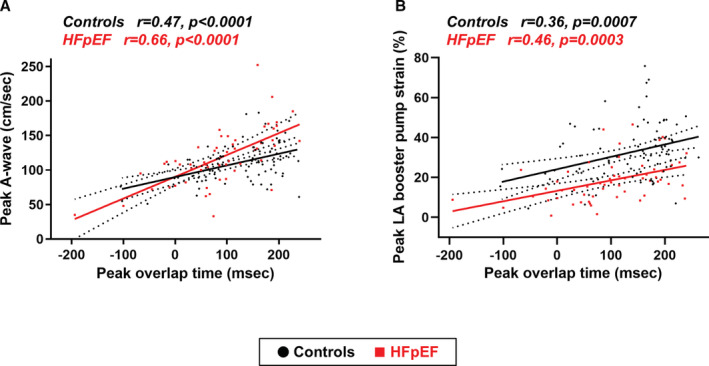
A, The overlap time was correlated with higher mitral A‐wave velocity and (B) left atrial (LA) booster pump strain during peak exercise both in patients with heart failure with preserved ejection fraction (HFpEF) and controls.
Impact of β‐Blocker Use, LV Diastolic Dysfunction, and LA Reservoir Strain
We performed sensitivity analyses to separate patients with HFpEF according to the use of β‐blockers, LV diastolic dysfunction, or LA reservoir strain. Compared with patients with HFpEF without β‐blockers (n=44), those with β‐blockers (n=22) had lower heart rates at rest and during exercise (Figure S1A). Peak overlap time was also shorter (that is, diastolic filling time was longer) in the patients on β‐blockers than those without (Figure S1B). Otherwise, the sensitivity analyses demonstrated similar results regardless of β‐blocker use, with correlations of overlap time with heart rates and LA booster pump function during peak exercise (Figures S1C, S1D and S2A through S2D). Sensitivity analyses separating patients with HFpEF according to the American Society of Echocardiography/European Association of Cardiovascular Imaging–recommended LV diastolic dysfunction grade (grade I [n=45] or grade II–III [n=21]) showed that changes in heart rates throughout the exercise were similar between the groups while overlap time tended to be shorter in patients with grade II‐III diastolic dysfunction than those in grade I diastolic dysfunction (Figure S3A through S3C). The relationship between overlap time and heart rates during peak exercise was similar between the groups (Figure S3D). We also found similar relationships between peak overlap time and LA booster pump function in patients with grade I and grade II‐III diastolic dysfunction (Figure S4A through S4D). Similar results were obtained when separating patients with HFpEF according to the median value of resting LA reservoir strain (≤24.0% or >24.0%) (Figures S5 and S6).
Intraobserver and Interobserver Reproducibility
The intra‐observer ICCs for the overlap time at rest and during exercise were 0.98 and 0.91, respectively. The corresponding interobserver ICCs for the overlap time were 0.98 and 0.90, respectively.
Discussion
In this study, we evaluated, for the first time, the association between heart rate and diastolic filling period as assessed by overlap time, hemodynamics, and exercise capacity during ergometry exercise in patients with HFpEF with sinus rhythm and controls without HF. We demonstrated that overlap time was tightly coupled with heart rate during exercise, and its relationship from rest to exercise was similar between patients with HFpEF and controls. Shorter diastolic filling time, as evidenced by longer overlap time, was associated with higher CO and greater exercise capacity but was unrelated to echocardiographic markers of LV filling pressure and abnormal RV‐PA coupling. These data suggest that shortening the diastolic filling interval in tandem with increased heart rate during exercise does not limit CO reserve or exercise capacity, even in patients with HFpEF. We also demonstrated a moderate association between overlap time and LA booster pump strain and mitral A velocity. This indicates a compensatory mechanism for the reduction in the diastolic filling period by enhancing LA contractile function. The use of β‐blockers lowered peak heart rate but did not affect the relationships of overlap time with heart rates and LA booster pump function during exercise. These data may provide new insights into the pathophysiology of HFpEF and an opportunity to reconsider the use of β‐blockers in patients with HFpEF.
Reduction in Diastolic Filling Time, Chronotropic Incompetence, and Exercise Intolerance in HFpEF
Exercise capacity is reduced in patients with HFpEF and is associated with symptoms of dyspnea and poor quality of life. 1 , 2 , 3 , 38 There has been a concern that, in the presence of LV diastolic dysfunction, an excessive increase in heart rate during exercise may shorten the diastolic filling time, leading to impairments in LV filing and CO. 6 This premise could be one of the reasons why clinicians prescribe β‐blockers to patients with HFpEF and may be the rationale of clinical trials that tested the effects of heart rate–lowering agents in HFpEF. 9 , 10 , 11 On the other hand, an increase in heart rate is the strongest determinant in augmenting CO to meet the metabolic demands during exercise. 36 During maximal exercise in healthy individuals, VO2 increases ≈7.7‐fold, with a 2.5‐fold increase in heart rate and only 1.4‐fold increase in stroke volume. 16 Many patients with HFpEF display chronotropic incompetence, which is associated with reduced exercise capacity, poor quality of life, as well as worse clinical outcomes. 2 , 13 , 14 , 15 , 17 , 39 , 40 In the current study, patients with HFpEF displayed severe chronotropic incompetence (heart rate reserve [HRR] 50±23%) despite near maximal effort intensity. The contradictory effects of the increase in heart rate and the accompanying reduction in the diastolic filling time have long been debated in patients with HFpEF. 13 , 41 , 42 The failure of clinical trials to identify beneficial effects of heart rate–lowering drugs on exercise capacity, quality of life, or clinical outcomes in patients with HFpEF has raised questions about their use in HFpEF. 7 , 8 , 9 , 10 , 11 , 41 , 42 A very recent study reported that β‐blocker withdrawal improved maximal functional capacity in patients with HFpEF and chronotropic incompetence. 43 On the basis of this background, we hypothesized that reduction in the diastolic filling time because of increased heart rate during exercise would not compromise CO reserve and exercise capacity in patients with HFpEF.
The present study examined diastolic filling time at rest and during exercise in patients with HFpEF and demonstrated that reduced diastolic filling time, as assessed by mitral overlap time, was tightly coupled with heart rate during exercise, and their relationship from rest to peak exercise was similar between patients with HFpEF and controls. Although previous studies have demonstrated the importance of chronotropic response in patients with HFpEF, 13 , 14 very little is known regarding how diastolic filling time contributes to exercise intolerance in patients with HFpEF. The current data suggest that reduction in the diastolic filling time relative to an increase in heart rate during exercise is not abnormal in patients with HFpEF but simply depends on heart rate augmentation. We also demonstrated that shorter diastolic filling intervals were associated with greater CO and exercise capacity in patients with HFpEF, without reaching a plateau where the overlap time was high (Figure 3C and 3D). Our findings suggest that reduction in the diastolic filling time associated with an increase in the heart rate does not limit LV filling and subsequent enhancement in CO, even in patients with HFpEF and sinus rhythm (Figure S7). Furthermore, the observed relationships between heart rate, overlap time, and peak VO2 indicate that a greater heart rate response is beneficial for achieving greater oxygen consumption even if the diastolic filling time is shortened.
We also demonstrated that overlap time was unrelated to echocardiographic markers of LV filling pressure and RV‐PA uncoupling during exercise. Our results are in accordance with previous data showing a decrease in LV end‐diastolic pressures with increasing heart rate by atrial pacing in patients with HFpEF. 44 , 45 A recent study showing a reduction in N‐terminal pro‐B‐type natriuretic peptide levels, a serum marker of LV filling pressure, following β‐blocker cessation, might support these data. Taken together, the current results may support the recent clinical trial demonstrating the benefit of β‐blocker withdrawal in patients with HFpEF, all of whom had chronotropic incompetence. 43
Relationship Between Diastolic Filling Time and Atrial Pump Function in HFpEF
It is of note that physiological (or pathophysiological) changes that occur during the stress of exercise are substantially different from those produced by atrial pacing. 44 , 45 The primary difference can be increased sympathetic nervous activity, which enhances LV contractility as well as venous return through the combined actions of skeletal muscle pump and venoconstriction in the splanchnic capacitance veins. 46 , 47 In contrast to the reduction in the LV end‐diastolic volume by pacing‐induced tachycardia in HFpEF, 44 , 45 we observed a similar increase in the LV end‐diastolic volume between patients with HFpEF and controls. This might be related to the enhancement in LV preload with exercise despite the relatively higher heart rate. Importantly, LA reservoir and booster pump function also improve during exercise, possibly through enhancement of LA relaxation and activation of the LA Frank‐Starling mechanism. 48 , 49 In the current study, LA booster pump strain increased during exercise even in patients with HFpEF, although its degree was lower than that in controls. We further demonstrated a moderate association of overlap time with LA booster pump strain and mitral A velocity (Figure 4). An experimental study has shown that A velocity increased with increasing heart rate by atrial pacing in dogs. 50 These data suggest a compensatory response to a reduction in the diastolic filling period during exercise by enhancing LA contractile function. Sensitivity analyses indicate that the severity of LV diastolic dysfunction or LA dysfunction would not significantly influence the LA compensatory mechanisms in HFpEF. On the other hand, the current study excluded patients with AF rhythm during the examination, and the patients with HFpEF enrolled had on average normal LA function with a modestly enlarged LA. Further study is warranted to validate the current findings in patients with LA dysfunction or AF, which is common in HFpEF. 51
Clinical Implications
β‐Blockers are commonly prescribed (up to 80%) to patients with HFpEF given the high prevalence of comorbidities such as AF, systemic hypertension, and coronary artery disease and possibly on the premise that heart rate lowering may enhance diastolic filling time and exercise capacity. 7 , 8 , 9 , 10 , 11 In the current study, β‐blocker use was associated with lower peak heart rate, but it did not affect the relationships of overlap time with heart rates and LA booster pump function during exercise. This indicates that the effects of β‐blockers might be mediated by maximal heart rate limitation rather than through modification of diastolic filling time. The present results support recent notions that raise questions about the use of β‐blockers in patients with HFpEF. 41 , 42 These agents may worsen chronotropic response to exercise that limits CO reserve and, thus, exercise capacity, as observed in recent clinical trials. 9 , 10 , 11 They also delay relaxation and increase ventricular volumes and pressures, leading to an increase in LV wall stress. 13 Our results and the recent clinical trials suggest that β‐blocker withdrawal might be an option for patients with reduced exercise capacity and chronotropic incompetence. 43 , 52 Further studies are required to advance our understanding of the underlying pathophysiological mechanisms and to explore optimal treatment in this syndrome.
Limitations
This was a single‐center study from a tertiary referral center, and all participants were referred for exercise stress echocardiography, introducing selection and referral bias. In the current study, the prevalence of AF was low because patients with AF rhythm during echocardiography were excluded because of the requirement of A‐wave for the measurement of overlap time. Another explanation could be the inclusion of patients with earlier disease. AF is very common in patients with HFpEF, and its exclusion is the primary limitation of the current study, which limits the generalizability of the results. The control participants were not normal, given that they had shortness of breath, poor exercise capacity, and multiple comorbidities including interstitial lung disease. However, the fact that the control population was more diseased than a truly normal healthy control population only biases our data toward the null. Participants in this study might have less severe HF status, with low natriuretic peptide levels, preserved renal function, a low prevalence of AF, and a relatively low frequency of diuretic use. Thus, the current results may not be applied to patients with more advanced phases of the disease. This was a hypothesis‐generating study and multiple testing correction was not performed.
Conclusions
The diastolic filling time, as assessed by mitral overlap time, was tightly coupled with heart rate during exercise, and their relationship from rest to exercise was similar between patients with HFpEF and controls. A shorter diastolic filling interval, as evident by longer overlap time, was associated with higher CO and greater exercise capacity, but was unrelated to echocardiographic markers of LV filling pressure and abnormal RV‐PA coupling. Our data suggest that shortening the diastolic filling interval in tandem with increased heart rate during exercise does not limit CO reserve or exercise capacity in patients with HFpEF.
Sources of Funding
Dr Obokata received research grants from the Fukuda Foundation for Medical Technology, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, Nippon Shinyaku, Takeda Science Foundation, the Japanese Circulation Society, and the Japanese College of Cardiology. This research was supported by grants from the Takeda Science Foundation.
Disclosures
None.
Supporting information
Table S1
Figures S1–S7
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.026009
For Sources of Funding and Disclosures, see page 10 and 11.
References
- 1. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–573. doi: 10.1038/s41569-020-0363-2 [DOI] [PubMed] [Google Scholar]
- 2. Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39:2810–2821. doi: 10.1093/eurheartj/ehy268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD. Exercise intolerance in heart failure with preserved ejection fraction. Circulation. 2018;137:148–161. doi: 10.1161/CIRCULATIONAHA.117.029058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging. 2020;13:245–257. doi: 10.1016/j.jcmg.2018.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borlaug BA, Jaber WA, Ommen SR, Lam CSP, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Runte KE, Bell SP, Selby DE, Häußler TN, Ashikaga T, Lewinter MM, Palmer BM, Meyer M. Relaxation and the role of calcium in isolated contracting myocardium from patients with hypertensive heart disease and heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10:1–15. doi: 10.1161/CIRCHEARTFAILURE.117.004311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J‐DHF). Eur J Heart Fail. 2013;15:110–118. doi: 10.1093/eurjhf/hfs141 [DOI] [PubMed] [Google Scholar]
- 9. Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka‐Kosmala M, Marwick TH. Effect of If‐channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62:1330–1338. doi: 10.1016/j.jacc.2013.06.043 [DOI] [PubMed] [Google Scholar]
- 10. Pal N, Sivaswamy N, Mahmod M, Yavari A, Rudd A, Singh S, Dawson DK, Francis JM, Dwight JS, Watkins H, et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation. 2015;132:1719–1725. doi: 10.1161/CIRCULATIONAHA.115.017119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Komajda M, Isnard R, Cohen‐Solal A, Metra M, Pieske B, Ponikowski P, Voors AA, Dominjon F, Henon‐Goburdhun C, Pannaux M, et al. Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo‐controlled trial. Eur J Heart Fail. 2017;19:1495–1503. doi: 10.1002/ejhf.876 [DOI] [PubMed] [Google Scholar]
- 12. Silverman DN, Plante TB, Infeld M, Callas PW, Juraschek SP, Dougherty GB, Meyer M. Association of β‐blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw Open. 2019;2:e1916598. doi: 10.1001/jamanetworkopen.2019.16598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745 [DOI] [PubMed] [Google Scholar]
- 14. Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Domínguez E, Palau P, Núñez E, Ramón JM, López L, Melero J, Bellver A, Santas E, Chorro FJ, Núñez J. Heart rate response and functional capacity in patients with chronic heart failure with preserved ejection fraction. ESC Heart Fail. 2018;5:579–585. doi: 10.1002/ehf2.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.RES.58.2.281 [DOI] [PubMed] [Google Scholar]
- 17. Pandey A, Khera R, Park B, Haykowsky M, Borlaug BA, Lewis GD, Kitzman DW, Butler J, Berry JD. Relative impairments in hemodynamic exercise reserve parameters in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:117–126. doi: 10.1016/j.jchf.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mesquita T, Zhang R, Cho JH, Zhang R, Lin Y‐N, Sanchez L, Goldhaber JI, Yu JK, Liang JA, Liu W, et al. Mechanisms of sinoatrial node dysfunction in heart failure with preserved ejection fraction. Circulation. 2022;145:45–60. doi: 10.1161/CIRCULATIONAHA.121.054976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarma S, Stoller D, Hendrix J, Howden E, Lawley J, Livingston S, Adams‐Huet B, Holmes C, Goldstein DS, Levine BD. Mechanisms of chronotropic incompetence in heart failure with preserved ejection fraction. Circ Heart Fail. 2020;13:e006331. doi: 10.1161/CIRCHEARTFAILURE.119.006331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obokata M, Kane GC, Reddy YNV, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pieske B, Tschöpe C, De Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, et al. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 22. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082 [DOI] [PubMed] [Google Scholar]
- 23. Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, Garcia G, Grünig E, Howard L, Humbert M, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50:1700578. doi: 10.1183/13993003.00578-2017 [DOI] [PubMed] [Google Scholar]
- 25. Herve P, Lau EM, Sitbon O, Savale L, Montani D, Godinas L, Lador F, Jaïs X, Parent F, Günther S, et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J. 2015;46:728–737. doi: 10.1183/09031936.00021915 [DOI] [PubMed] [Google Scholar]
- 26. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 27. Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, Tange S, Arai M, Kurabayashi M. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6:749–758. doi: 10.1016/j.jcmg.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 28. Reddy YNV, Obokata M, Egbe A, Yang JH, Pislaru S, Lin G, Carter R, Borlaug BA. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21:891–900. doi: 10.1002/ejhf.1464 [DOI] [PubMed] [Google Scholar]
- 29. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. doi: 10.1093/ehjci/jey042 [DOI] [PubMed] [Google Scholar]
- 30. Yang JH, Harada T, Choi KH, Kato T, Kim D, Takama N, Park TK, Kurabayashi M, Chang S‐A, Obokata M. Peripheral venous pressure‐assisted exercise stress echocardiography in the evaluation of pulmonary hypertension during exercise in patients with suspected heart failure with preserved ejection fraction. Circ Heart Fail. 2022;15:e009028. doi: 10.1161/CIRCHEARTFAILURE.121.009028 [DOI] [PubMed] [Google Scholar]
- 31. Chemla D, Castelain V, Humbert M, Hébert J‐L, Simonneau G, Lecarpentier Y, Hervé P. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–1317. doi: 10.1378/chest.126.4.1313 [DOI] [PubMed] [Google Scholar]
- 32. Kusunose K, Arase M, Zheng R, Hirata Y, Nishio S, Ise T, Yamaguchi K, Fukuda D, Yagi S, Yamada H, et al. Clinical utility of overlap time for incomplete relaxation to predict cardiac events in heart failure. J Card Fail. 2021;27:1222–1230. doi: 10.1016/j.cardfail.2021.05.018 [DOI] [PubMed] [Google Scholar]
- 33. Izumida T, Imamura T, Nakamura M, Fukuda N, Kinugawa K. How to consider target heart rate in patients with systolic heart failure. ESC Heart Fail. 2020;7:3231–3234. doi: 10.1002/ehf2.12814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kagami K, Harada T, Yamaguchi K, Kouno S, Ikoma T, Yoshida K, Kato T, Tomono J, Wada N, Adachi T, et al. Association between lung ultrasound B‐lines and exercise‐induced pulmonary hypertension in patients with connective tissue disease. Echocardiography. 2021;38:1297–1306. doi: 10.1111/echo.15141 [DOI] [PubMed] [Google Scholar]
- 35. Harada T, Araki T, Sunaga H, Kagami K, Yoshida K, Kato T, Kawakami R, Tomono J, Wada N, Iso T, et al. Pathophysiological and diagnostic importance of fatty acid‐binding protein 1 in heart failure with preserved ejection fraction. Sci Rep. 2021;11:21175. doi: 10.1038/s41598-021-00760-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, Dunlay S, McNulty S, Chakraborty H, Stevenson LW, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009–1018. doi: 10.1002/ejhf.1788 [DOI] [PubMed] [Google Scholar]
- 39. Santos M, West E, Skali H, Forman DE, Nadruz W, Shah AM. Resting heart rate and chronotropic response to exercise: prognostic implications in heart failure across the left ventricular ejection fraction spectrum. J Card Fail. 2018;24:753–762. doi: 10.1016/j.cardfail.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolsk E, Kaye DM, Komtebedde J, Shah SJ, Borlaug BA, Burkhoff D, Kitzman DW, Cleland JG, Hasenfuß G, Hassager C, et al. Determinants and consequences of heart rate and stroke volume response to exercise in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2021;23:754–764. doi: 10.1002/ejhf.2146 [DOI] [PubMed] [Google Scholar]
- 41. Meyer M, Rambod M, LeWinter M. Pharmacological heart rate lowering in patients with a preserved ejection fraction—review of a failing concept. Heart Fail Rev. 2018;23:499–506. doi: 10.1007/s10741-017-9660-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meyer M, LeWinter MM. Heart rate and heart failure with preserved ejection fraction. Circ Heart Fail. 2019;12:1–5. doi: 10.1161/CIRCHEARTFAILURE.119.006213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palau P, Seller J, Domínguez E, Sastre C, Ramón JM, de La Espriella R, Santas E, Miñana G, Bodí V, Sanchis J, et al. Effect of β‐blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78:2042–2056. doi: 10.1016/j.jacc.2021.08.073 [DOI] [PubMed] [Google Scholar]
- 44. Wachter R, Schmidt‐Schweda S, Westermann D, Post H, Edelmann F, Kasner M, Luers C, Steendijk P, Hasenfuss G, Tschope C, et al. Blunted frequency‐dependent upregulation of cardiac output is related to impaired relaxation in diastolic heart failure. Eur Heart J. 2009;30:3027–3036. doi: 10.1093/eurheartj/ehp341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silverman DN, Rambod M, Lustgarten DL, Lobel R, LeWinter MM, Meyer M. Heart rate–induced myocardial Ca2+ retention and left ventricular volume loss in patients with heart failure with preserved ejection fraction. J Am Heart Assoc. 2020;9:e017215. doi: 10.1161/JAHA.120.017215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sorimachi H, Burkhoff D, Verbrugge FH, Omote K, Obokata M, Reddy YNV, Takahashi N, Sunagawa K, Borlaug BA. Obesity, venous capacitance, and venous compliance in heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:1648–1658. doi: 10.1002/ejhf.2254 [DOI] [PubMed] [Google Scholar]
- 47. Fudim M, Hernandez AF, Felker GM. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc. 2017;6:e006817. doi: 10.1161/JAHA.117.006817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sugimoto T, Bandera F, Generati G, Alfonzetti E, Bussadori C, Guazzi M. Left atrial function dynamics during exercise in heart failure: pathophysiological implications on the right heart and exercise ventilation inefficiency. JACC Cardiovasc Imaging. 2017;10:1253–1264. doi: 10.1016/j.jcmg.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 49. Nishikawa Y, Roberts JP, Tan P, Klopfenstein CE, Klopfenstein HS. Effect of dynamic exercise on left atrial function in conscious dogs. J Physiol. 1994;481:457–468. doi: 10.1113/jphysiol.1994.sp020454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamamoto K, Masuyama T, Tanouchi J, Doi Y, Kondo H, Hori M, Kitabatake A, Kamada T. Effects of heart rate on left ventricular filling dynamics: assessment from simultaneous recordings of pulsed Doppler transmitral flow velocity pattern and haemodynamic variables. Cardiovasc Res. 1993;27:935–941. doi: 10.1093/cvr/27.6.935 [DOI] [PubMed] [Google Scholar]
- 51. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. 2020;76:1051–1064. doi: 10.1016/j.jacc.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meyer M, Du Fay LJ, Benson L, Savarese G, Dahlström U, Lund LH. Association between β‐blockers and outcomes in heart failure with preserved ejection fraction: current insights from the SwedeHF registry. J Card Fail. 2021;27:1165–1174. doi: 10.1016/j.cardfail.2021.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S7


