Abstract
Background
In daily hospital practice, antibiotic therapy is commonly prescribed for longer than recommended in guidelines. Understanding the key drivers of prescribing behaviour is crucial to generate meaningful interventions to bridge this evidence-to-practice gap.
Objectives
To identify behavioural determinants that might prevent or enable improvements in duration of antibiotic therapy in daily practice.
Methods
We systematically searched PubMed, Embase, PsycINFO and Web of Science for relevant studies that were published between January 2000 and August 2021. All qualitative, quantitative and mixed-method studies in adults in a hospital setting that reported determinants of antibiotic therapy duration were included.
Results
Twenty-two papers were included in this review. A first set of studies provided 82 behavioural determinants that shape how health professionals make decisions about duration; most of these were related to individual health professionals’ knowledge, skills and cognitions, and to professionals’ interactions. A second set of studies provided 17 determinants that point to differences in duration regarding various pathogens, diseases, or patient, professional or hospital department characteristics, but do not explain why or how these differences occur.
Conclusions
Limited literature is available describing a wide range of determinants that influence duration of antibiotic therapy in daily practice. This review provides a stepping stone for the development of stewardship interventions to optimize antibiotic therapy duration, but more research is warranted. Stewardship teams must develop complex improvement interventions to address the wide variety of behavioural determinants, adapted to the specific pathogen, disease, patient, professional and/or hospital department involved.
Introduction
Antimicrobial resistance (AMR) is a serious threat to public health. One way of tackling AMR is the appropriate use of antibiotics in hospitals for the prevention and treatment of infections. To reduce the density of antibiotic pressure there are merely two options: refrain from starting therapy and shorten the duration of antibiotic therapy by stopping as soon as safely possible.1
Evidence supports the use of shorter durations for a range of infections.2–6 Short courses are therefore advocated in (inter)national guidelines.4,7 In daily hospital practice, however, antibiotic therapy is commonly used for longer than recommended.1,8–11 A French multicentre ICU study showed, for example, that the median antibiotic therapy duration in the control arm of a procalcitonin (PCT) study was 13.3 days (SD 7.6), where the guideline advocates a maximum of 7–10 days.11
Professional societies strongly recommend introducing strategies to reduce antibiotic therapy to the shortest effective duration.4,7 Efforts to shorten the duration of antibiotic therapy in hospital practice are a growing area of focus for stewardship initiatives and programmes. A recent study by Langford et al.12 showed, however, that stewardship advice to stop antibiotics or reduce their duration was less often accepted than advice to start or increase antibiotic exposure.
To effectively bridge the evidence-to-practice gap, an understanding of the key drivers of prescribing behaviour is crucial to generate ideas for the planning of meaningful improvement interventions.13–17 Currently, there is insufficient knowledge about the individual and contextual factors that shape how prescribers make decisions about the duration of antibiotic therapy. Therefore, the aim of this systematic scoping review was to identify behavioural determinants influencing (prolonged) duration of antibiotic therapy in the hospital setting.
Methods
This systematic scoping review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines statement (Table S1, available as Supplementary data at JAC Online).18
Data sources and searches
We searched PubMed, Embase, PsycINFO and Web of Science for literature between January 2000 and 18 August 2021. We combined search terms addressing ‘antibiotic use’, ‘duration of therapy’, ‘determinants’ and ‘hospital setting’ (Figure S1). References of included studies were also reviewed.
Eligibility criteria
Studies that met the following criteria were eligible for inclusion: (1) the study provides original data on determinants influencing duration of antibiotic therapy or antibiotic prophylaxis; and (2) the study was conducted in the hospital setting. Inclusion of studies was not restricted by the type of study (qualitative or quantitative), study design or language, or to those reporting the assessment of determinants influencing duration as their main objective.
Studies conducted in the paediatric setting and studies that focused only on the effectiveness of an intervention (including procalcitonin studies) were excluded.
Study selection
Two reviewers (R.M.E.J. and J.A.S.) independently screened and discussed the title and abstract of all articles to determine whether the studies met the inclusion criteria. Articles where doubt remained, as well as all potentially eligible articles, were full-text screened by both reviewers independently. In a consensus meeting involving both reviewers and a third reviewer (M.E.J.L.H.), any disagreement was resolved by discussion.
Data extraction
Data on author, year of publication, country, topic, setting, population, sample size and study objectives were extracted in a standardized table to provide an overview of the characteristics of each included study (Table S2). Subsequently, all determinants were extracted from the included articles (Table S3).
Regarding quantitative studies on the presence of potential determinants of duration, a factor was considered a determinant when it was statistically significant (P < 0.05) in either univariate or multivariate analysis or when it was selected by ≥25% of the respondents (i.e. when ≥25% of respondents indicated that the suggested determinant hindered or helped them to adhere to the recommended duration). The 25% cut-off point was chosen by our research team for pragmatic reasons (i.e. to distinguish relevant, common determinants from the entire set of suggested determinants). In Table S4, all data are shown. In the case of qualitative research, a statement or comment made by a participant was included as a determinant in this review when: (1) it was directly related to the duration, discontinuation or continuation of antibiotics; and (2) when it was an identifiable result mentioned in the results section of the original article (either in the text or in the tables).
Classification of determinants
Determinants were extracted from studies exploring behavioural determinants that influenced how prescribers make decisions about the duration of antibiotic therapy (e.g. professionals’ lack of knowledge) and studies exploring between-group differences (e.g. surgical departments versus general medical departments). In extracting the data from the original studies, the original description was maintained but categorized to ensure an in-depth understanding of the subject.
Determinants extracted from studies exploring behavioural determinants were categorized using a comprehensive checklist, published by Flottorp et al.,14 distinguishing seven categories of determinants of practice:
Guideline factors (e.g. the clarity of the recommendation, the evidence supporting the recommendation);
Individual health professional factors (e.g. awareness and familiarity with the recommendation, or the skills needed to adhere);
Patient factors (patient preferences, or real or perceived needs and demands of the patient);
Professional interactions (e.g. opinions and communication among professionals or referral processes);
Incentives and resources (e.g. availability of necessary resources, or extent to which the information system influences adherence);
Capacity for organizational change (e.g. capable leadership, or the relative priority given to making necessary changes); and
Social, political and legal factors (e.g. payer or funder policies).
Flottorp and colleagues14 developed this comprehensive, integrated checklist of determinants of practice through a systematic review and synthesis of frameworks and taxonomies of determinants of practice, followed by a consensus process among implementation researchers. The work included 12 published checklists, frameworks, taxonomies and classifications of determinants of healthcare professional practice, including for example the consolidated framework for advancing implementation science (CFIR)19 and the work by Michie et al.,20 where 128 explanatory constructs drawing on 33 psychological theories were identified and categorized into 12 domains.
Determinants extracted from studies exploring between-group differences, i.e. describing the extent to which differences exist in duration based on specific characteristics of the pathogen, disease, patient, professional or hospital department, were classified as pathogen, disease, patient, professional or hospital department factors.
Quality assessment
Two reviewers (R.M.E.J. and A.J.M.O.) independently assessed the methodological quality of the included studies, using the Mixed-Method Appraisal Tool (MMAT) version 2018.21 Each article was assessed using seven questions based on study type. Methodological quality was scored as ‘strong’, ‘moderate’ or ‘weak’ (Table S5).
Results
Study selection
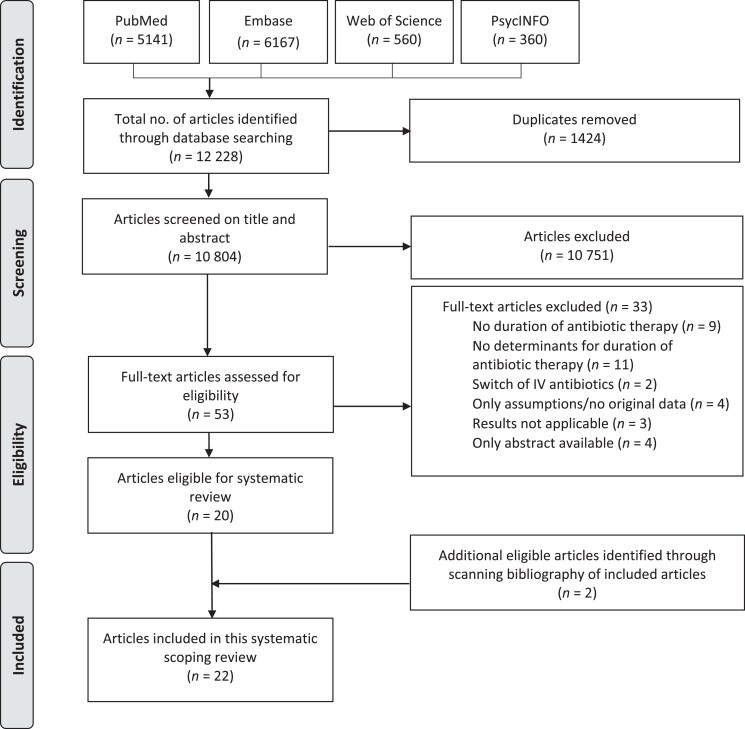
The search strategy resulted in 10 804 articles. Based on title and abstract, 10 751 articles were excluded. After assessing the full text of the remaining 53 articles, 33 articles were excluded. References of the 20 included articles were checked for potential additional eligible studies, resulting in the inclusion of 2 additional articles. Ultimately, 22 articles were included in this review (Figure 1).22–43
Figure 1.
PRISMA flowchart of study selection.
Characteristics of selected studies
All included articles were published between 2002 and 2021. Studies were performed in Europe (n = 7),22,25,36–38,40,43 North America (n = 6),23,28,31,39,41,42 Asia (n = 4),26,29,32,33 the Middle East (n = 3),27,30,34 Australia (n = 1)24 and Africa (n = 1).35 Of the 22 included articles, there were 7 qualitative studies23,24,32,36,37,40,41 and 15 quantitative studies.22,25–31,33–35,38,39,42,43 The following types of studies were found: seven survey studies,26,28,29,35,38,39,42 five interview studies,23,24,36,37,41 four prospective observational cohort studies,25,31,33,34 two retrospective chart reviews,27,30 one prospective audit of records and charts,43 one mixed-methods study (survey and interviews),32 one stakeholder consultation study40 and one study that combined data from two pre-existing data sources.22 Ten studies focused on duration of surgical antimicrobial prophylaxis.22–24,26,27,29,30,32,34,43 The remaining 12 studies focused on duration of treatment in general or specific patient groups, e.g. patients with urinary tract infections or ventilator-associated pneumonia (VAP).25,28,31,33,35–42 For all characteristics of the included studies, see Table S2.
The results from the quality appraisal are shown in Table S5. The methodology of seven studies was scored as strong,22,24,25,33,34,36,38 six as moderate,26,27,30,31,41,43 and nine were considered weak.23,28,29,32,35,37,39,40,42 The non-randomized studies scored better compared with the descriptive studies, which were often moderate or weak, mostly because of a risk of social desirability bias. Another frequent weakness of these survey studies was that the study sample was not representative of the target population or that information on representativeness was missing.
Eleven studies explored behavioural determinants influencing how prescribers make decisions about duration.22–24,29,32,36,37,39–41,43 For one study29 we were unable to categorize the determinant in the Flottorp checklist. In this study, the relationship between the suggested rationale for determining the duration of therapy (either literature, guidelines, personal preferences or similar practices as colleagues) and the actual duration prescribed was unclear.29
Nine studies explored between-group differences in duration.25–27,30,31,33–35,42 We were unable to categorize the determinant of one study showing that broad-spectrum antibiotics tend to be continued over weekends or holidays.33
Two studies explored both behavioural determinants and between-group differences.28,38
In most included studies, duration was examined and analysed amongst other aspects of antibiotic use; one study explicitly set out to understand the drivers of antibiotic therapy duration.38
Main findings
In total, 99 determinants were derived from the studies: 82 from the studies exploring behavioural determinants and 17 from studies exploring between-group differences. Almost all determinants resulted in prolonged duration. An overview of determinants extracted per article can be found in Table S3. We could not find any specific patterns with regard to determinants when comparing studies with different levels of methodological quality, i.e. studies scored as weak did not necessary yield different types of determinants than the methodologically strong studies.
Studies exploring behavioural determinants
Table 1 provides an overview of the behavioural determinants.22–24,28,32,36,37,39–41,43 Determinants were found for all seven Flottorp categories but were mostly related to the individual health professional (n = 44) and professional interactions (n = 19). Some determinants were classified into multiple categories (n = 10).
Table 1.
Behavioural determinants of duration of antibiotic therapy, classified according to the checklist of Flottorp et al. (2013)14
| Category | Determinants |
|---|---|
| 1. Guideline factors (N = 5) |
Recommendation (N = 5) |
| ‘Quality of evidence supporting the recommendation’ (n = 1) | |
| |
| ‘Clarity’ (n = 1) | |
| |
| ‘Cultural appropriateness’ (n = 1) | |
| |
| ‘Consistency with other guidelines’ (n = 2) | |
| |
| |
| 2. Individual health professional factors (N = 44) |
Knowledge and skills (N = 7) |
| Combination of two determinants: ‘Domain knowledge’ and ‘Skills needed to adhere’ (n = 6) | |
| |
| |
| |
| |
| |
| |
| ‘Awareness and familiarity with the recommendation’ (n = 1) | |
| |
| Cognitions (including attitudes) (N = 35) | |
| ‘Agreement with the recommendation’ (n = 1) | |
| |
| ‘Expected outcome’ (n = 24) | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| ‘Emotions’ (n = 6) | |
| |
| |
| |
| |
| |
| |
| ‘Self-efficacy’ (n = 3) | |
| |
| |
| |
| ‘Intention and motivation’ (n = 1) | |
| |
| Professional behaviour (N = 2) | |
| ‘Nature of behaviour: habit’ (n = 2) | |
| |
| |
| 3. Patient factors (N = 7) |
‘Patient needs’ (n = 7) |
| |
| |
| |
| |
| |
| |
| |
| 4. Professional interactions (N = 19) |
Communication and influence (N = 13) |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Team process (N = 3) | |
| |
| |
| |
| Referral process (N = 3) | |
| |
| |
| |
| 5. Incentives and resources (N = 3) |
‘Information system’ (n = 2) |
| |
| |
| ‘Quality assurance and patient safety systems’ (n = 1) | |
| |
| 6. Capacity for organizational change (N = 2) |
‘Capable leadership’ (n = 1) |
| |
| ‘Priority of necessary change’ (n = 1) | |
| |
| 7. Social, political and legal factors (N = 2) |
‘Malpractice liability’ (n = 2) |
| |
|
CIED, cardiovascular implantable electronic device; SSI, surgical site infection; SAP, surgical antimicrobial prophylaxis; (+), longer duration of antibiotic treatment; (−), shorter duration of antibiotic treatment. Text in Roman type denotes antibiotic therapy studies; text in italic type denotes antibiotic prophylaxis studies.
1. Guideline factors
Five studies provided five determinants related to guideline factors.23,32,38,40,43
One study showed that, according to the healthcare professionals, there was a lack of evidence that stopping antibiotics immediately after skin closure is safe.23Cultural appropriateness32 and clarity of the recommendation38 also influenced the duration of antibiotic therapy.
Two studies showed that inconsistency between guidelines influenced duration. For example, van Kasteren et al.43 described that prolonged use of surgical antimicrobial prophylaxis was caused by surgeons feeling insecure about the duration recommended in the hospital guideline compared with their department protocol.
2. Individual health professional factors
Eleven studies reported a total of 44 determinants related to individual professional factors.22–24,28,32,36–40,43
Four studies (n = 7) described that knowledge and skills and, more specifically, healthcare professionals’ knowledge or expertise about the targeted condition/recommendation and skills needed to adhere influenced duration.28,32,37,40 For example, diagnostic or prognostic uncertainty resulted in longer antibiotic treatment duration than recommended.28,40
Ten studies (n = 35) described how cognitions (the processes of acquiring and absorbing knowledge) and attitudes of individual professionals influenced prescribing behaviour.22–24,28,32,36,38–40,43 Especially, expected outcome (how professionals expect their actions to have an effect on patient outcome) (n = 24) and emotions (how fear, anticipated regret and empathy affect prescribing) (n = 6) were reported to influence antibiotic therapy duration in general,22–24,32,36,38–40,43 or more specifically in an end-of-life setting.28 Expectations of self-efficacy (i.e. self-perceived competence or confidence in abilities) also influenced duration (n = 3).36,39,40 Salsgiver et al.,39 for example, reported that 31% of 409 surveyed participants lacked confidence in determining appropriate duration. One study described the lack of intention and motivation (i.e. the extent to which the healthcare professional intends to adhere to a recommendation and is motivated to do so) as a determinant of longer duration (n = 1).28
Lastly, concerning professional behaviour (n = 2), one study by Santillo et al.40 described how habits may prevent choosing appropriate durations.
3. Patient factors
Three studies reported seven determinants related to real or perceived needs and demands of the patient or his/her family.28,38,41
One study on antimicrobial treatment at the end of life reported, for example, that according to healthcare professionals, the decision to (dis)continue antimicrobial treatment was dependent on the patients’ requests, family members’ requests, or family members’ expectations.28 Similarly, two studies described how specific patient conditions/situations (e.g. symptoms or comorbidities of patients) may prolong duration.38,41
4. Professional interactions
Seven studies described 19 determinants regarding professional interactions.24,28,36–38,40,41
Most determinants (n = 13) were related to communication and influence, i.e. how professionals were influenced by their peers’ opinions and communication. For example, professionals were not sure if they were allowed to change prescriptions when they did not know why they were initially prescribed, resulting in continuation of therapy.40 In contrast, input from external team members (e.g. an infectious disease physician, clinical microbiologist or pharmacist) could be a reason to discontinue antibiotic treatment.28,36
Two studies reported determinants related to team processes and interaction skills (n = 3).36,37 Stopping antibiotics was perceived as a decision made by seniors to which junior doctors were not expected to contribute.37 If trainees felt unable to access consultant support, they were likely to continue antibiotics.36 Moreover, heterogeneity in the seniors’ approach to stop treatment, together with a lack of feedback, discouraged trainees even more from making or suggesting changes to therapy.37
Lastly, two studies described determinants regarding referral processes and related communication (n = 3).28,41 Gaw et al.28 described that 67.3% of professionals indicated that in transfer situations at the end of life, antibiotic treatment was discontinued because the facility or home to which the patient is to be transferred prohibits or is unable to continue administration of antibiotics. The study by Sharara and colleagues41 showed that poor communication across care settings, including the lack of knowledge of the preadmission course, resulted in prolonged antibiotic courses.
5. Incentives and resources
Three studies reported three determinants regarding the availability of incentives and necessary resources.28,41,43
All studies described determinants related to the availability of adequate hospital systems, either information systems41,43 or quality assurance/patient safety systems.28 For example, van Kasteren et al.43 mentioned that the unintentional deviation from the hospital guideline regarding duration of surgical antimicrobial prophylaxis was a result of inaccurate stop orders issued within a patient safety system at the ward.
6. Capacity for organizational change
One study described two determinants related to capacity for organizational change.40
The aim of this study was to identify determinants of stopping antibiotics during a stakeholder workshop. One determinant mentioned was the lack of formal encouragement to stop antibiotic treatment (capable leadership).40 They also found that stopping antibiotic treatment might be hard to sustain as a priority in competition with other ward-round decisions (priority of necessary change).40
7. Social, political and legal factors
Two studies reported two determinants related to social, political and legal factors.24,36
Both studies referred to real or perceived risks of malpractice liability and described that physicians felt that prescribing antibiotics for a longer duration was necessary to protect them against litigation or adverse consequences of a complication.24,36
Studies exploring between-group differences
Table 2 provides an overview of the 17 determinants reported in 10 papers describing the extent to which differences exist in duration, based on specific characteristics of pathogen, disease, patient, professional or hospital department.25–28,30,31,34,35,38,42
Table 2.
Determinants describing group differences in antibiotic therapy duration
| Category | Determinants |
|---|---|
| 1. Pathogen factors | Resistant (+) versus non-resistant pathogens42 |
| 2. Disease factors | Type of infection (either + or −)35 |
| Type of infection in palliative patient/end-of-life patient (either + or −)28 | |
| Severity of infection (either + or −)35 | |
| Clinical infectious disease not meeting certified diagnostic criteria (+) (e.g. VAP criteria)31 | |
| Type of surgery [emergency (+) versus elective surgery]30 | |
| 3. Patient factors | Age of patient (+)31 |
| End-of-life vignette (either + or −)28 | |
| Age of patient (−)30 | |
| 4. Professional factors | Being a consultant (−) versus other occupations38 |
| Prescriber personality traits [extraversion, more likely to choose to continue antibiotics (+); agreeableness, less likely to continue antibiotics (−)]38 | |
| Profession of healthcare provider [nurse (+) versus aesthetic technician]34 | |
| Academic career [orthopaedic surgeon (+) versus (associate) professor]26 | |
| Number of arthroplasties per month [1–10 (+) versus >10]26 | |
| 5. Hospital department factors | Type of medical specialty [surgical (+) versus general medical]25 |
| Type of surgical (sub)specialty/surgical procedure [orthopaedic, neurological, urological and gastroenterology (+)]27 | |
| Patient care department [orthopaedic surgery (+) versus obstetrics & gynaecology]34 |
(+), longer duration of antibiotic treatment; (−), shorter duration of antibiotic treatment. Text in Roman type denotes antibiotic therapy studies; text in italic type denotes antibiotic prophylaxis studies.
1. Pathogen factors
One study pointed to the influence of the resistance profile of the pathogen.42
A survey among 1461 members of the North American Emerging Infections Network (IDSA EIN) showed that 28% preferred longer durations for MDR organisms compared with a susceptible isolate of the same species.42
2. Disease factors
Four studies reported a total of five determinants related to disease factors.28,30,31,35
Infection type was reported by two studies to influence duration of antibiotic therapy.28,35 Also, severity of infection seemed to influence duration.35 Jalil et al.30 found that, compared with elective Caesarean section surgery, patients undergoing emergency surgery had a 6-fold higher risk of receiving prolonged surgical prophylaxis. Kalanuria et al.31 reported antibiotic treatment for >8 days was significantly more common in patients not meeting predefined CDC diagnostic criteria for VAP (i.e. patients with an unclear diagnosis were treated for longer than patients with a clear diagnosis).
3. Patient factors
Three patient factor determinants were described in three studies.28,30,31
Two studies reported the relation between duration and age; however, they pointed in opposite directions.30,31 Patients with VAP receiving >8 days of antibiotic treatment were older than the group receiving ≤8 days of treatment,31 while for Caesarean sections, younger mothers more often received extended prophylaxis.30 The study by Gaw et al.28 reported end-of-life vignette as a determinant of antibiotic therapy duration (e.g. 97.3% of physicians would stop antimicrobials at the end of life in patients with ‘care not deemed medically futile, not enrolled in hospice’ versus 29.3% in patients ‘enrolled in hospice, death imminent’).
4. Professional factors
Three studies reported five determinants of antibiotic therapy duration related to characteristics of the healthcare professional.26,34,38
Duration of antibiotic therapy appeared to differ between healthcare professionals’ professions,26,34,38 their personality traits38 and work experience.26
5. Hospital department factors
Three studies reported three determinants related to the type of hospital department.25,27,34
Charani and colleagues25 showed that, for acutely admitted patients, antibiotics were prescribed for longer in surgical specialties than in general medical specialties. The two other studies concluded that the orthopaedic surgery department most frequently did not adhere to guidelines for antibiotic therapy duration compared with other hospital departments.27,34
Discussion
This systematic scoping review aimed to identify behavioural factors that might prevent or enable improvements in the duration of antibiotic therapy in daily hospital practice. Although only a small number of articles were available, a wide range of determinants were found to influence the duration of antibiotic therapy in daily hospital practice.
Twenty-two papers were included in this review, providing determinants of antibiotic therapy duration derived from two types of studies. A first set of studies yielded 82 behavioural determinants; most were related to the individual healthcare professional and to the way professionals interact, previously labelled by Charani as ‘prescribing etiquette’.44 A second set of studies described differences between groups based on 17 determinants related to the type of pathogen, disease, patient, professional and/or hospital department. These determinants were mostly related to disease and patient factors.
The first type of study provides the behavioural determinants that help develop improvement interventions to effectively address these drivers of duration. The literature reported a wide range of determinants covering all domains of the comprehensive determinant checklist; improvement interventions should be equally diverse. For example, the belief that guideline-recommended therapy duration will not lead to desired patient outcomes was found to be an evident factor influencing duration. This can be addressed by offering compelling evidence on this issue, preferably by a well-respected peer or role model. Social influence also played a role; for example, junior doctors were often reluctant to alter initial prescriptions from senior doctors. This can be addressed by mobilizing support among colleagues and encouraging communication and agreement on the appropriate duration of therapy for specific patient populations, providing an opportunity for all doctors to compare their performance and for junior doctors to develop the skills to resist social pressure and to speak up.45 Other examples linking determinants and interventions are provided in Table 3.
Table 3.
Potential improvement interventions tailored to determinants: some examplesa
| Category | Determinant | Target population AND potential improvement intervention by stewardship teams |
|---|---|---|
| 1. Guideline factors | Consistency with other guidelines | Guideline developers |
| ||
| ||
| ||
| Individual health professionals | ||
| ||
| 2. Individual health professional factors | Skills needed to adhere | Individual health professionals |
| ||
| ||
| ||
| Expected outcome | Individual health professionals | |
| ||
| ||
| ||
| 3. Patient factors | (Perceived) patient and family needs/demands | Individual health professionals |
| ||
| ||
| ||
| 4. Professional interactions | Communication and influence | Professional team/wards (as a group) |
| ||
| ||
| ||
| ||
| ||
| ||
| Referral process | Professional team/wards (as a group) | |
| ||
| ||
| ||
| ||
|
Many determinants were related to ‘individual healthcare professionals’ and ‘professional interactions’, while the other Flottorp domains were less represented. The question remains: are these other domains less important or just less studied? To answer this question, future high-quality empirical studies, particularly qualitative studies, should explicitly focus on understanding the drivers of antibiotic therapy duration to achieve the most appropriate and effective improvement interventions. In this effort, overviews of determinants of professional practice, such as the Flottorp checklist, should be used to ensure that all domains are explicitly and thoroughly explored.14,19,20
The second set of studies points to specific pathogens, diseases, patients, professionals and/or hospital departments on which improvement interventions should be focused. These studies tested 50 potentially important factors influencing duration (Table S4), of which only 17 were actually related to duration. Moreover, there was little overlap in the variables tested; few determinants were tested in multiple studies. Patient age, for example, was tested in two studies,30,31 which pointed in opposite directions. Two studies showed that antibiotics were prescribed for longer than recommended in the orthopaedics department compared with other surgical departments.27,34 The difference in duration of antibiotic therapy between emergency and scheduled surgery was statistically significantly in one study30 but not in a second study.34 Overall, this second group of determinants clearly describes where duration of antibiotic therapy mainly deviates, or where it should be improved. However, the limitation of these determinants is that they do not enhance our understanding of the underlying drivers of prolonged prescribing. Therefore, there is an urgent need for studies of the first type reported in this review.
The determinants included in this article were extracted from studies conducted in different regions in the world (with differences in culture, policies and healthcare systems) and from different types of studies (e.g. prophylaxis versus non-prophylaxis studies and qualitative versus quantitative studies). A closer look at these determinants, comparing the determinants from different regions (Figure S2a), revealed that studies from Africa, the Middle East, Asia and Australia provided us with almost no behavioural determinants. Only for Europe and North America were a variety of behavioural determinants found. When comparing European studies with North American studies, we noticed a higher variation in determinants extracted from the European studies. However, for both European and North American studies, determinants were mostly found for the category ‘individual health professional factors’, followed by the category ‘professional interactions’. A closer look at the latter two categories (Figure S2b) reveals several similarities and differences. For both European and North American studies, determinants related to the subcategory communication and influence were most prevalent. However, determinants related to the team process were found only in European studies, while determinants related to the referral process were found only in the North American studies. As interesting as these observations may seem, no firm conclusion can be drawn on why different determinants were found for different regions in the world. As mentioned above, not finding determinants for certain categories does not necessarily mean that those categories play no role in the prescribing process; perhaps those domains have simply been studied less. In order to truly be able to compare determinants of different regions, new studies should be performed, using generic overviews of determinants of professional practice to ensure that all domains are explored.
Notably, a substantial proportion of studies (10/22) focused on surgical antimicrobial prophylaxis. A possible explanation is that there is sufficient evidence available on (the appropriate duration of) surgical prophylaxis, resulting in clear guidelines and protocols promoting very short durations. Therefore, it is more feasible to investigate (determinants of) adherence to these recommendations. Nevertheless, surgical prophylaxis studies have provided us with fewer behavioural determinants than therapeutic studies. When comparing the type of determinants that emerged from the prophylaxis versus non-prophylaxis studies (Figure S3a), it was noteworthy that there was a higher variation in determinants extracted from the non-prophylaxis studies. Moreover, all but one of the determinants in the ‘professional interactions’ category and all determinants in the ‘patient factors’ category came from the non-prophylaxis studies. This, as described above, has implications for the intervention to be chosen. No other relevant differences in determinants were found between prophylaxis and non-prophylaxis studies (Tables 1 and 2, Figure S3a).
When comparing quantitative studies with qualitative studies (see Figure S3b), it was most noteworthy that although there were fewer qualitative studies compared with quantitative studies, they provided us with more behavioural determinants. Also, more variation in determinants was found in qualitative studies. For both types of studies, most determinants were related to the category ‘individual health professional factors’. Determinants relating to ‘professional interactions’ were more common, however, in qualitative studies, while determinants relating to ‘patient factors’ were more common in quantitative studies.
The current study has several strengths. This is the first systematic scoping review to focus specifically on determinants that influence the duration of antibiotic therapy, providing a first step for the development of effective behavioural interventions to improve duration by targeting these determinants. To ensure that all relevant articles were included, four databases were systematically searched and articles were independently screened by two researchers. Categorization of determinants was also done by two independent researchers, with a third researcher consulted in case of ambiguity, seeking consensus. Another strength is that both quantitative and qualitative studies were included to obtain all available information on determinants affecting antibiotic therapy duration.
This study also has some limitations. We may have missed relevant studies for several reasons. First, we likely missed publications describing between-group differences as our search strategy, in line with our research question, specifically focused on finding behavioural determinants to inform improvement interventions. Second, we did not include studies prior to the year 2000 because our review was performed with the perspective of antimicrobial stewardship programmes and behavioural sciences, which are fairly new concepts.46 Moreover, the professionals and hospital context have changed significantly over the years, making results prior to the year 2000 less applicable to current practice. A second limitation is that, for pragmatic reasons, only studies in adults were included. A study similar to the current one, focusing on children, should be conducted. Third, categorizing determinants from the literature into the Flottorp checklist was sometimes challenging. We tried to solve this problem by performing the data extraction and classification independently, after which a third experienced researcher was consulted. Finally, the heterogeneity of the included studies (with respect to indication, study aims, type of analysis etc.) made it impossible to further quantify our results (e.g. in a meta-analysis). However, the main goal of this scoping review was to explore the drivers that underpin duration of antibiotic therapy, not to quantify their relative importance.
In conclusion, this systematic scoping review provides a stepping stone for the development of effective stewardship interventions to optimize antibiotic duration in daily practice, but more research is warranted. Despite the paucity of research, a wide variety of determinants have been found, showing that there are many different factors that might prevent or enable improvements in both the duration of prophylaxis and therapy, making it a challenging topic for stewardship teams. Not only must they address a wide variety of behavioural determinants by developing complex stewardship interventions to improve duration appropriateness, these interventions must also be adapted to the specific pathogen, disease, patient, professional and/or hospital department involved.
Supplementary Material
Acknowledgements
We thank Elmie Peters for her help in developing the search strategy.
Contributor Information
Robin M E Janssen, Department of Intensive Care Medicine, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Scientific Center for Quality of Healthcare (IQ healthcare), Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Radboud Center for Infectious Diseases (RCI), Radboud University Medical Center, Nijmegen, The Netherlands.
Anke J M Oerlemans, Scientific Center for Quality of Healthcare (IQ healthcare), Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Johannes G Van Der Hoeven, Department of Intensive Care Medicine, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Jaap Ten Oever, Radboud Center for Infectious Diseases (RCI), Radboud University Medical Center, Nijmegen, The Netherlands; Department of Internal Medicine, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Jeroen A Schouten, Department of Intensive Care Medicine, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Scientific Center for Quality of Healthcare (IQ healthcare), Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Radboud Center for Infectious Diseases (RCI), Radboud University Medical Center, Nijmegen, The Netherlands.
Marlies E J L Hulscher, Scientific Center for Quality of Healthcare (IQ healthcare), Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Radboud Center for Infectious Diseases (RCI), Radboud University Medical Center, Nijmegen, The Netherlands.
Funding
This work was supported in part by a research grant from the Investigator Initiated Studies Program of Merck Sharp & Dohme (grant number 58288). The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Transparency declarations
None to declare.
Author contributions
M.E.J.L.H. and J.A.S. designed the study. R.M.E.J., M.E.J.L.H. and J.A.S. developed the search strategy. R.M.E.J. and J.A.S. screened the articles. R.M.E.J., M.E.J.L.H. and J.A.S. extracted the data. Methodological quality appraisal of the included articles was performed by R.M.E.J. and A.J.M.O. All authors were involved in interpretation of the data and the writing of the report.
Supplementary data
Tables S1 to S5 and Figures S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011; 52: 1232–40. [DOI] [PubMed] [Google Scholar]
- 2. el Moussaoui R, de Borgie CA, van den Broek Pet al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. Br Med J 2006; 332: 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klompas M, Li L, Menchaca JTet al. Ultra-short-course antibiotics for patients with suspected ventilator-associated pneumonia but minimal and stable ventilator settings. Clin Infect Dis 2016; 64: 870–6. [DOI] [PubMed] [Google Scholar]
- 4. Lee RA, Centor RM, Humphrey LLet al. Appropriate use of short-course antibiotics in common infections: best practice advice from the American College of Physicians. Ann Intern Med 2021; 174: 822–7. [DOI] [PubMed] [Google Scholar]
- 5. Sawyer RG, Claridge JA, Nathens ABet al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med 2015; 372: 1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molina J, Montero-Mateos E, Praena-Segovia Jet al. Seven versus 14-days course of antibiotics for the treatment of bloodstream infections by Enterobacterales: a randomized, controlled trial. Clin Microbiol Infect 2022; 28: 550–7. [DOI] [PubMed] [Google Scholar]
- 7. Barlam TF, Cosgrove SE, Abbo LMet al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: 51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Madaras-Kelly KJ, Burk M, Caplinger Cet al. Total duration of antimicrobial therapy in veterans hospitalized with uncomplicated pneumonia: results of a national medication utilization evaluation. J Hosp Med 2016; 11: 832–9. [DOI] [PubMed] [Google Scholar]
- 9. De Santis V, Gresoiu M, Corona Aet al. Bacteraemia incidence, causative organisms and resistance patterns, antibiotic strategies and outcomes in a single university hospital ICU: continuing improvement between 2000 and 2013. J Antimicrob Chemother 2014; 70: 273–8. [DOI] [PubMed] [Google Scholar]
- 10. Tabah A, Koulenti D, Laupland Ket al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 2012; 38: 1930–45. [DOI] [PubMed] [Google Scholar]
- 11. Bouadma L, Luyt C-E, Tubach Fet al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375: 463–74. [DOI] [PubMed] [Google Scholar]
- 12. Langford BJ, Nisenbaum R, Brown KAet al. Antibiotics: easier to start than to stop? Predictors of antimicrobial stewardship recommendation acceptance. Clin Microbiol Infect 2020; 26: 1638–43. [DOI] [PubMed] [Google Scholar]
- 13. Chiotos K, Tamma PD. Antibiotics: how can we make it as easy to stop as it is to start? Clin Microbiol Infect 2020; 26: 1600–1. [DOI] [PubMed] [Google Scholar]
- 14. Flottorp SA, Oxman AD, Krause Jet al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci 2013; 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet 2003; 362: 1225–30. [DOI] [PubMed] [Google Scholar]
- 16. Hulscher ME, Grol RP, van der Meer JW. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis 2010; 10: 167–75. [DOI] [PubMed] [Google Scholar]
- 17. Hulscher ME, Prins JM. Antibiotic stewardship: does it work in hospital practice? A review of the evidence base. Clin Microbiol Infect 2017; 23: 799–805. [DOI] [PubMed] [Google Scholar]
- 18. Tricco AC, Lillie E, Zarin Wet al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–73. [DOI] [PubMed] [Google Scholar]
- 19. Damschroder LJ, Aron DC, Keith REet al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009; 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michie S, Johnston M, Abraham Cet al. Making psychological theory useful for implementing evidence based practice: a consensus approach. BMJ Qual Saf 2005; 14: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong QN, Fàbregues S, Bartlett Get al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf 2018; 34: 285–91. [Google Scholar]
- 22. Borg MA. Prolonged perioperative surgical prophylaxis within European hospitals: an exercise in uncertainty avoidance? J Antimicrob Chemother 2013; 69: 1142–4. [DOI] [PubMed] [Google Scholar]
- 23. Branch-Elliman W, Gupta K, Elwy AR. Factors influencing uptake of evidence-based antimicrobial prophylaxis guidelines for electrophysiology procedures. Am J Infect Control 2020; 48: 668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broom J, Broom A, Kirby Eet al. Improvisation versus guideline concordance in surgical antibiotic prophylaxis: a qualitative study. Infection 2018; 46: 541–8. [DOI] [PubMed] [Google Scholar]
- 25. Charani E, de Barra E, Rawson TMet al. Antibiotic prescribing in general medical and surgical specialties: a prospective cohort study. Antimicrob Resist Infect Control 2019; 8: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Çimen O, Azboy N, Çatal Bet al. Assessment of periprosthetic joint infection prevention methods amongst Turkish orthopedic surgeons in total joint replacement: a survey. Jt Dis Relat Surg 2020; 31: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Hassan M, Elnour AA, Farah FHet al. Clinical pharmacists’ review of surgical antimicrobial prophylaxis in a tertiary hospital in Abu Dhabi. Int J Clin Pharm 2015; 37: 18–22. [DOI] [PubMed] [Google Scholar]
- 28. Gaw CE, Hamilton KW, Gerber JSet al. Physician perceptions regarding antimicrobial use in end-of-life care. Infect Control Hosp Epidemiol 2018; 39: 383–90. [DOI] [PubMed] [Google Scholar]
- 29. Gul YA, Lian LH, Jabar FMet al. Antibiotic prophylaxis in elective colorectal surgery. ANZ J Surg 2002; 72: 275–8. [DOI] [PubMed] [Google Scholar]
- 30. Jalil MHA, Hammour KA, Alsous Met al. Noncompliance with surgical antimicrobial prophylaxis guidelines: a Jordanian experience in cesarean deliveries. Am J Infect Control 2018; 46: 14–9. [DOI] [PubMed] [Google Scholar]
- 31. Kalanuria AA, Fellerman D, Nyquist Pet al. Variability in diagnosis and treatment of ventilator-associated pneumonia in neurocritical care patients. Neurocrit Care 2015; 23: 44–53. [DOI] [PubMed] [Google Scholar]
- 32. Khan F, Chaudhary B, Sultan Aet al. Qualitative thematic analysis of knowledge and practices of surgical antimicrobial prophylaxis at a tertiary care teaching hospital. Surg Infect 2021; 22: 434–41. [DOI] [PubMed] [Google Scholar]
- 33. Kokado R, Hagiya H, Morii Det al. Broad-spectrum antibiotic prescriptions are discontinued unevenly throughout the week. J Hosp Infect 2019; 101: 471–4. [DOI] [PubMed] [Google Scholar]
- 34. Musmar SM, Baba H. Adherence to guidelines of antibiotic prophylactic use in surgery: a prospective cohort study in North West Bank, Palestine. BMC Surg 2014; 14: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogunleye OO, Fadare JO, Yinka-Ogunleye AFet al. Determinants of antibiotic prescribing among doctors in a Nigerian urban tertiary hospital. Hosp Pract 2019; 47: 53–8. [DOI] [PubMed] [Google Scholar]
- 36. Pandolfo AM, Horne R, Jani Yet al. Understanding decisions about antibiotic prescribing in ICU: an application of the Necessity Concerns Framework. BMJ Qual Saf 2021; 31: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rawson TM, Charani E, Moore LSPet al. Mapping the decision pathways of acute infection management in secondary care among UK medical physicians: a qualitative study. BMC Med 2016; 14: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roope LS, Buchanan J, Morrell Let al. Why do hospital prescribers continue antibiotics when it is safe to stop? Results of a choice experiment survey. BMC Med 2020; 18: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salsgiver E, Bernstein D, Simon MSet al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol 2018; 39: 316–22. [DOI] [PubMed] [Google Scholar]
- 40. Santillo M, Sivyer K, Krusche Aet al. Intervention planning for Antibiotic Review Kit (ARK): a digital and behavioural intervention to safely review and reduce antibiotic prescriptions in acute and general medicine. J Antimicrob Chemother 2019; 74: 3362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharara SL, Arbaje AI, Cosgrove SEet al. A healthcare worker and patient-informed approach to oral antibiotic decision making during the hospital-to-home transition. Infect Control Hosp Epidemiol 2021; 42: 1266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trevino SE, Babcock HM, Henderson JPet al. Perceptions and behaviours of infectious diseases physicians when managing urinary tract infections due to MDR organisms. J Antimicrob Chemother 2015; 70: 3397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Kasteren M, Kullberg BJ, De Boer Aet al. Adherence to local hospital guidelines for surgical antimicrobial prophylaxis: a multicentre audit in Dutch hospitals. J Antimicrob Chemother 2003; 51: 1389–96. [DOI] [PubMed] [Google Scholar]
- 44. Charani E, Castro-Sanchez E, Sevdalis Net al. Understanding the determinants of antimicrobial prescribing within hospitals: the role of “prescribing etiquette”. Clin Infect Dis 2013; 57: 188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kok G, Gottlieb NH, Peters G-JYet al. A taxonomy of behaviour change methods: an intervention mapping approach. Health Psychol Rev 2016; 10: 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schouten J, De Waele J. Antimicrobial stewardship in ICU. In: Pulcini C, et al. (eds). Antimicrobial Stewardship. Elsevier Academic Press, 2017; 193–203. [Google Scholar]
- 47. Johnston M, Carey RN, Connell Bohlen LEet al. Development of an online tool for linking behavior change techniques and mechanisms of action based on triangulation of findings from literature synthesis and expert consensus. Transl Behav Med 2021; 11: 1049–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michie S, Atkins L, West R. The behaviour change wheel: a guide to designing interventions. Silverback Publishing, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.