Abstract
Background
In the population with cardiac sarcoidosis (CS), approximately one third lacks extracardiac involvement and is considered to have isolated CS. Recently, the Japanese Circulation Society updated the diagnostic criteria for CS, providing a methodology for diagnosing isolated CS. We aimed to assess the characteristics of isolated CS diagnosed using a multimodal imaging approach according to the updated Japanese Circulation Society guidelines.
Methods and Results
We retrospectively identified 161 consecutive patients who underwent 18F‐fluorodeoxyglucose positron emission tomography for suspected CS between 2012 and 2019. According to the guidelines, patients were classified as having CS with extracardiac involvement, isolated CS, or no CS. We compared the characteristics of multimodality imaging and the prevalence of major adverse cardiovascular events. The Japanese Circulation Society criteria classified 28 patients (17%) as having CS with 4 (2%) with histological confirmation, 21 (13%) as isolated CS, and 112 (70%) as no CS. Compared with CS, isolated CS showed higher left ventricular volume and reduced left ventricular ejection fraction (P<0.01 for all). During the median follow‐up period of 522 days, 24 patients had major adverse cardiovascular events. Isolated CS (hazard ratio, 3.35; [95% CI, 1.08–10.39], P=0.036) was independently associated with major adverse cardiovascular events after adjusting for reduced left ventricular ejection fraction and steroid. In the subgroup of 41 patients with serial 18F‐fluorodeoxyglucose positron emission tomography evaluation, only updated CS criteria were associated with improvement in myocardial inflammation on 18F‐fluorodeoxyglucose positron emission tomography.
Conclusions
Isolated CS detected using the updated Japanese Circulation Society guidelines was associated with poor event‐free survival and should be managed with caution.
Keywords: echocardiography, FDG‐PET, isolated cardiac sarcoidosis, multimodality imaging
Subject Categories: Imaging, Cardiomyopathy
Nonstandard Abbreviations and Acronyms
- CS
cardiac sarcoidosis
- FDG
18F‐fluorodeoxyglucose
- HRS
Heart Rhythm Society
- IVS
interventricular septum
- JCS
Japanese Circulation Society
- JMHW
Japanese Ministry of Health and Welfare
- LGE
late gadolinium enhancement
- SUVmax
maximum standardized uptake value
Clinical Perspective.
What Is New?
The current study shows that Japanese Circulation Society diagnostic criteria diagnosed 43% of patients with isolated cardiac sarcoidoisis (CS). Patients with isolated CS detected using updated Japanese Circulation Society guidelines were characterized as having more advanced left ventricular dysfunction and focal on diffuse accumulation with a lower maximum standardized uptake value value in 18F‐fluorodeoxyglucose positron emission tomography than in patients with CS and extracardiac involvement.
Patients with isolated CS experienced a higher incidence of cardiovascular events than patients with CS or those without CS.
Steroid therapy led to the improvement of 18F‐fluorodeoxyglucose positron emission tomography findings in most patients with isolated CS, suggesting the reliability of the Japanese Circulation Society criteria.
What Are the Clinical Implications?
Our results document the reliability of the updated Japanese Circulation Society diagnostic criteria using a multimodal imaging approach with considerable applicability.
Our study shows that isolated CS might be a more advanced stage of the disease course and needs to be managed with caution.
Detection of cardiac sarcoidosis (CS) is challenging because of the limited sensitivity of endomyocardial biopsy and the lack of applicable guidelines, especially in patients without extracardiac involvement or isolated CS. A prior study reported that approximately one third of CS cases are considered isolated CS. 1 , 2 , 3 , 4 Nevertheless, existing diagnostic criteria from both the Japanese Ministry of Health and Welfare (JMHW) and Heart Rhythm Society (HRS) require the presence of extracardiac sarcoidosis for clinical diagnosis (Tables S1 and S2). 5 , 6 Therefore, isolated CS is not diagnosable in the absence of a positive histological finding, and there is a lack of data. Recently, the Japanese Circulation Society (JCS) updated the diagnostic criteria for CS and provided a methodology to diagnose isolated CS. 7
In this study, we aimed to assess the characteristics of isolated CS diagnosed using a multimodality imaging approach according to the updated JCS guidelines. We also aimed to evaluate the reliability of the updated CS guidelines for diagnosing CS compared with the prior guidelines.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Sample
The study protocol was approved by the Ethical Review Committee of the University of Tsukuba Hospital (H30‐325). Data were de‐identified, and the requirement for informed consent was waived.
We identified consecutive patients who underwent 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) evaluation for suspected CS from 2012 through December 2019 at the University of Tsukuba Hospital. All patients underwent clinical evaluation, including a history of arrhythmic events, a 12‐lead ECG, 2‐dimensional and Doppler echocardiography, baseline blood test, FDG‐PET imaging, and gadolinium‐enhanced cardiac magnetic resonance imaging (CMR). Patients were classified as with and without evidence of CS using the diagnostic criteria updated by the JCS in 2016. 7 Because of the complexity of the original guidelines, which requires evaluation of 8 criteria including minor findings, we simplified the criteria and used only 5 major criteria to determine the presence of cardiac involvement in sarcoidosis, as shown in Figure 1. According to this simplified diagnostic flow, patients were classified as having CS with extracardiac involvement, isolated CS, or no CS. In patients with extracardiac sarcoidosis, when an endomyocardial biopsy revealed noncaseating epithelioid granulomas, the patient was histologically diagnosed with CS. If CS was not confirmed using endomyocardial biopsy, the presence of major criteria for cardiac involvement was investigated. Patients who met ≥2/5 major criteria were clinically diagnosed with CS. In patients with no evidence of extracardiac involvement, patients were diagnosed with isolated CS if they had FDG‐PET uptake and ≥3 positive major criteria other than FDG‐PET findings.
Figure 1. Simplified diagnostic flow of cardiac sarcoidosis using 2016 Japanese Circulation Society guidelines.
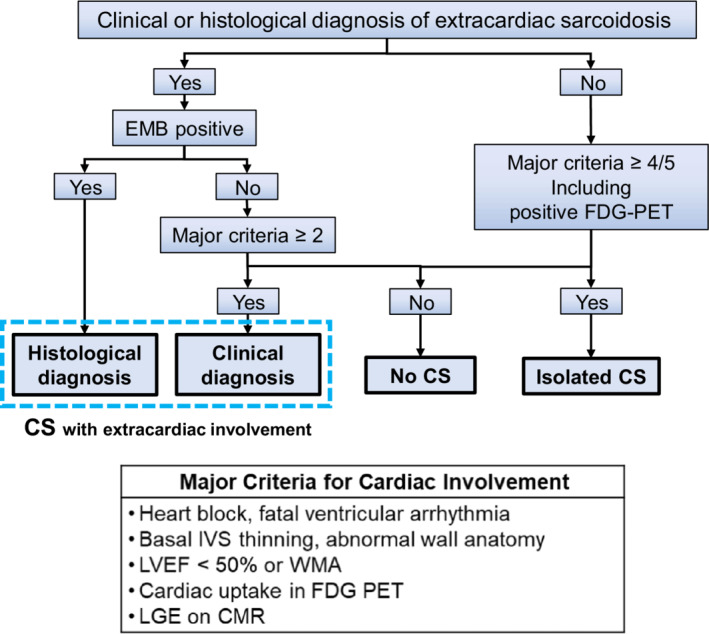
Patients were classified as having CS with extracardiac involvement, isolated CS, or no CS. In patients with evidence of a clinical or histological diagnosis of extracardiac sarcoidosis, when an endomyocardial biopsy revealed noncaseating epithelioid granulomas, patients were histologically diagnosed with CS. If CS was not confirmed on endomyocardial biopsy, the presence of major criteria for cardiac involvement was investigated. Patients meeting ≥2/5 major criteria were clinically diagnosed with CS. In patients with no evidence of extracardiac involvement, patients were diagnosed with isolated CS if they had FDG‐PET uptake and ≥3 positive major criteria other than FDG‐PET findings. CMR indicates cardiac magnetic resonance imaging; CS, cardiac sarcoidosis; EMB, endomyocardial biopsy; FDG‐PET, 18F‐fluorodeoxyglucose positron emission tomography; IVS, interventricular septum; LVEF, left ventricular ejection fraction; LGE, late gadolinium enhancement; and WMA, wall motion abnormality.
We obtained demographic and clinical data at the time of FDG‐PET evaluation via manual extraction from electronic medical records. The survival status and presence of other clinical outcomes, including ventricular arrhythmia, heart failure hospitalization, or other cardiovascular events, were also collected. Patients were followed via chart review with either the date of the last follow‐up or the date of death recorded. Mortality data were obtained from medical records and were last queried on August 30, 2020. We considered major adverse cardiovascular events (MACEs) as the primary outcomes, which included ventricular arrhythmia, heart failure hospitalization, and advanced heart block.
Echocardiographic Measurement
All patients underwent comprehensive echocardiographic assessments using commercial ultrasound systems. All echocardiographic data were reviewed and measured by experienced readers blinded to clinical information according to the current guidelines. 8 , 9 The parameters measured included left ventricular (LV) dimension, wall thickness, ejection fraction, LV end‐diastolic volume), LV end‐systolic volume, left atrial volume index, mitral LV inflow peak early and late diastolic velocities, peak early diastolic velocity of the septal and lateral mitral annulus, and peak tricuspid regurgitation velocity. LVEF, LV end‐diastolic volume, and LV end‐systolic volume were measured using the biplane method of disks. Basal interventricular septum (IVS) thinning was defined as previously reported. Basal IVS thinning was defined as basal IVS thickness ≤4 mm or basal IVS/IVS ratio ≤0.6. 10
FDG‐PET Imaging
All patients underwent dietary preparation for 24 hours before PET scanning with a high‐fat diet, which requires avoidance of carbohydrates, sugars, dairy, and starchy foods. Furthermore, patients were required to fast for 12 hours to suppress physiological myocardial FDG uptake. FDG‐PET imaging was performed according to guidelines. 7 LV myocardial FDG uptake was categorized visually into 4 patterns: none, diffuse, focal, and focal on diffuse. Focal or focal on diffuse was recognized as a positive scan and none or diffuse uptake as a negative scan. 11 , 12 We also evaluated the maximum standardized uptake value (SUVmax) as a semiquantitative measure of myocardial FDG uptake using commercially available software (Siemens SyngoVia software, Siemens Medical Solutions USA, Inc., Malvern, PA).
CMR Imaging
CMR studies were performed on a 1.5 or 3 T magnetic resonance imaging scanner, and all imaging was performed using commercially available software, electrographic triggering, and dedicated phased‐array receiver coils. CMR data will be evaluated to obtain LV geometry data such as LV end‐diastolic volume, LV end‐systolic volume, LV stroke volume, LV mass, and LVEF. Late gadolinium enhancement (LGE) images were obtained in long‐ and short‐axis orientations after the intravenous injection of gadolinium‐diethylenetriamine penta‐acetic acid (0.1–0.2 mmol/kg body weight) using a phase‐sensitive inversion recovery technique, and inversion time was selected for optimal nulling of the myocardium.
Statistical Analysis
Continuous data are expressed as mean ± SD when normally distributed, or median (interquartile range); normality was assessed using the Kolmogorov–Smirnov test. Categorical data were presented as absolute numbers and percentages. We used the Mann–Whitney test, Kruskal–Wallis test, and chi‐square test to compare the data between 2 or more groups, as appropriate. Significance values were adjusted using Bonferroni correction for multiple comparisons. We performed Cox proportional hazards analysis to assess the association between diagnosis and MACEs. The assumptions of proportional hazards were assessed using Schoenfeld residuals. In a multivariable model, relevant variables and possible confounding factors were selected because of their known prognostic value (LVEF and steroid therapy) were entered into the model. Kaplan–Meier curves were created to assess major adverse cardiovascular events‐free survival between groups and were compared using the log‐rank test. To assess changes in SUVmax after steroid therapy over time and the possible impact of diagnosis, we applied longitudinal data analysis using a mixed‐effect model with unstructured covariance for random and fixed effects and with individual patients treated as random effects. Statistical significance was set at P<0.05. All statistical analyses were performed using SPSS software (version 26.0; SPSS Inc., Chicago, IL).
Results
Study Population
A total of 161 patients (78 women [48%]; median age 62 [interquartile range 50, 69] years; median LVEF 49 [33–62] %) with clinically suspected CS were identified. CMR evaluation was available for 95 patients (59%) at baseline. The leading episode to receive work‐up for CS was an arrhythmic event in 36 patients (conduction abnormality 19; ventricular fibrillation or ventricular tachycardia 17), heart failure in 19 patients, diagnosis of extracardiac sarcoidosis in 24 patients, echocardiographic abnormalities including reduced LVEF in 51 patients, and detection of ECG abnormality at an annual checkup in 30 patients. Table 1 shows the baseline characteristics of the 161 patients stratified by diagnosis using the simplified JCS criteria. Extracardiac involvement was detected in 38 patients (24%), and 14 (9%) had histological confirmation of extracardiac sarcoidosis.
Table 1.
Baseline Characteristics
| No. | All patients N (%) | No CS N=112 (70%) | CS N=28 (17%) | Isolated CS N=21 (13%) | P value | |
|---|---|---|---|---|---|---|
| Age, y, median (interquartile range) | 161 | 62 (50–69) | 62 (47–69) | 65 (58–70) | 63 (56–72) | 0.06 |
| Women, n (%) | 161 | 78 (48) | 50 (45) | 21 (75)*,† | 7 (33) | 0.005 |
| Hypertension, n (%) | 161 | 39 (24) | 31 (28) | 5 (18) | 3 (14) | 0.29 |
| Hyperlipidemia, n (%) | 161 | 29 (18) | 18 (16) | 6 (21) | 5 (24) | 0.61 |
| Diabetes, n (%) | 161 | 23 (14) | 15 (13) | 5 (18) | 3 (14) | 0.83 |
| New York Heart Association III/IV, n (%) | 161 | 4/1 (3/1) | 3/1 (3/1) | 0/0 | 1/0 (5/0) | 0.56 |
| Medication at the time of diagnosis | ||||||
| ACE inhibitor/angiotensin II receptor blocker, n (%) | 158 | 84 (52) | 58 (53) | 12 (43) | 14 (67) | 0.26 |
| Mineralocorticoid receptor antagonist, n (%) | 158 | 50 (31) | 33 (30) | 7 (25) | 10 (48) | 0.21 |
| Beta blocker, n (%) | 158 | 98 (61) | 67 (62) | 13 (46)† | 18 (86) | 0.019 |
| Extracardiac sarcoidosis, n (%) | 161 | 38 (24) | 10 (9) | 28 (100)*,† | 0 | <0.001 |
| Eye, n (%) | 161 | 9 (6) | 3 (3) | 6 (21)* | 0 | <0.001 |
| Lung, n (%) | 161 | 29 (18) | 5 (5) | 24 (86)*,† | 0 | <0.001 |
| Skin, n (%) | 161 | 5 (3) | 2 (2) | 3 (11) | 0 | 0.035 |
| Histologically confirmed extracardiac sarcoidosis, n (%) | 161 | 14 (9) | 5 (5) | 9 (32)*,† | 0 | <0.001 |
| Serum ACE level, mg/mL, median (interquartile range) | 120 | 14.7 (10.2–19.9) | 13.3 (9.3–17.5) | 18.6 (13.0–26.1)* | 15.7 (9.1–19.3) | 0.004 |
| ACE elevation, n (%) | 120 | 7 (6) | 3 (4) | 3 (12) | 1 (6) | 0.31 |
| Serum sIL‐2R level, U/mL, median (interquartile range) | 75 | 363 (269–587) | 328 (244–479) | 481 (362–897)* | 329 (283–408) | 0.032 |
| Elevated sIL‐2R, n (%) | 75 | 14 (19) | 6 (14) | 7 (37) | 1 (8) | 0.06 |
| Brain natriuretic peptide level, pg/mL, median (interquartile range) | 146 | 113 (45–287) | 93 (40–285) | 173 (79–326) | 117 (60–373) | 0.22 |
| Bilateral hilar lymphadenopathy, n (%) | 161 | 36 (22) | 11 (10) | 25 (89)*,† | 0 | <0.001 |
| Bronchoalveolar lavage positive, n (%) | 161 | 5 (3) | 2 (2) | 3 (11) | 0 | 0.035 |
| CS by Japanese Ministry of Health and Welfare criteria, n (%) | 161 | 22 (14) | 1 (1) | 21 (75) | 0 | <0.001 |
| CS by Heart Rhythm Society criteria, n (%) | 161 | 12 (8) | 0 | 12 (43) | 0 | <0.001 |
Normal range: sIL‐2R 121–613 U/mL, ACE 7.7–29.4 IU/L. ACE indicates angiotensin‐converting enzyme; CS, cardiac sarcoidosis; and sIL‐2R, soluble interleukin‐2 receptor.
P≤0.01 for no CS.
P≤0.01 for isolated CS.
The simplified JCS criteria classified 28 patients (17%) as having CS and 21 patients (13%) as having isolated CS (Figure S1). Among the 112 patients classified into no CS group, 38% were diagnosed with other cardiomyopathies (dilated cardiomyopathy 37, hypertrophic cardiomyopathy 3, ischemic cardiomyopathy 3) after a comprehensive evaluation. Endomyocardial biopsy was performed in 91 patients, and 4 patients were histologically confirmed to have CS. In contrast, the JMHW criteria defined 22 patients (14%) as having CS and HRS criteria classified 12 patients (7%) as having CS. When we compared the patients with CS with the groups with no CS and with isolated CS, patients with CS showed a higher proportion of female patients and higher angiotensin‐converting enzyme or soluble interleukin‐2 receptor levels than those with no CS (Table 1). Patients with isolated CS showed a higher proportion of men and patients on beta blockers. Table 2 shows the comparison of patient characteristics associated with diagnostic criteria stratified by JCS diagnosis. Patients with isolated CS showed a higher prevalence of arrhythmic events, especially sustained ventricular tachycardia, compared with the group with no CS . LV geometrical abnormality and LGE on CMR were more common in patients with isolated CS and CS than in the group with no CS. Regional wall motion abnormality was more common in the group with isolated CS than in the other groups, whereas patients with CS tended to show higher LVEF than others. Furthermore, FDG‐PET abnormality and LGE on CMR were more prevalent in the groups with isolated CS and CS than in the group with no CS. Of note, LGE was observed in 15 patients (100%) in the isolated CS group, 18 patients (95%) in the CS group, and 34 patients (56%) in the no CS group among patients undergoing CMR at baseline.
Table 2.
Clinical or imaging findings associated with cardiac sarcoidosis
| No. | All patients N (%) | No CS N=112 (70%) | CS N=28 (17%) | Isolated CS N=21 (13%) | P value | |
|---|---|---|---|---|---|---|
| Arrhythmic event, n (%) | 161 | 57 (36) | 30 (27) | 15 (54)* | 12 (57)† | 0.003 |
| Advanced atrioventricular block, n (%) | 161 | 26 (16) | 11 (10) | 10 (36)* | 5 (24) | 0.002 |
| Sustained ventricular tachycardia, n (%) | 161 | 34 (21) | 19 (17) | 6 (21) | 9 (43)† | 0.030 |
| Ventricular fibrillation, n (%) | 161 | 6 (4) | 3 (3) | 2 (7) | 1 (5) | 0.52 |
| LV geometrical abnormality, n (%) | 161 | 77 (48) | 36 (32) | 21 (75)* | 20 (95)† | <0.001 |
| Basal interventricular septum thinning, n (%) | 161 | 43 (27) | 25 (22) | 10 (36) | 8 (38) | 0.16 |
| Thinning of LV wall, n (%) | 161 | 37 (23) | 15 (13) | 11 (39)* | 11 (52)† | <0.001 |
| Aneurysm, n (%) | 161 | 26 (16) | 10 (9) | 8 (29)* | 8 (38)† | 0.001 |
| Thickening of LV wall, n (%) | 161 | 11 (7) | 5 (5) | 3 (11) | 3 (14) | 0.18 |
| LV wall motion abnormality, n (%) | 161 | 120 (75) | 78 (70) | 21 (75)‡ | 21 (100)† | 0.014 |
| Regional wall motion abnormality, n (%) | 161 | 108 (67) | 67 (60) | 20 (71)‡ | 20 (100)† | 0.001 |
| LV ejection fraction, %, median (interquartile range) | 161 | 49 (33–62) | 49 (32–63) | 54 (44–64) | 45 (27–51) | 0.06 |
| Ejection fraction<50%, n (%) | 161 | 82 (51) | 57 (51) | 11 (39) | 14 (67) | 0.17 |
| 18F‐fluorodeoxyglucose positron emission tomography or Gallium‐67 scintigraphy abnormality, n (%) | 161 | 71 (44) | 22 (20) | 28 (100)* | 21 (100)† | <0.001 |
| Late gadolinium enhancement on cardiac magnetic resonance imaging, n (%) | 95 | 67 (71) | 34/61 (56) | 18/19 (95)* | 15/15 (100)† | <0.001 |
CS indicates cardiac sarcoidoisis; and LV, left ventricular.
P≤0.01 for no CS.
P≤0.01 for no CS.
P≤0.01 for isolated CS.
Imaging Characteristics of Isolated CS According to the Updated CS Guideline
When we compared the echocardiographic parameters of isolated CS with CS, patients with isolated CS had higher LV dimension at end‐diastole, LV end‐systolic dimension, and LV volume than patients with CS (Table 3). LVEF was significantly lower in patients with isolated CS. Mitral LV inflow peak early/late diastolic velocity was higher in isolated CS, whereas mitral LV inflow peak early/early diastolic velocity of mitral annulus was similar in both groups.
Table 3.
Comparison of Imaging Parameters Between CS and Isolated CS
| CS N=28 | Isolated CS N=21 | P value | |
|---|---|---|---|
| Height, cm, median (interquartile range) | 155 (153–165) | 167 (158–171) | 0.007 |
| Weight, kg, median (interquartile range) | 56 (49–70) | 63 (54–73) | 0.12 |
| Body surface area, m2, median (interquartile range) | 1.60 (1.40–1.70) | 1.70 (1.60–1.80) | 0.029 |
| Systolic blood pressure, mm Hg, median (interquartile range) | 129 (113–144) | 119 (108–134) | 0.17 |
| Diastolic blood pressure, mm Hg, median (interquartile range) | 75 (65–83) | 66 (59–76) | 0.036 |
| Heart rate, bpm, median (interquartile range) | 65 (61–80) | 62 (53–73) | 0.31 |
| Echocardiographic findings | |||
| IVSd, mm, median (interquartile range) | 9.9 (7.9–11.5) | 9.2 (8.5–12.0) | 0.89 |
| Basal IVSd, mm, median (interquartile range) | 8.1 (5.6–10.0) | 6.7 (4.6–10.3) | 0.57 |
| Posterior wall thickness at end‐diastole, mm, median (interquartile range) | 9.2 (7.9–10.5) | 9.0 (8.4–10.7) | 0.59 |
| LV dimension at end‐diastole, mm, median (interquartile range) | 52 (47–56) | 59 (56–67) | <0.001 |
| LV end‐systolic dimension, mm, median (interquartile range) | 38 (30–44) | 45 (39–54) | 0.001 |
| LV end‐diastolic volume, mL, median (interquartile range) | 101 (84–139) | 132 (108–181) | 0.006 |
| LV end‐systolic volume, mL, median (interquartile range) | 48 (33–68) | 75 (58–109) | 0.003 |
| LV ejection fraction, %, median (interquartile range) | 54 (44–64) | 45 (27–51) | 0.005 |
| Left atrial volume index, mL/m2, median (interquartile range) | 38 (25–47) | 38 (29–46) | 0.79 |
| Mitral regurgitation moderate, n (%) | 8 (29) | 7 (33) | 0.72 |
| Tricuspid regurgitation moderate, n (%) | 2 (7) | 3 (14) | 0.41 |
| Mitral LV inflow peak early/late diastolic velocity, median (interquartile range) | 0.8 (0.7–1.1) | 1.2 (0.8–1.5) | 0.017 |
| Mitral LV inflow peak early/early diastolic velocity of mitral annulus, median (interquartile range) | 11.0 (8.6–14.5) | 10.1 (6.8–13.3) | 0.39 |
| Tricuspid regurgitation pressure gradient, mm Hg, median (interquartile range) | 20 (16–25) | 22 (19–25) | 0.50 |
| 18F‐fluorodeoxyglucose positron emission tomography abnormality | |||
| Focal, n (%) | 7 (25) | 1 (5) | 0.06 |
| Focal on diffuse, n (%) | 21 (75) | 20 (95) | |
| Maximum standardized uptake value, median (interquartile range) | 7.8 (4.5–10.7) | 4.1 (3.4–6.5) | 0.023 |
| Late gadolinium enhancement on cardiac magnetic resonance imaging, n (%) | 18/19 (95) | 15/15 (100) | 0.26 |
CS indicates cardiac sarcoidosis; IVSd, interventricular septum thickness at end‐diastole; and LV, left ventricular.
All patients with CS or isolated CS showed an abnormal accumulation of FDG‐PET. Among 21 patients with isolated CS, only 1 patient (5%) showed focal uptake and the remaining 20 patients showed focal on diffuse uptake. In the patient group with CS, 7 patients (25%) showed focal uptake and 21 patients showed focal on diffuse uptake. Patients with isolated CS showed lower SUVmax values than the group with CS (4.1[3.4–6.5] versus 7.8 [4.5–10.7], P=0.023).
Patient Outcomes
During a median follow‐up duration of 522 days (interquartile range 180–959), 24 patients (15%) experienced MACEs, 12 patients had symptomatic sustained ventricular tachyarrhythmia leading to ablation or increased medication, 11 were hospitalized for decompensated heart failure, and 1 developed atrioventricular block requiring pacemaker implantation. In the multivariable Cox proportional hazards model analysis, isolated CS (hazard ratio [HR], 3.35 [95% CI, 1.08–10.39], P=0.036) was independently associated with MACEs after adjusting for reduced ejection fraction (HR, 2.20 [95% CI, 0.89–5.41], P=0.088) and steroid therapy (HR, 0.56 [95% CI, 0.19–1.65] P=0.30) (Table 4). Clinical outcomes according to the diagnosis based on the JCS criteria are illustrated using Kaplan–Meier survival curves (Figure 2). The group with isolated CS showed significantly lower event‐free survival than the groups with CS (P=0.033) and with no CS (P=0.034).
Table 4.
Univariable and Multivariable Cox Proportional Hazards Model Analysis of Adverse Event (n=161)
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Age | 1.02 (0.99–1.05) | 0.26 | ||
| Female sex | 0.69 (0.30–1.57) | 0.37 | ||
| LV ejection fraction<50% | 2.63 (1.08–6.37) | 0.033 | 2.20 (0.89–5.41) | 0.088 |
| LV geometrical abnormality | 1.73 (0.76–3.95) | 0.20 | ||
| LV wall motion abnormality | 8.59 (1.16–63.60) | 0.04 | ||
| Isolated CS by JCS criteria | 2.82 (1.16–6.83) | 0.022 | 3.35 (1.08–10.39) | 0.036 |
| CS by JCS criteria | 0.54 (0.16–1.82) | 0.32 | ||
| CS or isolated CS by JCS criteria | 1.41 (0.63–3.18) | 0.41 | ||
| CS by Heart Rhythm Society criteria | 0.39 (0.05–2.92) | 0.36 | ||
| CS by Japanese Ministry of Health and Welfare criteria | 0.93 (0.28–3.11) | 0.90 | ||
| 18F‐fluorodeoxyglucose positron emission tomography positive findings | 1.43 (0.63–3.21) | 0.39 | ||
| Presence of late gadolinium enhancement on cardiac magnetic resonance imaging | 0.99 (0.44–2.28) | 0.99 | ||
| Steroid therapy | 0.98 (0.42–2.29) | 0.96 | 0.56 (0.19–1.65) | 0.30 |
CS indicates cardiac sarcoidosis; JCS, Japanese Circulation Society; and LV, left ventricular.
Figure 2. Impact of isolated CS on outcomes in patients who had multimodality imaging evaluation for suspected cardiac sarcoidosis (n=161).
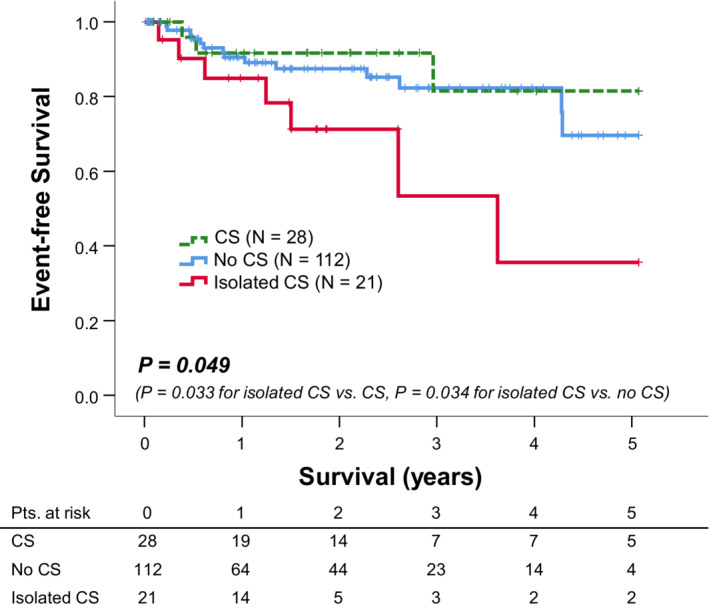
Kaplan–Meier curves demonstrated MACE‐free survival in our study population stratified by diagnosis according to the JCS guidelines. Patients diagnosed with isolated CS had lower MACE‐free survival than those in the other groups (P=0.049). CS indicates cardiac sarcoidosis; JCS, Japanese Circulation Society; and MACE, major adverse cardiovascular event.
When we performed a comparable analysis according to the HRS and JMHW criteria, CS by HRS criteria (HR, 0.39 [95% CI, 0.05–2.92], P=0.36) or JMHW criteria (HR, 0.93 [95% CI, 0.28–3.11], P=0.90) was not associated with outcomes (Figure S2).
Serial FDG‐PET Evaluation During Steroid Therapy
Forty‐six patients were treated with steroids according to the clinician's decision, including 26 patients with CS, 16 with isolated CS, and 4 patients without CS. Of the 46 patients who underwent steroid therapy, 41 patients, including 23 patients with CS and 14 with isolated CS, underwent serial FDG‐PET evaluation during steroid therapy. At follow‐up, 19 patients (83%) in the CS group showed improvement in FDG‐PET findings, 11 patients with isolated CS (79%) showed improvement, and 2 patients (50%) in the no CS group showed improvement (Table S3, Figure 3). When we applied the HRS criteria, among 41 patients who underwent serial PET evaluation, 9 patients were diagnosed with CS, and PET findings were improved in 8 patients with CS (89%) and in 24 patients (75%) with no CS (P=0.37). Similarly, applying the JMHW criteria defined 14 patients as CS and 27 patients as no CS. PET findings were improved in 11 patients (78%) in the CS defined by the JMHW criteria, whereas 21 patients (78%) with no CS according to the JMHW criteria also showed improvement (P=0.95). In logistic regression analysis, CS or isolated CS defined by the JCS criteria was significantly associated with improvement of PET findings at follow‐up (odds ratio [OR], 8.9 [95% CI, 1.3–58.3], P=0.023). CS defined by HRS criteria (OR, 3.2 [95% CI, 0.4–29.0], P=0.30) and JMHW criteria (OR, 1.5 [95% CI, 0.34–6.9], P=0.58) did not show a significant association with subsequent PET findings.
Figure 3. FDG‐PET, echocardiogram, and CMR images from patients with isolated CS ( A through F) and CS with extracardiac involvement (G through J).
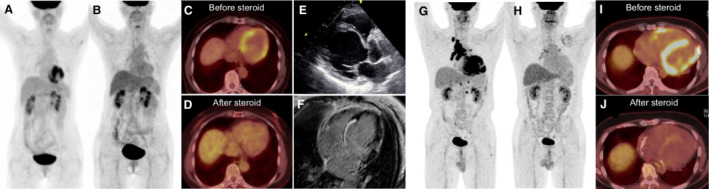
Panels A through F are images from a patient diagnosed with isolated CS. Maximum intensity projection (MIP; A) and axial image (C) of FDG‐PET demonstrate focal on diffuse myocardial uptake with SUVmax of 7.4. There was no FDG uptake in other organs. Echocardiogram also revealed prominent basal interventricular thinning with reduced left ventricular ejection fraction (E). CMR showed LGE in the basal interventricular septum and lateral wall (F). Follow‐up FDG‐PET evaluation after steroid therapy revealed improvement in myocardial uptake in MIP (B) and axial image (D) with SUVmax of 2.9. Panels G‐J show MIP (G and H) and axial images (I and J) of FDG‐PET images in another patient with CS with extracardiac involvement. Baseline FDG‐PET evaluation showed intense focal myocardial uptake (SUVmax 14.9; G and I) with hypermetabolic lymph nodes in the mediastinum and hilar regions. Administration of steroid therapy led to improvement in myocardial uptake (SUVmax 2.0; H and J). CMR indicates cardiac magnetic resonance imaging; CS, cardiac sarcoidosis; FDG, 18F‐fluorodeoxyglucose; FDG‐PET, 18F‐fluorodeoxyglucose positron emission tomography; LGE, late gadolinium enhancement; and SUVmax, maximum standardized uptake value.
SUVmax was significantly reduced in CS patients from 9.29 (6.85–13.56) to 2.54 (1.85–4.25) at follow‐up (P<0.001; Figure 4A). Similarly, SUVmax was significantly improved in isolated CS patients from 6.39 (3.99–12.46) to 2.54 (1.85–4.25) at subsequent follow‐up (P=0.024; Figure 4B). In the mixed‐effect model, there was no significant difference in the SUVmax change between the groups with CS and isolated CS (P=0.35).
Figure 4. Changes in SUVmax during steroid therapy in patients with subsequent FDG‐PET evaluation.
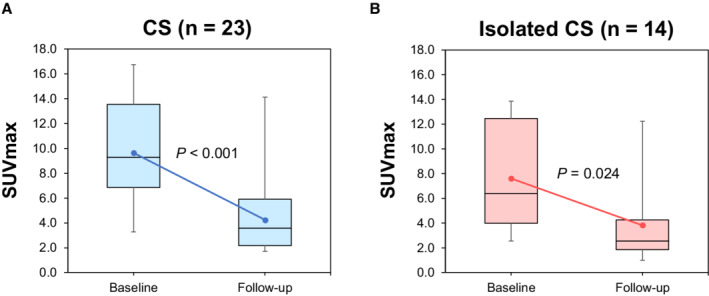
Box plots with median and interquartile range of the observed data obtained at baseline and subsequent evaluation after steroid therapy. Error bars represent 95% CIs. The regression line was obtained using a mixed‐model approach. SUVmax significantly improved at subsequent follow‐up in CS (P<0.001; A) and isolated CS (P=0.024; B). CS indicates cardiac sarcoidosis, and SUVmax, maximum standardized uptake value.
Discussion
In this study, we showed that the simplified JCS diagnostic criteria diagnosed 43% of patients with CS as isolated CS. Patients with isolated CS were characterized as having more advanced LV dysfunction and focal on diffuse accumulation with a lower SUVmax value in FDG‐PET than in patients with CS. We also showed that patients with isolated CS experienced a higher incidence of cardiovascular events than patients with CS or those without CS. Furthermore, even though patients with isolated CS showed lower SUVmax on FDG‐PET, steroid therapy led to the improvement of FDG‐PET findings in most patients with isolated CS, suggesting the reliability of the JCS criteria.
Characteristics of Isolated CS Detected by JCS 2016 Criteria
Because of the lack of definitive diagnostic criteria for isolated CS, the prevalence of isolated CS in prior studies ranged from 27% to 54%. 4 In the present study, we found that among 49 patients who were diagnosed with CS using the simplified JCS criteria, 43% were classified as isolated CS. Previous studies have also reported that patients with isolated CS have impaired LV systolic function compared with those with CS with systemic sarcoidosis, 1 , 3 , 4 which is in line with our findings, suggesting that the JCS criteria enabled us to detect patients with isolated CS. It is known that echocardiographic manifestations can vary in patients with CS, and small cardiac regions may not show any clinical abnormalities at an early stage of the disease. However, if left untreated, the extension of cardiac involvement can cause heart failure along with deterioration of LV function or ventricular arrhythmia. 13 Our results suggest that isolated CS detected by the JCS guideline may represent a more advanced stage of the disease course and may be relevant to poor outcomes. There is a paucity of data to explain this characteristic difference between isolated CS and CS with systemic involvement, and whether it is solely related to delayed recognition or whether isolated CS has distinct disease entities. Interestingly, we also found that patients with isolated CS had a lower SUVmax than the CS population. In the advanced stages of the disease in CS, scar tissue may be present without any inflammation. 14 These imaging characteristics also indicated that isolated CS as an advanced stage of the disease with extended scar formation.
Prognostic Importance to Differentiate Isolated CS
We also found that isolated CS defined by the JCS criteria was associated with a higher incidence of cardiovascular events for the first time. Isolated CS cases have also been reported to have a higher prevalence of sudden cardiac death attributable to CS. 4 Furthermore, Kron et al. reported that isolated CS had more implantable cardioverter defibrillator therapy than CS with systemic involvement. 15 Our data are consistent with those prior findings and suggest a need to provide special attention and care to patients with isolated CS, including device therapy or aggressive medical therapy, to reduce the risk of ventricular arrhythmia or heart failure.
Reliability of JCS Criteria
As we have a limited number of patients who underwent endomyocardial biopsy and its low sensitivity, 16 we used MACEs and FDG‐PET responses at subsequent evaluation to compare the reliability of each diagnostic criterion. Only the JCS criteria showed an association with MACEs and response on FDG‐PET at follow‐up, indicating the reliability and superiority of the updated JCS criteria.
At the same time, the JCS criteria do not necessitate histological confirmation or other organ involvement for the diagnosis of CS and rely more on integrated imaging evaluation. More than twice the number of patients were diagnosed with CS using the JCS criteria compared with the HRS and JMHW criteria, which showed its great applicability. However, at the same time, it is possible that a false‐positive case may occur, and careful interpretation of FDG‐PET images is required to make a proper diagnosis. A prolonged fasting preparation protocol is essential to suppress physiological myocardial FDG uptake, and inadequate preparation may lead to false‐positive FDG‐PET findings. 11 A previous report showed that altered myocardial glucose metabolism in myocardial ischemia or cardiomyopathies also causes FDG uptake in cardiac muscle. 17 , 18 Therefore, it might be quite difficult to distinguish whether myocardial FDG uptake truly reflects active CS involvement just by single evaluation. Serial PET evaluation in the present study showed that patients with isolated CS also showed similar improvements in FDG uptake and SUVmax levels in patients with CS after steroid therapy. Nonetheless, further prospective studies are needed to confirm the applicability and accuracy of these updated CS criteria. Our data suggest that the JCS criteria adequately diagnose patients with isolated CS who have active sarcoidosis involvement that requires anti‐inflammatory medication.
Limitations
This was a single‐center, retrospective, observational study conducted at a tertiary referral center. In addition, we evaluated only patients who underwent FDG‐PET. Limited availability of CMR evaluation may affect the prognostic value of LGE in the present study. Further investigation is required to confirm the generalizability of our findings in a larger prospective study. Furthermore, we have a limited number of endomyocardial biopsies and histologically confirmed cases; thus, we only used clinical outcomes to compare the reliability of the diagnostic criteria. Moreover, therapeutic decisions were made by physicians who were aware of all the clinical data. Because of the retrospective nature of our study, physicians can bring together all clinical information and recommend treatment accordingly. One can argue that the beneficial effect of steroid therapy in a population with isolated CS may be, at least in part, attributed to the relevant selection bias. Further prospective studies with serial FDG‐PET evaluation, including patients with no CS, are required. Lastly, we used only 5 major criteria to determine the presence of cardiac involvement to simplify the JCS guidelines and neglected the minor criteria. However, in our population, we were able to classify CS with extracardiac involvement using only the major criteria, and omitting the minor criteria did not affect our results.
Conclusions
Patients with isolated CS, as detected by updated JCS guidelines, showed a higher rate of poor event‐free survival, characterized by more advanced cardiac dysfunction and ventricular arrhythmia than patients with CS and extracardiac involvement. These patients needed to be cautiously managed.
Sources of Funding
This work was supported by JSPS KAKENHI Grant Number JP22K16127.
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S2
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025565
For Sources of Funding and Disclosures, see page 11.
References
- 1. Kandolin R, Lehtonen J, Graner M, Schildt J, Salmenkivi K, Kivistö SM, Kupari M. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–468. doi: 10.1111/j.1365-2796.2011.02396.x [DOI] [PubMed] [Google Scholar]
- 2. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/circulationaha.114.011522 [DOI] [PubMed] [Google Scholar]
- 3. Tezuka D, Terashima M, Kato Y, Toriihara A, Hirasawa K, Sasaoka T, Yoshikawa S, Maejima Y, Ashikaga T, Suzuki J, et al. Clinical characteristics of definite or suspected isolated cardiac sarcoidosis: application of cardiac magnetic resonance imaging and 18F‐Fluoro‐2‐deoxyglucose positron‐emission tomography/computerized tomography. J Card Fail. 2015;21:313–322. doi: 10.1016/j.cardfail.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 4. Okada DR, Bravo PE, Vita T, Agarwal V, Osborne MT, Taqueti VR, Skali H, Chareonthaitawee P, Dorbala S, Stewart G, et al. Isolated cardiac sarcoidosis: a focused review of an under‐recognized entity. J Nucl Cardiol. 2018;25:1136–1146. doi: 10.1007/s12350-016-0658-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hiraga H, Yuwai K, Hiroe M. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102. [Google Scholar]
- 6. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 7. Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, Inomata T, Ishibashi‐Ueda H, Eishi Y, Kitakaze M, et al. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis ‐ Digest version. Circ J. 2019;83:2329–2388. doi: 10.1253/circj.CJ-19-0508 [DOI] [PubMed] [Google Scholar]
- 8. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 10. Nagano N, Nagai T, Sugano Y, Morita Y, Asaumi Y, Aiba T, Kanzaki H, Kusano K, Noguchi T, Yasuda S. Association between basal thinning of interventricular septum and adverse long‐term clinical outcomes in patients with cardiac sarcoidosis. Circ J. 2015;79:1601–1608. doi: 10.1253/circj.CJ-14-1217 [DOI] [PubMed] [Google Scholar]
- 11. Ishida Y, Yoshinaga K, Miyagawa M, Moroi M, Kondoh C, Kiso K, Kumita S. Recommendations for 18 F‐fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med. 2014;28:393–403. doi: 10.1007/s12149-014-0806-0 [DOI] [PubMed] [Google Scholar]
- 12. Skali H, Schulman AR, Dorbala S. 18 F‐FDG PET/CT for the assessment of myocardial sarcoidosis. Curr Cardiol Rep. 2013;15:370. doi: 10.1007/s11886-013-0370-6 [DOI] [PubMed] [Google Scholar]
- 13. Danwade TA, Devidutta S, Shelke AB, Saggu DK, Yalagudri SD, Sridevi C, Reddy NK, Narasimhan C. Prognostic value of fluorine‐18 fluoro‐2‐deoxyglucose positron emission computed tomography in patients with unexplained atrioventricular block. Heart Rhythm. 2018;15:234–239. doi: 10.1016/j.hrthm.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 14. Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9:e000867. doi: 10.1161/circimaging.113.000867 [DOI] [PubMed] [Google Scholar]
- 15. Kron J, Sauer W, Mueller G, Schuller J, Bogun F, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D, et al. Outcomes of patients with definite and suspected isolated cardiac sarcoidosis treated with an implantable cardiac defibrillator. J Interv Card Electrophysiol. 2015;43:55–64. doi: 10.1007/s10840-015-9978-3 [DOI] [PubMed] [Google Scholar]
- 16. Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/circulationaha.109.851352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Protonotarios A, Wicks E, Ashworth M, Stephenson E, Guttmann O, Savvatis K, Sekhri N, Mohiddin SA, Syrris P, Menezes L, et al. Prevalence of (18)F‐fluorodeoxyglucose positron emission tomography abnormalities in patients with arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol. 2019;284:99–104. doi: 10.1016/j.ijcard.2018.10.083 [DOI] [PubMed] [Google Scholar]
- 18. Masuda A, Takeishi Y. Current status and future direction of PET/MR in cardiology. Ann Nucl Cardiol. 2017;3:73–79. doi: 10.17996/anc.17-00018 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2


