Abstract
Background
The association between alcohol consumption, atrial substrate, and outcomes after atrial fibrillation (AF) ablation remains controversial. This study evaluated the impacts of drinking on left atrial substrate and AF recurrence after ablation.
Methods and Results
We prospectively enrolled 110 patients with AF without structural heart disease (64±12 years) from 2 institutions. High‐density left atrial electroanatomic mapping was performed using a high‐density grid multipolar catheter. We investigated the impact of alcohol consumption on left atrial voltage, left atrial conduction velocity, and AF ablation outcome. Patients were classified as abstainers (<1 drink/wk), mild drinkers (1–7 drinks/wk), or moderate‐heavy drinkers (>7 drinks/wk). High‐density mapping (mean 2287±600 points/patient) was performed on 49 abstainers, 27 mild drinkers, and 34 moderate‐heavy drinkers. Low‐voltage zone and slow‐conduction zone were identified in 39 (35%) and 54 (49%) patients, respectively. There was no significant difference in the proportions of low‐voltage zone and slow‐conduction zone among the 3 groups. The success rate after a single ablation was significantly lower in drinkers than in abstainers (79.3% versus 95.9% at 12 months; mean follow‐up, 18±8 months; P=0.013). The success rate after a single or multiple ablations was not significantly different among abstainers and drinkers. In multivariate analysis, alcohol consumption (P=0.02) and the presence of a low‐voltage zone (P=0.032) and slow‐conduction zone (P=0.02) were associated with AF recurrence after a single ablation, while low‐voltage zone (P=0.023) and slow‐conduction zone (P=0.024) were associated with AF recurrence after a single or multiple ablations.
Conclusions
Alcohol consumption was associated with AF recurrence after a single ablation but not changes in atrial substrate.
Keywords: ablation, alcohol, atrial fibrillation, high‐density mapping, left atrial substrate
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- HDG
high‐density grid multipolar catheter
- LVZ
low‐voltage zone
- PV
pulmonary vein
- PVI
pulmonary vein isolation
- SC
slow conduction
- SCZ
slow‐conduction zone
Clinical Perspective.
What Is New?
Alcohol consumption is not associated with changes in atrial substrate, such as low‐voltage zone or slow conduction zone.
It is associated with atrial fibrillation recurrence after a single ablation but not after a single or multiple ablations.
The presence of a low‐voltage zone and slow‐conduction zone are significantly associated with atrial fibrillation recurrence both after a single and a single or multiple ablations.
What Are the Clinical Implications?
When discussing the success rate of a single ablation for atrial fibrillation, drinking habits should be taken into consideration preoperatively.
In patients with changes in atrial substrate, such as low‐voltage zone or slow‐conduction zone, pulmonary vein isolation alone may not provide sufficient sinus rhythm maintenance; therefore, additional ablations for atrial substrate that may be beneficial should be discussed.
Catheter ablation is a standard therapy for nonvalvular atrial fibrillation (AF); pulmonary vein isolation (PVI) is the cornerstone of AF ablation procedures. 1 , 2 , 3 However, 20% to 30% of patients with paroxysmal AF and 40% to 50% of patients with persistent AF undergoing PVI experience AF recurrence during a 1‐year follow‐up after the procedure. 4 , 5 , 6 Several studies have reported that the low‐voltage zone (LVZ), intracardiac conduction delay, or late gadolinium‐enhanced magnetic resonance imaging findings in the atrium are associated with AF recurrence after ablation. 7 , 8 , 9 LVZ and conduction delay in the atrium are also significantly related to late gadolinium‐enhanced magnetic resonance imaging, which are considered to indicate the atrial substrate progression. 7 , 10 , 11 Recently, the relationship between alcohol and AF has attracted attention. Alcohol consumption is known to be associated with episodes of AF 12 and changes in atrial substrate, such as LVZ or slow conduction (SC). 13 , 14 Moreover, habitual alcohol consumption has been reported to be a risk factor for recurrence after AF ablation. 13 , 15 More specifically, Takigawa et al 15 reported that alcohol consumption may be a predictor of recurrence after a single ablation, but it may not be a predictor of recurrence after single or multiple ablations. Most previous reports examining the impacts of alcohol consumption on atrial substrate and AF ablation outcomes are retrospective studies; therefore, more prospective studies are required. Furthermore, the mapping method in previous studies investigating the relationship between alcohol and atrial mapping findings included limitations related to the configuration of the mapping catheter and lower sampling density. 16 , 17
Therefore, this study investigated the influence of alcohol consumption on atrial voltage and conduction velocity obtained by high‐density mapping using a high‐density grid multipolar catheter (HDG; Abbott Technologies, Minneapolis, MN) and to prospectively examine the relationship between alcohol consumption and AF recurrence after ablation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
This multicenter, prospective, observational study was conducted between October 2018 and March 2020 at the Japanese Red Cross Musashino Hospital in Tokyo and the Cardiovascular Center of Tsuchiura Kyodo Hospital in Tsuchiura. A total of 110 consecutive patients with AF without structural heart disease who underwent high‐density left atrial (LA) electroanatomic mapping using an HDG during initial AF ablation were enrolled in this study. AF ablation was performed using a 3‐dimensional electroanatomic mapping system (Ensite NavX, Abbott Technologies, Minneapolis, MN). Patients with an LA mapping number <1000 points (including insufficient LA mapping attributable to immediate recurrence of AF), significant structural heart disease (left ventricular ejection fraction <40% or previous myocardial infarction), severe renal impairment (including dialysis), previous AF ablation, and patients who had open heart surgery were excluded from this study. The study was approved by the local research ethics committees of the 2 institutions and was conducted in accordance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Alcohol Consumption Assessment
Alcohol consumption was assessed during baseline clinical visits. Patients were asked whether they regularly consumed alcohol and about their average alcohol consumption per week over the preceding 12 months. The quantification of the amount of alcohol was calculated with reference to Takahashi et al. 18 In this study, 1 standard drink was defined as 12 g of alcohol, while the cutoff values for weekly alcohol consumption were set in accordance with past studies. 14 , 19 We classified the patients’ level of consumption as abstainers (<1 drink/wk), mild drinkers (1–7 drinks/wk), and moderate‐heavy drinkers (>7 drinks/wk).
Electroanatomic Mapping Protocol and Ablation Procedure
After written informed consent was obtained from all patients, antiarrhythmic drugs were discontinued at least 5 half‐lives before the procedure. A multielectrode catheter was positioned in the coronary sinus via the right internal jugular vein. An ablation catheter, circular mapping catheter, and an HDG were introduced into the left atrium through 3 transseptal sheaths. In all cases, detailed endocardial voltage and activation maps were created using an HDG through a deflectable sheath (Agilis, Abbott Technologies) during stable pacing from the distal coronary sinus at 600‐ms cycle length before ablation. All patients with persistent AF had sinus rhythm that was successfully restored by intracardiac cardioversion before mapping. All procedures were performed with the assistance of the Ensite NavX system. Points were acquired after careful evaluation of tissue contact based on fluoroscopic motion of an HDG, deformation of the catheter caused by contact with the wall, catheter icon‐to‐surface feature of the mapping system, and the presence of constant electrogram characteristics. Surface color projection with an interpolation fill threshold of 7 mm was used to ensure a minimum number of points in an even distribution throughout the left atrium. Points acquired following ectopic beats were excluded from the analysis. An activated clotting time of 300 to 400 seconds was maintained with a continuous infusion of heparin during the procedure. PVI was performed using an open‐irrigated 3.5‐mm tip electrode catheter (TactiCath SE, Abbott Technologies), or a cryoballoon (Arctic Front Advance; Medtronic, Minneapolis, MN). When a radiofrequency catheter was used, we set power ≤30 W at the posterior wall and ≤35 W elsewhere to perform PVI; an upper temperature limit of 40 °C was set, as was a flow rate of 17 mL/min in ≤30 W and 30 mL/min in 35 W. PVI was performed using point‐by‐point application of radiofrequency energy at the antrum of the pulmonary veins. When using a cryoballoon, 180‐second freezing was applied using a second‐generation 28‐mm cryoballoon catheter. The end point of the ablation procedure was isolation of the pulmonary veins with proof of both the exit and entrance block. Subsequently, a cavotricuspid isthmus linear ablation was performed at the physician’s discretion. Isoproterenol (5–20 μg/min) was injected intravenously after PVI and cavotricuspid isthmus linear ablation. If sustained or nonsustained AF was reproducibly induced from non–pulmonary vein (PV) foci, they were focally ablated. When non‐PV foci were located in the superior vena cava, the superior vena cava was electrically isolated. If spontaneous AF did not occur, atrial burst pacing was performed to induce AF. After pacing‐induced AF was sustained, internal cardioversion was attempted to convert the AF to sinus rhythm and confirm whether spontaneous reinitiation of AF occurred.
Analysis of LA Voltage and Local Conduction
Voltage maps were created for each patient during constant pacing from the distal coronary sinus at 600‐ms cycle length. Mean LA voltage was evaluated by a mean of the highest voltage at a sampling density of a 10‐mm tag. Low voltage was defined as a bipolar peak‐to‐peak voltage amplitude of <0.5 mV, and the %LVZ was defined as the total low‐voltage area divided by the entire LA surface area. LVZ positive was defined as %LVZ >5%, in accordance with previous studies. 7 , 20 The left atrium was divided into 6 segments (anterior wall, septal wall, roof, posterior wall, inferior wall, and lateral wall; all depicted in Figure 1A) to compare mean LA voltage and LVZ distribution. 21 , 22 Mean LA voltage and the proportion of patients with LVZ both in the whole left atrium and in different segments of the left atrium was investigated, respectively. The activation map was also constructed during distal coronary sinus pacing, and the local activation time for each point was annotated using the algorithm based on bipolar signals using the maximum negative slope. Local conduction in the left atrium was assessed using an isochronal map. SC was defined as <30 cm/s with reference to previous studies, 23 and slow‐conduction zone (SCZ) positive was defined as SC with a length of 16 mm. An example of the SC measurement is shown in Figure 1B, C, and D. Similar to the LVZ analysis, the proportion of patients with SCZ in the whole left atrium and the distribution of SCZ in different segments of the left atrium was investigated. The investigators were blinded to patient drinking status during electrogram analysis.
Figure 1.
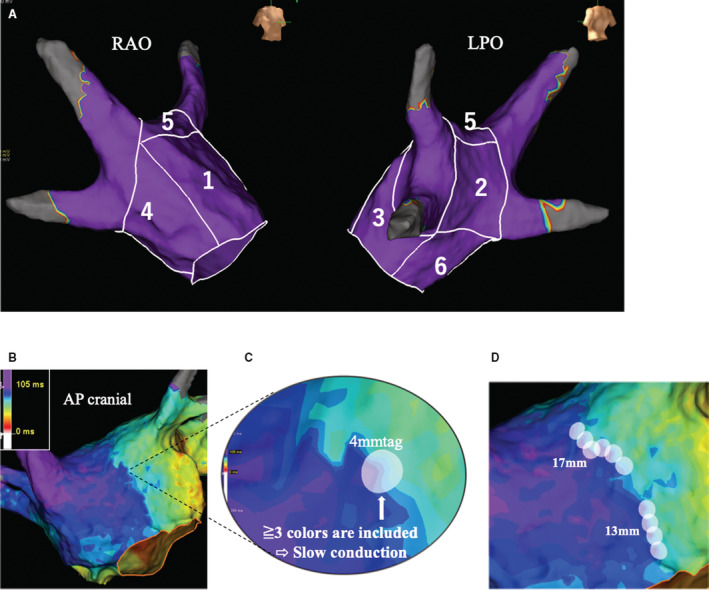
Details of LA segment and slow‐conduction length measurement.
A, Numbers 1 to 6 indicate the anterior wall (1), posterior wall (2), lateral wall (3), septal wall (4), roof (5), and inferior wall (6), respectively. B, Isochronal activation map. First, slow conduction was defined as <30 cm/s (≒4 mm/15 ms). It is set to color contour 3, and 1 color is calculated as 5 ms. C, Slow‐conduction analysis. If a 4‐mm tag is attached and ≥3 colors (=15 ms) are included in the tag, that section is thought to exhibit slow conduction with reference to the above definition. D, Slow‐conduction length. In this case, the slow‐conduction length was 30 mm. AP indicates anteroposterior; LA, left atrium; LPO, left posterior oblique; and RAO, right anterior oblique.
Follow‐Up Strategy
All patients were monitored in the hospital for 48 hours after the procedure, and patients who were prescribed antiarrhythmic drugs continued the drugs for 3 months after discharge; antiarrhythmic drugs were discontinued if no AF recurrence was observed. All patients were prospectively followed up at 1, 3, 6, 9, and 12 months after the procedure, with 12‐lead ECGs performed at each visit. In addition, 24‐hour Holter monitoring was performed 3 and 12 months after the procedure. Thereafter, a follow‐up was performed for patients every 1 to 3 months at our institutions or by a general physician, and 24‐hour Holter monitoring was performed every 6 to 12 months at our institutions. When recurrence was suspected on the basis of symptoms, additional 24‐hour Holter monitoring was performed. A 3‐month blanking period was introduced both after the first and second ablation. After this period, the occurrence of atrial tachyarrhythmias lasting >30 seconds was defined as AF recurrence.
Statistical Analysis
Categorical variables are summarized as numbers and percentages and continuous variables as mean±SD or median and interquartile range. The Shapiro‐Wilk test was performed to confirm that the data were normally distributed. The differences in clinical characteristics among the 3 variables were analyzed using 1‐way ANOVA or the Kruskal‐Wallis test, as appropriate. Clinical characteristics expressed as categorical variables were compared using the χ2 test. Multiple linear regression was performed to clarify the factors related to mean LA voltage, %LVZ, and SC length. A multivariate Cox proportional analysis was performed to identify significant risk factors of AF recurrence after a single, and a single or multiple ablations. All variables with P<0.05 in the univariate analysis were included in a multivariate analysis. The follow‐up period was defined as the period from the date of the procedure (the first session or the second session) to the date of the first AF recurrence or censoring events (death and end of follow‐up). The estimated event‐free survival probabilities were calculated using the Kaplan‐Meier analysis, and log‐rank statistics were used for group comparisons. All analyses were performed using JMP software version 12.2.0 (SAS Institute Inc., Cary, NC). Statistical significance was defined as a 2‐sided P value <0.05.
Results
Patient and Procedural Characteristics
High‐density mapping (mean 2287±600 points/patient) was performed on 49 abstainers, 27 mild drinkers, and 34 moderate‐heavy drinkers. The baseline clinical and procedural characteristics of all 3 groups are shown in Table 1. Baseline characteristics were balanced among the 3 groups except for the proportion of women and hypertension (women: 47% versus 41% versus 21%; P=0.039; hypertension: 49% versus 67% versus 79%; P=0.015). The number of mapping points did not differ significantly among the 3 groups (abstainers, 2300±591 points versus mild drinkers, 2178±550 points versus moderate‐heavy drinkers, 2355±655 points; P=0.514), and there was no significant difference in the rate of cavotricuspid isthmus linear ablation and superior vena cava isolation among the 3 groups (cavotricuspid isthmus linear ablation: 82% versus 74% versus 85%; P=0.571; superior vena cava isolation: 18% versus 15% versus 18%; P=0.922).
Table 1.
Baseline Characteristics
| All | Abstainers | Mild drinkers | Moderate‐heavy drinkers | P value | |
|---|---|---|---|---|---|
| n=110 | n=49 | n=27 | n=34 | ||
| Age, y | 67 (56–73) | 70 (61–75) | 61 (56–72) | 62 (55–72) | 0.195 |
| Female sex, n (%) | 41 (37) | 23 (47) | 11 (41) | 7 (21) | 0.039 |
| BMI, kg/m2 | 24.6±3.5 | 24.4±3.8 | 25.0±3.5 | 24.8±3.2 | 0.770 |
| Paroxysmal AF, n (%) | 67 (61) | 32 (65) | 18 (67) | 17 (50) | 0.294 |
| Duration of AF history, y | 1 (0–2) | 1 (0–2) | 1 (0–1.5) | 1 (0–2) | 0.879 |
| AF duration, mo | 3 (1–18) | 3 (1–19.5) | 2 (1–2) | 5 (2–27) | 0.085 |
| Hypertension, n (%) | 69 (63) | 24 (49) | 18 (67) | 27 (79) | 0.015 |
| Diabetes, n (%) | 16 (15) | 8 (16) | 1 (4) | 7 (21) | 0.105 |
| TIA/stroke, n (%) | 7 (6) | 4 (8) | 2 (7) | 1 (3) | 0.572 |
| Heart failure, n (%) | 17 (15) | 6 (12) | 3 (11) | 8 (24) | 0.310 |
| Dyslipidemia, n (%) | 40 (36) | 19 (39) | 7 (26) | 14 (41) | 0.408 |
| Chronic kidney disease, n (%) | 9 (8) | 4 (8) | 2 (7) | 3 (9) | 0.980 |
| Antiarrhythmic drug, nos. | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1 (1–1.25) | 0.117 |
| CHA2DS2‐VASc score | 2 (1–3) | 2 (1–3.5) | 2 (1–3) | 2 (1–3) | 0.630 |
| Alcohol intake, standard drinks/wk | 1.7 (0–10) | 0 | 3.5 (2.2–5.8) | 15 (10.6–23.7) | <0.001 |
| Brain natriuretic peptide, pg/mL | 50.3 (16.6–76.8) | 41.5 (14.1–81.7) | 48.3 (17.2–72.5) | 61.6 (17.1–85.6) | 0.455 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 66.8±15.2 | 64.5±15.4 | 66.7±12.9 | 69.9±16.5 | 0.293 |
| LA diameter, mm | 38±6 | 38±6 | 38±5 | 39±5 | 0.420 |
| LV ejection fraction, % | 64±10 | 64±11 | 64±8 | 64±11 | 0.936 |
| Mapping | |||||
| LA mapping time, s | 464 (368–558) | 472 (354–563) | 421 (360–510) | 483 (427–574) | 0.217 |
| LA mapping point, nos. | 2287±600 | 2300±591 | 2178±550 | 2355±655 | 0.514 |
| Pulmonary vein isolation modality | |||||
| Radiofrequency ablation, n (%) | 100 (91) | 46 (94) | 22 (81) | 32 (94) | 0.186 |
| Cryoballoon ablation, n (%) | 10 (9) | 3 (6) | 5 (19) | 2 (6) | |
| Additional ablation procedure | |||||
| CTI linear ablation, n (%) | 88 (81) | 40 (82) | 20 (74) | 28 (85) | 0.571 |
| SVC isolation, n (%) | 19 (17) | 9 (18) | 4 (15) | 6 (18) | 0.922 |
P values were determined by 1‐way ANOVA, Kruskal‐Wallis test, or χ2 test, as appropriate. AF indicates atrial fibrillation; BMI, body mass index; CTI, cavotricuspid isthmus; LA, left atrial; LV, left ventricular; SVC, superior vena cava; and TIA, transient ischemic attack.
LA Voltage Analysis
The overall mean LA voltage was 5.75±1.69 mV, with no significant difference among the 3 groups (P=0.502). In addition, the mean LA regional voltage for each LA segment did not differ significantly among the 3 groups in all segments (Figure 2A). LVZ was identified in 39 (35%) patients (Table 2), and low voltage was predominant in the lateral wall (37%), anterior wall (29%), and septal wall (28%). There was no significant difference in the prevalence of low voltage in each segment among the 3 groups (Figure 2B). Univariate and multivariate analyses of mean LA voltage and %LVZ are shown in Table S1 and S2, respectively. Age (P < 0.001), female sex (P < 0.001), and nonparoxysmal AF (P=0.015) were significantly associated with mean LA voltage. Female sex (P=0.013) and LA diameter (P=0.018) were significantly associated with %LVZ. Alcohol consumption was not associated with either mean LA voltage or %LVZ.
Figure 2.
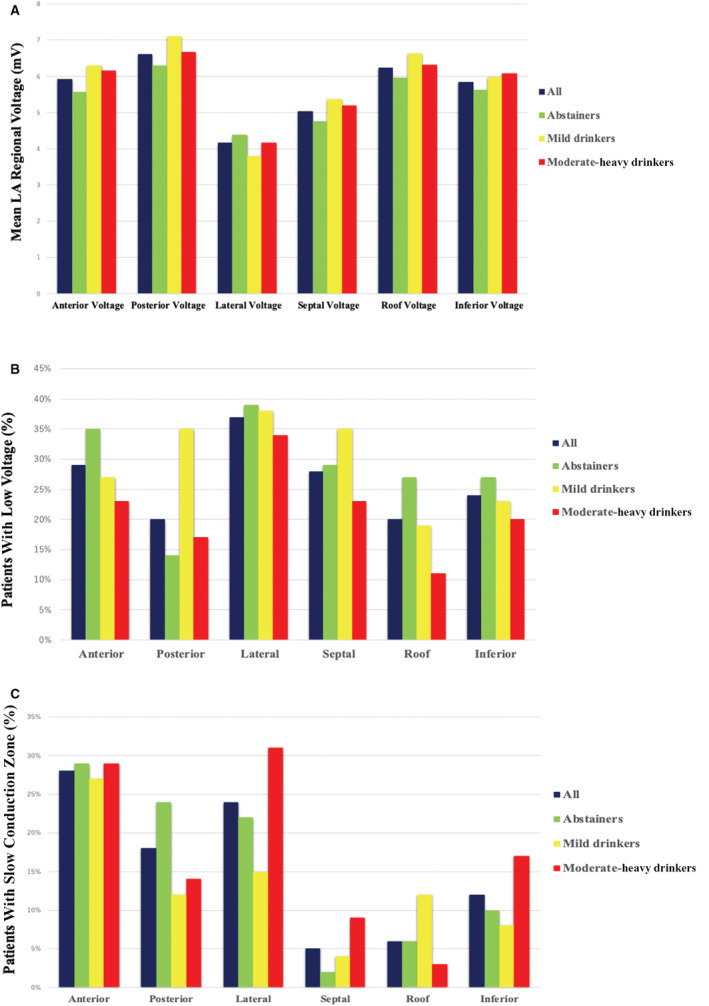
Mean LA regional voltage and distribution of low voltage and SCZ in different LA segments.
A, Mean LA regional voltage in abstainers, mild drinkers, and moderate‐heavy drinkers. No significant difference in mean LA regional voltage in each segment was observed among the 3 groups. The differences in mean LA regional voltage were analyzed using 1‐way ANOVA, or the Kruskal‐Wallis test, as appropriate. B, Distribution of low voltage in different LA segments in abstainers, mild drinkers, and moderate‐heavy drinkers. No significant difference in the prevalence of low voltage in each segment was observed among the 3 groups. C, Distribution of SCZ in different LA segments in abstainers, mild drinkers, and moderate‐heavy drinkers. No significant difference in the prevalence of SCZ in each segment was observed among the 3 groups. The differences in the prevalence of low voltage or SCZ were analyzed using χ2 test. LA indicates left atrium; and SCZ, slow‐conduction zone.
Table 2.
LA Voltage, Conduction Analysis and Non‐PV AF Trigger
| Parameters | All | Abstainers | Mild drinkers | Moderate‐heavy drinkers | P value |
|---|---|---|---|---|---|
| n=110 | n=49 | n=27 | n=34 | ||
| LA surface area, cm2 | 64.9±15.3 | 63.0±14.3 | 66.2±16.6 | 66.6±15.8 | 0.512 |
| Mean LA voltage, mV | 5.75±1.69 | 5.54±1.58 | 5.98±2.11 | 5.86±1.47 | 0.502 |
| Low‐voltage area, cm2 | 1.25 (0–4.1) | 1.3 (0–3.8) | 1.1 (0–4.2) | 3.05 (0–4.675) | 0.948 |
| %LVZ, % | 1.87 (0–6.27) | 2.32 (0–6.13) | 1.5 (0–5.9) | 3.91 (0–6.8) | 0.956 |
| LVZ, n (%) | 39 (35) | 16 (33) | 8 (30) | 15 (44) | 0.435 |
| SC length, mm | 16 (0–36.25) | 12 (0–31.5) | 0 (0–35) | 19.5 (0–43.25) | 0.235 |
| SCZ, n (%) | 54 (49) | 22 (45) | 11 (41) | 21 (62) | 0.192 |
| Non‐PV AF trigger, n (%) | 22 (20) | 12 (24) | 5 (19) | 5 (15) | 0.531 |
P values were determined by 1‐way ANOVA, Kruskal‐Wallis test, or χ2 test, as appropriate. AF indicates atrial fibrillation; LA, left atrial; LVZ, low voltage zone; PV, pulmonary vein; SC, slow conduction; and SCZ, slow‐conduction zone ablation.
LA Conduction Analysis
SCZ was identified in 54 (49%) patients (Table 2). SC was predominantly found in the anterior wall (28%), lateral wall (24%), and posterior wall (18%). No significant difference in the prevalence of SC in each segment was observed among the 3 groups (Figure 2C). Univariate and multivariate analyses of SC length are shown in Table S3. Age was significantly associated with SC length (P=0.045).
AF Recurrence After Catheter Ablation
During the median follow‐up period of 549±245 days (365–749 days), 19 (17.3%) patients experienced AF recurrence after a single ablation. The sinus rhythm maintenance rates at 12 months after a single ablation were 95.9% and 79.3% in abstainers and drinkers, respectively. The sinus rhythm maintenance rate in drinkers was significantly lower than that in abstainers (log‐rank, P=0.013) (Figure 3A). In addition, in multivariate analysis, alcohol consumption (hazard ratio [HR], 3.77; 95% CI, 1.23–11.5; P=0.02) and the presence of LVZ (HR, 2.73; 95% CI, 1.09–6.86; P=0.032) and SCZ (HR, 3.58; 95% CI, 1.22–10.5; P=0.02) were significantly associated with AF recurrence after a single ablation (Table 3). Alcohol consumption was significantly associated with AF recurrence after a single ablation, even after adjustment for female sex and hypertension with significant differences in proportions among the 3 groups (Table S4). Among the 19 patients who had AF recurrence, 9 patients underwent a second ablation; the details of patients with a second ablation are presented in Table 4. During a median follow‐up period of 564±235 days (372–751 days), 14 (12.7%) patients had AF recurrence after a single or multiple ablations. The sinus rhythm maintenance rates at 12 months after a single or multiple ablations were 95.9% and 87.7% in abstainers and drinkers, respectively. No significant difference in the sinus rhythm maintenance rate was observed between abstainers and drinkers (Figure 3B). In multivariate analysis, the presence of LVZ (HR, 3.66; 95% CI, 1.20–11.1; P=0.023) and SCZ (HR, 4.52; 95% CI, 1.22–16.8; P=0.024) was significantly associated with AF recurrence after a single or multiple ablations (Table 5). However, alcohol consumption was not associated with AF recurrence after a single or multiple ablations.
Figure 3.
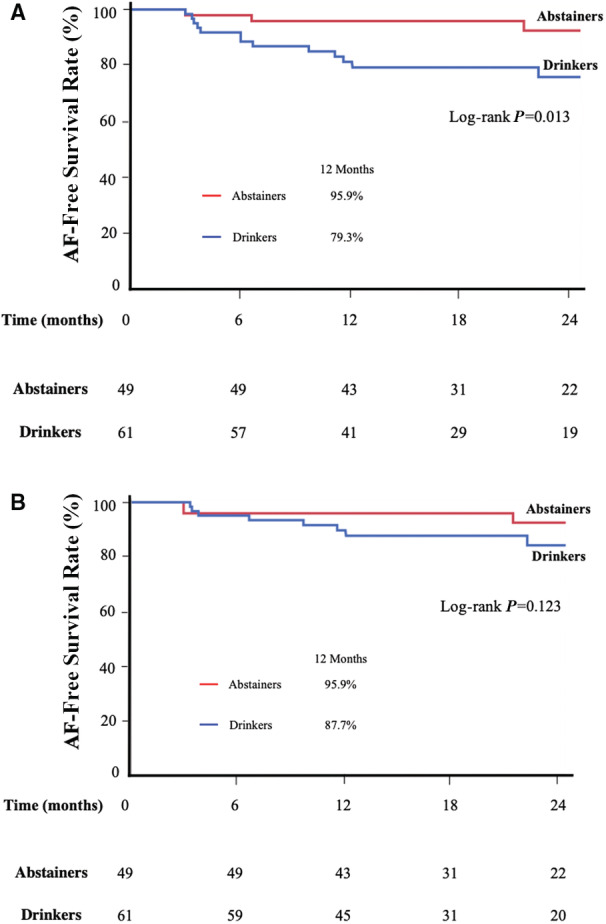
AF recurrence‐free survival after a single and a single or multiple ablations.
A, Kaplan‐Meier curves for AF recurrence‐free survival after a single ablation in abstainers and drinkers. B, Kaplan‐Meier curves for AF recurrence‐free survival after a single or multiple ablations in abstainers and drinkers. Log‐rank statistics were used to make comparisons between the two groups. AF indicates atrial fibrillation.
Table 3.
Univariate and Multivariate Analyses of AF Recurrence After a Single Ablation
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| P value | HR | 95% CI | P value | |
| Age, y | 0.931 | |||
| Female, n (%) | 0.517 | |||
| BMI, kg/m2 | 0.434 | |||
| Alcohol consumption, n (%) | 0.021 | 3.77 | 1.23–11.5 | 0.020 |
| AF type, n (%) | 0.572 | |||
| Duration of AF history, y | 0.151 | |||
| Hypertension, n (%) | 0.173 | |||
| Diabetes, n (%) | 0.660 | |||
| Heart failure, n (%) | 0.129 | |||
| Chronic kidney disease, n (%) | 0.910 | |||
| LV ejection fraction, % | 0.590 | |||
| LA diameter, mm | 0.469 | |||
| Antiarrhythmic drugs, nos. | 0.118 | |||
| Non‐PV AF foci, n (%) | 0.220 | |||
| Low‐voltage zone, n (%) | 0.026 | 2.73 | 1.09–6.86 | 0.032 |
| Slow‐conduction zone, n (%) | 0.024 | 3.58 | 1.22–10.5 | 0.020 |
P values, HRs, and 95% CIs were determined by a multivariate Cox proportional analysis. AF indicates atrial fibrillation; BMI, body mass index; HR, hazard ratio; LA, left atrial; LV, left ventricular; and PV, pulmonary vein.
Table 4.
Details of Patients Undergoing Second AF Ablation
| No | Alcohol group | Age, y | Sex | AF type | LAD, mm | EF, % | Mean LA voltage, mV | %LVZ, % | SC length, mm | Ablation strategy during first session | PVI modality | PV reconnections during second session | Ablation strategy during second session |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Moderate‐heavy | 75 | Male | Non‐PAF | 46 | 56 | 5.46 | 5.1 | 13 | PVI + CTIABL | RF | − | reCTIABL, SVCI |
| 2 | Mild | 50 | Female | PAF | 30 | 73 | 5.83 | 0 | 0 | PVI + CTIABL | RF | + | rePVI (RS, RI), SVCI |
| 3 | Moderate‐heavy | 75 | Male | PAF | 40 | 67 | 5.09 | 4.7 | 37 | PVI | RF | + | rePVI (RS), SVCI, focal ABL |
| 4 | Mild | 58 | Male | PAF | 33 | 69 | 6.00 | 1.5 | 51 | PVI + CTIABL | Cryo | + | rePVI (RS, RI, LI), CTIABL |
| 5 | Mild | 48 | Male | Non PAF | 28 | 49 | 5.04 | 7.9 | 43 | PVI + CTIABL | RF | + | rePVI (RS,RI), LAPWI |
| 6 | Abstainer | 76 | Female | PAF | 44 | 69 | 4.85 | 0 | 96 | PVI + CTIABL | RF | + | rePVI (LS, RS), SVCI |
| 7 | Abstainer | 80 | Female | PAF | 46 | 65 | 2.31 | 20.3 | 36 | PVI + CTIABL | Cryo | − | focal ABL |
| 8 | Moderate‐heavy | 57 | Male | Non PAF | 46 | 50 | 4.34 | 7.1 | 20 | PVI | RF | + | rePVI (LS, LI, RS, RI), CTIABL, SVCI |
| 9 | Abstainer | 60 | Female | Non PAF | 51 | 71 | 1.85 | 16.9 | 17 | PVI | RF | − | LAPWI, MIABL, CTIABL, focal ABL |
CTIABL indicates cavotricuspid isthmus ablation; EF, ejection fraction; LA, left atrial; LAD, left atrial diameter; LAPWI, left atrial posterior wall isolation; LI, left inferior; LS, left superior; LVZ, low‐voltage zone; MIABL, mitral isthmus ablation; PAF, paroxysmal atrial fibrillation; PV, pulmonary vein; PVI, pulmonary vein isolation; RF, radiofrequency; RI, right inferior; RS, right superior; SC, slow conduction; and SVCI, superior vena cava isolation.
Table 5.
Univariate and multivariate analyses of AF recurrence after a single or multiple ablations
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| P value | HR | 95% CI | P value | |
| Age, y | 0.719 | |||
| Female, n (%) | 0.223 | |||
| BMI, kg/m2 | 0.291 | |||
| Alcohol consumption, n (%) | 0.135 | |||
| AF type, n (%) | 0.516 | |||
| Duration of AF history, y | 0.055 | |||
| Hypertension, n (%) | 0.103 | |||
| Diabetes, n (%) | 0.697 | |||
| Heart failure, n (%) | 0.124 | |||
| Chronic kidney disease, n (%) | 0.616 | |||
| LV ejection fraction, % | 0.429 | |||
| LA diameter, mm | 0.132 | |||
| Antiarrhythmic drugs, nos | 0.192 | |||
| Non‐PV AF foci, n (%) | 0.039 | 2.79 | 0.91–8.54 | 0.073 |
| Low‐voltage zone, n (%) | 0.018 | 3.66 | 1.20–11.1 | 0.023 |
| Slow‐conduction zone, n (%) | 0.025 | 4.52 | 1.22–16.8 | 0.024 |
P values, HRs, and 95% CIs were determined by a multivariate Cox proportional analysis. AF indicates atrial fibrillation; BMI, body mass index; HR, hazard ratio; LA, left atrial; LV, left ventricular; and PV, pulmonary vein.
Discussion
Main Findings
The main findings of this study are as follows: (1) There was no significant difference in the mean LA voltage and the prevalence of an LVZ among abstainers, mild drinkers, and moderate‐heavy drinkers in the whole left atrium and each LA segment; in multivariate analyses, age, female sex, and nonparoxysmal AF were significantly associated with mean LA voltage, while female sex and LA diameter were significantly associated with %LVZ; (2) no significant difference in the prevalence of an SCZ in the whole left atrium and each LA segment was observed among the 3 groups. In multivariate analyses, only age was significantly associated with SC length; and (3) the sinus rhythm maintenance rate after a single ablation was significantly lower in drinkers than in abstainers, while no significant difference in the sinus rhythm maintenance rate after a single or multiple ablations was observed between abstainers and drinkers. In multivariate analyses, alcohol consumption was significantly associated with AF recurrence after a single ablation; however, alcohol consumption was not associated with AF recurrence after a single or multiple ablations, and the presence of an LVZ and SCZ was significantly associated with AF recurrence both after a single ablation and a single or multiple ablations.
Influence of Alcohol on Atrial Substrate
Previous studies have reported that alcohol is associated with LVZ and SC, 13 , 14 but this study was unable to identify a correlation. Differences in the shape of the mapping catheter and the total number of mapping points may have contributed to the discrepancy in results between this study and others. The value of the atrial voltage easily changes depending on how the catheter contacts the atrial wall, the electrode size of the catheter, and the distance between the electrodes. Most mapping catheters used in past studies were ablation catheters or circular mapping catheters; however, in those studies, there were limitations, as the mapping catheter did not come into close contact with the atrial wall because of the circular shape. It was also difficult to determine how much force was pushing against the wall surface. In contrast, the high‐density grid multipolar catheter used in this study was able to make flat contact against the atrial wall because of its high flexibility, with the contact with the wall surface easily visualized using fluoroscopy. Because the potential in the long‐ and short‐axis directions of the catheter can be recorded in HDG, pseudo low voltage caused by the direction of propagation can be excluded, and a high‐quality map can be created, particularly in the LVZ. In this study, the prevalence of an LVZ was 35%, consistent with that in previous reports, but the median low‐voltage area was 1.25 cm2, which was smaller than that in previous reports. 13 This is probably because the number of mapping points in this study was larger than that in previous reports, and the pseudo low voltage was reduced because of the characteristics of HDG. A recent study comparing the effects of alcohol in the acute phase on conduction velocity with placebo showed that alcohol did not affect atrial conduction time. 24 However, there are limited data regarding the effects of chronic alcohol consumption on conduction velocity. 14 There are also no comparative studies with a placebo; therefore, the association between chronic alcohol consumption and conduction velocity remains unclear. Regarding the association between alcohol, atrial remodeling, and new onset of AF, some large‐scale studies have reported that alcohol does not affect LA diameter, and mild‐moderate drinking is not correlated with AF development. 13 , 15 , 19 , 25 , 26 Moreover, a recent study shows that mild drinkers have a lower risk of developing AF than abstainers. 27 Consequently, low levels of alcohol consumption do not significantly affect the atrial substrate and, as a result, may not correlate with the onset of AF. The relationship between alcohol and atrial substrate should continue to be discussed, and further investigation is required, especially on the cutoff value of alcohol consumption that can affect atrial substrate.
Alcohol Consumption and Clinical Outcomes After Ablation
In this study, alcohol consumption was associated with AF recurrence after a single ablation, even after adjustment for covariates with significant differences among the 3 groups, and drinkers had a significantly higher AF recurrence rate than abstainers; however, alcohol consumption was not associated with AF recurrence after a single or multiple ablations. Alcohol is associated with increased levels of serum catecholamines, impaired vagal tone, and transient triggers. 28 , 29 , 30 , 31 , 32 Furthermore, alcohol has been reported to shorten the effective refractory period, particularly the effective refractory period of the PV. 24 Alcohol consumption generates a short‐coupled PV trigger in patients with AF, which may lead to an increase in AF initiation. If PVI is completed and can be maintained for a long time, the PV triggers are blocked, and as a result, the incidence of alcohol‐related AF is expected to decrease. However, there are some cases where durable PVI is not completed with only a single ablation, and it was reported that 62% of patients with AF undergoing contact force–guided PVI 33 and 22% of patients undergoing ablation index–guided PVI 34 had PV reconnections during the follow‐up period. Alcohol may be associated with AF recurrence after a single ablation because it is difficult to maintain the permanent block of PV‐LA conduction in all PVs after a single ablation. In fact, in this study, among the 19 patients who exhibited AF recurrence, 9 underwent a second ablation, and among them, PV reconnection was observed in 33% (1/3 patients) of abstainers versus 83% (5/6 patients) of drinkers, which is consistent with the above estimation. The probability of durable PVI being maintained after a single or multiple ablations is higher than that after a single ablation, so it is presumed that alcohol consumption was not associated with AF recurrence after a single or multiple ablations, while factors related to the “progression of atrial substrate,” such as LVZ or SCZ, were significantly associated with AF recurrence. Takigawa et al 15 reported that alcohol consumption was strongly correlated with AF recurrence after a single ablation, but not with AF recurrence after a single or multiple ablations. If alcohol consumption affects atrial substrate, it is expected to correlate with AF recurrence even after a single or multiple ablations; however, herein, alcohol consumption was not involved in the development of LVZ and SCZ, suggesting that alcohol consumption was not significantly associated with AF recurrence after a single or multiple ablations. Further studies are needed to confirm whether alcohol consumption increases the incidence of AF derived from PV triggers. Regarding LVZ and SCZ obtained from high‐density mapping, they were significantly associated with AF recurrence both after a single ablation and after a single or multiple ablations. Both of these mapping findings are considered noteworthy for the evaluation of the atrial substrate. Kurata et al 8 reported that in addition to LVZ, decreased conduction velocity in LA is also an important predictor of AF recurrence after ablation. LVZ and SCZ in the atrium were significantly associated with late gadolinium‐enhanced magnetic resonance imaging, 10 , 11 and they are a likely consequence of atrial fibrosis, which can also cause promoting micro‐reentry 35 and macro‐reentry. 36 Moreover, atrial fibrosis also causes triggered activity, enhanced automaticity, and shortening of the atrial effective refractory period. Because the strategy of the first ablation in this study is basically PVI, and the modification on atrial substrate such as LVZ and SCZ is not performed, the presence of fibrosis with many electrophysiological characteristics may be involved in AF recurrence in patients following ablation. In patients with LVZ or SCZ, PVI alone cannot provide sufficient sinus rhythm maintenance, and additional ablations are often needed, one of which is to homogenize the regions in cases with LVZ or scar areas. LVZ homogenization has been reported to contribute to sinus rhythm maintenance. 37 While there are no reports regarding the influence of additional ablation, such as linear ablation or homogenization of SCZ on clinical outcomes, it has also been reported that the effect of LVZ homogenization on outcomes is limited. 38 , 39 Therefore, further studies are needed to investigate whether atrial substrate modification is beneficial for patients with AF recurrence despite the completion and maintenance of PVI.
Limitations
First, there remains a concern that the amount of alcohol is underreported; however, alcohol consumption was recorded in a detailed interview and calculated with reference to a previous report. Second, alcohol consumption was not monitored throughout the follow‐up period. If the amount of alcohol changes during follow‐up, it may affect the episodes of AF and make a difference in ablation outcome. Third, the overall study population may have been too small to detect significant differences in clinical outcomes. Fourth, only mapping during distal coronary sinus pacing was performed. Wavefront propagation in different directions can affect the conduction velocity and voltage. Fifth, although the right atrium also plays an important role in AF development and maintenance in some patients, right atrial mapping was not performed. Sixth, all patients underwent 24‐hour Holter monitoring in regular intervals and when they exhibited AF symptoms; 24‐hour Holter monitoring was less capable of detecting AF recurrence than the implanted loop recorder. This may have led to an underestimation of the recurrence rates and is likely to be one of the factors behind the low rates of AF recurrence, which itself could blunt differences in clinical outcomes.
Conclusions
In this prospective observational study, alcohol consumption was not associated with changes in atrial substrate as confirmed by high‐density mapping. Alcohol consumption was associated with AF recurrence after a single ablation, but AF recurrence after a single or multiple ablations did not have an association with alcohol consumption. The presence of an LVZ and SCZ was associated with AF recurrence both after a single and a single or multiple ablations.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S4
For Sources of Funding and Disclosures, see page 11.
References
- 1. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003 [DOI] [PubMed] [Google Scholar]
- 2. Takahashi A, Iesaka Y, Takahashi Y, Takahashi R, Kobayashi K, Takagi K, Kuboyama O, Nishimori T, Takei H, Amemiya H, et al. Electrical connections between pulmonary veins: implication for ostial ablation of pulmonary veins in patients with paroxysmal atrial fibrillation. Circulation. 2002;105:2998–3003. doi: 10.1161/01.cir.0000019585.91146.ab [DOI] [PubMed] [Google Scholar]
- 3. Di Biase L, Elayi CS, Fahmy TS, Martin DO, Ching CK, Barrett C, Bai R, Patel D, Khaykin Y, Hongo R, et al. Atrial fibrillation ablation strategies for paroxysmal patients: randomized comparison between different techniques. Circ Arrhythm Electrophysiol. 2009;2:113–119. doi: 10.1161/CIRCEP.108.798447 [DOI] [PubMed] [Google Scholar]
- 4. Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, Vidal B, Arriagada G, Méndez F, Matiello M, et al. Pre‐procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–841. doi: 10.1093/eurheartj/ehm027 [DOI] [PubMed] [Google Scholar]
- 5. Takigawa M, Takahashi A, Kuwahara T, Okubo K, Takahashi Y, Watari Y, Takagi K, Fujino T, Kimura S, Hikita H, et al. Long‐term follow‐up after catheter ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:267–273. doi: 10.1161/CIRCEP.113.000471 [DOI] [PubMed] [Google Scholar]
- 6. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288 [DOI] [PubMed] [Google Scholar]
- 7. Yamaguchi T, Tsuchiya T, Nagamoto Y, Miyamoto K, Murotani K, Okishige K, Takahashi N. Long‐term results of pulmonary vein antrum isolation in patients with atrial fibrillation: An analysis in regards to substrates and pulmonary vein reconnections. Europace. 2014;16:511–520. doi: 10.1093/europace/eut265 [DOI] [PubMed] [Google Scholar]
- 8. Kurata N, Masuda M, Kanda T, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, Tsujimura T, Matsuda Y, et al. Slow whole left atrial conduction velocity after pulmonary vein isolation predicts atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2020;31:1942–1949. doi: 10.1111/jce.14582 [DOI] [PubMed] [Google Scholar]
- 9. Chelu MG, King JB, Kholmovski EG, Ma J, Gal P, Marashly Q, MA AJ, Kaur G, Silver MA, Johnson KA, et al. Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5‐year follow‐up data. J Am Heart Assoc. 2018;7:e006313. doi: 10.1161/JAHA.117.006313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caixal G, Alarcón F, Althoff TF, Nuñez‐Garcia M, Benito EM, Borràs R, Perea RJ, Prat‐González S, Garre P, Soto‐Iglesias D, et al. Accuracy of left atrial fibrosis detection with cardiac magnetic resonance: Correlation of late gadolinium enhancement with endocardial voltage and conduction velocity. Europace. 2021;8(23):380–388. doi: 10.1093/europace/euaa313 [DOI] [PubMed] [Google Scholar]
- 11. Fukumoto K, Habibi M, Ipek EG, Zahid S, Khurram IM, Zimmerman SL, Zipunnikov V, Spragg D, Ashikaga H, Trayanova N, et al. Association of left atrial local conduction velocity with late gadolinium enhancement on cardiac magnetic resonance in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9:e002897. doi: 10.1161/CIRCEP.115.002897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcus GM, Vittinghoff E, Whitman IR, Joyce D, Yang V, Nah G, Gerstenfeld EP, Moss JD, Lee RJ, Lee BK, et al. Acute consumption of alcohol and discrete atrial fibrillation events. Ann Intern Med. 2021;174:1503–1509. doi: 10.7326/M21-0228 [DOI] [PubMed] [Google Scholar]
- 13. Qiao Y, Shi R, Hou B, Wu L, Zheng L, Ding L, Chen G, Zhang S, Yao Y. Impact of alcohol consumption on substrate remodeling and ablation outcome of paroxysmal atrial fibrillation. J Am Heart Assoc. 2015;4:e002349. doi: 10.1161/JAHA.115.002349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voskoboinik A, Wong G, Lee G, Nalliah C, Hawson J, Prabhu S, Sugumar H, Ling LH, McLellan A, Morton J, et al. Moderate alcohol consumption is associated with atrial electrical and structural changes: insights from high‐density left atrial electroanatomic mapping. Heart Rhythm. 2019;16:251–259. doi: 10.1016/j.hrthm.2018.10.041 [DOI] [PubMed] [Google Scholar]
- 15. Takigawa M, Takahashi A, Kuwahara T, Takahashi Y, Okubo K, Nakashima E, Watari Y, Nakajima J, Yamao K, Osaka Y, et al. Impact of alcohol consumption on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation. J Am Heart Assoc. 2016;5:e004149. doi: 10.1161/JAHA.116.004149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huemer M, Qaiyumi D, Attanasio P, Parwani A, Pieske B, Blaschke F, Boldt LH, Haverkamp W, Wutzler A. Does the extent of left atrial arrhythmogenic substrate depend on the electroanatomical mapping technique: Impact of pulmonary vein mapping catheter vs. ablation catheter. Europace. 2017;19:1293–1301. doi: 10.1093/europace/euw185 [DOI] [PubMed] [Google Scholar]
- 17. Anter E, Tschabrunn CM, Josephson ME. High‐resolution mapping of scar‐related atrial arrhythmias using smaller electrodes with closer interelectrode spacing. Circ Arrhythmia Electrophysiol. 2015;8:537–545. doi: 10.1161/CIRCEP.114.002737 [DOI] [PubMed] [Google Scholar]
- 18. Takahashi Y, Nitta J, Kobori A, Sakamoto Y, Nagata Y, Tanimoto K, Matsuo S, Yamane T, Morita N, Satomi K, et al. Alcohol consumption reduction and clinical outcomes of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2021;14:e009770. doi: 10.1161/CIRCEP.121.009770 [DOI] [PubMed] [Google Scholar]
- 19. Di Castelnuovo A, Costanzo S, Bonaccio M, Rago L, De Curtis A, Persichillo M, Bracone F, Olivieri M, Cerletti C, Donati MB, et al. Moderate alcohol consumption is associated with lower risk for heart failure but not atrial fibrillation. JACC Heart Fail. 2017;5:837–844. doi: 10.1016/j.jchf.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 20. Mahnkopf C, Badger TJ, Burgon NS, Daccarett M, Haslam TS, Badger CT, McGann CJ, Akoum N, Kholmovski E, Macleod RS, et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed‐enhanced MRI: Implications for disease progression and response to catheter ablation. Heart Rhythm. 2010;7:1475–1481. doi: 10.1016/j.hrthm.2010.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamaguchi T, Tsuchiya T, Nakahara S, Fukui A, Nagamoto Y, Murotani K, Eshima K, Takahashi N. Efficacy of left atrial voltage‐based catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:1055–1063. doi: 10.1111/jce.13019 [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi T, Fukui A, Node K. Bipolar voltage mapping for the evaluation of atrial substrate: can we overcome the challenge of directionality? J Atr Fibrillation. 2019;11:2116. doi: 10.4022/jafib.2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neuberger HR, Schotten U, Verheule S, Eijsbouts S, Blaauw Y, van Hunnik A, Allessie M. Development of a substrate of atrial fibrillation during chronic atrioventricular block in the goat. Circulation. 2005;111:30–37. doi: 10.1161/01.CIR.0000151517.43137.97 [DOI] [PubMed] [Google Scholar]
- 24. Marcus GM, Dukes JW, Vittinghoff E, Nah G, Badhwar N, Moss JD, Lee RJ, Lee BK, Tseng ZH, Walters TE, et al. A randomized, double‐blind, placebo‐controlled trial of intravenous alcohol to assess changes in atrial electrophysiology. JACC Clin Electrophysiol. 2021;7:662–670. doi: 10.1016/j.jacep.2020.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gémes K, Malmo V, Laugsand LE, Loennechen JP, Ellekjaer H, László KD, Ahnve S, Vatten LJ, Mukamal KJ, Janszky I. Does moderate drinking increase the risk of atrial fibrillation? The Norwegian HUNT (Nord‐Trøndelag health) study. J Am Heart Assoc. 2017;6:e007094. doi: 10.1161/JAHA.117.007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barmano N, Charitakis E, Kronstrand R, Walfridsson U, Karlsson JE, Walfridsson H, Nystrom FH. The association between alcohol consumption, cardiac biomarkers, left atrial size and re‐ablation in patients with atrial fibrillation referred for catheter ablation. PLoS One. 2019;14:e0215121. doi: 10.1371/journal.pone.0215121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tu SJ, Gallagher C, Elliott AD, Linz D, Pitman BM, Hendriks JML, Lau DH, Sanders P, Wong CX. Risk thresholds for total and beverage‐specific alcohol consumption and incident atrial fibrillation. JACC Clin Electrophysiol 2021;7:1561–1569. doi: 10.1016/j.jacep.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 28. Denison H, Jern S, Jagenburg R, Wendestam C, Wallerstedt S. Influence of increased adrenergic activity and magnesium depletion on cardiac rhythm in alcohol withdrawal. Br Heart J. 1994;72:554–560. doi: 10.1136/hrt.72.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mäki T, Toivonen L, Koskinen P, Näveri H, Härkönen M, Leinonen H. Effect of ethanol drinking, hangover, and exercise on adrenergic activity and heart rate variability in patients with a history of alcohol‐induced atrial fibrillation. Am J Cardiol. 1998;82:317–322. doi: 10.1016/s0002-9149(98)00299-9 [DOI] [PubMed] [Google Scholar]
- 30. Koskinen P, Virolainen J, Kupari M. Acute alcohol intake decreases short‐term heart rate variability in healthy subjects. Clin Sci (Lond). 1994;87:225–230. doi: 10.1042/cs0870225 [DOI] [PubMed] [Google Scholar]
- 31. Rossinen J, Viitasalo M, Partanen J, Koskinen P, Kupari M, Nieminen MS. Effects of acute alcohol ingestion on heart rate variability in patients with documented coronary artery disease and stable angina pectoris. Am J Cardiol. 1997;79:487–491. doi: 10.1016/s0002-9149(96)00790-4 [DOI] [PubMed] [Google Scholar]
- 32. Marcus GM, Smith LM, Whiteman D, Tseng ZH, Badhwar N, Lee BK, Lee RJ, Scheinman MM, Olgin JE. Alcohol intake is significantly associated with atrial flutter in patients under 60years of age and a shorter right atrial effective refractory period. Pacing Clin Electrophysiol. 2008;31:266–272. doi: 10.1111/j.1540-8159.2008.00985.x [DOI] [PubMed] [Google Scholar]
- 33. Das M, Wynn GJ, Morgan M, Ronayne C, Waktare JE, Todd DM, Hall MC, Snowdon RL, Modi S, Gupta D. Reablated sites of acute reconnection after pulmonary vein isolation do not predict sites of late reconnection at repeat electrophysiology study. J Cardiovasc Electrophysiol. 2016;27:381–389. doi: 10.1111/jce.12933 [DOI] [PubMed] [Google Scholar]
- 34. Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni A, Shaw M, Todd D, Hall M, Modi S, et al. Use of ablation index‐guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: The PRAISE study results. Circ Arrhythm Electrophysiol. 2018;11:e006576. doi: 10.1161/CIRCEP.118.006576 [DOI] [PubMed] [Google Scholar]
- 35. Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87 [DOI] [PubMed] [Google Scholar]
- 36. Derakhchan K, Li D, Courtemanche M, Smith B, Brouillette J, Pagé PL, Nattel S. Method for simultaneous epicardial and endocardial mapping of in vivo canine heart: application to atrial conduction properties and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:548–555. doi: 10.1046/j.1540-8167.2001.00548.x [DOI] [PubMed] [Google Scholar]
- 37. Al‐Kaisey AM, Parameswaran R, Joseph SA, Kistler PM, Morton JB, Kalman JM. Extensive right atrial free wall low‐voltage zone as the substrate for atrial fibrillation: Successful ablation by scar homogenization. Europace. 2021;23:59–64. doi: 10.1093/europace/euaa233 [DOI] [PubMed] [Google Scholar]
- 38. Mohanty S, Mohanty P, Di Biase L, Trivedi C, Morris EH, Gianni C, Santangeli P, Bai R, Sanchez JE, Hranitzky P, et al. Long‐term follow‐up of patients with paroxysmal atrial fibrillation and severe left atrial scarring: Comparison between pulmonary vein antrum isolation only or pulmonary vein isolation combined with either scar homogenization or trigger ablation. Europace. 2017;19:1790–1797. doi: 10.1093/europace/euw338 [DOI] [PubMed] [Google Scholar]
- 39. Yamaguchi T, Tsuchiya T, Fukui A, Kawano Y, Otsubo T, Takahashi Y, Hirota K, Murotani K, Eshima K, Takahashi N. Impact of the extent of low‐voltage zone on outcomes after voltage‐based catheter ablation for persistent atrial fibrillation. J Cardiol. 2018;72:427–433. doi: 10.1016/j.jjcc.2018.04.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


